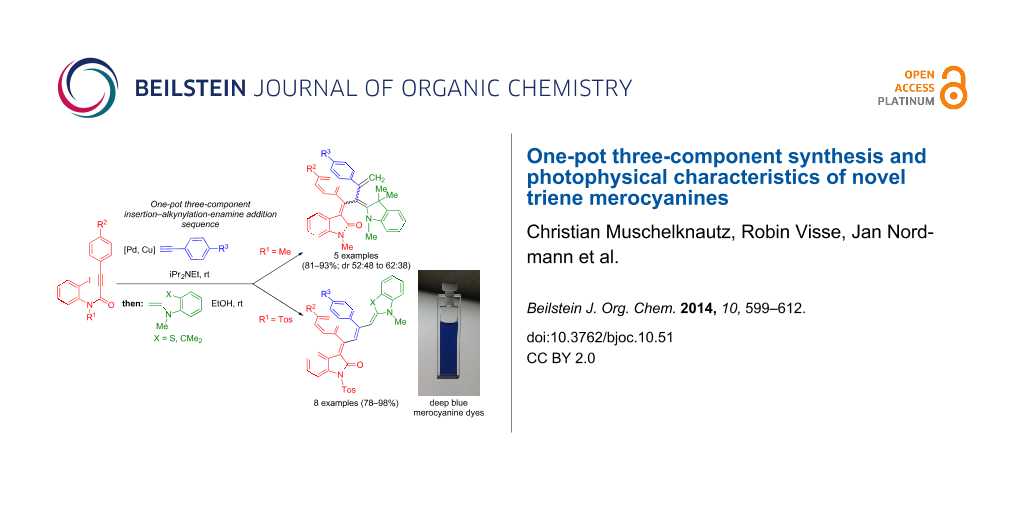
Abstract
Novel triene merocyanines, i.e. 1-styryleth-2-enylidene and 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones are obtained in good to excellent yields in a consecutive three-component insertion Sonogashira coupling–addition sequence. The selectivity of either series is remarkable and has its origin in the stepwise character of the terminal addition step as shown by extensive computations on the DFT level. All merocyanines display intense absorption bands in solution and the film spectra indicate J-aggregation. While 1-styryleth-2-enylideneindolones show an intense deep red emission in films, 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones are essentially nonemissive in films or in the solid state. TD-DFT computations rationalize the charge-transfer nature of the characteristic broad long-wavelength absorptions bands.
Graphical Abstract
Introduction
Functional organic materials [1], such as chromophores, fluorophores, and electrophores, constitute the active components in molecular electronics [2], photonics [3], and bioanalytics [4-6]. Among many chromophores the class of merocyanines [7-9], i.e. α-donor-ω-acceptor-substituted polyenes, has become increasingly interesting due to their fine-tunable electronic distribution [10]. For instance, merocyanines are perfectly suited for developing molecule-based non-linear optical materials and photovoltaic chromophores [11]. Classically, these push–pull chromophores always have been accessed by Knoevenagel condensations [12-14] or substitution reactions [15-18]. Still the quest for new synthetic strategies, novel substitution patterns, and eventually unusual properties and effects has become an ongoing challenge for organic synthesis, physical organic chemistry, and photophysics.
Inspired by the concept of a diversity-oriented synthetic approach to chromophores [19-26] we have launched a program to apply transition metal-catalyzed processes as an entry to consecutive multicomponent [27,28] and domino reactions [29]. These highly convergent strategies paved the way to luminescent push–pull dienes 1–4 with conformationally flexible and fixed acceptor units (Figure 1) [30-32], pyrazoles [33,34], benzodiazepines [35], furans and pyrroles [36,37] by consecutive multicomponent reactions and to highly emissive spirocycles [38-40] and pyranoindoles [41] via domino sequences. Interestingly, our versatile three-component enaminone synthesis [42,43] could be readily extended in a vinylogous fashion with enamines furnishing orange or deep red diene chromophores 2 and 3 that display aggregation induced emission [31].
Figure 1: Linear push–pull solid-state diene lumophores with conformationally flexible and fixed acceptor moieties.
Figure 1: Linear push–pull solid-state diene lumophores with conformationally flexible and fixed acceptor moi...
In particular, the electrophilic enyne intermediate [32,40], which is trespassed in the three-component synthesis of solid-state luminescent push–pull indolones 4, intrigued our interest for accessing even triene push–pull systems and to study their electronic properties. Here we report our findings on the diversity-oriented and highly selective three-component synthesis of a new class of deeply colored triene merocyanines in a one-pot fashion. Furthermore, their absorption and emission characteristics are investigated.
Results and Discussion
After the coupling of the N-methyl-substituted alkynoyl o-iodoanilides 5a and terminal arylalkynes 6 at room temperature under Sonogashira conditions forming ynylideneindolones as intermediates (the reaction was monitored by TLC to ensure complete conversion) [30-32], which were not isolated, an ethanolic solution of Fischer’s base (7) was added and reacted at reflux temperature to give 1-styryleth-2-enylideneindolones 8 in good to excellent yields as violet solids with a metallic luster (Scheme 1, Table 1). In contrary to the reaction with secondary amines, where the E,E-configured butadiene chromophores are formed with excellent stereoselectivity [30-32], Fischer’s base gives rise to the formation of a mixture of E,E- and E,Z-configured push–pull dienes with 3-styryl substituents in narrow diastereomeric ratios ranging from 52:48 to 62:38 (Table 1, entries 1–5) as indicated by the appearance of a second set of many signals in the proton and carbon NMR spectra.
Scheme 1: Three-component synthesis of 1-styryleth-2-enylideneindolones 8.
Scheme 1: Three-component synthesis of 1-styryleth-2-enylideneindolones 8.
Table 1: Three-component synthesis of 1-styryleth-2-enylidene indolones 8.
| Entry | Alkyne 6 | 1-Styryleth-2-enylideneindolones 8 | Yield [%]a |
|---|---|---|---|
| 1 | R3 = H (6a) |
8a |
93 (d.r. = 56:44)b |
| 2 | R3 = OMe (6b) |
8b |
81 (d.r. = 62:38)b |
| 3 | R3 = Cl (6c) |
8c |
82 (d.r. = 56:44)b |
| 4 | R3 = CN (6d) |
8d |
87 (d.r. = 52:48)b |
| 5 | R3 = NO2 (6e) |
8e |
91 (d.r. = 52:48)b |
aThe yields were determined after chromatography on silica gel. bThe diastereomeric ratios were determined by 1H NMR spectroscopy after chromatography on silica gel.
The structures of the 1-styryleth-2-enylideneindolones 8 were assigned by NMR, mass spectrometry and by combustion analysis. The diastereomeric ratios of the E,E- and E,Z-configured push–pull dienes 8 were determined by integration of the distinct signals of the corresponding pairs of geminal olefinic protons appearing at δ 4.8–5.2 and δ 5.5–5.8 for the major diastereomer and at δ 4.5–4.8 and δ 5.3–5.5 for the minor diastereomer in the 1H NMR spectra. The signals of the corresponding carbon nuclei are accordingly identified in the 13C NMR spectra as methylene signals at δ 114.7–117.5 for the major diastereomer and at δ 118.1–123.5 for the minor diastereomer.
Based upon computations (B3LYP functional, 6-31G* basis set) [44] on geometry-optimized diastereomers 2Z,4Z-8a and 2Z,4E-8a the former is energetically favored by 1.5 kcal mol−1 over the latter (Figure 2), nicely reproducing the experimentally determined very similar diastereomeric distribution of the 1-styryleth-2-enylideneindolones 8.
Figure 2: DFT-computed energy differences of the stereoisomers of 2Z,4Z-8a and 2Z,4E-8a.
Figure 2: DFT-computed energy differences of the stereoisomers of 2Z,4Z-8a and 2Z,4E-8a.
Interestingly, upon coupling of the N-tosyl-substituted alkynoyl o-iodoanilides 5b and 5c with terminal arylalkynes 6 at room temperature under Sonogashira conditions and reacting the ynylideneindolone intermediates (complete conversion monitored by TLC) [30-32] under the same conditions as above with an ethanolic solution of Fischer’s base (7), the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10a–g (X = CMe2) were isolated in good to excellent yields as bluish-black solids with a metallic luster (Scheme 2, Table 2, entries 1–7). The sequence starting from the N-methyl-substituted alkynoyl o-iodoanilide 5a surprisingly furnishes after coupling and reaction with an ethanolic solution of benzothiazolium iodide 9 in the presence of diisopropylethylamine (DIPEA) the 4-(3-methylbenzo[d]thiazol-2(3H)-ylidene)but-2-en-1-ylideneindolone 10h (X = S) as a greenish-black solid and not the corresponding 1-styryleth-2-enylideneindolones 8 (Table 2, entry 8).
Scheme 2: Three-component synthesis of 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10.
Scheme 2: Three-component synthesis of 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10.
Table 2: Three-component synthesis of of 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10.
| Entry | Alkynoyl o-iodoanilides 5 | Alkyne 6 | Enamine 7 or benzothiazolium salt 9 | 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10 | Yield [%]a |
|---|---|---|---|---|---|
| 1 | R1 = pTos, R2 = H (5b) | 6a | 7 |
10a |
98 |
| 2 | 5b | 6e | 7 |
10b |
90b |
| 3 | 5b | 6c | 7 |
10c |
78 |
| 4 | 5b | 6d | 7 |
10d |
82 |
| 5 | R1 = pTos, R2 = Cl (5c) | R3 = t-Bu (6e) | 7 |
10e |
82 |
| 6 | 5c | 6c | 7 |
10f |
92 |
| 7 | 5c | 6d | 7 |
10g |
90 |
| 8 | 5a | 6c | 9c |
10h |
84 |
aThe yields were determined after chromatography on silica gel. bThe nitro group was reduced to an amino group under the reaction conditions. cDiisopropylethylamine was added for in situ generation of the S,N-ketene acetal.
The structures of the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10 were unambiguously assigned by NMR, mass spectrometry and by combustion analysis. The appearance of only a single set of signals in the NMR spectra indicates that the process is highly stereoselective. The occurrence of deep-colored products indicates the presence of a chromophore with extended π-electron conjugation where the terminal acceptor and donor functionalities are connected via an essentially coplanar methine bridge. Based upon analogy to the 4-aminoprop-3-enylideneindolones [30-32] and computations (B3LYP functional, 6-31G* basis set) [44] on geometry-optimized 2E,4Z,6Z- and 2E,4Z,6E-diastereomers of 10a and 10h the stereochemistry of the most stable isomers of these novel triene merocyanines was assigned to be 2E,4Z,6Z for both the Fischer’s base derivatives 10a–g (X = CMe2) and the benzothiazole derivative 10h (X = S) (Figure 3). Since the stereoconvergent-product formation (only single sets of signals are obtained in the NMR spectra for all representatives of 10) occurs at elevated temperatures (boiling ethanol in the terminal step) it can be assumed that the assigned structures represent the thermodynamically and kinetically controlled products in this series.
Figure 3: DFT-computed energy differences of the stereoisomers of 10a and 10h.
Figure 3: DFT-computed energy differences of the stereoisomers of 10a and 10h.
Mechanistically the observed unusual selectivity for the formation of 1-styryleth-2-enylideneindolones 8 vs 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10 obviously originates from minute electronic differences in the ynylideneindolone intermediate 11. This species was previously isolated and unambiguously structurally identified [40]. In the case of amine additions to ynylideneindolone intermediates 11 we recently could show by experimental and computational studies that the terminal Michael addition proceeds in a stepwise fashion with the intermediacy of an allenyl enol that undergoes a rapid, irreversible 1,5-sigmatropic hydride shift triggering the allenyl enol–dienone tautomerism [30]. Therefore, we propose a similar mechanism for the formation of the merocyanines 8 and 10 (Scheme 3). The sequences commence after oxidative addition of the Pd species in the carbon–iodine bond of 5 with a 5-exo-dig insertion of the appended alkynoyl moiety, which is coupled by transmetallation with the alkyne 6 and reductive elimination to give the ynylideneindolone intermediate 11. The ynylideneindolone 11 is a vinylogous Michael system, and therefore, it is reasonable to assume a 1,4-addition of the nucleophilic enamine 12, which is employed directly (as in the case of Fischer’s base (7)) or generated in situ by deprotonation of the benzothiazolium salt 9. Hence, in both cases a resonance-stabilized iminium–allenyl enolate 13 is formed. Here, the bifurcation of the sequences takes over. Based upon product analysis the pathway to the formation of the merocyanines 8 begins with a 1,4-dipolar cyclization of 13 furnishing the highly substituted cyclobutene intermediate 14. Finally, the conrotatory electrocyclic ring opening of the cyclobutene occurs under thermodynamic control, which is obviously only governed by steric effects as reflected by very similar levels of diastereoselectivity of the double-bond formation. In contrast the formation of the merocyanines 10 starts with a proton transfer from the CH-acidic α-position of the iminium moiety of 13 to the amide enolate part. The resulting enol 15 is part of an allenyl enol, which is just perfectly suited for undergoing a 1,5-sigmatropic H-shift, giving directly rise to the formation of the conjugated push–pull triene 10.
Scheme 3: Mechanistic rationale of the three-component sequence furnishing the 1-styryleth-2-enylideneindolones 8 and 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10.
Scheme 3: Mechanistic rationale of the three-component sequence furnishing the 1-styryleth-2-enylideneindolon...
This mechanistic rationale suggests that the observed remarkable chemoselectivity in the formation of two different triene merocyanines could originate from minute electronic distributions in the zwitterionic key intermediate, which is controlled in the allenyl enolate moiety by the indolyl nitrogen substituent R1 and by the fragment X on the iminium part, which participates in the stabilization on that side of the zwitterion. The former hypothesis is supported by the fact that only methyl-substituted ynylideneindolone intermediates 11 enable the cyclobutene pathway, whereas all N-tosyl derivatives exclusively give the merocyanines 10 via the allenyl enol pathway. All other remote substituents on the ynylideneindolone intermediates 11 do not influence the outcome of the sequence. The latter hypothesis of the iminium-ion stabilization obviously influences the lifetime of the zwitterion 13. Furthermore the enamine formed by deprotonation of 9 is a S,N-ketene acetal, which is significantly more nucleophilic than Fischer’s base (7). The higher reactivity of the latter enamine and the higher thermodynamic stability of the iminium intermediate both account for a stepwise pathway that proceeds via the intermediacy of zwitterion 13. Therefore, if intermediate 13 is long lived enough to undergo the prototropy the merocyanines 10 will be the obvious product. Therefore, the local charge density in 13 is most crucial for the bifurcation and, hence, for the product formation.
For scrutinizing this rationale we computed the electrostatic charges on the atoms 1–7 of the allenyl enolate moiety of model zwitterions 16, which only differ by the N-substituent, on the density functional level of theory (B3LYP functional, 6-31G* basis set) [40]. For a stepwise cyclobutene formation via 1,4-dipolar cyclization the negative partial charge on the central allenyl carbon atom 6 should be relatively high. The computations of the electrostatic charges for an N-methyl (16a) and an N-tosyl intermediate (16b) clearly shows charge density differences at five distinct atoms (Table 3).
All merocyanines 8 and 10 are expectedly deeply colored both in the solid state and in solution. For further investigation of the photophysical properties the absorption spectra were recorded in dichloromethane solution and of thin films prepared by dropcasting of concentrated dichloromethane solutions on glass-probe cuvettes (Table 4). For the powders and films of the merocyanines 8 intense deep-red luminescence was detected upon eyesight. The emission spectra of the films of 8 were recorded, whereas the films of the merocyanines 10 did not display emission upon eyesight.
Table 4: Selected absorption and emission data of the 1-styryleth-2-enylidene indolones 8 and the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylidene indolones 10.
| Entry | Compound | Absorption | Emission | Stokes shift | |
|---|---|---|---|---|---|
|
λmax,abs [nm]
(ε, L·mol−1·cm−1)a |
λmax,abs [nm] (film)b | λmax,em [nm] (film)b |
Δ |
||
| 1 | 8a |
510 (23600)
330 (20100) 290 (33200) |
519 | 655 | 4000 |
| 2 | 8b |
513 (24200)
327 (25800) 267 (56300) |
523 | 662 | 4000 |
| 3 | 8c |
513 (17900)
259 (40200) |
525 | 665 | 4000 |
| 4 | 8d |
517 (21500)
298 (39700) 265 (51300) |
527 | 665 | 3900 |
| 5 | 8e |
522 (14900)
317 (29300) |
532 | 665 | 3800 |
| 6 | 10a |
587 (33600)
290 (20400) |
601 | – | – |
| 7 | 10b |
592 (57500)
276 (51400) |
623 | – | – |
| 8 | 10c |
577 (30200)
257 (66400) |
599 | – | – |
| 9 | 10d |
592 (34000)
375 (15200) 269 (36100) |
604 | – | – |
| 10 | 10e |
597 (50900)
373 (40300) 266 (37300) |
609 | – | – |
| 11 | 10f | 591 (28700) | 604 | – | – |
| 12 | 10g |
597 (39400)
376 (20400) 276 (40700) |
607 | – | – |
| 13 | 10h |
563 (68900)
345 (24500) |
617
572 (sh) |
– | – |
aRecorded in dichloromethane. bPrepared by dropcasting. cΔ = λmax,abs−1 − λmax,em−1 [cm−1].
The 1-styryleth-2-enylideneindolones 8 are deep red in solution and in the films with intense, broad unstructured absorption bands between 510 and 522 nm in dichloromethane solutions, whereas the maxima of the films are slightly red-shifted and appear between 519 and 532 nm as a result of a J-aggregation [45]. Apparently, the aryl substitution on the triene moiety only affects the absorption bands to a minor extent. The merocyanine character of the 1-styryleth-2-enylideneindolones 8 is additionally supported by quantum chemical calculations on the DFT level (B3LYP functional, 6-31G* basis set) [40]. The computed Kohn–Sham frontier molecular orbitals of compound 8a clearly indicate a charge transfer from the aminovinyl dominated donor fragment in the HOMO to the indolone-centered LUMO, which is generally responsible for the intense longest-wavelength absorption band upon optical excitation (Figure 4).
![[1860-5397-10-51-4]](/bjoc/content/figures/1860-5397-10-51-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: DFT-computed (B3LYP functional, 6-31G* basis set) HOMO (left) and LUMO (right) of merocyanine 8a.
Figure 4: DFT-computed (B3LYP functional, 6-31G* basis set) HOMO (left) and LUMO (right) of merocyanine 8a.
In contrast to the deep-red luminescence of both amorphous films and dyes in the solid state with sharp bands between 644 and 665 nm (Figure 5), the emission in solution is completely quenched. As already witnessed for the film absorption maxima of the 4-amino-prop-3-enylideneindolones 4 [30,32] the J-aggregation results in an induced emission [46,47] by Davydov splitting of the vibrationally relaxed lowest excited-state energy level [48,49] and causes a significant red shift of the sharp emission bands.
![[1860-5397-10-51-5]](/bjoc/content/figures/1860-5397-10-51-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Absorption and emission spectrum of the dropcasted film of compound 8a (recorded at room temperature, normalized spectra, λmax,exc = 490 nm).
Figure 5: Absorption and emission spectrum of the dropcasted film of compound 8a (recorded at room temperatur...
The 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10a–g are dark-blue to black solids and display in dichloromethane solutions broad unstructured longest wavelength absorption bands in a range from 577 to 597 nm with high molar extinction coefficients. As a consequence of J-aggregation the absorption bands of the films are red-shifted and appear between 599 and 623 nm (Table 4, Figure 6). The benzothiazol-terminated merocyanine 10h displays in dichloromethane solution a hypsochromically shifted absorption band at 563 nm with the highest molar extinction coefficient (Table 4, entry 13), yet for the amorphous film the most pronounced bathochromic shift in the series of the merocyanines 10 (Figure 7). In the solid state a merocyanine characteristic metallic green luster can be seen. Again, the aryl substitution on the triene moiety only affects the absorption bands to a minor extent.
![[1860-5397-10-51-6]](/bjoc/content/figures/1860-5397-10-51-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Absorption spectrum of the dropcasted film of compound 10d (recorded at room temperature, normalized spectrum).
Figure 6: Absorption spectrum of the dropcasted film of compound 10d (recorded at room temperature, normalize...
![[1860-5397-10-51-7]](/bjoc/content/figures/1860-5397-10-51-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: Absorption spectra of compound 10h in dichloromethane (right trace) and of the dropcasted film (left trace) (recorded at room temperature, normalized spectrum).
Figure 7: Absorption spectra of compound 10h in dichloromethane (right trace) and of the dropcasted film (lef...
For elucidation of the absorption characteristics of the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones 10, a thorough geometry optimization of the ground-state structure of compound 10a was performed using Gaussian09 [50] with the B3LYP functional [51-55] and the Pople 6-311G(d,p) basis set [56]. For a better comparison with the experimentally determined solution spectrum the calculation was carried out using the Polarizable Continuum Model (PCM) applying dichloromethane as a solvent [57]. The minimum structure of 10a was unambiguously confirmed by an analytical frequency analysis.
The optimized structure of 10a was submitted to a TD-DFT calculation for assigning the experimentally determined absorption characteristics (Table 5). Therefore, the hybrid exchange–correlation functional CAM-B3LYP [58] was implemented and a non-equilibrium solvation [59-63] for the state-specific solvation of the vertical excitation was included.
Table 5: Experimental and TD-DFT computed (CAM-B3LYP 6-311G(d,p)) absorption maxima of the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolone 2E,4Z,6Z-10a.
| Structure | Experimental λmax,abs [nm]a | Computed λmax,abs [nm] | Dominant contributions |
|---|---|---|---|
| 2E,4Z,6Z-10a | 290 | 286 | HOMO → LUMO+2 (56%) |
| – | 345 | HOMO−1 → LUMO (89%) | |
| 587 | 541 | HOMO → LUMO (96%) | |
aRecorded in dichloromethane.
The computations reveal that the longest wavelength absorption maximum appears at 541 nm, i.e., at a comparable energy as in the experimental spectrum. This transition is exclusively dominated by the HOMO–LUMO transition. The computed Kohn–Sham frontier molecular orbitals of structure 10a clearly indicate the charge-transfer character from HOMO to LUMO along the triene axis in the molecule, which generates the intense longest wavelength absorption band upon optical excitation (Figure 8). While the phenyl substituents on the triene chromophore largely contribute to shorter wavelength absorption bands, the tosyl moiety does not display any coefficient density in the FMOs and, therefore, qualifies as a favorable electronic innocent bridge for ligating other chromophores to this novel class of merocyanines.
![[1860-5397-10-51-8]](/bjoc/content/figures/1860-5397-10-51-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: DFT-computed (B3LYP functional, 6-311G(d,p) basis set) FMOs (HOMO, bottom; LUMO (center), and LUMO+2 (top)) of merocyanine 2E,4Z,6Z-10a.
Figure 8: DFT-computed (B3LYP functional, 6-311G(d,p) basis set) FMOs (HOMO, bottom; LUMO (center), and LUMO+...
Conclusion
In conclusion, we enabled the diversity-oriented synthesis of novel triene merocyanines with intense bathochromic absorption by a consecutive three-component insertion–coupling–addition sequence in good to excellent yields. While the N-substituent on the indolone moiety exerts minute electronic differences in the dipolar intermediate, which is responsible for the bifurcation as supported by computational studies, enamine nucleophiles favorably lead to 1-styryleth-2-enylideneindolones diastereomers for N-methyl-substituted anilides. The S,N-ketene acetal derived from dimethyl benzothiazolium favors the formation of the corresponding 4-(3-methylbenzo[d]thiazol-2(3H)-ylidene)but-2-en-1-ylideneindolone. 4-(1,3,3-Trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones are also the exclusive products for N-tosylanilides as starting materials. As a result of aggregation-induced luminescence, 1-styryleth-2-enylideneindolones display in films and in the solid state distinct and intensive deep-red emission upon excitation of the longest wavelength absorption band. 4-(1,3,3-Trimethylindolin-2-ylidene)but-2-en-1-ylideneindolones neither luminesce in solution nor in the solid state upon electronic excitation, yet, they display broad absorption bands and computations suggest, that novel types of panchromatic absorbing bichromophores should be readily available by ligating the second chromophore via the electronically nonperturbing N-sulfonyl moiety. Synthetic, photophysical, and computational studies addressing aggregating broad-band absorbing bichromophores are currently underway.
Experimental
8c: In a flame-dried and argon-flushed Schlenk tube the iodophenylanilide 5a (361 mg, 1.00 mmol), alkyne 6c (150 mg, 1.10 mmol), and dry, degassed THF (5 mL) were placed. After the addition of PdCl2(PPh3)2 (35 mg, 0.05 mmol), and CuI (10 mg, 0.05 mmol), diisopropylethylamine (1.7 mL, 10 mmol) was added and the reaction mixture was stirred at rt for 16 h. Then, Fischer’s base (7, 346 mg, 2.00 mmol), and EtOH (2 mL) were added. The sealed reaction vessel was placed in a thermostatted oil bath at 80 °C and stirred for 48 h. After cooling to rt the solvents were removed in vacuo and the residue was chromatographed on silica gel (hexane/EtOAc 9:1) to give the 1-styryleth-2-enylideneindolone 8c (441 mg, 0.82 mmol, 82%) as violet solid, dr = 56:44. Mp 228 °C; 1H NMR (300 MHz, CDCl3) δ 1.46–1.58 (m, 6H), 2.75–3.29 (m, 6H), 4.92 (0.56H), 5.61 (s, 0.56H), 5.78 (s, 0.56H), 6.25 (d, J = 7.8 Hz, 0.56H), 6.40–6.56 (m, 2H), 6.60–6.67 (m, 1H), 6.80–7.29 (m, 11H), 7.32 (d, J = 7.4 Hz, 0.56H); additional signals for the minor diastereomer: δ 4.61 (s, 0.44H), 5.33 (s, 0.44H), 6.73 (d, J = 7.5 Hz, 0.88H), 7.49 (d, J = 7.1 Hz, 0.44H); 13C NMR (75 MHz, CDCl3) δ 25.2 (CH3), 26.2 (CH3), 36.2 (CH3), 49.4 (Cquat), 106.8 (Cquat), 107.1 (CH), 107.9 (CH), 115.0 (CH2), 120.4 (CH), 121.3 (CH), 122.2 (CH), 124.3 (Cquat), 124.8 (Cquat), 126.4 (CH), 127.4 (CH), 127.8 (CH), 128.0 (CH), 128.9 (CH), 129.5 (CH), 130.0 (CH), 131.2 (CH), 132.9 (Cquat), 133.2 (Cquat), 140.2 (Cquat), 140.4 (Cquat), 140.5 (Cquat), 141.4 (Cquat), 144.4 (Cquat), 145.5 (Cquat), 153.9 (Cquat), 165.4 (Cquat); additional signals for the minor diastereomer: δ 26.3 (CH3), 37.4 (CH3), 50.1 (Cquat), 107.2 (Cquat), 107.2 (CH), 120.1 (CH2), 120.8 (CH), 121.6 (CH), 122.5 (CH), 126.5 (CH), 127.5 (CH), 128.2 (CH), 128.3 (CH), 128.4 (CH), 128.6 (CH), 129.0 (CH), 129.5 (CH), 132.5 (CH), 154.4 (Cquat), 167.5 (Cquat); EIMS (70 eV) m/z (% relative intensity): 544 ([37Cl–M]+, 23), 542 ([35Cl–M]+, 100), 382 ([C25H1835ClNO]+, 22), 158 ([C11H12N]+, 38); IR (KBr) : 3080, 3053, 2968, 2924, 2862, 1654, 1598, 1541, 1471, 1456, 1438, 1413, 1373, 1352, 1336, 1288, 1263, 1236, 1203, 1138, 1122, 1085, 1074, 1024, 1009, 958, 925, 893, 846, 825, 799, 732, 711, 693, 650, 619 cm−1; UV–vis (CH2Cl2) λmax, nm (ε): 259 (40200), 513 (17900); HRMS (m/z) calcd for C36H3135ClN2O: 542.2125; found: 542.2119; Anal. calcd for C36H31ClN2O (543.1): C, 79.61; H, 5.75; N, 5.16; found: C, 79.80; H, 6.02; N, 5.16.
10a: In a flame-dried and argon-flushed Schlenk tube iodophenylanilide 5b (501 mg, 1.00 mmol), alkyne 6a (112 mg, 1.10 mmol), and dry, degassed THF (5 mL) were placed. After the addition of PdCl2(PPh3)2 (35 mg, 0.05 mmol), and CuI (10 mg, 0.05 mmol), diisopropylethylamine (1.7 mL, 10 mmol) was added and the reaction mixture was stirred at rt for 16 h. Then, the enamine 7 (346 mg, 2.00 mmol) and EtOH (2 mL) were added. The sealed reaction vessel was placed in a thermostatted oil bath at 80 °C and stirred for 48 h. After cooling to rt the solvents were removed in vacuo and the residue was chromatographed on silica gel (hexane/EtOAc 4:1) to give the 4-(1,3,3-trimethylindolin-2-ylidene)but-2-en-1-ylideneindolone 10a (636 mg, 98%) as bluish-black solid. Mp 141 °C; 1H NMR (300 MHz, CDCl3) δ 0.85 (s, 6H), 2.30 (s, 3H), 2.31 (s, 3H), 4.68 (d, J = 1.4 Hz, 1H), 5.70 (d, J = 7.3 Hz, 1H), 6.27 (d, J = 7.8 Hz, 1H), 6.56 (t, J = 7.8 Hz, 1H), 6.74 (dt, J = 7.3, 0.7 Hz, 1H), 6.90–7.22 (m, 12H), 7.32 (dd, J = 7.7, 1.8 Hz, 2H), 7.66 (d, J = 1.4 Hz, 1H), 7.80 (dd, J = 8.2, 2.2 Hz, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 21.9 (CH3), 29.4 (CH3), 34.4 (CH3), 46.7 (Cquat), 94.3 (CH), 107.4 (CH), 113.0 (CH), 118.2 (Cquat), 120.8 (CH), 121.8 (CH), 122.7 (CH), 123.4 (CH), 124.6 (CH), 125.4 (Cquat), 127.3 (CH), 127.8 (CH), 127.9 (CH), 128.1 (CH), 128.7 (CH), 128.9 (CH), 129.1 (CH), 129.3 (CH), 129.5 (CH), 129.8 (CH), 136.4 (Cquat), 137.1 (Cquat), 138.2 (Cquat), 140.7 (Cquat), 144.4 (Cquat), 145.1 (Cquat), 146.4 (Cquat), 153.9 (Cquat), 155.9 (Cquat), 161.8 (Cquat), 165.9 (Cquat). EIMS (70 eV) m/z (% relative intensity): 648 ([M]+, 4), 493 ([C35H29N2O]+, 6), 334 (31), 321 (11), 306 (11), 291 (12), 222 (11), 218 (37), 144 (33), 142 (47), 132 (27), 127 (22), 117 (16), 105 (53), 91 (100); IR (KBr) : 3051, 2964, 2924, 2862, 1691, 1577, 1504, 1485, 1465, 1454, 1442, 1400, 1367, 1334, 1315, 1290, 1246, 1217, 1176, 1161, 1130, 1161, 1130, 1116, 1085, 1076, 1060, 1018, 1001, 960, 943, 925, 873, 854, 815, 773, 742, 725, 686, 663, 651, 611 cm−1; UV–vis (CH2Cl2) λmax, nm (ε): 290 nm (20400), 587 (33600); Anal. calcd for C42H36N2O3S (648.8): C, 77.75; H, 5.59; N, 4.32; found: C, 77.58; H, 5.41; N, 4.29.
Supporting Information
| Supporting Information File 1: Experimental procedures, spectroscopic and analytical data, and copies of NMR spectra of compounds 8 and 10. | ||
| Format: PDF | Size: 1.8 MB | Download |
References
-
Müller, T. J. J.; Bunz, U. H. F., Eds. Functional Organic Materials. Syntheses, Strategies, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2007. doi:10.1002/9783527610266
Return to citation in text: [1] -
Müllen, K.; Wegner, G., Eds. Electronic Materials: The Oligomer Approach; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 1998. doi:10.1002/9783527603220
Return to citation in text: [1] -
Müllen, K.; Scherf, U., Eds. Organic Light-Emitting Diodes – Synthesis, Properties, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2006. doi:10.1002/3527607986
See for a recent monograph.
Return to citation in text: [1] -
Kim, E.; Park, S. B. Chem.–Asian J. 2009, 4, 1646–1658. doi:10.1002/asia.200900102
Return to citation in text: [1] -
Cairo, C. W.; Key, J. A.; Sadek, C. M. Curr. Opin. Chem. Biol. 2010, 14, 57–63. doi:10.1016/j.cbpa.2009.09.032
Return to citation in text: [1] -
Wagenknecht, H.-A. Ann. N. Y. Acad. Sci. 2008, 1130, 122–130. doi:10.1196/annals.1430.001
Return to citation in text: [1] -
Hamer, F. M. The Cyanine Dyes and Related Compounds; Interscience: New York, London, 1964.
Return to citation in text: [1] -
Mishra, A.; Behera, R. K.; Behera, P. K.; Mishra, B. K.; Behera, G. B. Chem. Rev. 2000, 100, 1973–2012. doi:10.1021/cr990402t
Return to citation in text: [1] -
Kulinich, A. V.; Ishchenko, A. A. Russ. Chem. Rev. 2009, 78, 141–164. doi:10.1070/RC2009v078n02ABEH003900
Return to citation in text: [1] -
Peng, X.-H.; Zhou, X.-F.; Carroll, S.; Geise, H. J.; Peng, B.; Dommisse, R.; Esmans, E.; Carleer, R. J. Mater. Chem. 1996, 6, 1325–1333. doi:10.1039/JM9960601325
Return to citation in text: [1] -
Shirinian, V. Z.; Shimkin, A. A. Top. Heterocycl. Chem. 2008, 14, 75–105. doi:10.1007/7081_2007_110
Return to citation in text: [1] -
Kovtun, Yu. P.; Prostota, Ya. O.; Shandura, M. P.; Poronik, Ye. M.; Tolmachev, A. I. Dyes Pigm. 2004, 60, 215–221. doi:10.1016/S0143-7208(03)00152-9
Return to citation in text: [1] -
Kovtun, Y. P.; Prostota, Y. O.; Tolmachev, A. I. Dyes Pigm. 2003, 58, 83–91. doi:10.1016/S0143-7208(03)00038-X
Return to citation in text: [1] -
Yagi, S.; Maeda, K.; Nakazumi, H. J. Mater. Chem. 1999, 9, 2991–2997. doi:10.1039/A905098A
Return to citation in text: [1] -
Kay, A. J.; Woolhouse, A. D.; Gainsford, G. J.; Haskell, T. G.; Barnes, T. H.; McKinnie, I. T.; Wyss, C. P. J. Mater. Chem. 2001, 11, 996–1002. doi:10.1039/B008316J
Return to citation in text: [1] -
Würthner, F. Synthesis 1999, 2103–2113. doi:10.1055/s-1999-3635
Return to citation in text: [1] -
Würthner, F.; Yao, S. J. Org. Chem. 2003, 68, 8943–8949. doi:10.1021/jo0351670
Return to citation in text: [1] -
Yao, S.; Beginn, U.; Gress, T.; Lysetska, M.; Würthner, F. J. Am. Chem. Soc. 2004, 126, 8336–8348. doi:10.1021/ja0496367
Return to citation in text: [1] -
Müller, T. J. J. Diversity-oriented Synthesis of Chromophores by Combinatorial Strategies and Multi-component Reactions. In Functional Organic Materials. Syntheses, Strategies, and Applications; Müller, T. J. J.; Bunz, U. H. F., Eds.; Wiley-VCH: Weinheim, 2007; pp 179–223. doi:10.1002/9783527610266.ch5
Return to citation in text: [1] -
Müller, T. J. J.; D'Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609
Return to citation in text: [1] -
Yi, C.; Blum, C.; Liu, S.-X.; Frei, G.; Neels, A.; Stoeckli-Evans, H.; Leutwyler, S.; Decurtins, S. Tetrahedron 2008, 64, 9437–9441. doi:10.1016/j.tet.2008.07.084
Return to citation in text: [1] -
Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.-T. Chem. Commun. 2010, 46, 7406–7408. doi:10.1039/C0CC02366C
Return to citation in text: [1] -
Vendrell, M.; Lee, J.-S.; Chang, Y.-T. Curr. Opin. Chem. Biol. 2010, 14, 383–389. doi:10.1016/j.cbpa.2010.02.020
Return to citation in text: [1] -
Main, M.; Snaith, J. S.; Meloni, M. M.; Jauregui, M.; Sykes, D.; Faulkner, S.; Kenwright, A. M. Chem. Commun. 2008, 5212–5214. doi:10.1039/B810083G
Return to citation in text: [1] -
Briehn, C. A.; Bäuerle, P. Chem. Commun. 2002, 1015–1023. doi:10.1039/B108846G
Return to citation in text: [1] -
Briehn, C. A.; Schiedel, M.-S.; Bonsen, E. M.; Schuhmann, W.; Bäuerle, P. Angew. Chem., Int. Ed. 2001, 40, 4680–4683. doi:10.1002/1521-3773(20011217)40:24<4680::AID-ANIE4680>3.0.CO;2-X
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. Curr. Org. Chem. 2009, 13, 1777–1790. doi:10.2174/138527209789630479
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. ARKIVOC 2008, No. i, 195–208. doi:10.3998/ark.5550190.0009.107
Return to citation in text: [1] -
Müller, T. J. J. Synthesis 2012, 159–174. doi:10.1055/s-0031-1289636
Return to citation in text: [1] -
Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s
Return to citation in text: [1] [2] [3] [4] [5] [6] -
D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Willy, B.; Müller, T. J. J. Org. Lett. 2011, 13, 2082–2085. doi:10.1021/ol2004947
Return to citation in text: [1] -
Willy, B.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4157–4168. doi:10.1002/ejoc.200800444
Return to citation in text: [1] -
Willy, B.; Dallos, T.; Rominger, F.; Schönhaber, J.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4796–4805. doi:10.1002/ejoc.200800619
Return to citation in text: [1] -
Braun, R. U.; Zeitler, K.; Müller, T. J. J. Org. Lett. 2001, 3, 3297–3300. doi:10.1021/ol0165185
Return to citation in text: [1] -
Braun, R. U.; Müller, T. J. J. Synthesis 2004, 2391–2406. doi:10.1055/s-2004-831192
Return to citation in text: [1] -
Schönhaber, J.; Müller, T. J. J. Org. Biomol. Chem. 2011, 9, 6196–6199. doi:10.1039/C1OB05703K
Return to citation in text: [1] -
D'Souza, D. M.; Kiel, A.; Herten, D. P.; Rominger, F.; Müller, T. J. J. Chem.–Eur. J. 2008, 14, 529–547. doi:10.1002/chem.200700759
Return to citation in text: [1] -
D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489
Return to citation in text: [1] [2] [3] [4] [5] -
Schönhaber, J.; Frank, W.; Müller, T. J. J. Org. Lett. 2010, 12, 4122–4125. doi:10.1021/ol101709p
Return to citation in text: [1] -
Karpov, A. S.; Müller, T. J. J. Org. Lett. 2003, 5, 3451–3454. doi:10.1021/ol035212q
Return to citation in text: [1] -
Karpov, A. S.; Müller, T. J. J. Synthesis 2003, 2815–2826. doi:10.1055/s-2003-42480
Return to citation in text: [1] -
Spartan ’08, Version 1.2.0; Wavefunction Inc.: Irvine, CA, 2008.
As implemented in the software.
Return to citation in text: [1] [2] -
Möbius, D. Adv. Mater. 1995, 7, 437–444. doi:10.1002/adma.19950070503
Return to citation in text: [1] -
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361–5388. doi:10.1039/C1CS15113D
Return to citation in text: [1] -
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Commun. 2009, 4332–4353. doi:10.1039/B904665H
Return to citation in text: [1] -
Davydov, A. S. Theory of Molecular Excitons; Plenum: New York, 1971.
Return to citation in text: [1] -
Pope, M.; Swenberg, C. E. Electronic Processes in Organic Crystals; Clarendon Press: Oxford, 1982.
Return to citation in text: [1] -
Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2009.
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/PhysRevB.37.785
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 1372–1377. doi:10.1063/1.464304
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913
Return to citation in text: [1] -
Kim, K.; Jordan, K. D. J. Phys. Chem. 1994, 98, 10089–10094. doi:10.1021/j100091a024
Return to citation in text: [1] -
Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001
Return to citation in text: [1] -
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955
Return to citation in text: [1] -
Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110. doi:10.1063/1.3359469
Return to citation in text: [1] -
Yanai, T.; Tew, D. P.; Handy, N. C. Chem. Phys. Lett. 2004, 393, 51–57. doi:10.1016/j.cplett.2004.06.011
Return to citation in text: [1] -
Berezhkovskii, A. M. Chem. Phys. 1992, 164, 331–339. doi:10.1016/0301-0104(92)87072-H
Return to citation in text: [1] -
Cammi, R.; Tomasi, J. Int. J. Quantum Chem., Quantum Chem. Symp. 1995, 56 (Suppl. 29), 465–474. doi:10.1002/qua.560560850
Return to citation in text: [1] -
Mennucci, B.; Cammi, R.; Tomasi, J. J. Chem. Phys. 1998, 109, 2798–2807. doi:10.1063/1.476878
Return to citation in text: [1] -
Li, X.-Y.; Fu, K.-X. J. Comput. Chem. 2004, 25, 500–509. doi:10.1002/jcc.10377
Return to citation in text: [1] -
Cammi, R.; Corni, S.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 2005, 122, 104513. doi:10.1063/1.1867373
Return to citation in text: [1]
| 40. | D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489 |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 40. | D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489 |
| 1. | Müller, T. J. J.; Bunz, U. H. F., Eds. Functional Organic Materials. Syntheses, Strategies, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2007. doi:10.1002/9783527610266 |
| 7. | Hamer, F. M. The Cyanine Dyes and Related Compounds; Interscience: New York, London, 1964. |
| 8. | Mishra, A.; Behera, R. K.; Behera, P. K.; Mishra, B. K.; Behera, G. B. Chem. Rev. 2000, 100, 1973–2012. doi:10.1021/cr990402t |
| 9. | Kulinich, A. V.; Ishchenko, A. A. Russ. Chem. Rev. 2009, 78, 141–164. doi:10.1070/RC2009v078n02ABEH003900 |
| 35. | Willy, B.; Dallos, T.; Rominger, F.; Schönhaber, J.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4796–4805. doi:10.1002/ejoc.200800619 |
| 51. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/PhysRevB.37.785 |
| 52. | Becke, A. D. J. Chem. Phys. 1993, 98, 1372–1377. doi:10.1063/1.464304 |
| 53. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913 |
| 54. | Kim, K.; Jordan, K. D. J. Phys. Chem. 1994, 98, 10089–10094. doi:10.1021/j100091a024 |
| 55. | Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001 |
| 4. | Kim, E.; Park, S. B. Chem.–Asian J. 2009, 4, 1646–1658. doi:10.1002/asia.200900102 |
| 5. | Cairo, C. W.; Key, J. A.; Sadek, C. M. Curr. Opin. Chem. Biol. 2010, 14, 57–63. doi:10.1016/j.cbpa.2009.09.032 |
| 6. | Wagenknecht, H.-A. Ann. N. Y. Acad. Sci. 2008, 1130, 122–130. doi:10.1196/annals.1430.001 |
| 36. | Braun, R. U.; Zeitler, K.; Müller, T. J. J. Org. Lett. 2001, 3, 3297–3300. doi:10.1021/ol0165185 |
| 37. | Braun, R. U.; Müller, T. J. J. Synthesis 2004, 2391–2406. doi:10.1055/s-2004-831192 |
| 56. | Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955 |
| 3. |
Müllen, K.; Scherf, U., Eds. Organic Light-Emitting Diodes – Synthesis, Properties, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2006. doi:10.1002/3527607986
See for a recent monograph. |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 48. | Davydov, A. S. Theory of Molecular Excitons; Plenum: New York, 1971. |
| 49. | Pope, M.; Swenberg, C. E. Electronic Processes in Organic Crystals; Clarendon Press: Oxford, 1982. |
| 2. | Müllen, K.; Wegner, G., Eds. Electronic Materials: The Oligomer Approach; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 1998. doi:10.1002/9783527603220 |
| 33. | Willy, B.; Müller, T. J. J. Org. Lett. 2011, 13, 2082–2085. doi:10.1021/ol2004947 |
| 34. | Willy, B.; Müller, T. J. J. Eur. J. Org. Chem. 2008, 4157–4168. doi:10.1002/ejoc.200800444 |
| 15. | Kay, A. J.; Woolhouse, A. D.; Gainsford, G. J.; Haskell, T. G.; Barnes, T. H.; McKinnie, I. T.; Wyss, C. P. J. Mater. Chem. 2001, 11, 996–1002. doi:10.1039/B008316J |
| 16. | Würthner, F. Synthesis 1999, 2103–2113. doi:10.1055/s-1999-3635 |
| 17. | Würthner, F.; Yao, S. J. Org. Chem. 2003, 68, 8943–8949. doi:10.1021/jo0351670 |
| 18. | Yao, S.; Beginn, U.; Gress, T.; Lysetska, M.; Würthner, F. J. Am. Chem. Soc. 2004, 126, 8336–8348. doi:10.1021/ja0496367 |
| 27. | Willy, B.; Müller, T. J. J. Curr. Org. Chem. 2009, 13, 1777–1790. doi:10.2174/138527209789630479 |
| 28. | Willy, B.; Müller, T. J. J. ARKIVOC 2008, No. i, 195–208. doi:10.3998/ark.5550190.0009.107 |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 12. | Kovtun, Yu. P.; Prostota, Ya. O.; Shandura, M. P.; Poronik, Ye. M.; Tolmachev, A. I. Dyes Pigm. 2004, 60, 215–221. doi:10.1016/S0143-7208(03)00152-9 |
| 13. | Kovtun, Y. P.; Prostota, Y. O.; Tolmachev, A. I. Dyes Pigm. 2003, 58, 83–91. doi:10.1016/S0143-7208(03)00038-X |
| 14. | Yagi, S.; Maeda, K.; Nakazumi, H. J. Mater. Chem. 1999, 9, 2991–2997. doi:10.1039/A905098A |
| 46. | Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361–5388. doi:10.1039/C1CS15113D |
| 47. | Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Commun. 2009, 4332–4353. doi:10.1039/B904665H |
| 11. | Shirinian, V. Z.; Shimkin, A. A. Top. Heterocycl. Chem. 2008, 14, 75–105. doi:10.1007/7081_2007_110 |
| 10. | Peng, X.-H.; Zhou, X.-F.; Carroll, S.; Geise, H. J.; Peng, B.; Dommisse, R.; Esmans, E.; Carleer, R. J. Mater. Chem. 1996, 6, 1325–1333. doi:10.1039/JM9960601325 |
| 19. | Müller, T. J. J. Diversity-oriented Synthesis of Chromophores by Combinatorial Strategies and Multi-component Reactions. In Functional Organic Materials. Syntheses, Strategies, and Applications; Müller, T. J. J.; Bunz, U. H. F., Eds.; Wiley-VCH: Weinheim, 2007; pp 179–223. doi:10.1002/9783527610266.ch5 |
| 20. | Müller, T. J. J.; D'Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609 |
| 21. | Yi, C.; Blum, C.; Liu, S.-X.; Frei, G.; Neels, A.; Stoeckli-Evans, H.; Leutwyler, S.; Decurtins, S. Tetrahedron 2008, 64, 9437–9441. doi:10.1016/j.tet.2008.07.084 |
| 22. | Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.-T. Chem. Commun. 2010, 46, 7406–7408. doi:10.1039/C0CC02366C |
| 23. | Vendrell, M.; Lee, J.-S.; Chang, Y.-T. Curr. Opin. Chem. Biol. 2010, 14, 383–389. doi:10.1016/j.cbpa.2010.02.020 |
| 24. | Main, M.; Snaith, J. S.; Meloni, M. M.; Jauregui, M.; Sykes, D.; Faulkner, S.; Kenwright, A. M. Chem. Commun. 2008, 5212–5214. doi:10.1039/B810083G |
| 25. | Briehn, C. A.; Bäuerle, P. Chem. Commun. 2002, 1015–1023. doi:10.1039/B108846G |
| 26. | Briehn, C. A.; Schiedel, M.-S.; Bonsen, E. M.; Schuhmann, W.; Bäuerle, P. Angew. Chem., Int. Ed. 2001, 40, 4680–4683. doi:10.1002/1521-3773(20011217)40:24<4680::AID-ANIE4680>3.0.CO;2-X |
| 40. | D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489 |
| 42. | Karpov, A. S.; Müller, T. J. J. Org. Lett. 2003, 5, 3451–3454. doi:10.1021/ol035212q |
| 43. | Karpov, A. S.; Müller, T. J. J. Synthesis 2003, 2815–2826. doi:10.1055/s-2003-42480 |
| 38. | Schönhaber, J.; Müller, T. J. J. Org. Biomol. Chem. 2011, 9, 6196–6199. doi:10.1039/C1OB05703K |
| 39. | D'Souza, D. M.; Kiel, A.; Herten, D. P.; Rominger, F.; Müller, T. J. J. Chem.–Eur. J. 2008, 14, 529–547. doi:10.1002/chem.200700759 |
| 40. | D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489 |
| 57. | Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110. doi:10.1063/1.3359469 |
| 41. | Schönhaber, J.; Frank, W.; Müller, T. J. J. Org. Lett. 2010, 12, 4122–4125. doi:10.1021/ol101709p |
| 58. | Yanai, T.; Tew, D. P.; Handy, N. C. Chem. Phys. Lett. 2004, 393, 51–57. doi:10.1016/j.cplett.2004.06.011 |
| 59. | Berezhkovskii, A. M. Chem. Phys. 1992, 164, 331–339. doi:10.1016/0301-0104(92)87072-H |
| 60. | Cammi, R.; Tomasi, J. Int. J. Quantum Chem., Quantum Chem. Symp. 1995, 56 (Suppl. 29), 465–474. doi:10.1002/qua.560560850 |
| 61. | Mennucci, B.; Cammi, R.; Tomasi, J. J. Chem. Phys. 1998, 109, 2798–2807. doi:10.1063/1.476878 |
| 62. | Li, X.-Y.; Fu, K.-X. J. Comput. Chem. 2004, 25, 500–509. doi:10.1002/jcc.10377 |
| 63. | Cammi, R.; Corni, S.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 2005, 122, 104513. doi:10.1063/1.1867373 |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 44. |
Spartan ’08, Version 1.2.0; Wavefunction Inc.: Irvine, CA, 2008.
As implemented in the software. |
| 44. |
Spartan ’08, Version 1.2.0; Wavefunction Inc.: Irvine, CA, 2008.
As implemented in the software. |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 30. | Muschelknautz, C.; Mayer, B.; Rominger, F.; Müller, T. J. J. Chem. Heterocycl. Compd. 2013, 49, 860–871. doi:10.1007/s10593-013-1320-3 |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 31. | Muschelknautz, C.; Frank, W.; Müller, T. J. J. Org. Lett. 2011, 13, 2556–2559. doi:10.1021/ol200655s |
| 32. | D'Souza, D. M.; Muschelknautz, C.; Rominger, F.; Müller, T. J. J. Org. Lett. 2010, 12, 3364–3367. doi:10.1021/ol101165m |
| 40. | D'Souza, D. M.; Rominger, F.; Müller, T. J. J. Angew. Chem., Int. Ed. 2005, 44, 153–158. doi:10.1002/anie.200461489 |
© 2014 Muschelknautz et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)