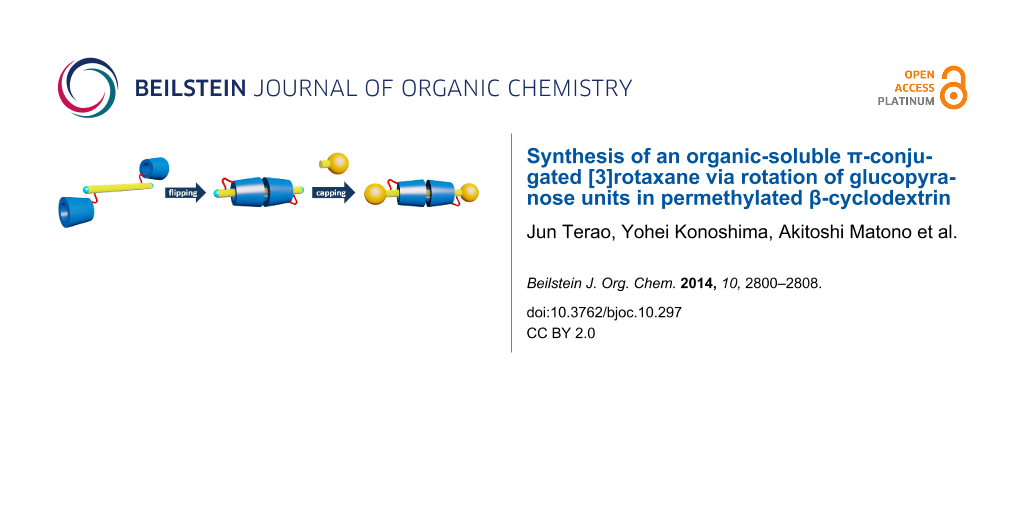
Abstract
We synthesized symmetrically insulated oligo(para-phenylene) and oligothiophene with a pseudo-linked [3]rotaxane structure by full rotation of glucopyranose units via a flipping (tumbling) mechanism in the π-conjugated guest having two permethylated β-cyclodextrin units at both ends. We also succeeded in the synthesis of an organic-soluble fixed [3]rotaxane by a cross-coupling or complexation reaction of thus formed pseudo linked [3]rotaxane. Oligo(para-phenylene), oligothiophene, and porphyrin derivatives were used as π-conjugated guests with stopper groups.

Graphical Abstract
Introduction
Insulated molecular wires (IMWs) [1,2], which feature π-conjugated polymer chains covered by protective sheaths, have attracted considerable attention as next-generation mono-molecular electronic devices because of their potential conductivity and luminescent properties [3-5]. Cyclodextrin (CD) derivatives are widely used as a protective sheath for the synthesis of IMWs because they are easily obtainable and efficient in the inclusion of various polymers into their cavity via hydrophobic interactions in water [6,7]. General methods for the synthesis of π-conjugated polymer-based IMWs using CD derivatives involve (I) threading π-conjugated polymers through CD [8,9] using a method developed by Harada for the synthesis of a polyrotaxane [10], (II) polymerization of pseudo [2]rotaxane monomer formed by self-inclusion of a π-conjugated guest with CD [11], and (III) copolymerization of the thus-formed pseudorotaxane with linker molecules [12]. These polymers are soluble in water but generally insoluble in organic solvents; this is because the hydrophilic CD covers the organic soluble π-conjugated polymer chains [13]. Uncovered sites are also randomly present because of shuttling of CDs along the π-conjugated chain and remnant water molecules; these affect the charge-transport ability and are disadvantageous for the use of these IMWs as electronic materials. Therefore, we decided to use permethylated cyclodextrin (PMCD) provided by permethylation of all the hydroxy groups of CD. However, the low solubility of PMCD in water as compared with native CDs or other randomly methylated CDs such as 2,6-dimethyl-β-cyclodextrin impedes the formation of self-inclusion complexes via hydrophobic interactions in water. To rise above this problem, we prepared organic-soluble host–guest-linked PMCD derivatives that can undergo intramolecular self-inclusion to form an insulated molecule with an [1]rotaxane structure in methanolic aqueous solutions, where PMCD derivatives are soluble in. We recently developed a new method for synthesizing π-conjugated IMWs with polyrotaxane structures via polymerization of π-conjugated [1]rotaxane monomers bearing PMCDs as macrocycles [14]. Further, we confirmed that such IMWs with poly[1]rotaxane structure are highly soluble in a variety of organic solvents and have good rigidity, photoluminescence efficiency [15], and charge mobility [16,17]. A key step for the synthesis of these IMWs is the preparation of insulated π-conjugated monomers with [1]rotaxane structures by self-inclusion of π-conjugated monomer-linked PMCDs followed by elongation of the π-conjugated units via cross-coupling in a hydrophilic solvent, such as an aqueous 50% methanol solution [18]. For this method, it is necessary that the molecular length of the π-conjugated guest is less than the internal diameter of the CD and similar to the depth of PMCD in order to form the self-inclusion complex (Figure 1b). When the π-conjugated guest is shorter than the depth of PMCD, the cross-coupling reaction is strongly inhibited because the reaction has to occur in the PMCD cavity (Figure 1a). On the other hand, a self-inclusion complex does not form if the guest is longer than the internal diameter of PMCD (Figure 1c). Thus far, we have succeeded in the synthesis of insulated π-conjugated monomers with [1]rotaxane structures using oligo(phenylene–ethylene) units, which have the appropriate length, as the π-conjugated guests for the fixation of self-inclusion complexes by elongation of the guest unit via cross-coupling (Figure 1b). However, this method can only be applied to the synthesis of insulated oligo(phenylene–ethylene) monomers. To synthesize other insulated π-conjugated molecules, we became interested in another insulation technique to replace this self-inclusion: full rotation of the glucopyranose units of methylated cyclodextrin by alteration of the relative orientation of the D-glucopyranoside rings via a flipping (tumbling) mechanism as shown in Figure 2 [19-22]. From the results of our study on the synthesis of organic-soluble π-conjugated rotaxanes, we report herein the synthesis of insulated oligo(para-phenylene) [23] and oligothiophene [24] with linked [3]rotaxane structures via the flipping phenomenon [25].
![[1860-5397-10-297-1]](/bjoc/content/figures/1860-5397-10-297-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Synthesis of [1]rotaxane by self-inclusion of a host–guest-linked molecule: a) short molecular length, b) appropriate molecular length, c) long molecular length.
Figure 1: Synthesis of [1]rotaxane by self-inclusion of a host–guest-linked molecule: a) short molecular leng...
![[1860-5397-10-297-2]](/bjoc/content/figures/1860-5397-10-297-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Synthesis of an insulated molecule via flipping phenomenon.
Figure 2: Synthesis of an insulated molecule via flipping phenomenon.
Results and Discussion
Kano and co-workers reported the synthesis of a linked pseudo [3]rotaxane involving a water-soluble porphyrin unit with carboxylate groups as the guest unit by double self-inclusion via sequential flipping of permethylated β-cyclodextrin (PM β-CD) in water [26]. To synthesize an organic-soluble insulated π-conjugated rotaxane, we applied this technique to the synthesis of an insulated porphyrin without water-soluble functional groups. The synthetic route to the PM β-CD based linked [3]rotaxane precursor with a 5,15-di([1,1'-biphenyl]-4-yl)porphyrin backbone as the guest unit is shown in Scheme 1. In order to fix the pseudo [3]rotaxane structure, we introduced two bromo groups (cross-coupling reaction point) at both terminal positions of the 5,15-di([1,1'-biphenyl]-4-yl)porphyrin unit and two PM β-CD groups at meta position to the bromo groups. 7-O-Monotosyl PM β-CD 1 was synthesized from a native β-cyclodextrin in two steps using an established protocol [27]. Reaction of 1 with 5-bromo-2-iodophenol (2) afforded benzene-linked PM β-CD 3. The 2:1 Suzuki cross-coupling reaction of thus-formed 3 with dipinacolborane porphyrin derivative 4 gave rise to precursor of pseudo [3]rotaxane 5. It should be noted that pseudo-linked [3]rotaxane 6 formed quantitatively via double self-inclusion through flipping of 5 in an aqueous 50% methanol solution.
![[1860-5397-10-297-i1]](/bjoc/content/inline/1860-5397-10-297-i1.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 1: The synthetic route to the PMβ-CD based linked [3]rotaxane with a 5,15-di([1,1'-biphenyl]-4-yl)porphyrin backbone.
Scheme 1: The synthetic route to the PMβ-CD based linked [3]rotaxane with a 5,15-di([1,1'-biphenyl]-4-yl)porp...
This unique phenomenon has been characterized by 1H NMR in different solvents (Figure 3). The 1H NMR spectrum in CD2Cl2 reveals that the 5,15-di([1,1'-biphenyl]-4-yl)porphyrin moiety is excluded from the cavity of the PM β-CD, while the 1H NMR spectrum in CD3OD reveals a mixture of exclusion and inclusion complexes. On increasing the hydrophilicity of the solvent, the formation of double inclusion complex 6 predominated. When we added D2O in order to increase hydrophilicity, the exclusion complex completely converted into inclusion complex 6 in CD3OD:D2O = 1:1 solutions.
![[1860-5397-10-297-3]](/bjoc/content/figures/1860-5397-10-297-3.png?scale=1.5&max-width=1024&background=FFFFFF)
Figure 3: The aromatic region of the 1H NMR spectra of 5 at 25 °C: 1) CDCl3, 2) CD3OD, and 3) CD3OD:D2O 1:1.
Figure 3: The aromatic region of the 1H NMR spectra of 5 at 25 °C: 1) CDCl3, 2) CD3OD, and 3) CD3OD:D2O 1:1.
To synthesize an organic-soluble linked [3]rotaxane, we then fixed this pseudo rotaxane structure, which was only present in 50% methanol aqueous solution. The capping reaction by Suzuki cross-coupling with pinacolboron derivative 7 bearing PM α-CD as the bulky stopper group was carried out under the same hydrophilic solvent conditions as in the formation of 6 (Scheme 2). The desired fixed [3]rotaxane 9 was isolated in 31% yield by preparative size exclusion chromatography using CHCl3 as the eluent. The corresponding uninsulated compound 8 as a reference was also intentionally synthesized by the reaction of 5 with 7 in hydrophobic solvent (toluene/H2O 5:1) instead of hydrophilic solvent (CH3OH/H2O 1:1). Although the MALDI–TOF mass spectrum of 8 and 9 exhibited the same signal at m/z = 6097 corresponding to [8 or 9 + Na]+, each 1H NMR spectrum of those showed the pure single product but completely different, respectively. These results suggest that we succeeded in the selective synthesis of 8 and 9 by simply changing the reaction solvent.
![[1860-5397-10-297-i2]](/bjoc/content/inline/1860-5397-10-297-i2.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 2: Selective synthesis of fixed [3]rotaxane by Suzuki cross-coupling reaction.
Scheme 2: Selective synthesis of fixed [3]rotaxane by Suzuki cross-coupling reaction.
We next applied this synthetic method to other π-conjugated guests. Scheme 3 shows the synthetic routes to precursor of PM β-CD based insulated oligothiophene. Mono-6-desmethyl PM β-CD alcohol 10 was synthesized from 1 using sodium naphthalenide [27]. The reaction of the thus-formed monoalcohol with 2-bromo-3-(bromomethyl)thiophene (11) afforded thiophene-linked PM β-CD 12. Pd-catalyzed Stille cross-coupling of 12 with bithiophene 13 bearing a silyl-protected alkynyl group afforded trithiophene-linked PM β-CD 14. After the deprotection of the triisopropyl group of 14 and the Glaser dimerization reaction of 15, symmetrical oligothiophene 16 bearing two PM β-CDs was obtained. Treatment of 16 with Na2S and KOH followed by dibromination by N-bromosuccinimide formed dibromo-heptathiophene 17 with two PMCDs.
![[1860-5397-10-297-i3]](/bjoc/content/inline/1860-5397-10-297-i3.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 3: The synthetic routes to precursor of PM β-CD based insulated oligothiophene.
Scheme 3: The synthetic routes to precursor of PM β-CD based insulated oligothiophene.
Scheme 4 shows the synthetic route to the precursor of PM β-CD-based insulated oligo(para-phenylene) 20. Sequential Suzuki cross-coupling of 3 with unsymmetrically protected benzene-1,4-diboronic acid derivative 18 and diiodobiphenyl gave rise to hexa(para-phenylene) derivative 20.
![[1860-5397-10-297-i4]](/bjoc/content/inline/1860-5397-10-297-i4.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 4: Synthesis of dibromohexa(para-phenylene) with two PMCDs 20.
Scheme 4: Synthesis of dibromohexa(para-phenylene) with two PMCDs 20.
Pseudo linked [3]rotaxanes 21 and 22 were also formed quantitatively in an aqueous 50% methanol solution via double self-inclusion through flipping of 17 and 20, respectively (Scheme 5). The formation of these inclusion complexes were also confirmed by 1H NMR spectroscopy in different solvents (Figure 4 and Figure 5, Supporting Information File 1, Figure S2). These linked [3]rotaxanes can be key monomers for synthesizing organic soluble IMWs bearing polythiophene or poly(para-phenylene) as backbone units.
![[1860-5397-10-297-i5]](/bjoc/content/inline/1860-5397-10-297-i5.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 5: Synthesis of pseudo-linked [3]rotaxanes via double self-inclusion through flipping.
Scheme 5: Synthesis of pseudo-linked [3]rotaxanes via double self-inclusion through flipping.
![[1860-5397-10-297-4]](/bjoc/content/figures/1860-5397-10-297-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: The aromatic region of the 1H NMR spectra of 5 at 25 °C: 1) CDCl3, 2) CD3OD, and CD3OD/D2O 1:1.
Figure 4: The aromatic region of the 1H NMR spectra of 5 at 25 °C: 1) CDCl3, 2) CD3OD, and CD3OD/D2O 1:1.
![[1860-5397-10-297-5]](/bjoc/content/figures/1860-5397-10-297-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The aromatic region of the 1H NMR spectra of 20 at 25 °C: 1) CDCl3, 2) CD3OD, and CD3OD/D2O 1:1.
Figure 5: The aromatic region of the 1H NMR spectra of 20 at 25 °C: 1) CDCl3, 2) CD3OD, and CD3OD/D2O 1:1.
We next fixed the pseudo rotaxane structure by complexation instead of cross-coupling reaction (Scheme 6). First, we introduced pyridyl groups at the terminal positions of hexa(para-phenylene) precursor 20 by Suzuki cross-coupling with para-pyridylboronic acid to generate 23. After confirmation of the formation of pseudo [3]rotaxane 24 via flipping of 23 in CD3OD/D2O 1:2 solution by 1H NMR analysis, we capped this pseudo [3]rotaxane structure by reacting 24 with rhodium porphyrin complex 25 in chloroform to form fixed [3]rotaxane 26 in 17% isolated yield [28]. The structure of 26 was confirmed by 1H NMR analyses in CDCl3. The results suggest that 26 maintains a [3]rotaxane structure even in organic solvents because it is capped with sterically bulky porphyrin units, which impede the reverse flipping of the PM β-CD units.
![[1860-5397-10-297-i6]](/bjoc/content/inline/1860-5397-10-297-i6.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 6: Synthesis of fixed [3]rotaxane via complexation with rhodium porphyrin.
Scheme 6: Synthesis of fixed [3]rotaxane via complexation with rhodium porphyrin.
As shown in Figure 6, the unique fixed [3]rotaxane structure of 26 was confirmed by 2D 1H ROESY NMR analysis: there are obvious nuclear Overhauser enhancements (NOEs) between the protons on the hexa(para-phenylene) unit and the H3 and H5 protons on the interior of the permethylated cyclodextrin [29]. These experimental results indicated that the rotaxane structure was constructed between PM β-CDs and para-phenylene as hosts and guest, respectively.
![[1860-5397-10-297-6]](/bjoc/content/figures/1860-5397-10-297-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Partial ROESY NMR spectrum of 26 (400 MHz, CDCl3) showing the NOEs between aromatic protons of the axial hexa(para-phenylene) and inner protons of cyclodextrins.
Figure 6: Partial ROESY NMR spectrum of 26 (400 MHz, CDCl3) showing the NOEs between aromatic protons of the ...
Conclusion
We developed the synthesis of symmetrically insulated π-conjugated molecules with pseudo linked [3]rotaxane structure via flipping of a glucopyranose unit of permethylated β-cyclodextrin. In this method, oligo(para-phenylene), oligothiophene, and porphyrin derivatives were used as π-conjugated guests. After cross-coupling or complexation of the pseudo linked [3]rotaxane with capping molecules, the fixed [3]rotaxane was isolated in chloroform solution. The formation of the fixed [3]rotaxane was confirmed by NMR analysis. Experiments are now in progress towards synthesizing IMWs bearing polythiophene or poly(para-phenylene) as backbone units by polymerization of these linked [3]rotaxanes as monomers.
Supporting Information
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 569.8 KB | Download |
References
-
Wenz, G. Inclusion Polymers; Springer Verlag, 2009. doi:10.1007/978-3-642-01410-9
Return to citation in text: [1] -
Frampton, M. J.; Anderson, H. L. Angew. Chem., Int. Ed. 2007, 46, 1028–1064. doi:10.1002/anie.200601780
Return to citation in text: [1] -
Petty, M. C. In Molecular Electronics from Principles to Practice, John Wiley & Sons: Chippenham 2007.
Return to citation in text: [1] -
Jalabert, A.; Amara, A.; Clermidy, F. In Molecular Electronics Materials, Devices and Applications, Springer: New York 2008.
Return to citation in text: [1] -
Taniguchi, M.; Nojima, Y.; Yokota, K.; Terao, J.; Sato, K.; Kambe, N.; Kawai, T. J. Am. Chem. Soc. 2006, 128, 15062–15063. doi:10.1021/ja065806z
Return to citation in text: [1] -
Wenz, G.; Han, B.-H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+
Return to citation in text: [1] -
Harada, A.; Takashima, Y.; Yamaguchi, H. Chem. Soc. Rev. 2009, 38, 875–882. doi:10.1039/b705458k
Return to citation in text: [1] -
Yoshida, K.-i.; Shimomura, T.; Ito, K.; Hayakawa, R. Langmuir 1999, 15, 910–913. doi:10.1021/la9812471
Return to citation in text: [1] -
Yamaguchi, I.; Kashiwagi, K.; Yamamoto, T. Macromol. Rapid Commun. 2004, 25, 1163–1166. doi:10.1002/marc.200400024
Return to citation in text: [1] -
Harada, A.; Li, J.; Kamachi, M. Nature 1992, 356, 325–327. doi:10.1038/356325a0
Return to citation in text: [1] -
Shinohara, K.-i.; Suzuki, T.; Kitami, T.; Yamaguchi, S. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 801–809. doi:10.1002/pola.21175
Return to citation in text: [1] -
Michels, J. J.; O'Connell, M. J.; Taylor, P. N.; Wilson, J. S.; Cacialli, F.; Anderson, H. L. Chem. – Eur. J. 2003, 9, 6167–6176. doi:10.1002/chem.200305245
Return to citation in text: [1] -
Frampton, M. J.; Sforazzini, G.; Brovelli, S.; Latini, G.; Townsend, E.; Williams, C. C.; Charas, A.; Zalewski, L.; Kaka, N. S.; Sirish, M.; Parrott, L. J.; Wilson, J. S.; Cacialli, F.; Anderson, H. L. Adv. Funct. Mater. 2008, 18, 3367–3376.
Return to citation in text: [1] -
Terao, J. Polym. Chem. 2011, 2, 2444–2452. doi:10.1039/c1py00243k
Return to citation in text: [1] -
Terao, J.; Tsuda, S.; Tanaka, Y.; Okoshi, K.; Fujihara, T.; Tsuji, Y.; Kambe, N. J. Am. Chem. Soc. 2009, 131, 16004–16005. doi:10.1021/ja9074437
Return to citation in text: [1] -
Terao, J.; Tanaka, Y.; Tsuda, S.; Kambe, N.; Taniguchi, M.; Kawai, T.; Saeki, A.; Seki, S. J. Am. Chem. Soc. 2009, 131, 18046–18047. doi:10.1021/ja908783f
Return to citation in text: [1] -
Terao, J.; Wadahama, A.; Matono, A.; Tada, T.; Watanabe, S.; Seki, S.; Fujihara, T.; Tsuji, Y. Nat. Commun. 2013, 4, 1691. doi:10.1038/ncomms2707
Return to citation in text: [1] -
Terao, J. Chem. Rec. 2011, 11, 269–285. doi:10.1002/tcr.201100009
Return to citation in text: [1] -
Jullien, L.; Canceill, J.; Lacombe, L.; Lehn, J.-M. J. Chem. Soc., Perkin Trans. 2 1994, 989–1002. doi:10.1039/p29940000989
Return to citation in text: [1] -
Yamada, T.; Fukuhara, G.; Kaneda, T. Chem. Lett. 2003, 534–535. doi:10.1246/cl.2003.534
Return to citation in text: [1] -
Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Org. Lett. 2010, 12, 1284–1286. doi:10.1021/ol1001736
Return to citation in text: [1] -
Liu, Y.; Chipot, C.; Shao, X.; Cai, W. J. Phys. Chem. C 2014, 118, 19380–19386. doi:10.1021/jp503866q
Return to citation in text: [1] -
Taylor, P. N.; O’Connell, M. J.; McNeill, L. A.; Hall, M. J.; Aplin, R. T.; Anderson, H. L. Angew. Chem., Int. Ed. 2000, 39, 3456–3460.
Return to citation in text: [1] -
Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A. J. Org. Chem. 2007, 72, 459–465.
Return to citation in text: [1] -
Yerin, A.; Wilks, E. S.; Moss, G. P.; Harada, A. Pure Appl. Chem. 2008, 80, 2041–2068.
Return to citation in text: [1] -
Nishiyabu, R.; Kano, K. Eur. J. Org. Chem. 2004, 24, 4985–4988. doi:10.1002/ejoc.200400309
Return to citation in text: [1] -
Kaneda, T.; Fujimoto, T.; Goto, J.; Asano, K.; Yasufuku, Y.; Jung, J. H.; Hosono, C.; Sakata, Y. Chem. Lett. 2002, 31, 514–515. doi:10.1246/cl.2002.514
Return to citation in text: [1] [2] -
Masai, H.; Terao, J.; Seki, S.; Nakashima, S.; Kiguchi, M.; Okoshi, K.; Fujihara, T.; Tsuji, Y. J. Am. Chem. Soc. 2014, 136, 1742–1745. doi:10.1021/ja411665k
Return to citation in text: [1] -
Fujimoto, T.; Sakata, Y.; Kaneda, T. Chem. Commun. 2000, 2143–2145. doi:10.1039/b006758j
Return to citation in text: [1]
| 27. | Kaneda, T.; Fujimoto, T.; Goto, J.; Asano, K.; Yasufuku, Y.; Jung, J. H.; Hosono, C.; Sakata, Y. Chem. Lett. 2002, 31, 514–515. doi:10.1246/cl.2002.514 |
| 26. | Nishiyabu, R.; Kano, K. Eur. J. Org. Chem. 2004, 24, 4985–4988. doi:10.1002/ejoc.200400309 |
| 27. | Kaneda, T.; Fujimoto, T.; Goto, J.; Asano, K.; Yasufuku, Y.; Jung, J. H.; Hosono, C.; Sakata, Y. Chem. Lett. 2002, 31, 514–515. doi:10.1246/cl.2002.514 |
| 1. | Wenz, G. Inclusion Polymers; Springer Verlag, 2009. doi:10.1007/978-3-642-01410-9 |
| 2. | Frampton, M. J.; Anderson, H. L. Angew. Chem., Int. Ed. 2007, 46, 1028–1064. doi:10.1002/anie.200601780 |
| 10. | Harada, A.; Li, J.; Kamachi, M. Nature 1992, 356, 325–327. doi:10.1038/356325a0 |
| 24. | Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A. J. Org. Chem. 2007, 72, 459–465. |
| 8. | Yoshida, K.-i.; Shimomura, T.; Ito, K.; Hayakawa, R. Langmuir 1999, 15, 910–913. doi:10.1021/la9812471 |
| 9. | Yamaguchi, I.; Kashiwagi, K.; Yamamoto, T. Macromol. Rapid Commun. 2004, 25, 1163–1166. doi:10.1002/marc.200400024 |
| 25. | Yerin, A.; Wilks, E. S.; Moss, G. P.; Harada, A. Pure Appl. Chem. 2008, 80, 2041–2068. |
| 6. | Wenz, G.; Han, B.-H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+ |
| 7. | Harada, A.; Takashima, Y.; Yamaguchi, H. Chem. Soc. Rev. 2009, 38, 875–882. doi:10.1039/b705458k |
| 19. | Jullien, L.; Canceill, J.; Lacombe, L.; Lehn, J.-M. J. Chem. Soc., Perkin Trans. 2 1994, 989–1002. doi:10.1039/p29940000989 |
| 20. | Yamada, T.; Fukuhara, G.; Kaneda, T. Chem. Lett. 2003, 534–535. doi:10.1246/cl.2003.534 |
| 21. | Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Org. Lett. 2010, 12, 1284–1286. doi:10.1021/ol1001736 |
| 22. | Liu, Y.; Chipot, C.; Shao, X.; Cai, W. J. Phys. Chem. C 2014, 118, 19380–19386. doi:10.1021/jp503866q |
| 3. | Petty, M. C. In Molecular Electronics from Principles to Practice, John Wiley & Sons: Chippenham 2007. |
| 4. | Jalabert, A.; Amara, A.; Clermidy, F. In Molecular Electronics Materials, Devices and Applications, Springer: New York 2008. |
| 5. | Taniguchi, M.; Nojima, Y.; Yokota, K.; Terao, J.; Sato, K.; Kambe, N.; Kawai, T. J. Am. Chem. Soc. 2006, 128, 15062–15063. doi:10.1021/ja065806z |
| 23. | Taylor, P. N.; O’Connell, M. J.; McNeill, L. A.; Hall, M. J.; Aplin, R. T.; Anderson, H. L. Angew. Chem., Int. Ed. 2000, 39, 3456–3460. |
| 16. | Terao, J.; Tanaka, Y.; Tsuda, S.; Kambe, N.; Taniguchi, M.; Kawai, T.; Saeki, A.; Seki, S. J. Am. Chem. Soc. 2009, 131, 18046–18047. doi:10.1021/ja908783f |
| 17. | Terao, J.; Wadahama, A.; Matono, A.; Tada, T.; Watanabe, S.; Seki, S.; Fujihara, T.; Tsuji, Y. Nat. Commun. 2013, 4, 1691. doi:10.1038/ncomms2707 |
| 13. | Frampton, M. J.; Sforazzini, G.; Brovelli, S.; Latini, G.; Townsend, E.; Williams, C. C.; Charas, A.; Zalewski, L.; Kaka, N. S.; Sirish, M.; Parrott, L. J.; Wilson, J. S.; Cacialli, F.; Anderson, H. L. Adv. Funct. Mater. 2008, 18, 3367–3376. |
| 12. | Michels, J. J.; O'Connell, M. J.; Taylor, P. N.; Wilson, J. S.; Cacialli, F.; Anderson, H. L. Chem. – Eur. J. 2003, 9, 6167–6176. doi:10.1002/chem.200305245 |
| 28. | Masai, H.; Terao, J.; Seki, S.; Nakashima, S.; Kiguchi, M.; Okoshi, K.; Fujihara, T.; Tsuji, Y. J. Am. Chem. Soc. 2014, 136, 1742–1745. doi:10.1021/ja411665k |
| 11. | Shinohara, K.-i.; Suzuki, T.; Kitami, T.; Yamaguchi, S. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 801–809. doi:10.1002/pola.21175 |
| 15. | Terao, J.; Tsuda, S.; Tanaka, Y.; Okoshi, K.; Fujihara, T.; Tsuji, Y.; Kambe, N. J. Am. Chem. Soc. 2009, 131, 16004–16005. doi:10.1021/ja9074437 |
| 29. | Fujimoto, T.; Sakata, Y.; Kaneda, T. Chem. Commun. 2000, 2143–2145. doi:10.1039/b006758j |
© 2014 Terao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)