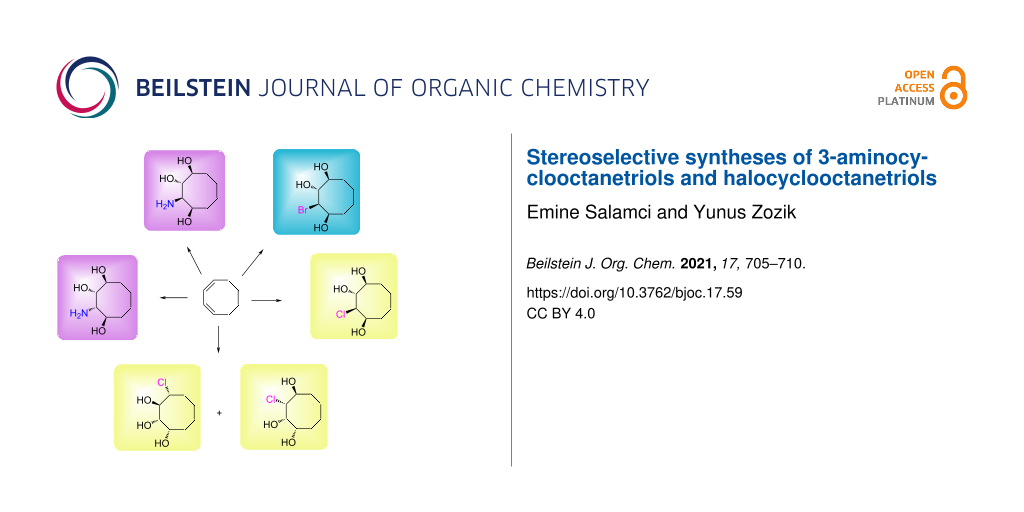
Abstract
The efficient synthesis of two new stereoisomeric 3-aminocyclooctanetriols and their new halocyclitol derivatives starting from cis,cis-1,3-cyclooctadiene are reported. Reduction of cyclooctene endoperoxide, obtained by photooxygenation of cis,cis-1,3-cyclooctadiene, with zinc yielded a cyclooctene diol followed by acetylation of the hydroxy group, which gave dioldiacetate by OsO4/NMO oxidation. The cyclooctane dioldiacetate prepared was converted to the corresponding cyclic sulfate via the formation of a cyclic sulfite in the presence of catalytic RuO4. The reaction of this cyclic sulfate with a nucleophilic azide followed by the reduction of the azide group provided the target, 3-aminocyclooctanetriol. The second key compound, bromotriol, was prepared by epoxidation of the cyclooctenediol with m-chloroperbenzoic acid followed by hydrolysis with HBr(g) in methanol. Treatment of bromotriol with NaN3 and the reduction of the azide group yielded the other desired 3-aminocyclooctanetriol. Hydrolysis of the epoxides with HCl(g) in methanol gave stereospecifically new chlorocyclooctanetriols.
Graphical Abstract
Introduction
The synthesis of aminocyclitols has attracted attention because they contain substructures of many biologically active natural products [1-3]. They have become important structural components for drug development with a modifying action as inhibitors of glycosidases [4-10]. Aminocyclitols are amino polyhydroxy cycloalkanes [2] formally derived from cyclitols [11-15], which are polyhydroxylated cycloalkanes, via replacement of one of the hydroxy groups with an amino group. Many aminocyclitols and their derivatives have been found to possess antibiotic properties, such as validamycins (1) [16]. Validamycin A (1) contains two aminocyclitol units, the one is valienamine (2) and the other is validamine (3, Figure 1).
Figure 1: Structures of some important aminocyclitols.
Figure 1: Structures of some important aminocyclitols.
One of the most important conduramines 4 is valienamine (3) [17], which is found as a building block in several aminoglycoside antibiotics [2]. Furthermore, conduramines 4 and their derivatives are used as both inhibitors of glycosidases and useful intermediates in organic synthesis [18]. Halocyclitols are also cyclitol derivatives, in which one of the hydroxy groups is replaced by a halogen. They have also attracted interest in the last decade because of their biological activities [11,19]. For instance, some brominated quercitol (cyclohexanepentol) derivatives and bromoconduritol-B act as strong inhibitors of α-glycosidases [11,19]. Recent reviews report on the latest synthetic methodologies for aminocyclitols and related compounds [1-3,16].
Many methods have been previously reported for the synthesis of aminocyclitols containing five- and six-membered rings, along with their diverse biological activities [1-3,16-26]. However, only a limited number of synthetic methods are available for the synthesis of seven- [27,28], eight- [29-38], and nine- [35] membered aminocyclitols. Therefore, we were inspired to work on the development of the first synthesis of some C8-amino- and chloro-substituted cyclitols. Recently, we developed the first synthesis of various C8-amino- [29,31] and diaminocyclitol derivatives [30]. As part of our work involving the synthesis of C8-cyclitols, we report the stereospecific syntheses of two new 3-aminocyclooctanetriols and some chlorinated C8-cyclitols starting from cis,cis-1,3-cyclooctadiene.
Results and Discussion
For the synthesis of amino- and chlorocyclitols and their derivatives, we first selected endoperoxide 5 as the starting molecule, which was prepared using a procedure described in the literature [33] (Scheme 1). Among the most relevant precursors for the synthesis of aminocyclitols are cyclic sulfates [36,37,39,40] and they have been also used in the synthesis of C8-aminocarbasugars [36,37] recently. We envisioned that aminotriol 12 could be prepared by the reaction with sodium azide of the corresponding cyclic sulfate intermediate 9, which contains the only stereocentre. The cyclic sulfate 9 could be synthesized from diacetatediol 7 [33].
For this purpose, the reduction of the endoperoxide 5 with zinc followed by acetylation of the hydroxy group and OsO4/NMO oxidation of the double bond gave diacetatediol 7 [33]. Treatment of diacetatediol 7 with thionyl chloride in pyridine gave the corresponding cyclic sulfite 8 in 95% yield (Scheme 1). Oxidation of the cyclic sulfite 8 with sodium periodate in the presence of ruthenium trichloride provided the corresponding cyclic sulfate 9 in 95% yield.
The cyclic sulfate moiety in 9 was reacted with sodium azide in DMF at 80 °C followed by acidic hydrolysis of the resulting acyclic sulfate ester to give azidotriol 10 as a single stereoisomer in 97% yield (Scheme 2). For further structural proof, the azidotriol 10 was converted into the corresponding triacetate 11 with acetic anhydride in pyridine and 4-(dimethylamino)pyridine (DMAP) (yield 76%). To determine the exact configurations of the substituents in 11, we made full assignments for the H-3 and the acetoxy protons with the help of the 1D and 2D NMR experiments. First, protons H-3 and H-1 in the triacetate 11 were irradiated separately at their resonance frequencies and the changes in the spectrum were observed. Upon irradiation at the resonance frequency of the proton H-3 at 3.87 ppm there is no change in the multiplet at 5.04–4.97 ppm. However, in the multiplet part at 5.24–5.15 ppm, some splittings disappeared. This experiment clearly shows that the proton H-3 has couplings to both acetoxy protons H-2 and H-4. Furthermore, the proton H-3 resonates as a doublet of doublets with coupling constants of J = 8.8 and 2.7 Hz, clearly indicating that H-3 and H-2 with a large coupling constant (J2,3 = 8.8 Hz) are trans to each other. The small coupling constant (J3,4 = 2.7 Hz) between H-3 and H-4 shows the cis relationship between those protons. The configuration of the azide group in 11 was also confirmed by the cross peak between the proton H-3 and the protons H-2 and H-4 in the COSY spectrum. Moreover, the fact that the proton H-3 gives positive NOE clearly indicates that the proton H-3 should have a cis configuration relative to the proton H-1. On the other hand, the fact that the proton H-1 gives a positive NOE’s clearly indicates that the proton H-1 should have a cis configuration relative to the protons H-3 and H-4. Next, the reduction of azidotriol 10 by hydrogenation afforded the target aminotriol 12 in 95% yield.
Scheme 2: Synthesis of aminocyclooctanetriol 12.
Scheme 2: Synthesis of aminocyclooctanetriol 12.
For the synthesis of the other aminocyclooctanetriol 18, the diol 6a [33] was reacted with m-CPBA to give trans-epoxide isomer 13 [33] (79% yield) as the sole product (Scheme 3). Ring opening of trans-epoxide 13 by HBr(g)–MeOH gave bromotriol 14, which is an ideal substrate for the synthesis of the aminocyclooctanetriol 18. For structural proof, bromotriol 14 was converted into the corresponding acetate 15 using Ac2O in pyridine and DMAP (81%).
Scheme 3: Synthesis of aminocyclooctanetriol 18.
Scheme 3: Synthesis of aminocyclooctanetriol 18.
Next, to introduce the azido group in a cis-configuration, the bromotriol 14 was treated with sodium azide in DMF at 100 °C to afford azidotriol 16 as a single product in 78% yield. Compound 16 was transformed into the corresponding triacetate 17 for full characterization of the structure (Scheme 3). The position of the azide group in 17 was confirmed by the help of the COSY spectrum. The diagonal peak at 3.95 ppm has cross peaks with the protons resonating at 4.96 and 5.47 ppm, respectively. Analysis of these cross peaks shows that the cross peak at 5.47 ppm is weaker. This weak correlation is due to the small coupling constant (J = 2.2 Hz). On the other hand, the resonance signal of H-3 appears as a doublet of doublets at 3.95 ppm with coupling constants of J = 8.6 and 2.2 Hz. The large coupling constant (J = 8.6 Hz) clearly supports the trans relation of the protons H-3 and H-4 and the small coupling constant (J = 2.2 Hz) the cis relation of the protons H-3 and H-2. Finally, the desired aminocyclooctanetriol 18 was obtained by hydrogenation of the azide functionality in compound 16 in 97% yield.
In the second part of this work, we turned our attention to the stereospecific synthesis of chlorocyclooctanetriol 19 starting from the trans-epoxide 13 (Scheme 4). The hydroxy groups in 19 were acetylated to give 20 for further characterization of the structure. The position of the chlorine atom in 20 was confirmed with the help of the COSY spectra. The resonance signal of H-3 appears as a doublet of doublets at 4.35 ppm with coupling constants of J = 9.0 and 2.5 Hz. The large coupling constant (J = 9.0 Hz) clearly supports the trans relation of the protons H-3 and H-2 and the small coupling constant (J = 2.5 Hz) the cis relation of the protons H-3 and H-4.
Scheme 4: Synthesis of chlorocyclooctanetriol 20.
Scheme 4: Synthesis of chlorocyclooctanetriol 20.
As an alternate method for the synthesis of a novel chlorocyclooctanetriol isomer, epoxy-diol 22, which was synthesized in our previous work [31], was hydrolysed by HCl(g) in MeOH, resulting in the formation of two chlorocyclooctanetriol isomers 23 and 24 in an 85:15 ratio (1H NMR) in 96% combined yield (Scheme 5). Chlorotriols 23 and 24 were transformed into the corresponding triacetates 25 and 26 for full characterization of their structures. A mixture of isomeric triacetates 25 and 26 was isolated by column chromatography in 74% and 12% yields, respectively. The structures and configurations of these compounds were assigned using 1H NMR and 2D NMR spectroscopic data. The position of the chlorine atom in 25 was confirmed with the help of the COSY spectra. The diagonal peak at 4.26 ppm has cross peaks with the protons resonating at 2.15 and 5.62 ppm, respectively. Analysis of these cross peaks shows that the cross peak at 5.62 ppm is strong. This strong correlation is due to the large coupling constant (J = 8.7 Hz). The fact that the proton H-4 appears as a doublet of doublet of doublets with coupling constants of J = 13.1, J = 8.7, and 3.3 Hz also supports the trans relation (J = 8.7 Hz) of the protons H-4 and H-3. Similarly, the configuration of the chlorine atom and the acetoxy groups in 26 was determined with 1H NMR and COSY spectra. Finally, deacetylation of chlorotriacetates 25 and 26 was carried out with HCl(g) in MeOH to give the free chlorotriols derivatives 23 and 24.
Scheme 5: Synthesis of chlorocyclooctanetriols 23 and 24.
Scheme 5: Synthesis of chlorocyclooctanetriols 23 and 24.
Conclusion
The synthesis of two stereoisomeric 3-aminocyclooctanetriols 12 and 18 and their halocyclitol derivatives 14, 19, 23, and 24 was achieved for the first time concisely and efficiently from cis,cis-1,3-cyclooctadiene. The nitrogen functionalities were introduced by the substitution with NaN3 of the corresponding cyclic sulfate and bromo groups, while the halogen functionality was introduced to the molecule by opening of the epoxide ring with HBr(g) or HCl(g) in MeOH.
Supporting Information
| Supporting Information File 1: Experimental section, 1H and 13C NMR spectra for all new compounds, as well as selected 2D NMR spectra. | ||
| Format: PDF | Size: 5.9 MB | Download |
References
-
Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728
Return to citation in text: [1] [2] [3] -
Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512
Return to citation in text: [1] [2] [3] [4] [5] -
Donaldson, W. A. ARKIVOC 2018, No. 4, 231–256. doi:10.24820/ark.5550190.p010.450
Return to citation in text: [1] [2] [3] -
Ashry, E. E.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 331–348.
Return to citation in text: [1] -
Donohoe, T. J.; Flores, A.; Bataille, C. J. R.; Churruca, F. Angew. Chem., Int. Ed. 2009, 48, 6507–6510. doi:10.1002/anie.200902840
Angew. Chem. 2009, 121, 6629–6632. doi:10.1002/ange.200902840
Return to citation in text: [1] -
Griffen, J. A.; White, J. C.; Kociok-Köhn, G.; Lloyd, M. D.; Wells, A.; Arnot, T. C.; Lewis, S. E. Tetrahedron 2013, 69, 5989–5997. doi:10.1016/j.tet.2013.04.033
Return to citation in text: [1] -
Ji, L.; Zhang, D.-f.; Zhao, Q.; Hu, S.-m.; Qian, C.; Chen, X.-Z. Tetrahedron 2013, 69, 7031–7037. doi:10.1016/j.tet.2013.06.046
Return to citation in text: [1] -
Trapero, A.; González-Bulnes, P.; Butters, T. D.; Llebaria, A. J. Med. Chem. 2012, 55, 4479–4488. doi:10.1021/jm300342q
Return to citation in text: [1] -
Lo, H.-J.; Chang, Y.-K.; Yan, T.-H. Org. Lett. 2012, 14, 5896–5899. doi:10.1021/ol3028237
Return to citation in text: [1] -
Ghosal, P.; Shaw, A. K. J. Org. Chem. 2012, 77, 7627–7632. doi:10.1021/jo300804d
Return to citation in text: [1] -
Aydin, G.; Savran, T.; Aktaş, F.; Baran, A.; Balci, M. Org. Biomol. Chem. 2013, 11, 1511–1524. doi:10.1039/c3ob26909d
Return to citation in text: [1] [2] [3] -
Gültekin, M. S.; Salamci, E.; Balci, M. Carbohydr. Res. 2003, 338, 1615–1619. doi:10.1016/s0008-6215(03)00256-8
Return to citation in text: [1] -
Salamci, E.; Seçen, H.; Sütbeyaz, Y.; Balci, M. J. Org. Chem. 1997, 62, 2453–2457. doi:10.1021/jo962092+
Return to citation in text: [1] -
Salamci, E.; Seçen, H.; Sütbeyaz, Y.; Balci, M. Synth. Commun. 1997, 27, 2223–2234. doi:10.1080/00397919708003375
Return to citation in text: [1] -
Seçen, H.; Salamci, E.; Sütbeyaz, Y.; Balci, M. Synlett 1993, 609–610. doi:10.1055/s-1993-22550
Return to citation in text: [1] -
Delgado, A. Eur. J. Org. Chem. 2008, 3893–3906. doi:10.1002/ejoc.200800238
Return to citation in text: [1] [2] [3] -
Chen, X.; Fan, Y.; Zheng, Y.; Shen, Y. Chem. Rev. 2003, 103, 1955–1978. doi:10.1021/cr0102260
Return to citation in text: [1] [2] -
Kim, J.-S.; Kang, J.-C.; Yoo, G.-H.; Jin, X.; Myeong, I.-S.; Oh, C.-Y.; Ham, W.-H. Tetrahedron 2015, 71, 2772–2776. doi:10.1016/j.tet.2014.12.071
Return to citation in text: [1] [2] -
Cantekin, S.; Baran, A.; Çalışkan, R.; Balci, M. Carbohydr. Res. 2009, 344, 426–431. doi:10.1016/j.carres.2008.12.005
Return to citation in text: [1] [2] [3] -
Łysek, R.; Schütz, C.; Favre, S.; O’Sullivan, A. C.; Pillonel, C.; Krülle, T.; Vogel, P. Bioorg. Med. Chem. 2006, 14, 6255–6282. doi:10.1016/j.bmc.2006.05.080
Return to citation in text: [1] -
Harit, V. K.; Ramesh, N. G. J. Org. Chem. 2016, 81, 11574–11586. doi:10.1021/acs.joc.6b01790
Return to citation in text: [1] -
Trost, B. M.; Malhotra, S. Chem. – Eur. J. 2014, 20, 8288–8292. doi:10.1002/chem.201402175
Return to citation in text: [1] -
Rajender, A.; Rao, B. V. Tetrahedron Lett. 2013, 54, 2329–2331. doi:10.1016/j.tetlet.2013.02.046
Return to citation in text: [1] -
Łysek, R.; Favre, S.; Vogel, P. Tetrahedron 2007, 63, 6558–6572. doi:10.1016/j.tet.2007.03.149
Return to citation in text: [1] -
Li, Q. R.; Kim, S. I.; Park, S. J.; Yang, H. R.; Baek, A. R.; Kim, I. S.; Jung, Y. H. Tetrahedron 2013, 69, 10384–10390. doi:10.1016/j.tet.2013.09.098
Return to citation in text: [1] -
Ekmekci, Z.; Balci, M. Eur. J. Org. Chem. 2012, 4988–4995. doi:10.1002/ejoc.201200582
Return to citation in text: [1] -
Shing, T. K. M.; Wong, W. F.; Ikeno, T.; Yamada, T. Org. Lett. 2007, 9, 207–209. doi:10.1021/ol062621o
Return to citation in text: [1] -
Gravier-Pelletier, C.; Maton, W.; Dintinger, T.; Tellier, C.; Le Merrer, Y. Tetrahedron 2003, 59, 8705–8720. doi:10.1016/j.tet.2003.09.049
Return to citation in text: [1] -
Karavaizoglu, U. N.; Salamci, E. New J. Chem. 2020, 44, 17976–17983. doi:10.1039/d0nj02697b
Return to citation in text: [1] [2] -
Zozik, Y.; Salamci, E.; Kilic, A. Tetrahedron Lett. 2017, 58, 4822–4826. doi:10.1016/j.tetlet.2017.11.014
Return to citation in text: [1] [2] -
Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060
Return to citation in text: [1] [2] [3] -
Kaya, A. A.; Salamci, E.; Menzek, A.; Erdem, S. S.; Şahin, E.; Ecer, K. Tetrahedron 2017, 73, 5381–5388. doi:10.1016/j.tet.2017.07.040
Return to citation in text: [1] -
Salamci, E. Tetrahedron 2010, 66, 4010–4015. doi:10.1016/j.tet.2010.04.052
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Andriuzzi, O.; Gravier-Pelletier, C.; Vogel, P.; Le Merrer, Y. Tetrahedron 2005, 61, 7094–7104. doi:10.1016/j.tet.2005.05.066
Return to citation in text: [1] -
Mehta, G.; Mohanrao, R.; Katukojvala, S.; Landais, Y.; Sen, S. Tetrahedron Lett. 2011, 52, 2893–2897. doi:10.1016/j.tetlet.2011.03.141
Return to citation in text: [1] [2] -
Andriuzzi, O.; Gravier-Pelletier, C.; Le Merrer, Y. Tetrahedron Lett. 2004, 45, 8043–8046. doi:10.1016/j.tetlet.2004.08.172
Return to citation in text: [1] [2] [3] -
Andriuzzi, O.; Gravier-Pelletier, C.; Bertho, G.; Prange, T.; Le Merrer, Y. Beilstein J. Org. Chem. 2005, 1, No. 12. doi:10.1186/1860-5397-1-12
Return to citation in text: [1] [2] [3] -
Grabowski, S.; Armbruster, J.; Prinzbach, H. Tetrahedron Lett. 1997, 38, 5485–5488. doi:10.1016/s0040-4039(97)01227-6
Return to citation in text: [1] -
Byun, H.-S.; He, L.; Bittman, R. Tetrahedron 2000, 56, 7051–7091. doi:10.1016/s0040-4020(00)00494-4
Return to citation in text: [1] -
Zou, J.; Ni, G.; Tang, J.; Yu, J.; Jiang, L.; Ju, D.; Zhang, F.; Chen, S. Eur. J. Org. Chem. 2018, 5044–5053. doi:10.1002/ejoc.201800658
Return to citation in text: [1]
| 1. | Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728 |
| 2. | Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512 |
| 3. | Donaldson, W. A. ARKIVOC 2018, No. 4, 231–256. doi:10.24820/ark.5550190.p010.450 |
| 35. | Mehta, G.; Mohanrao, R.; Katukojvala, S.; Landais, Y.; Sen, S. Tetrahedron Lett. 2011, 52, 2893–2897. doi:10.1016/j.tetlet.2011.03.141 |
| 11. | Aydin, G.; Savran, T.; Aktaş, F.; Baran, A.; Balci, M. Org. Biomol. Chem. 2013, 11, 1511–1524. doi:10.1039/c3ob26909d |
| 12. | Gültekin, M. S.; Salamci, E.; Balci, M. Carbohydr. Res. 2003, 338, 1615–1619. doi:10.1016/s0008-6215(03)00256-8 |
| 13. | Salamci, E.; Seçen, H.; Sütbeyaz, Y.; Balci, M. J. Org. Chem. 1997, 62, 2453–2457. doi:10.1021/jo962092+ |
| 14. | Salamci, E.; Seçen, H.; Sütbeyaz, Y.; Balci, M. Synth. Commun. 1997, 27, 2223–2234. doi:10.1080/00397919708003375 |
| 15. | Seçen, H.; Salamci, E.; Sütbeyaz, Y.; Balci, M. Synlett 1993, 609–610. doi:10.1055/s-1993-22550 |
| 29. | Karavaizoglu, U. N.; Salamci, E. New J. Chem. 2020, 44, 17976–17983. doi:10.1039/d0nj02697b |
| 31. | Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060 |
| 2. | Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512 |
| 27. | Shing, T. K. M.; Wong, W. F.; Ikeno, T.; Yamada, T. Org. Lett. 2007, 9, 207–209. doi:10.1021/ol062621o |
| 28. | Gravier-Pelletier, C.; Maton, W.; Dintinger, T.; Tellier, C.; Le Merrer, Y. Tetrahedron 2003, 59, 8705–8720. doi:10.1016/j.tet.2003.09.049 |
| 4. | Ashry, E. E.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 331–348. |
| 5. |
Donohoe, T. J.; Flores, A.; Bataille, C. J. R.; Churruca, F. Angew. Chem., Int. Ed. 2009, 48, 6507–6510. doi:10.1002/anie.200902840
Angew. Chem. 2009, 121, 6629–6632. doi:10.1002/ange.200902840 |
| 6. | Griffen, J. A.; White, J. C.; Kociok-Köhn, G.; Lloyd, M. D.; Wells, A.; Arnot, T. C.; Lewis, S. E. Tetrahedron 2013, 69, 5989–5997. doi:10.1016/j.tet.2013.04.033 |
| 7. | Ji, L.; Zhang, D.-f.; Zhao, Q.; Hu, S.-m.; Qian, C.; Chen, X.-Z. Tetrahedron 2013, 69, 7031–7037. doi:10.1016/j.tet.2013.06.046 |
| 8. | Trapero, A.; González-Bulnes, P.; Butters, T. D.; Llebaria, A. J. Med. Chem. 2012, 55, 4479–4488. doi:10.1021/jm300342q |
| 9. | Lo, H.-J.; Chang, Y.-K.; Yan, T.-H. Org. Lett. 2012, 14, 5896–5899. doi:10.1021/ol3028237 |
| 10. | Ghosal, P.; Shaw, A. K. J. Org. Chem. 2012, 77, 7627–7632. doi:10.1021/jo300804d |
| 29. | Karavaizoglu, U. N.; Salamci, E. New J. Chem. 2020, 44, 17976–17983. doi:10.1039/d0nj02697b |
| 30. | Zozik, Y.; Salamci, E.; Kilic, A. Tetrahedron Lett. 2017, 58, 4822–4826. doi:10.1016/j.tetlet.2017.11.014 |
| 31. | Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060 |
| 32. | Kaya, A. A.; Salamci, E.; Menzek, A.; Erdem, S. S.; Şahin, E.; Ecer, K. Tetrahedron 2017, 73, 5381–5388. doi:10.1016/j.tet.2017.07.040 |
| 33. | Salamci, E. Tetrahedron 2010, 66, 4010–4015. doi:10.1016/j.tet.2010.04.052 |
| 34. | Andriuzzi, O.; Gravier-Pelletier, C.; Vogel, P.; Le Merrer, Y. Tetrahedron 2005, 61, 7094–7104. doi:10.1016/j.tet.2005.05.066 |
| 35. | Mehta, G.; Mohanrao, R.; Katukojvala, S.; Landais, Y.; Sen, S. Tetrahedron Lett. 2011, 52, 2893–2897. doi:10.1016/j.tetlet.2011.03.141 |
| 36. | Andriuzzi, O.; Gravier-Pelletier, C.; Le Merrer, Y. Tetrahedron Lett. 2004, 45, 8043–8046. doi:10.1016/j.tetlet.2004.08.172 |
| 37. | Andriuzzi, O.; Gravier-Pelletier, C.; Bertho, G.; Prange, T.; Le Merrer, Y. Beilstein J. Org. Chem. 2005, 1, No. 12. doi:10.1186/1860-5397-1-12 |
| 38. | Grabowski, S.; Armbruster, J.; Prinzbach, H. Tetrahedron Lett. 1997, 38, 5485–5488. doi:10.1016/s0040-4039(97)01227-6 |
| 11. | Aydin, G.; Savran, T.; Aktaş, F.; Baran, A.; Balci, M. Org. Biomol. Chem. 2013, 11, 1511–1524. doi:10.1039/c3ob26909d |
| 19. | Cantekin, S.; Baran, A.; Çalışkan, R.; Balci, M. Carbohydr. Res. 2009, 344, 426–431. doi:10.1016/j.carres.2008.12.005 |
| 1. | Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728 |
| 2. | Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512 |
| 3. | Donaldson, W. A. ARKIVOC 2018, No. 4, 231–256. doi:10.24820/ark.5550190.p010.450 |
| 16. | Delgado, A. Eur. J. Org. Chem. 2008, 3893–3906. doi:10.1002/ejoc.200800238 |
| 18. | Kim, J.-S.; Kang, J.-C.; Yoo, G.-H.; Jin, X.; Myeong, I.-S.; Oh, C.-Y.; Ham, W.-H. Tetrahedron 2015, 71, 2772–2776. doi:10.1016/j.tet.2014.12.071 |
| 1. | Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728 |
| 2. | Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512 |
| 3. | Donaldson, W. A. ARKIVOC 2018, No. 4, 231–256. doi:10.24820/ark.5550190.p010.450 |
| 16. | Delgado, A. Eur. J. Org. Chem. 2008, 3893–3906. doi:10.1002/ejoc.200800238 |
| 17. | Chen, X.; Fan, Y.; Zheng, Y.; Shen, Y. Chem. Rev. 2003, 103, 1955–1978. doi:10.1021/cr0102260 |
| 18. | Kim, J.-S.; Kang, J.-C.; Yoo, G.-H.; Jin, X.; Myeong, I.-S.; Oh, C.-Y.; Ham, W.-H. Tetrahedron 2015, 71, 2772–2776. doi:10.1016/j.tet.2014.12.071 |
| 19. | Cantekin, S.; Baran, A.; Çalışkan, R.; Balci, M. Carbohydr. Res. 2009, 344, 426–431. doi:10.1016/j.carres.2008.12.005 |
| 20. | Łysek, R.; Schütz, C.; Favre, S.; O’Sullivan, A. C.; Pillonel, C.; Krülle, T.; Vogel, P. Bioorg. Med. Chem. 2006, 14, 6255–6282. doi:10.1016/j.bmc.2006.05.080 |
| 21. | Harit, V. K.; Ramesh, N. G. J. Org. Chem. 2016, 81, 11574–11586. doi:10.1021/acs.joc.6b01790 |
| 22. | Trost, B. M.; Malhotra, S. Chem. – Eur. J. 2014, 20, 8288–8292. doi:10.1002/chem.201402175 |
| 23. | Rajender, A.; Rao, B. V. Tetrahedron Lett. 2013, 54, 2329–2331. doi:10.1016/j.tetlet.2013.02.046 |
| 24. | Łysek, R.; Favre, S.; Vogel, P. Tetrahedron 2007, 63, 6558–6572. doi:10.1016/j.tet.2007.03.149 |
| 25. | Li, Q. R.; Kim, S. I.; Park, S. J.; Yang, H. R.; Baek, A. R.; Kim, I. S.; Jung, Y. H. Tetrahedron 2013, 69, 10384–10390. doi:10.1016/j.tet.2013.09.098 |
| 26. | Ekmekci, Z.; Balci, M. Eur. J. Org. Chem. 2012, 4988–4995. doi:10.1002/ejoc.201200582 |
| 2. | Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393–2418. doi:10.2174/092986710791698512 |
| 17. | Chen, X.; Fan, Y.; Zheng, Y.; Shen, Y. Chem. Rev. 2003, 103, 1955–1978. doi:10.1021/cr0102260 |
| 11. | Aydin, G.; Savran, T.; Aktaş, F.; Baran, A.; Balci, M. Org. Biomol. Chem. 2013, 11, 1511–1524. doi:10.1039/c3ob26909d |
| 19. | Cantekin, S.; Baran, A.; Çalışkan, R.; Balci, M. Carbohydr. Res. 2009, 344, 426–431. doi:10.1016/j.carres.2008.12.005 |
| 36. | Andriuzzi, O.; Gravier-Pelletier, C.; Le Merrer, Y. Tetrahedron Lett. 2004, 45, 8043–8046. doi:10.1016/j.tetlet.2004.08.172 |
| 37. | Andriuzzi, O.; Gravier-Pelletier, C.; Bertho, G.; Prange, T.; Le Merrer, Y. Beilstein J. Org. Chem. 2005, 1, No. 12. doi:10.1186/1860-5397-1-12 |
| 39. | Byun, H.-S.; He, L.; Bittman, R. Tetrahedron 2000, 56, 7051–7091. doi:10.1016/s0040-4020(00)00494-4 |
| 40. | Zou, J.; Ni, G.; Tang, J.; Yu, J.; Jiang, L.; Ju, D.; Zhang, F.; Chen, S. Eur. J. Org. Chem. 2018, 5044–5053. doi:10.1002/ejoc.201800658 |
| 30. | Zozik, Y.; Salamci, E.; Kilic, A. Tetrahedron Lett. 2017, 58, 4822–4826. doi:10.1016/j.tetlet.2017.11.014 |
| 31. | Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060 |
| 36. | Andriuzzi, O.; Gravier-Pelletier, C.; Le Merrer, Y. Tetrahedron Lett. 2004, 45, 8043–8046. doi:10.1016/j.tetlet.2004.08.172 |
| 37. | Andriuzzi, O.; Gravier-Pelletier, C.; Bertho, G.; Prange, T.; Le Merrer, Y. Beilstein J. Org. Chem. 2005, 1, No. 12. doi:10.1186/1860-5397-1-12 |
© 2021 Salamci and Zozik; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)