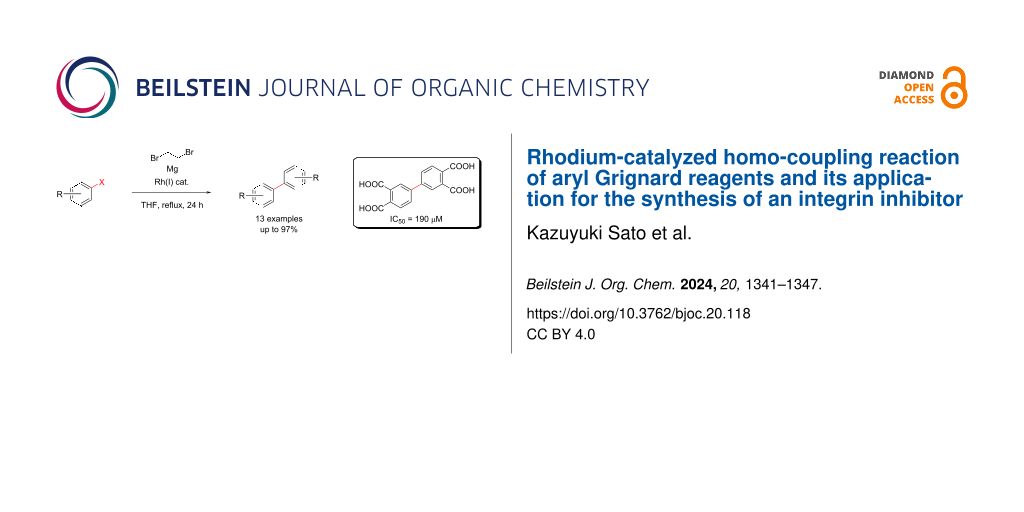
Abstract
A novel Rh-catalyzed one-pot homo-coupling reaction of aryl Grignard reagents was achieved. The reaction with bromobenzenes having an electron-donating group or a halogen substituent gave the corresponding homo-coupling products in good yields, although the reaction using heterocyclic or aliphatic bromides scarcely proceeded. A Rh(III)–bis(aryl) complex, which might be formed from RhCl(PPh3)3 and the aryl Grignard reagents, plays an important role in giving the homo-coupling products in this reaction. Furthermore, we applied the reaction to the synthesis of a novel inhibitor for integrins which is critical for several diseases.
Graphical Abstract
Introduction
The Ullmann reaction is a coupling reaction of aryl halides using copper, traditionally using metallic copper-bronze alloy, and has been used as one of the methods for obtaining homo-coupling biaryl compounds (Scheme 1) [1,2]. Starting from these works, various modified Ullmann-type coupling reactions have been developed [3,4]. However, the reaction usually required high temperatures and the yield was not very high. Therefore, alternative procedures for homo-coupling reactions using other transition-metal catalysts such as palladium, nickel, manganese, and iron have been developed [5-13]. In recent years, transition-metal-free coupling reactions have also been developed for environmentally benign synthetic applications [14-18].
Scheme 1: Ullmann and Ullmann-type homo-coupling reactions.
Scheme 1: Ullmann and Ullmann-type homo-coupling reactions.
We have successfully achieved various reactions using a Rh complex easily derived from dialkylzinc (R2Zn) and RhCl(PPh3)3 [19,20]. Additionally, in the related study, we also reported a new Rh-catalyzed C(sp3)–C(sp3) homo-coupling reaction of benzyl halides, which involved a rhodium-bis(benzyl) complex (Scheme 2) [21]. Following these outcomes, as part of a research program aimed at a wide range of Rh-catalyzed C–C bond-formation reactions, in this paper, we report a Rh-catalyzed Ullmann-type homo-coupling reaction of aryl Grignard reagents.
Scheme 2: Rh-catalyzed homo-coupling reactions.
Scheme 2: Rh-catalyzed homo-coupling reactions.
Results and Discussion
Methodology development
In our work towards Rh-catalyzed homo-coupling reactions of benzyl halides, we observed that a similar rhodium–bis(benzyl) complex can also be formed from benzyl halide by using a Grignard reagent instead R2Zn in the presence of RhCl(PPh3)3 to subsequently give the desired dibenzyl product. For example, the reaction gave the desired dibenzyl product 2a in 25% yield along with the corresponding biphenyl product 3a in 68% yield, when methyl 4-(bromomethyl)benzoate (1a) was treated with 2.0 equiv of 3-methoxyphenylmagnesium bromide in the presence of 2 mol % of RhCl(PPh3)3 in THF as shown in Scheme 3. In addition, similar reactions using 4-fluorobenzyl bromide (1b) or 4-bromobenzyl bromide (1c) gave the desired dibenzyl products 2b (85%) or 2c (90%) along with 3a in 64% or 54% yield, respectively.
Scheme 3: Rh-catalyzed homo-coupling reaction by using Grignard reagents.
Scheme 3: Rh-catalyzed homo-coupling reaction by using Grignard reagents.
Mechanistically, the benzyl halide works as an oxidizing agent, so various alkyl halides to replace the benzyl halides were investigated in this reaction (Table 1). Allyl bromide, propargyl bromide, and carbon tetrabromide did not work well as shown in entries 1, 2, and 6 in Table 1. On the other hand, other alkyl halides gave the product in moderate to good yields, especially 1,2-dibromoethane was the best oxidant. The reaction proceeded in good yield, even if 0.5 equiv of 1,2-dibromoethane were used in this reaction, as shown in Table 1, entries 7 and 8. However, it has been confirmed that this reaction did not proceed when no alkyl halide was added to the reaction mixture (Table 1, entry 10).
Next, we investigated various Rh catalysts and solvents, and the results are summarized in Table 2. All Rh catalysts that were examined in this reaction gave the product in good yields, although in the absence of a Rh catalyst the reaction failed as shown in Table 2, entries 1–8. In addition, there was no need to prolong the reaction time (entry 3 in Table 2). Therefore, a Rh catalyst is essential in this reaction, and RhCl(PPh3)3 was chosen as the best catalyst because of its availability. In the examination of the reaction solvents, the use of THF led to the highest yield. It is interesting that the reaction proceeded without adding the oxidant (1,2-dibromoethane) when 1,2-dichloroethane was used as the solvent, although a slight decrease in yield was observed (Table 2, entry 14). Based on these results, we selected RhCl(PPh3)3 as the Rh catalyst and THF as the solvent as the best conditions (Table 2, entry 1).
Table 2: Examination of various Rh catalysts and solvents.
|
|
|||||||
| entry | Rh cat (mol %) | solv. | yield (%) | entry | Rh cat. | solv. | yield (%) |
| 1 | RhCl(PPh3)3 | THF | 73 | 9 | RhCl(PPh3)3 | Et2O | 48 |
| 2 | RhCl(PPh3)3 | THF | 67 | 10 | RhCl(PPh3)3 | 1,4-dioxane | 48 |
| 3a | RhCl(PPh3)3 | THF | 67 | 11 | RhCl(PPh3)3 | toluene | 65 |
| 4 | RhCl(PPh3)3 | THF | 64 | 12 | RhCl(PPh3)3 | DCM | 66 |
| 5 | RhCl(CO)(PPh3)2 | THF | 61 | 13 | RhCl(PPh3)3 | DCE | 67 |
| 6 | [Rh(cod)Cl]2 | THF | 65 | 14b | RhCl(PPh3)3 | DCE | 58 |
| 7 | Rh(CO)2(acac) | THF | 65 | 15 | RhCl(PPh3)3 | DMF | trace |
| 8 | none | THF | trace | ||||
aThe reaction was carried out for 13 h. bThe reaction was carried out without oxidant.
As shown above, the commercially available Grignard reagent 4a gave the corresponding homo-coupled product 3a in a short reaction time at room temperature. Subsequently, for the purpose of expanding the scope of substrates, we examined the in situ preparation of the Grignard reagent followed by the homo-coupling reaction. Various conditions were examined and biphenyl (3b) was obtained in 85% yield in a one-pot reaction, when bromobenzene (5b) was treated with 1.5 equiv of Mg (turnings, grade for Grignard reaction) under reflux conditions of THF for 24 h in the presence of RhCl(PPh3)3 (Scheme 4).
Scheme 4: Rh-catalyzed one-pot Ullmann-type reaction with bromobenzene under optimized reaction conditions.
Scheme 4: Rh-catalyzed one-pot Ullmann-type reaction with bromobenzene under optimized reaction conditions.
Next, we investigated the Rh-catalyzed one-pot Ullmann-type reaction conditions by using various substrates, and the results are summarized in Figure 1. In the reaction with bromobenzenes bearing an electron-donating group such as bromotoluene and bromoanisole, the corresponding homo-coupling products 3a, 3c–3e were obtained in good yields. In addition, reactions with substrates having halogen substituents also gave the products (3h and 3i) in moderate yields. It is interesting to note, that the reaction using 3-bromofluorobenzene (5h) gave a small amount of 3,3''-difluoro-1,1':3',1''-terphenyl (6h) as side product, that might derive from an SNAr reaction of 3h with the Grignard reagent of 5h. Moreover, bromoxylenes 5m–o also gave the corresponding products 3m–o, respectively, although the position of substituents affected the yields. On the other hand, bromothiophenes 5f and 5g, bromobenzonitriles 5j and 5k, or cyclohexyl bromide (5l) were no suitable substrates for this reaction.
Figure 1: Scope and limitations for the Rh-catalyzed one-pot Ullmann-type reaction. Conditions: a) The reaction was carried out at rt for 1–3 h without Mg. b) The side product 6h by SNAr reaction onto 3h was obtained in 8%.
Figure 1: Scope and limitations for the Rh-catalyzed one-pot Ullmann-type reaction. Conditions: a) The reacti...
Since this reaction did not proceed in the absence of a Rh catalyst, it was evident that a Rh complex is involved in this reaction, as already indicated by our previous results [21,22]. Consequently, we propose the reaction mechanism as shown in Figure 2. In the initial step, the Rh catalyst reacts with the Grignard reagent 4 to give the Rh(I)–aryl complex 7. Oxidative addition of 1,2-dibromoethane onto complex 7 then generates Rh(III)–aryl complex 8 along with elimination of ethylene. Further transmetalation between the complex 8 and another Grignard reagent gives Rh(III)–bis(aryl) complex 9. Finally, reductive elimination affords the desired homo-coupling product 3 and regenerates the Rh catalyst. We did not identify any cross-coupling products such as (2-bromoethyl)arenes or styrenes in this reaction. Unfortunately, we have not clarified the reason why a cross-coupling reaction did not proceed. At this stage, we speculate that the elimination rate of ethylene and reductive elimination rate of 3 might be fast in this reaction.
Medicinal chemistry application
Integrins are transmembrane heterodimers, each consisting of α and β subunits, that mediate cell–cell and cell–matrix adhesion involved in normal and pathological processes. Bidirectional signaling through integrins regulate cell shape, motility, and cell cycle progression [23]. The integrin complexes are highly important for performing various cellular functions, such as cell proliferation, migration, and morphological changes [24,25]. Consequently, integrin inhibitors have received great attention as novel candidates for the treatment of several intractable diseases [26-28].
In the process of developing novel inhibitors of integrin function, we identified a drug candidate (10n) through high throughput screening (HTS) that inhibits the integrin complex formation, which is an important step for integrin activation. The binding inhibitor 10n was effective as IC50 of 190 μM in AlphaScreen system, and we thought that our homo-coupling reaction would be optimal for the synthesis of compound 10n. Indeed, compound 5n was successfully synthesized by the Rh-catalyzed homo-coupling reaction and subsequently oxidized by KMnO4 to give the desired product 10n in 38% yield over two steps (Scheme 5) [29].
Scheme 5: Synthesis of compound 10n as a candidate for an integrin inhibitor.
Scheme 5: Synthesis of compound 10n as a candidate for an integrin inhibitor.
Conclusion
In conclusion, we have developed a novel Rh-catalyzed one-pot Ullmann-type homo-coupling reaction of Grignard reagents. The use of commercially available Grignard reagents gave the corresponding homo-coupling products even when carried out at room temperature and short reaction time. However, since the preparation of Grignard reagents derived from haloarenes with Mg in situ requires heating for a long time, we decided to use heating conditions for the one-pot homo-coupling reaction. Most homo-coupling products were obtained in this reaction, although the product yield decreased when using substrates that are difficult to react with Mg to give the corresponding Grignard reagents. Furthermore, we successfully synthesized a candidate of integrin inhibitor by using the herein developed Rh-catalyzed Ullmann-type homo-coupling reaction as the key step. The integrin inhibitor was effective at an IC50 of 190 μM in an AlphaScreen system, and we believe that further QSAR studies of analogs of the inhibitor will lead to the discovery of novel potential therapeutics for the treatment of several intractable diseases.
Supporting Information
| Supporting Information File 1: General procedures and analytical data, including copies of 1H NMR and 13C NMR spectra. | ||
| Format: PDF | Size: 1.3 MB | Download |
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Ullmann, F.; Bielecki, J. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. doi:10.1002/cber.190103402141
Return to citation in text: [1] -
Fanta, P. E. Synthesis 1974, 9–21. doi:10.1055/s-1974-23219
Return to citation in text: [1] -
Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r
Return to citation in text: [1] -
Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c
Return to citation in text: [1] -
Yu, M.; Tang, R.-Y.; Li, J.-H. Tetrahedron 2009, 65, 3409–3416. doi:10.1016/j.tet.2009.02.040
Return to citation in text: [1] -
Zhou, Z.; Xue, W. J. Organomet. Chem. 2009, 694, 599–603. doi:10.1016/j.jorganchem.2008.12.021
Return to citation in text: [1] -
Toummini, D.; Ouazzani, F.; Taillefer, M. Org. Lett. 2013, 15, 4690–4693. doi:10.1021/ol401987s
Return to citation in text: [1] -
Karimi, B.; Behzadnia, H.; Vali, H. ChemCatChem 2014, 6, 745–748. doi:10.1002/cctc.201300893
Return to citation in text: [1] -
Bhat, A. P. I.; Bhat, B. R. Appl. Organomet. Chem. 2014, 28, 383–388. doi:10.1002/aoc.3130
Return to citation in text: [1] -
Li, X.; Li, D.; Li, Y.; Chang, H.; Gao, W.; Wei, W. RSC Adv. 2016, 6, 86998–87002. doi:10.1039/c6ra17859f
Return to citation in text: [1] -
Lv, L.; Qiu, Z.; Li, J.; Liu, M.; Li, C.-J. Nat. Commun. 2018, 9, 4739. doi:10.1038/s41467-018-07198-7
Return to citation in text: [1] -
Liu, Y.; Bergès, J.; Zaid, Y.; Chahdi, F. O.; Van Der Lee, A.; Harakat, D.; Clot, E.; Jaroschik, F.; Taillefer, M. J. Org. Chem. 2019, 84, 4413–4420. doi:10.1021/acs.joc.8b02834
Return to citation in text: [1] -
Bao, F.; Liu, Z.; Bai, H.; Zhang, H.; Liu, P.; Zhang, Q.; Chai, G. Synlett 2020, 31, 1501–1506. doi:10.1055/s-0040-1707214
Return to citation in text: [1] -
Maji, M. S.; Pfeifer, T.; Studer, A. Angew. Chem., Int. Ed. 2008, 47, 9547–9550. doi:10.1002/anie.200804197
Return to citation in text: [1] -
Maji, M. S.; Studer, A. Synthesis 2009, 2467–2470. doi:10.1055/s-0029-1216859
Return to citation in text: [1] -
Korenaga, T.; Nitatori, K.; Muraoka, H.; Ogawa, S.; Shimada, K. Org. Lett. 2015, 17, 5500–5503. doi:10.1021/acs.orglett.5b02887
Return to citation in text: [1] -
Amaya, T.; Suzuki, R.; Hirao, T. Chem. Commun. 2016, 52, 7790–7793. doi:10.1039/c6cc03053j
Return to citation in text: [1] -
Korenaga, T.; Nitatori, K.; Muraoka, H.; Ogawa, S.; Shimada, K. J. Org. Chem. 2018, 83, 4835–4839. doi:10.1021/acs.joc.8b00299
Return to citation in text: [1] -
Sato, K.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Synthesis 2010, 1865–1882. doi:10.1055/s-0029-1218745
Return to citation in text: [1] -
Sato, K.; Isoda, M.; Tarui, A.; Omote, M. Eur. J. Org. Chem. 2020, 6503–6511. doi:10.1002/ejoc.202001041
Return to citation in text: [1] -
Sato, K.; Inoue, Y.; Mori, T.; Sakaue, A.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Org. Lett. 2014, 16, 3756–3759. doi:10.1021/ol501619w
Return to citation in text: [1] [2] -
Sato, K.; Yuki, T.; Yamaguchi, R.; Hamano, T.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. J. Org. Chem. 2009, 74, 3815–3819. doi:10.1021/jo9004348
Return to citation in text: [1] -
Ojaniemi, M.; Vuori, K. J. Biol. Chem. 1997, 272, 25993–25998. doi:10.1074/jbc.272.41.25993
Return to citation in text: [1] -
Kawai, K.; Tomonou, M.; Machida, Y.; Karuo, Y.; Tarui, A.; Sato, K.; Ikeda, Y.; Kinashi, T.; Omote, M. Mol. Inf. 2021, 40, 2060040. doi:10.1002/minf.202060040
Return to citation in text: [1] -
Lu, F.; Zhu, L.; Bromberger, T.; Yang, J.; Yang, Q.; Liu, J.; Plow, E. F.; Moser, M.; Qin, J. Nat. Commun. 2022, 13, 2362. doi:10.1038/s41467-022-30117-w
Return to citation in text: [1] -
Fox, K. M.; Krikler, D. M. B. Br. Heart J. 1986, 56, 398–399. doi:10.1136/hrt.56.5.398
Return to citation in text: [1] -
Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N. V.; Monjezi, M. R.; Ilbeigi, S.; Alahari, S. K. Oncogene 2021, 40, 1043–1063. doi:10.1038/s41388-020-01588-2
Return to citation in text: [1] -
Chen, Y.; Yang, S.; Tavormina, J.; Tampe, D.; Zeisberg, M.; Wang, H.; Mahadevan, K. K.; Wu, C.-J.; Sugimoto, H.; Chang, C.-C.; Jenq, R. R.; McAndrews, K. M.; Kalluri, R. Cancer Cell 2022, 40, 818–834. doi:10.1016/j.ccell.2022.06.011
Return to citation in text: [1] -
Hayashi, T. Method for producing biphenyl-3,4,3’,4’-tetracarboxylic acid. Japanese Patent Application JP 2009079008 A, April 16, 2009.
Return to citation in text: [1]
| 1. | Ullmann, F.; Bielecki, J. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. doi:10.1002/cber.190103402141 |
| 2. | Fanta, P. E. Synthesis 1974, 9–21. doi:10.1055/s-1974-23219 |
| 19. | Sato, K.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Synthesis 2010, 1865–1882. doi:10.1055/s-0029-1218745 |
| 20. | Sato, K.; Isoda, M.; Tarui, A.; Omote, M. Eur. J. Org. Chem. 2020, 6503–6511. doi:10.1002/ejoc.202001041 |
| 14. | Maji, M. S.; Pfeifer, T.; Studer, A. Angew. Chem., Int. Ed. 2008, 47, 9547–9550. doi:10.1002/anie.200804197 |
| 15. | Maji, M. S.; Studer, A. Synthesis 2009, 2467–2470. doi:10.1055/s-0029-1216859 |
| 16. | Korenaga, T.; Nitatori, K.; Muraoka, H.; Ogawa, S.; Shimada, K. Org. Lett. 2015, 17, 5500–5503. doi:10.1021/acs.orglett.5b02887 |
| 17. | Amaya, T.; Suzuki, R.; Hirao, T. Chem. Commun. 2016, 52, 7790–7793. doi:10.1039/c6cc03053j |
| 18. | Korenaga, T.; Nitatori, K.; Muraoka, H.; Ogawa, S.; Shimada, K. J. Org. Chem. 2018, 83, 4835–4839. doi:10.1021/acs.joc.8b00299 |
| 5. | Yu, M.; Tang, R.-Y.; Li, J.-H. Tetrahedron 2009, 65, 3409–3416. doi:10.1016/j.tet.2009.02.040 |
| 6. | Zhou, Z.; Xue, W. J. Organomet. Chem. 2009, 694, 599–603. doi:10.1016/j.jorganchem.2008.12.021 |
| 7. | Toummini, D.; Ouazzani, F.; Taillefer, M. Org. Lett. 2013, 15, 4690–4693. doi:10.1021/ol401987s |
| 8. | Karimi, B.; Behzadnia, H.; Vali, H. ChemCatChem 2014, 6, 745–748. doi:10.1002/cctc.201300893 |
| 9. | Bhat, A. P. I.; Bhat, B. R. Appl. Organomet. Chem. 2014, 28, 383–388. doi:10.1002/aoc.3130 |
| 10. | Li, X.; Li, D.; Li, Y.; Chang, H.; Gao, W.; Wei, W. RSC Adv. 2016, 6, 86998–87002. doi:10.1039/c6ra17859f |
| 11. | Lv, L.; Qiu, Z.; Li, J.; Liu, M.; Li, C.-J. Nat. Commun. 2018, 9, 4739. doi:10.1038/s41467-018-07198-7 |
| 12. | Liu, Y.; Bergès, J.; Zaid, Y.; Chahdi, F. O.; Van Der Lee, A.; Harakat, D.; Clot, E.; Jaroschik, F.; Taillefer, M. J. Org. Chem. 2019, 84, 4413–4420. doi:10.1021/acs.joc.8b02834 |
| 13. | Bao, F.; Liu, Z.; Bai, H.; Zhang, H.; Liu, P.; Zhang, Q.; Chai, G. Synlett 2020, 31, 1501–1506. doi:10.1055/s-0040-1707214 |
| 3. | Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r |
| 4. | Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c |
| 24. | Kawai, K.; Tomonou, M.; Machida, Y.; Karuo, Y.; Tarui, A.; Sato, K.; Ikeda, Y.; Kinashi, T.; Omote, M. Mol. Inf. 2021, 40, 2060040. doi:10.1002/minf.202060040 |
| 25. | Lu, F.; Zhu, L.; Bromberger, T.; Yang, J.; Yang, Q.; Liu, J.; Plow, E. F.; Moser, M.; Qin, J. Nat. Commun. 2022, 13, 2362. doi:10.1038/s41467-022-30117-w |
| 29. | Hayashi, T. Method for producing biphenyl-3,4,3’,4’-tetracarboxylic acid. Japanese Patent Application JP 2009079008 A, April 16, 2009. |
| 23. | Ojaniemi, M.; Vuori, K. J. Biol. Chem. 1997, 272, 25993–25998. doi:10.1074/jbc.272.41.25993 |
| 21. | Sato, K.; Inoue, Y.; Mori, T.; Sakaue, A.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Org. Lett. 2014, 16, 3756–3759. doi:10.1021/ol501619w |
| 22. | Sato, K.; Yuki, T.; Yamaguchi, R.; Hamano, T.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. J. Org. Chem. 2009, 74, 3815–3819. doi:10.1021/jo9004348 |
| 21. | Sato, K.; Inoue, Y.; Mori, T.; Sakaue, A.; Tarui, A.; Omote, M.; Kumadaki, I.; Ando, A. Org. Lett. 2014, 16, 3756–3759. doi:10.1021/ol501619w |
| 26. | Fox, K. M.; Krikler, D. M. B. Br. Heart J. 1986, 56, 398–399. doi:10.1136/hrt.56.5.398 |
| 27. | Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N. V.; Monjezi, M. R.; Ilbeigi, S.; Alahari, S. K. Oncogene 2021, 40, 1043–1063. doi:10.1038/s41388-020-01588-2 |
| 28. | Chen, Y.; Yang, S.; Tavormina, J.; Tampe, D.; Zeisberg, M.; Wang, H.; Mahadevan, K. K.; Wu, C.-J.; Sugimoto, H.; Chang, C.-C.; Jenq, R. R.; McAndrews, K. M.; Kalluri, R. Cancer Cell 2022, 40, 818–834. doi:10.1016/j.ccell.2022.06.011 |
© 2024 Sato et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.