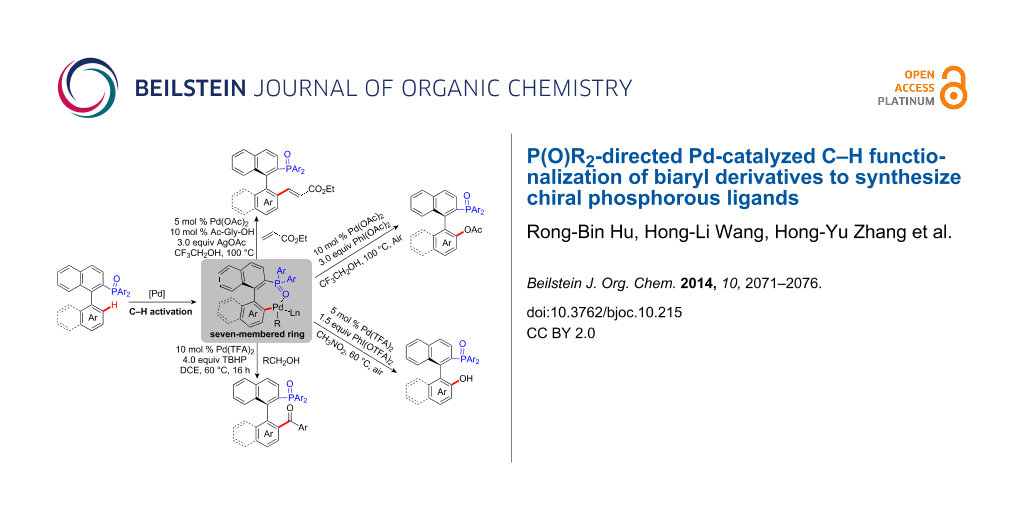
Abstract
Chiral phosphorus ligands have been widely used in transition metal-catalyzed asymmetric reactions. Herein, we report a new synthesis approach of chiral biaryls containing a phosphorus moiety using P(O)R2-directed Pd-catalyzed C–H activation; the functionalized products are produced with good enantioselectivity.
Graphical Abstract
Introduction
In the past decades, phosphorus ligands have been demonstrated to be efficient ligands in many metal-catalyzed organic reactions [1-4]. In particular, their special effects of enhancing the metal-catalyst efficiency and of controlling chiral induction has continually prompted synthetic chemists to probe efficient methods generating access to chiral, enantiomerically pure phosphorus compounds used in pharmaceutical, agrochemical and perfume industries [5-10]. However, the difficulty of synthesizing such ligands hampered their wide application, mainly due to the challenging formation of P–X (X = N, O, C…), especially C–P bond formation.
At present, the traditional strategy to introduce phosphorus atoms requires prefunctionalization or lithiation of substrate. However, these methods are not compatible for some activated functional groups in precursor compounds. Over the past several years, we have achieved reactions of C–P bond formation with new and efficient protocols via transition metal-catalysis [11-16]. Despite the progress in this area, only limited development has been accomplished through metal-catalyzed C–H activation to build C–P bonds [17,18]. As an alternative, we disclosed a novel protocol of palladium-catalyzed C–H functionalization by using the P(O)R2 moiety as a new directing group to synthesize a series of phosphorus-containing compounds in a straightforward and atom-efficient way (Scheme 1) [19-23]. In our system, we proposed a seven-membered cyclopalladium transition state instead of the common five or six-membered transition state [24-30]. The P(O)R2 group not only achieved the directing role but also acts as an important component unit of the C–H functionalized products. In this paper, we use the axially chiral biaryl phosphine oxides as substrates and report the synthesis of various chiral phosphorus ligands with high enantiomeric selectivity using palladium-catalyzed C–H functionalization.
Scheme 1: C–H functionalization of P(O)R2 directed through a seven-membered cyclopalladium transition state.
Scheme 1: C–H functionalization of P(O)R2 directed through a seven-membered cyclopalladium transition state.
Results and Discussion
To obtain the axially chiral phosphorus compounds, we first synthesized the special chiral-bridged atropisomeric monophosphorus ligand L-1 through an eight-step reaction sequence starting from 1,3-dimethoxybenzene. According to the reported operation, the substrates of biaryl derivatives that contained phosphate with axial chirality were obtained in high yields using the Suzuki–Miyaura coupling reaction with the assistance of this versatile chiral ligand [31-34]. We used substituted naphthylboronic acid or ortho-substituted-phenylboronic acid to synthesize the corresponding substituted binaphthyl or phenyl-naphthyl skeleton substrates with axial chirality. To maintain the axial chirality within the substrates, a steric hindrance effect at the ortho position of phenylboronic acids was required, rendering the non-ortho substituted-phenylboronic acid that is not applicable in these reactions. As the P(O)Ar2 group showed a better directing ability in the process of C–H activation, the axial chiral P(O)(OEt)2 4a was transformed into P(O)Ar2 by reacting with an arylgrignard reagent (Scheme 2) [32]. At the same time, the racemic substrates were produced using the non-chiral S-phos ligand. By using 2-chlorophenylboronic acid as coupling component, we demonstrated that we could obtain the phosphate compound, but it failed to yield the P(O)Ph2 group in the arylation step. In addition, in the processes of hydroxylation, arylation, alkenylation, the P(O)(iPr)2 group showed a good guiding ability, but the corresponding substrates could not be obtained because the phosphate moiety did not react with the (iPr)MgBr.
Scheme 2: Synthesis of chiral and racemic substrates.
Scheme 2: Synthesis of chiral and racemic substrates.
Under the optimized conditions, we started to investigate the scope and applicability of our strategies. Initially, we used chiral [1,1'-binaphthalen]-2-yldiphenylphosphine oxide as a substrate [35]. In the process of alkenylation and acetoxylation, the corresponding products 2a and 2b were obtained in moderate yields and high enantioselectivities. Next, we examined the substituent effect with P(O)(p-Tol)2 as a directing group: The reactions of alkenylation, acetoxylation, hydroxylation and acylation occurred smoothly. Even if the products were obtained in low to moderate yields, they were optically pure (Figure 1, 2c–f). For the substrate of 4-methoxy substituted binaphthyl, we could achieve the alkenylation product 2g in moderate yield and with high ee. When a fluorine substituent was used, the acetoxylated product 2h was obtained in moderate yield and high ee. Even if the alkenylation product 2i was obtained when the substituent was methyl, we failed to produce the desired chromatogram; however, it did exhibit a good optical rotation. Those results showed that the products of C–H functionalization were maintained with high enantioselectivities when the substrates were optically pure, even when these reactions were carried out in air atmosphere and at high temperature. Herein, we provided a method to synthesize the substituted axially chiral binaphthyl compounds with a phosphorus moiety. Moreover, these products can be further transformed into other functional groups.
Next, the substrates of the phenyl-naphthyl framework were examined. For the ortho-OMe substituted substrate, we achieved the products of alkenylation, acetoxylation and hydroxylation. The OMe group is a relatively small group, so the ee was not very high. If the substituent was OEt, the products of alkenylation and acetoxylation (Figure 1, 2m and 2n) were obtained in moderate yield and the results showed good enantioselectivities. Although the yields were not very high in these processes, the starting materials were completely converted except for the acylation reaction, presumably due to partial decomposition of the starting materials. These functionalized products showed that the axially chiral substrates could be well maintained in our system of P(O)R2-directed Pd-catalyzed C–H activation. These compounds could be transformed to trivalent phosphorus compounds by silane to obtain the corresponding phosphorous ligands.
Figure 1: C–H functionalization of axially chiral phosphorus substrates. The yields are isolated yields and the ee values are determind by HPLC. aReaction conditions: substrate (0.3 mmol), ethyl acrylate (1.5 mmol), Pd(OAc)2 (10 mol %), Ac-Gly-OH (20 mol %), AgOAc (1.5 mmol), TFE (3.0 mL), 100 °C, 24 h, air atmosphere; bSubstrate (0.3 mmol), PhI(OAc)2 (0.9 mmol), Pd(OAc)2 (10 mol %), TFE (3.0 mL), 100 °C, 24 h, air atmosphere; cSubstrate (0.3 mmol), TBHP (1.2 mmol), benzyl alcohol (0.75 mmol), Pd(TFA)2 (10 mol %), DCE (3.0 mL), 60 °C, air atmosphere; dSubstrate (0.3 mmol), PhI(TFA)2 (0.45 mmol), Pd(OAc)2 (10 mol %), MeNO2 (3.0 mL), 60 °C, 24 h, air atmosphere.
Figure 1: C–H functionalization of axially chiral phosphorus substrates. The yields are isolated yields and t...
Conclusion
In summary, a series of substrates with axially chiral biaryl compounds containing a P(O)Ar2 directing group were successfully synthesized using the Suzuki–Miyaura coupling reaction under the assistance of a chiral ligand. Moreover, the substrates were further C–H functionalized using the P(O)Ar2 directing role with Pd salt as catalyst. Notably, the reactions took place in air atmosphere and at high temperature and the corresponding functionalized products exhibited good enantioselectivities. We propose a unique seven-membered cyclopalladium transition state for this transformation and provide a new and efficient route to synthesize the substituted axially-chiral oxygen–phosphine or alkene–phosphine ligand analogues.
Supporting Information
| Supporting Information File 1: Experimental details, characterization data (1H, 13C, 31P spectra) of products. | ||
| Format: PDF | Size: 3.4 MB | Download |
References
-
Kamer, P. C. J.; van Leeuwen, P. W. N. M. Phosphorus (III) Ligands in Homogeneous Catalysis: Design and Synthesis; John Wiley & Sons: Chichester, 2012.
Return to citation in text: [1] -
Tolman, C. A. Chem. Rev. 1977, 77, 313–348. doi:10.1021/cr60307a002
Return to citation in text: [1] -
Fest, C.; Schmidt, K.-J. The chemistry of organophosphorus pesticide; Springer: Berlin, Heidelberg, 1982.
Return to citation in text: [1] -
de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, 2004.
Return to citation in text: [1] -
Jacobsen, E. N.; Pfaltz, A.; Yamamoto, H., Eds. Comprehensive Asymmetric Catalysis I- III; Springer: Berlin, 1999.
Return to citation in text: [1] -
Fernández-Pérez, H.; Etayo, P.; Panossian, A.; Vidal-Ferran, A. Chem. Rev. 2011, 111, 2119–2176. doi:10.1021/cr100244e
Return to citation in text: [1] -
Lagasse, F.; Kagan, H. B. Chem. Pharm. Bull. 2000, 48, 315–324. doi:10.1248/cpb.48.315
Return to citation in text: [1] -
Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029–3070. doi:10.1021/cr020049i
Return to citation in text: [1] -
van Leeuwen, P. W. N. M.; Kamer, P. C. J.; Claver, C.; Pàmies, O.; Diéguez, M. Chem. Rev. 2011, 111, 2077–2118. doi:10.1021/cr1002497
Return to citation in text: [1] -
Cao, Z.; Liu, Y.; Liu, Z.; Feng, X.; Zhuang, M.; Du, H. Org. Lett. 2011, 13, 2164–2167. doi:10.1021/ol200602x
Return to citation in text: [1] -
Li, Y.-M.; Sun, M.; Wang, H.-L.; Tian, Q.-P.; Yang, S.-D. Angew. Chem., Int. Ed. 2013, 52, 3972–3976. doi:10.1002/anie.201209475
Return to citation in text: [1] -
Zhang, H.-Y.; Sun, M.; Ma, Y.-N.; Tian, Q.-P.; Yang, S.-D. Org. Biomol. Chem. 2012, 10, 9627–9633. doi:10.1039/C2OB26874D
Return to citation in text: [1] -
Sun, M.; Zhang, H.-Y.; Han, Q.; Yang, K.; Yang, S.-D. Chem. – Eur. J. 2011, 17, 9566–9570. doi:10.1002/chem.201101930
Return to citation in text: [1] -
Hu, J.; Zhao, N.; Yang, B.; Wang, G.; Guo, L.-N.; Liang, Y.-M.; Yang, S.-D. Chem. – Eur. J. 2011, 17, 5516–5521. doi:10.1002/chem.201003561
Return to citation in text: [1] -
Yang, B.; Yang, T.-T.; Li, X.-A.; Wang, J.-J.; Yang, S.-D. Org. Lett. 2013, 15, 5024–5027. doi:10.1021/ol402355a
Return to citation in text: [1] -
Yang, B.; Tian, Q. P.; Yang, S. D. Chin. J. Org. Chem. 2014, 34, 717–721.
Return to citation in text: [1] -
Feng, C.-G.; Ye, M.; Xiao, K.-J.; Li, S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 9322–9325. doi:10.1021/ja404526x
Return to citation in text: [1] -
Li, C.; Yano, T.; Ishida, N.; Murakami, M. Angew. Chem., Int. Ed. 2013, 52, 9801–9804. doi:10.1002/anie.201305202
Return to citation in text: [1] -
Wang, H.-L.; Hu, R.-B.; Zhang, H.; Zhou, A.-X.; Yang, S.-D. Org. Lett. 2013, 15, 5302–5305. doi:10.1021/ol402577p
Return to citation in text: [1] -
Zhang, H.-Y.; Yi, H.-M.; Wang, G.-W.; Yang, B.; Yang, S.-D. Org. Lett. 2013, 15, 6186–6189. doi:10.1021/ol403028a
Return to citation in text: [1] -
Hu, R.-B.; Zhang, H.; Zhang, X.-Y.; Yang, S.-D. Chem. Commun. 2014, 50, 2193–2195. doi:10.1039/C3CC49050E
Return to citation in text: [1] -
Zhang, H.; Hu, R.-B.; Zhang, X.-Y.; Li, S.-X.; Yang, S.-D. Chem. Commun. 2014, 50, 4686–4689. doi:10.1039/C4CC01238K
Return to citation in text: [1] -
Ma, Y.-N.; Tian, Q.-P.; Zhang, H.-Y.; Zhou, A.-X.; Yang, S.-D. Org. Chem. Front. 2014, 1, 284–288. doi:10.1039/C4QO00005F
Return to citation in text: [1] -
Chan, L. Y.; Kim, S.; Ryu, T.; Lee, P. H. Chem. Commun. 2013, 49, 4682–4684. doi:10.1039/c3cc41107a
Return to citation in text: [1] -
Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358–3361. doi:10.1021/ol401407v
Return to citation in text: [1] -
Chary, B. C.; Kim, S.; Park, Y.; Kim, J.; Lee, P. H. Org. Lett. 2013, 15, 2692–2695. doi:10.1021/ol4009987
Return to citation in text: [1] -
Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258–3261. doi:10.1021/ol4012794
Return to citation in text: [1] -
Zhao, D.; Nimphius, C.; Lindale, M.; Glorius, F. Org. Lett. 2013, 15, 4504–4507. doi:10.1021/ol402053n
Return to citation in text: [1] -
Meng, X.; Kim, S. J. Org. Chem. 2013, 78, 11247–11254. doi:10.1021/jo401716p
Return to citation in text: [1] -
Mo, J.; Lim, S.; Park, S.; Ryu, T.; Kim, S.; Lee, P. H. RSC Adv. 2013, 3, 18296–18299. doi:10.1039/c3ra43764g
Return to citation in text: [1] -
Wang, S.; Li, J.; Miao, T.; Wu, W.; Li, Q.; Zhuang, Y.; Zhou, Z.; Qiu, L. Org. Lett. 2012, 14, 1966–1969. doi:10.1021/ol300721p
Return to citation in text: [1] -
Yin, J.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 12051–12052. doi:10.1021/ja005622z
Return to citation in text: [1] [2] -
Uozumi, Y.; Matsuura, Y.; Arakawa, T.; Yamada, Y. M. A. Angew. Chem., Int. Ed. 2009, 48, 2708–2710. doi:10.1002/anie.200900469
Return to citation in text: [1] -
Sawai, K.; Tatumi, R.; Nakahodo, T.; Fujihara, H. Angew. Chem., Int. Ed. 2008, 47, 6917–6919. doi:10.1002/anie.200802174
Return to citation in text: [1] -
Uozumi, Y.; Suzuki, N.; Ogiwara, A.; Hayashi, T. Tetrahedron 1994, 50, 4293–4302. doi:10.1016/S0040-4020(01)89366-2
The [1, 1'-binaphthalen]-2-yldiphenylphosphine oxide compounds were synthesized following this reference.
Return to citation in text: [1]
| 1. | Kamer, P. C. J.; van Leeuwen, P. W. N. M. Phosphorus (III) Ligands in Homogeneous Catalysis: Design and Synthesis; John Wiley & Sons: Chichester, 2012. |
| 2. | Tolman, C. A. Chem. Rev. 1977, 77, 313–348. doi:10.1021/cr60307a002 |
| 3. | Fest, C.; Schmidt, K.-J. The chemistry of organophosphorus pesticide; Springer: Berlin, Heidelberg, 1982. |
| 4. | de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, 2004. |
| 19. | Wang, H.-L.; Hu, R.-B.; Zhang, H.; Zhou, A.-X.; Yang, S.-D. Org. Lett. 2013, 15, 5302–5305. doi:10.1021/ol402577p |
| 20. | Zhang, H.-Y.; Yi, H.-M.; Wang, G.-W.; Yang, B.; Yang, S.-D. Org. Lett. 2013, 15, 6186–6189. doi:10.1021/ol403028a |
| 21. | Hu, R.-B.; Zhang, H.; Zhang, X.-Y.; Yang, S.-D. Chem. Commun. 2014, 50, 2193–2195. doi:10.1039/C3CC49050E |
| 22. | Zhang, H.; Hu, R.-B.; Zhang, X.-Y.; Li, S.-X.; Yang, S.-D. Chem. Commun. 2014, 50, 4686–4689. doi:10.1039/C4CC01238K |
| 23. | Ma, Y.-N.; Tian, Q.-P.; Zhang, H.-Y.; Zhou, A.-X.; Yang, S.-D. Org. Chem. Front. 2014, 1, 284–288. doi:10.1039/C4QO00005F |
| 17. | Feng, C.-G.; Ye, M.; Xiao, K.-J.; Li, S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 9322–9325. doi:10.1021/ja404526x |
| 18. | Li, C.; Yano, T.; Ishida, N.; Murakami, M. Angew. Chem., Int. Ed. 2013, 52, 9801–9804. doi:10.1002/anie.201305202 |
| 11. | Li, Y.-M.; Sun, M.; Wang, H.-L.; Tian, Q.-P.; Yang, S.-D. Angew. Chem., Int. Ed. 2013, 52, 3972–3976. doi:10.1002/anie.201209475 |
| 12. | Zhang, H.-Y.; Sun, M.; Ma, Y.-N.; Tian, Q.-P.; Yang, S.-D. Org. Biomol. Chem. 2012, 10, 9627–9633. doi:10.1039/C2OB26874D |
| 13. | Sun, M.; Zhang, H.-Y.; Han, Q.; Yang, K.; Yang, S.-D. Chem. – Eur. J. 2011, 17, 9566–9570. doi:10.1002/chem.201101930 |
| 14. | Hu, J.; Zhao, N.; Yang, B.; Wang, G.; Guo, L.-N.; Liang, Y.-M.; Yang, S.-D. Chem. – Eur. J. 2011, 17, 5516–5521. doi:10.1002/chem.201003561 |
| 15. | Yang, B.; Yang, T.-T.; Li, X.-A.; Wang, J.-J.; Yang, S.-D. Org. Lett. 2013, 15, 5024–5027. doi:10.1021/ol402355a |
| 16. | Yang, B.; Tian, Q. P.; Yang, S. D. Chin. J. Org. Chem. 2014, 34, 717–721. |
| 5. | Jacobsen, E. N.; Pfaltz, A.; Yamamoto, H., Eds. Comprehensive Asymmetric Catalysis I- III; Springer: Berlin, 1999. |
| 6. | Fernández-Pérez, H.; Etayo, P.; Panossian, A.; Vidal-Ferran, A. Chem. Rev. 2011, 111, 2119–2176. doi:10.1021/cr100244e |
| 7. | Lagasse, F.; Kagan, H. B. Chem. Pharm. Bull. 2000, 48, 315–324. doi:10.1248/cpb.48.315 |
| 8. | Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029–3070. doi:10.1021/cr020049i |
| 9. | van Leeuwen, P. W. N. M.; Kamer, P. C. J.; Claver, C.; Pàmies, O.; Diéguez, M. Chem. Rev. 2011, 111, 2077–2118. doi:10.1021/cr1002497 |
| 10. | Cao, Z.; Liu, Y.; Liu, Z.; Feng, X.; Zhuang, M.; Du, H. Org. Lett. 2011, 13, 2164–2167. doi:10.1021/ol200602x |
| 35. |
Uozumi, Y.; Suzuki, N.; Ogiwara, A.; Hayashi, T. Tetrahedron 1994, 50, 4293–4302. doi:10.1016/S0040-4020(01)89366-2
The [1, 1'-binaphthalen]-2-yldiphenylphosphine oxide compounds were synthesized following this reference. |
| 32. | Yin, J.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 12051–12052. doi:10.1021/ja005622z |
| 31. | Wang, S.; Li, J.; Miao, T.; Wu, W.; Li, Q.; Zhuang, Y.; Zhou, Z.; Qiu, L. Org. Lett. 2012, 14, 1966–1969. doi:10.1021/ol300721p |
| 32. | Yin, J.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 12051–12052. doi:10.1021/ja005622z |
| 33. | Uozumi, Y.; Matsuura, Y.; Arakawa, T.; Yamada, Y. M. A. Angew. Chem., Int. Ed. 2009, 48, 2708–2710. doi:10.1002/anie.200900469 |
| 34. | Sawai, K.; Tatumi, R.; Nakahodo, T.; Fujihara, H. Angew. Chem., Int. Ed. 2008, 47, 6917–6919. doi:10.1002/anie.200802174 |
| 24. | Chan, L. Y.; Kim, S.; Ryu, T.; Lee, P. H. Chem. Commun. 2013, 49, 4682–4684. doi:10.1039/c3cc41107a |
| 25. | Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358–3361. doi:10.1021/ol401407v |
| 26. | Chary, B. C.; Kim, S.; Park, Y.; Kim, J.; Lee, P. H. Org. Lett. 2013, 15, 2692–2695. doi:10.1021/ol4009987 |
| 27. | Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258–3261. doi:10.1021/ol4012794 |
| 28. | Zhao, D.; Nimphius, C.; Lindale, M.; Glorius, F. Org. Lett. 2013, 15, 4504–4507. doi:10.1021/ol402053n |
| 29. | Meng, X.; Kim, S. J. Org. Chem. 2013, 78, 11247–11254. doi:10.1021/jo401716p |
| 30. | Mo, J.; Lim, S.; Park, S.; Ryu, T.; Kim, S.; Lee, P. H. RSC Adv. 2013, 3, 18296–18299. doi:10.1039/c3ra43764g |
© 2014 Hu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)