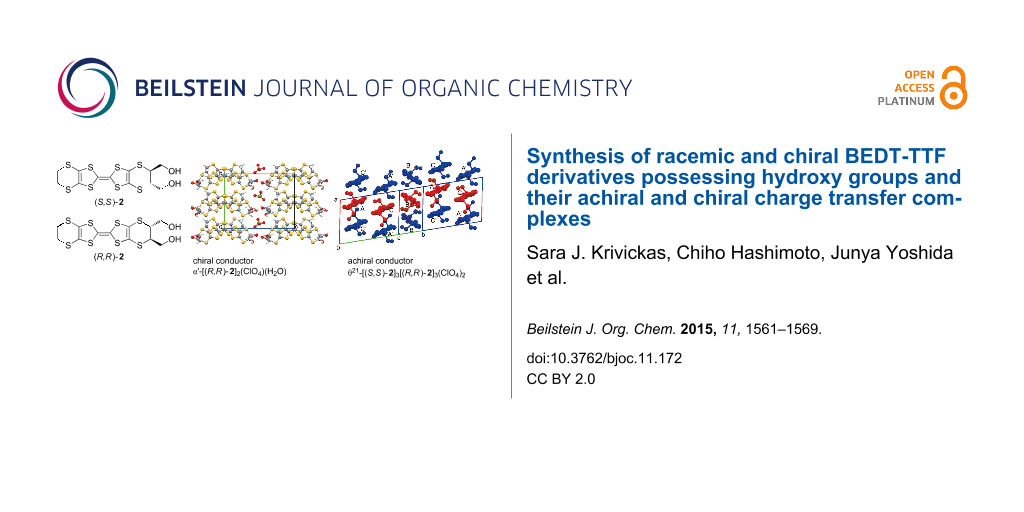
Abstract
Chiral molecular crystals built up by chiral molecules without inversion centers have attracted much interest owing to their versatile functionalities related to optical, magnetic, and electrical properties. However, there is a difficulty in chiral crystal growth due to the lack of symmetry. Therefore, we made the molecular design to introduce intermolecular hydrogen bonds in chiral crystals. Racemic and enantiopure bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) derivatives possessing hydroxymethyl groups as the source of hydrogen bonds were designed. The novel racemic trans-vic-(hydroxymethyl)(methyl)-BEDT-TTF 1, and racemic and enantiopure trans-vic-bis(hydroxymethyl)-BEDT-TTF 2 were synthesized. Moreover, the preparations, crystal structure analyses, and electrical resistivity measurements of the novel achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and the chiral salt α’-[(R,R)-2]ClO4(H2O) were carried out. In the former θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2, there are two sets of three crystallographically independent donor molecules [(S,S)-2]2[(R,R)-2] in a unit cell, where the two sets are related by an inversion center. The latter α’-[(R,R)-2]ClO4(H2O) is the chiral salt with included solvent H2O, which is not isostructural with the reported chiral salt α’-[(S,S)-2]ClO4 without H2O, but has a similar donor arrangement. According to the molecular design by introduction of hydroxy groups and a ClO4− anion, many intermediate-strength intermolecular hydrogen bonds (2.6–3.0 Å) were observed in these crystals between electron donor molecules, anions, and included H2O solvent, which improve the crystallinity and facilitate the extraction of physical properties. Both salts are semiconductors with relatively low resistivities at room temperature and activation energies of 1.2 ohm cm with Ea = 86 meV for θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and 0.6 ohm cm with Ea = 140 meV for α'-[(R,R)-2]2ClO4(H2O), respectively. The variety of donor arrangements, θ21 and two kinds of α’-types, and their electrical conductivities of charge transfer complexes based upon the racemic and enantiopure (S,S)-2, and (R,R)-2 donors originates not only from the chirality, but also the introduced intermolecular hydrogen bonds involving the hydroxymethyl groups, perchlorate anion, and the included solvent H2O.
Graphical Abstract
Introduction
The chiral crystals without an inversion center have attracted much interest recently. Non-centrosymmetric crystals can exhibit a variety of physical properties related to their crystal class [1]; optical and magneto-optical phenomena such as second-harmonic generation, Faraday and Kerr effects, magneto-chiral dichroism, and electrical and magneto-electrical phenomena such as piezoelectricity, pyroelectricity, ferroelectricity and electrical magnetochiral anisotropy. Rikken et al. observed magneto-chiral dichroism in a europium complex [2,3] and also electrical magneto-chiral anisotropy in carbon nanotubes where small changes in the resistance of the chiral carbon nanotubes in a magnetic field were observed between enantiomers [4-6].
One method of constructing chiral crystals is through the use of chiral molecules as building blocks. Tetrathiafulvalene derivatives such as TTF (3) and BEDT-TTF (4) have been investigated considerably due to their ability to form radical cation salts with interesting conductive and magnetic properties (Figure 1). The influence of chirality in the TTF molecules on the crystal structure and physical properties has been shown by Avarvari’s (R)-, (S)- and racemic (±)-(ethylenedithio(tetrathiafulvalene)methyloxazoline)2X, (52X, X = AsF6 [7], PF6 [8]) where disorder within the racemic salt leads to lower conductivity. Recently, Pop et al., have also reported electrical magnetochiral anisotropy in chiral molecular conductors, (R,R)- and (S,S)-[dimethyl-(ethylenedithio)tetrathiafulvalene]2ClO4 (62ClO4) [9,10].
Figure 1: Molecular structures of trans-vic-(hydroxymethyl)(methyl)-BEDT-TTF (1), trans-vic-bis(hydroxymethyl)-BEDT-TTF (2), TTF (3), BEDT-TTF (4), EDT-TTF-methyl-oxazoline 5, and trans-dimethyl-EDT-TTF (6).
Figure 1: Molecular structures of trans-vic-(hydroxymethyl)(methyl)-BEDT-TTF (1), trans-vic-bis(hydroxymethyl...
Although there is a relatively large number of chiral TTF derivatives, only a few conducting properties for chiral cation salts have been so far reported, for those based upon tetramethyl-4 [11-15], 5 [7,8], 6 [9,10], and X-dimethyl-(ethylenedithio)tetrathiafulvalene (X = ethylenedithio [16,17], ethylenedioxy [18], and pyrazino [19]), due to the difficulty of chiral-crystal growth. In order to improve the crystallinity, the inclusion of hydroxy groups in the BEDT-TTF molecule has been postulated to produce hydrogen bonding interactions between electron-donor molecules, electron-acceptor molecules, and anions in the subsequent radical cation salts [20-22]. This may lead to improved order in the crystalline state, which in turn may help the observation of physical properties of the salts. Previously, the synthesis of racemic-2 [21,22], the preliminary synthesis of enantiopure (R,R)- and (S,S)-2, and the preparation, and crystal structure of the radical cation salt α’-[(S,S)-2]2ClO4 [22] have been reported. In this article, we report the syntheses of novel racemic-1 and enantiopure (R,R)- and (S,S)-2 possessing one or two hydroxymethyl groups, and the preparations, crystal structures, and electrical resistivities of the achiral charge transfer complex θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and the chiral complex α’-[(R,R)-2]2ClO4(H2O), in comparison with those of α’-[(S,S)-2]2ClO4. The effects of introducing hydrogen bonds between hydroxymethyl groups of donors and ClO4− anions in charge transfer complexes are also discussed.
Results and Discussion
Syntheses of racemic-1, enantiopure (S,S)- and (R,R)-2 and evaluation of their electrochemical properties
The synthesis of the racemic trans-vic-(hydroxymethyl)(methyl)-BEDT-TTF (1) was performed in a similar manner to racemic 2 [22]. The trans-alkene 8 was reacted with trithione 7 under standard Diels–Alder cycloaddition conditions in refluxing toluene to afford a mixture of the trans-(S,S)- and (R,R)-9 in 56% yield (Scheme 1). The purchased alkene contained a small amount of the cis-isomer (trans-form:cis-form 96:4), but the cis-product can be removed by simple recrystallization of the thione 9 from hexane/dichloromethane. The racemic donor 1 could then be synthesized following procedures whereby the alcohol functionality is protected as acetate, 10, the thione 10 is then converted to the oxo-analogue 11 using mercuric acetate and acetic acid in chloroform. Oxo compound 11 was then cross-coupled with 1.2 equivalents of thione 12 in triethyl phosphite to afford the racemic protected donor 13 in reasonable yield (37%). Basic hydrolysis of the acetyl protecting group afforded the racemic donor 1 in an 81% yield. The syntheses of enatiopure donors 1 and the preparations of their charge transfer complexes are under way.
Moreover, enantiopure (S,S)-2 and (R,R)-2 were also synthesized as shown in Scheme 2. Chiral HPLC was performed using a JAIGEL-OA7500 column on a JAI LC-908 recycling preparative system using the solvent system methanol/water 7:3 to separate (S,S)- and (R,R)-14. The obtained dihydroxy-thione (S,S)-14 was protected as a diacetate to give (S,S)-15, which was converted to the oxo-form (S,S)-16, and coupled with 2 equivalents of 12 to give (S,S)-17. Deprotection under basic conditions afforded enantiopure (S,S)-2. The other enantiomer (R,R)-2 was synthesized in the same manner.
Scheme 2: Synthesis of enantiopure donor (S,S)-2.
Scheme 2: Synthesis of enantiopure donor (S,S)-2.
The cyclic voltammetry measurement on racemic-1 indicated the first and second oxidation potentials (E11/2, E21/2) and their difference ΔE (= E21/2 − E11/2) to be 0.52, 0.83, and 0.31 V by utilizing glassy carbon as working electode with 0.1 M tetrabutylammonium perchlorate in benzonitrile. These potentials are similar to those of (S,S)- and (R,R)-2 with E11/2, E21/2, and ΔE of 0.52, 0.80, and 0.28 V, respectively.
Preparations of single crystals for achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and chiral charge transfer salt α’-[(R,R)-2]2ClO4(H2O). The single brown plate crystals of θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 were grown by the oxidation of the racemic donor 2 (7 mg) in the presence of tetrabutylammonium perchlorate (44 mg) in dichloromethane (24 mL) at room temperature under a constant current of 0.5 μA under a N2 atmosphere during the course of 6 days. The other chiral brown plate crystal of α’-[(R,R)-2]2ClO4(H2O) was prepared electrochemically by utilizing (R,R)-2 (5 mg) and tetrabutylammonium perchlorate (40 mg) in dichloromethane (9 mL) at 0.5 μA for 5 days.
Crystal structures of achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and chiral charge transfer salt α’-[(R,R)-2]2ClO4(H2O). The crystal structure of θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 is depicted in Figure 2. The lattice parameters are listed in Table S1 in Supporting Information File 1. The crystallographically independent molecules are two (S,S)-2 (molecules A and C indicated by red in Figure 2b), one (R,R)-2 (molecules B indicated by blue), and one ClO4− anion. The unit cell contains six donor molecules, consisting of three (S,S)-2 (molecules A, B’, and C) and three (R,R)-2 (molecules A’, B and C’), and two ClO4− anions. Molecules in a pair, X and X’ (X = A, B, and C) are related by an inversion center, so that the space group is centrosymmetric P-1 (No. 2). The donor arrangement is the θ21-type, where transvers inclination pattern is ++−++−... (+ and − represent upward and downward slopes, respectively), whereas the usual θ11-type has the +−+−+−... pattern [23]. In every (+) and (−)-stacking column, the alternate (R,R)–(S,S)–(R,R)–(S,S)- chiral donors indicated by blue–red–blue–red are stacked such as C’AC’A..., A’CA’C..., or BB’BB’... in head-to-tail fashion (Figure 2b). Moreover, the molecules with the same chirality, (R,R) or (S,S), are arranged side-by-side along the b-axis. The charge of each molecule is estimated by bond analyses; as shown in Table S2 (Supporting Information File 1) [24], the charges of molecules A, B, and C are +0.59(8), +0.23(7), and +0.18(8), respectively. In the (+) stacking column, the charge rich A (+0.59(8)) and charge poor C (+0.18(8)) stack, whereas B with the medium charge (+0.23(7)) is arranged in the (−)-column, constructing the appropriate charge balance. The introduced hydroxymethyl groups are set to axial positions for donors A, B, and C (Figure 2b). Following the molecular design, many intermediate-strength intermolecular O···O hydrogen bonds (<2.6–3.0 Å) between hydroxymethyl groups in the donors (a = 2.89(1), b = 2.90(1), c = 2.879(8), and d = 2.837(8) Å in Figure 2c), and between a hydroxymethyl group in the donor and a ClO4− anion (e = 2.94(1) Å) were observed, presumably helping the crystallinity of the complex based upon chiral molecules.
![[1860-5397-11-172-2]](/bjoc/content/figures/1860-5397-11-172-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (a) Crystal structure, (b) θ21-type donor arrangement of molecules A and A’ [(S,S) and (R,R)-2 indicated by red and blue with charge of +0.59(8)], B and B’ [(R,R) and (S,S)-2, blue and red with +0.23(7)], and C and C’ [(S,S) and (R,R)-2, red and blue with +0.18(8)], and (c) O···O hydrogen bond contacts of achital charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2; a = 2.89(1), b = 2.90(1), c = 2.879(8), d = 2.837(8), e = 2.94(1) Å.
Figure 2: (a) Crystal structure, (b) θ21-type donor arrangement of molecules A and A’ [(S,S) and (R,R)-2 indi...
The crystal structure of the chiral salt α’-[(R, R)-2]2ClO4(H2O) is shown in Figure 3. The crystallographically independent molecules are two (R,R)-2 donors, one ClO4− anion, and one H2O molecule as an included solvent. There are four donors, two anions, and two H2O solvents in the unit cell. The enantiopure (R,R)-2 donors stack in a head-to-tail manner and twisted with respect to each other along the a-axis, namely the α’-type donor arrangement [25].
![[1860-5397-11-172-3]](/bjoc/content/figures/1860-5397-11-172-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Crystal structure (a) viewed along the a-axis, (b) donor arrangement, (c) viewed along the b-axis, and (d) intermolecular hydrogen bond contacts for chiral charge transfer salt α'-[(R,R)-2]2ClO4(H2O). The calculated hydrogen bonds are as follows; r(O1···O4) = 2.67(3), r(O1···O9) = 3.00(3), r(O2···O3) = 2.67(2), r(O2···O9) = 2.85(3), r(O5···O9) = 2.62(6), and r(O7···O9) = 2.90(6) Å.
Figure 3: Crystal structure (a) viewed along the a-axis, (b) donor arrangement, (c) viewed along the b-axis, ...
The hydroxymethyl groups project from the molecular BEDT-TTF plane in axial positions (Figure 3c). According to the molecular design, the intermolecular moderate hydrogen bonds between the oxygen atoms in hydroxymethyl groups of (R,R)-2 donors are observed such as r(O1···O4) = 2.67(3) and r(O2···O3) = 2.67(2) Å (Figure 3d). The other hydrogen bonds are found between the oxygen atoms of either included H2O solvent and hydroxymethyl groups such as r(O1···O9) = 3.00(3) and r(O2···O9) = 2.85(3) Å, or of a solvent H2O and an anion ClO4− such as r(O5···O9) = 2.62(6) and r(O7···O9) = 2.90(6) Å. These hydrogen bonds contribute to forming this chiral crystal.
This chiral crystal α’-[(R,R)-2]2ClO4(H2O) is not isostructural to the other enantiopure crystal α'-[(S,S)-2]2ClO4, previously reported (Table S1, Supporting Information File 1) [22]. The crystallographically independent donors are two for α’-[(R,R)-5]2ClO4(H2O) in the space group P21 (No. 4), but one for α'-[(S,S)-5]2ClO4 in P2 (No. 3). Although this α’-[(R,R)-2]2ClO4(H2O) crystal includes a H2O solvent molecule and α'-[(S,S)-2]2ClO4 does not in the same preparation conditions, both salts have the similar α’-type donor arrangements.
Electrical resistivities for the achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and the chiral salt α’-[(R,R)-2]2ClO4(H2O). Temperature dependences of electrical resistivites for the achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and the chiral salt α'-[(R,R)-2]2ClO4(H2O) are shown in Figure 4. The resistivities at room temperature are very similar, 1.2 and 0.6 ohm cm for θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and α'-[(R,R)-2]2ClO4(H2O), respectively. Both salts show semiconducting behaviour and the activation energy of θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 is Ea = 86 meV which is lower than that of α'-[(R,R)-2]2ClO4(H2O) which has Ea = 140 meV.
![[1860-5397-11-172-4]](/bjoc/content/figures/1860-5397-11-172-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Temperature dependences of electrical resistivities for (a) achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and (b) chiral salt α'-[(R,R)-2]2ClO4(H2O).
Figure 4: Temperature dependences of electrical resistivities for (a) achiral charge transfer salt θ21-[(S,S)-...
Conclusion
In summary, we have synthesized redox-active racemic and enantiopure donors of BEDT-TTF derivatives containing one or two hydroxymethyl groups, the novel racemic trans-vic-(hydroxymethyl)(methyl)-BEDT-TTF 1 and enantiopure vic-bis(hydroxymethyl)-BEDT-TTF 2. By successful molecular design to introduce intermolecular hydrogen bonds, the achiral charge transfer salt θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 and the chiral salt α’-[(R, R)-2]ClO4(H2O) could be obtained, and their crystal structure analyses and measurements of electrical resistivities were performed. In the racemic complex θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2, (S,S)-2 and (R,R)-2 donors stack alternately along the a-axis and the same chiral (S,S)-2 (or (R,R)-2) donors are arranged in the side-by-side interaction to construct the stripe chirality order. The latter chiral salt α’-[(R,R)-2]ClO4(H2O) is not isostructural with α’-[(S,S)-2]ClO4 without H2O, but has a similar α’-type donor arrangement. According to the molecular design, both crystals have many moderate-strength hydrogen bonds of 2.6–3.0 Å between donor molecules, ClO4− anions, and H2O, which contribute to crystallinity based upon chiral molecules and allow the investigation of physical properties. The promising strategy of chiral crystal growth will lead to the development of the versatile functionalities of molecular chiral crystals.
Experimental
General information
The parent racemic-2 (Figure 1) was synthesized according to the literature [22]. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were measured with a JEOL JNM-AL300 spectrometer with CDCl3 as solvent using Me4Si or residual solvent as an internal standard. Cyclic voltammetry (CV) measurements were performed on an ALS 610DB electrochemical analyzer in benzonitrile containing 0.1 M tetrabutylammonium perchlorate (working electrode: Pt, counter electrode: Pt wire, reference electrode: saturated calomel electrode (SCE)) [22]. EI-mass spectra were obtained with a JEOL JMS-AX500 spectrometer. X-ray crystallographic measurements were made on a Rigaku AFC-7R diffractometer (Mo Kα, λ = 0.71073 Å). The crystal structures were solved by direct methods and refined with full-matrix least-squares technique using Crystal Structure (ver. 4.0.1, Rigaku Co. and Rigaku Americas Co.). Anisotropic thermal parameters were applied to all non-hydrogen atoms. The hydrogen atoms were generated geometrically (C–H = 0.960 Å). The direct current electrical conductivity measurement was made by the conventional four-probe method using carbon paste and gold wires.
Synthesis of racemic-1
trans-5-(Hydroxymethyl)-6-methyl-5,6-dihydro[1,3]dithiolo[4,5-b][1,4]dithiine-2-thione (9). A solution of trithione 7 (1 g, 5.1 mmol, 1 equiv) and alkene 8 (0.52 mL, 6.12 mmol, 1.2 equiv, trans-form 96%) in toluene (50 mL) was refluxed overnight. The toluene was removed under reduced pressure and the crude material purified by column chromatography (silica gel, 2:1 hexane:ethyl acetate) to afford the trans-thione 9 as a brown powder (with small amounts of the cis derivative). This material was recrystallized from dichloromethane/hexane to give the trans-thione 9 as a brown powder (0.77g, 56%). 1H NMR (300 MHz, CDCl3) δ 1.55(d, J = 6.6 Hz, 3H), 1.98 (dd, J = 5.1, 6.6 Hz, 1H), 3.36 (ddd, J = 3.9, 6.3, 7.5 Hz, 1H), 3.64 (dq, J = 3.9, 6.9 Hz, 1H), 3.86 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 21.90, 37.07, 49.95, 64.89, 120.20, 120.46, 207.57.
trans-5-(Acetoxymethyl)-6-methyl-5,6-dihydro[1,3]dithiolo[4,5-b][1,4]dithiine-2-thione (10). To a solution of thione 9 (482 mg, 1.80 mmol, 1 equiv) in pyridine (5 mL) was added acetic anhydride (202 mg, 1.98 mmol, 1.1 equiv). The reaction mixture was stirred overnight under argon at room temperature. Dichloromethane (50 mL) was added, the organic phase was washed with 1 M HCl (2 × 50 mL) and water (50 mL), dried over MgSO4, filtered and reduced in vacuo to give the required product 10 as a brown oil (560 mg, 100%). The material was used in the next step without further purification. 1H NMR (300 MHz, CDCl3) δ 1.50 (d, J = 6.9 Hz, 3H), 2.03 (s, 3H), 3.42 (ddd, J = 3.9, 6.6, 7.8 Hz, 1H), 3.50 (dq, J = 3.9, 6.9 Hz, 1H), 4.22 (dd, J = 7.5, 11.7 Hz, 1H), 4.27 (dd, J = 6.0, 11.7 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 20.69, 21.82, 37.13, 45.86, 65.66, 119.44, 119.82, 170.33, 207.20.
trans-5-(Acetoxymethyl)-6-methyl-5,6-dihydro[1,3]dithiolo[4,5-b][1,4]dithiine-2-one (11). To a solution of thione 10 (560 mg, 1.81 mmol, 1 equiv) in chloroform (30 mL) was added mercuric acetate (843 mg, 2.71 mmol, 1.5 equiv) and acetic acid (5 mL). The reaction mixture was stirred for 3 hours at room temperature. The solution was filtered, washed with sat. NaHCO3 (2 × 50 mL) and water (50 mL), dried over MgSO4, filtered and reduced in vacuo to give the oxo compound 11 (480 mg, 90%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ 1.53 (d, J = 6.6 Hz, 3H), 2.03 (s, 3H), 3.40 (ddd, J = 3.9, 6.3, 7.2 Hz, 1H), 3.46 (dq, J = 3.9, 6.6 Hz, 1H), 4.28 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 20.73, 21.80, 38.50, 47.59, 65.82, 110.40, 110.61, 116.36, 170.43.
trans-vic-Acetoxymethyl-methyl-BEDT-TTF (13). A solution of thione 12 (439 mg, 1.96 mmol, 1.2 equiv) and the oxo-compound 11 (480 mg, 1.63 mmol, 1 equiv) in triethyl phosphite (10 mL) was stirred overnight at 90 °C. After cooling, hexane was added and the precipitate collected by vacuum filtration and purified by column chromatography (silica gel, 4:1 hexane/ethyl acetate) to afford the cross-coupled product 13 as a red-orange solid (280 mg, 37%). 1H NMR (300 MHz, CDCl3) δ 1.44 (d, J = 6.6 Hz, 3H), 2.05 (s, 3H), 3.26 (s, 4H), 3.38 (ddd, J = 3.9, 6.3, 7.5 Hz, 1H), 3.43 (dq, J = 3.9, 6.6 Hz, 1H), 4.18 (dd, J = 7.5, 11.4 Hz, 1H), 4.24 (dd, J = 6.3, 11.4 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 20.75, 21.58, 30.06, 37.80, 46.56, 65.80, 110.54, 110.77, 110.97, 111.86, 113.69, 170.35.
trans-vic-Hydroxymethyl-methyl-BEDT-TTF (1). A solution of the acetyl protected BEDT-TTF 13 (280 mg, 0.596 mmol, 1 equiv) and K2CO3 (95 mg, 0.894 mmol, 1.5 equiv) in MeOH/H2O/THF (5 mL/5 mL/5 mL) was stirred overnight at room temperature under an argon atmosphere. Water (50 mL) and dichloromethane (100 mL) were added, the organic layer separated and washed with water (2 × 50 mL). The organic layer was dried over MgSO4, filtered and evaporated to give the crude product that was purified by column chromatography (silica gel, ethyl acetate) to afford the trans-racemic BEDT-TTF derivative 1 as an orange powder (210 mg, 81%). 1H NMR (300 MHz, CDCl3) δ 1.43 (d, J = 6.6 Hz, 3H), 1.85 (br s, 1H), 3.22 (s, 4H), 3.24 (m, 1H), 3.46 (dq, J = 3.6, 6.6 Hz, 1H), 3.71 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ 21.29, 29.58, 37.39, 50.37, 63.98, 110.03, 110.11, 110.44, 111.62, 112.93, MS m/z: [M – OH]+ calcd for C12H11S8, 411; found, 410.8627.
Synthesis of (S,S)-2
(S,S)-trans-5,6-Bis(acetyloxymethyl)-5,6-dihydro-1,3-dithiolo[4,5-b]-1,4-dithiin-2-thione ((S,S)-15). A solution of the dihydroxythione (S,S)-14 (25 mg, 0.088 mmol, 1 equiv) in pyridine (1 mL) and acetic anhydride (0.02 mL, 1.96 mmol, 20 equiv) was stirred at room temperature overnight under an argon atmosphere. The reaction mixture was taken up in dichloromethane and washed briefly with 1 M HCl (2 × 50 mL) and water (50 mL). The organic layer was dried over MgSO4, filtered and the volatiles removed in vacuo to give the crude enantiopure diacetoxythione (S,S)-15 (32 mg, quantitative). The isolated product was used in the next step without further purification. 1H NMR (300 MHz, CDCl3) δ 2.12 (s, 6H), 3.79 (m, 2H), 4.32 (dd, J = 8.1, 11.4 Hz, 2H), 4.42 (dd, J = 6.0, 11.4 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 20.69, 40.06, 64.71, 118.79, 170.28, 206.49.
(S,S)-trans-5,6-Bis(acetoxymethyl)-5,6-dihydro-1,3-dithiolo[4,5-b]-1,4-dithiin-2-one ((S,S)-16). To a solution of thione (S,S)-15 (32 mg, 0.087 mmol, 1 equiv) in chloroform (10 mL) was added mercuric acetate (35 mg, 0.11 mmol, 1.3 equiv) and acetic acid (0.5 mL). The reaction mixture was stirred for 3 hours, after which it was filtered, washed with (sat.) NaHCO3 (3 × 50 mL) and water (50 mL). The organic layer was dried over MgSO4, filtered and the solvent removed under reduced pressure to afford the oxo compound (S,S)-16 (28 mg (93%). 1H NMR (300 MHz, CDCl3) δ 2.12 (s, 6H), 3.76 (m, 2H), 4.38 (dd, J = 7.5, 11.4 Hz, 2H), 4.45 (dd, J = 5.7, 11.4 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 20.69, 41.40, 64.84, 109.72, 170.35, 187.57.
(S,S)-trans-vic-Bis(acetyloxymethyl)-BEDT-TTF ((S,S)-17). A solution of thione 12 (64 mg, 0.16 mmol, 2 equiv) and oxo compound (S,S)-16 (50 mg, 0.14 mmol, 1 equiv) in triethyl phosphite (2 mL) was stirred at 90 °C for 5 hours. After cooling hexane was added, the precipitate collected and purified by column chromatography (silica gel, hexane/ethyl actetate) to afford the enantiopure cross coupled product (S,S)-17 (34 mg, 47%). 1H NMR (300 MHz, CDCl3) δ 2.09 (s, 6H), 3.29 (s, 4H), 3.69 (m, 2H), 4.25 (dd, J = 7.8, 11.4 Hz, 2H), 4.34 (dd, J = 6.0, 11.4 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 20.73, 30.17, 40.88, 64.93, 110.43, 113.92, 170.35.
(S,S)-trans-vic-Bis(hydroxymethyl)-BEDT-TTF ((S,S)-2). K2CO3 (27 mg, 0.193 mmol, 3 equiv) was added to a solution of the acetyl protected BEDT-TTF ((S,S)-17, 34 mg, 0.064 mmol) in MeOH/THF/H2O (1 mL/1 mL/1 mL). The reaction mixture was stirred for 3 hours under an argon atmosphere, after which no starting material remained (TLC control). Dichloromethane (100 mL) was added and the organic layer washed with water (2 × 20 mL). The organic layer was dried over MgSO4, filtered and the solvent was removed under reduced pressure. The crude material was purified by column chromatography (silica gel, 1:1 hexane/ethyl acetate) to afford the desired product (S,S)-2 (23 mg, 80%) as a red–pink solid. 1H NMR (300 MHz, DMSO-d6) δ 3.34 (s, 4H), 3.58 (m, 4H), 3.71 (m, 2H), 4.44 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ 30.83, 45.82, 67.40, 111.24, 111.38, 111.97, 114.44.
Supporting Information
| Supporting Information File 1: CD spectra of (S,S)-2 and (R,R)-2, crystal data for θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2, α’-[(R,R)-2]2ClO4(H2O), and α’-[(S,S)-2]2ClO4 (Table S1), charge estimation of θ21-[(S,S)-2]3[(R,R)-2]3(ClO4)2 (Table S2, Figure S2), and NMR data (Figures S3-1–S3-18). | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
Inoue, K.; Ohkoshi, S.; Imai, H. Chiral Molecule-Based Magnets. In Magnetism: Molecules to Materials V; Miller, J.; Drillon, M., Eds.; Wiley-VCH: Weinheim, 2005; pp 41–70.
Return to citation in text: [1] -
Rikken, G. L. J. A.; Raupach, E. Nature 1997, 390, 493–494. doi:10.1038/37323
Return to citation in text: [1] -
Rikken, G. L. J. A.; Raupach, E. Phys. Rev. E 1998, 58, 5081–5084. doi:10.1103/PhysRevE.58.5081
Return to citation in text: [1] -
Rikken, G. L. J. A.; Fölling, J.; Wyder, P. Phys. Rev. Lett. 2001, 87, 236602. doi:10.1103/PhysRevLett.87.236602
Return to citation in text: [1] -
Krstić, V.; Roth, S.; Burghard, M.; Kern, K.; Rikken, G. L. J. A. J. Chem. Phys. 2002, 117, 11315–11319. doi:10.1063/1.1523895
Return to citation in text: [1] -
Krstić, V.; Rikken, G. L. J. A. Chem. Phys. Lett. 2002, 364, 51–56. doi:10.1016/S0009-2614(02)01243-5
Return to citation in text: [1] -
Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. J. Am. Chem. Soc. 2005, 127, 5748–5749. doi:10.1021/ja0503884
Return to citation in text: [1] [2] -
Madalan, A. M.; Réthoré, C.; Fourmigué, M.; Canadell, E.; Lopes, E. B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Chem. – Eur. J. 2010, 16, 528–537. doi:10.1002/chem.200901980
Return to citation in text: [1] [2] -
Pop, F.; Auban-Senzier, P.; Frackowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J. D.; Canadell, E.; Avarvari, N. J. Am. Chem. Soc. 2013, 135, 17176–17186. doi:10.1021/ja408350r
Return to citation in text: [1] [2] -
Pop, F.; Auban-Senzier, P.; Canadell, E.; Rikken, G. L. J. A.; Avarvari, N. Nat. Commun. 2014, 5, 3757. doi:10.1038/ncomms4757
Return to citation in text: [1] [2] -
Wallis, J. D.; Karrer, A.; Dunitz, J. D. Helv. Chim. Acta 1986, 69, 69–70. doi:10.1002/hlca.19860690110
Return to citation in text: [1] -
Karrer, A.; Wallis, J. D.; Dunitz, J. D.; Hilti, B.; Mayer, C. W.; Bürkle, M.; Pfeiffer, J. Helv. Chim. Acta 1987, 70, 942–953. doi:10.1002/hlca.19870700405
Return to citation in text: [1] -
Wallis, J. D.; Dunitz, J. D. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1988, 44, 1037–1039. doi:10.1107/S0108270188001593
Return to citation in text: [1] -
Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y. Bull. Chem. Soc. Jpn. 1993, 66, 513–522. doi:10.1246/bcsj.66.513
Return to citation in text: [1] -
Avarvari, N.; Wallis, J. D. J. Mater. Chem. 2009, 19, 4061–4076. doi:10.1039/B820598A
Return to citation in text: [1] -
Zambounis, J. S.; Mayer, C. W.; Hauenstein, K.; Hilti, B.; Hofherr, W.; Pfeiffer, J.; Bürkle, M.; Rihs, G. Adv. Mater. 1992, 4, 33–35. doi:10.1002/adma.19920040106
Return to citation in text: [1] -
Krivickas, S. J.; Ichikawa, A.; Takahashi, K.; Tajima, H.; Wallis, J. D.; Mori, H. Synth. Met. 2011, 161, 1563–1565. doi:10.1016/j.synthmet.2011.05.019
Return to citation in text: [1] -
Konoike, T.; Iwashita, K.; Yoshino, H.; Murata, K.; Sasaki, T.; Papavassiliou, G. C. Phys. Rev. B 2002, 66, 245308. doi:10.1103/PhysRevB.66.245308
Return to citation in text: [1] -
Zambounis, J. S.; Pfeiffer, J.; Papavassiliou, G. C.; Lagouvardos, D. J.; Terzis, A.; Raptopoulou, C. P.; Delhaès, P.; Ducasse, L.; Fortune, N. A.; Murata, K. Solid State Commun. 1995, 95, 211–215. doi:10.1016/0038-1098(95)00231-6
Return to citation in text: [1] -
Leurquin, F.; Ozturk, T.; Pilkington, M.; Wallis, J. D. J. Chem. Soc., Perkin Trans. 1 1997, 3173–3177. doi:10.1039/A704364C
Return to citation in text: [1] -
Brown, R. J.; Brooks, A. C.; Griffiths, J.; Vital, B.; Day, P.; Wallis, J. D. Org. Biomol. Chem. 2007, 5, 3172–3182. doi:10.1039/b709823e
Return to citation in text: [1] [2] -
Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Mori, T.; Mori, H.; Tanaka, S. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. doi:10.1246/bcsj.72.179
Return to citation in text: [1] -
Guionneau, P.; Kepert, C. J.; Bravic, G.; Chasseau, D.; Truter, M. R.; Kurmoo, M.; Day, P. Synth. Met. 1997, 86, 1973–1974. doi:10.1016/S0379-6779(97)80983-6
Return to citation in text: [1] -
Mori, T. Bull. Chem. Soc. Jpn. 1999, 72, 2011–2027. doi:10.1246/bcsj.72.2011
Return to citation in text: [1]
| 23. | Mori, T.; Mori, H.; Tanaka, S. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. doi:10.1246/bcsj.72.179 |
| 24. | Guionneau, P.; Kepert, C. J.; Bravic, G.; Chasseau, D.; Truter, M. R.; Kurmoo, M.; Day, P. Synth. Met. 1997, 86, 1973–1974. doi:10.1016/S0379-6779(97)80983-6 |
| 1. | Inoue, K.; Ohkoshi, S.; Imai, H. Chiral Molecule-Based Magnets. In Magnetism: Molecules to Materials V; Miller, J.; Drillon, M., Eds.; Wiley-VCH: Weinheim, 2005; pp 41–70. |
| 8. | Madalan, A. M.; Réthoré, C.; Fourmigué, M.; Canadell, E.; Lopes, E. B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Chem. – Eur. J. 2010, 16, 528–537. doi:10.1002/chem.200901980 |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 7. | Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. J. Am. Chem. Soc. 2005, 127, 5748–5749. doi:10.1021/ja0503884 |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 4. | Rikken, G. L. J. A.; Fölling, J.; Wyder, P. Phys. Rev. Lett. 2001, 87, 236602. doi:10.1103/PhysRevLett.87.236602 |
| 5. | Krstić, V.; Roth, S.; Burghard, M.; Kern, K.; Rikken, G. L. J. A. J. Chem. Phys. 2002, 117, 11315–11319. doi:10.1063/1.1523895 |
| 6. | Krstić, V.; Rikken, G. L. J. A. Chem. Phys. Lett. 2002, 364, 51–56. doi:10.1016/S0009-2614(02)01243-5 |
| 20. | Leurquin, F.; Ozturk, T.; Pilkington, M.; Wallis, J. D. J. Chem. Soc., Perkin Trans. 1 1997, 3173–3177. doi:10.1039/A704364C |
| 21. | Brown, R. J.; Brooks, A. C.; Griffiths, J.; Vital, B.; Day, P.; Wallis, J. D. Org. Biomol. Chem. 2007, 5, 3172–3182. doi:10.1039/b709823e |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 2. | Rikken, G. L. J. A.; Raupach, E. Nature 1997, 390, 493–494. doi:10.1038/37323 |
| 3. | Rikken, G. L. J. A.; Raupach, E. Phys. Rev. E 1998, 58, 5081–5084. doi:10.1103/PhysRevE.58.5081 |
| 21. | Brown, R. J.; Brooks, A. C.; Griffiths, J.; Vital, B.; Day, P.; Wallis, J. D. Org. Biomol. Chem. 2007, 5, 3172–3182. doi:10.1039/b709823e |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 9. | Pop, F.; Auban-Senzier, P.; Frackowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J. D.; Canadell, E.; Avarvari, N. J. Am. Chem. Soc. 2013, 135, 17176–17186. doi:10.1021/ja408350r |
| 10. | Pop, F.; Auban-Senzier, P.; Canadell, E.; Rikken, G. L. J. A.; Avarvari, N. Nat. Commun. 2014, 5, 3757. doi:10.1038/ncomms4757 |
| 18. | Konoike, T.; Iwashita, K.; Yoshino, H.; Murata, K.; Sasaki, T.; Papavassiliou, G. C. Phys. Rev. B 2002, 66, 245308. doi:10.1103/PhysRevB.66.245308 |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 7. | Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. J. Am. Chem. Soc. 2005, 127, 5748–5749. doi:10.1021/ja0503884 |
| 8. | Madalan, A. M.; Réthoré, C.; Fourmigué, M.; Canadell, E.; Lopes, E. B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Chem. – Eur. J. 2010, 16, 528–537. doi:10.1002/chem.200901980 |
| 19. | Zambounis, J. S.; Pfeiffer, J.; Papavassiliou, G. C.; Lagouvardos, D. J.; Terzis, A.; Raptopoulou, C. P.; Delhaès, P.; Ducasse, L.; Fortune, N. A.; Murata, K. Solid State Commun. 1995, 95, 211–215. doi:10.1016/0038-1098(95)00231-6 |
| 11. | Wallis, J. D.; Karrer, A.; Dunitz, J. D. Helv. Chim. Acta 1986, 69, 69–70. doi:10.1002/hlca.19860690110 |
| 12. | Karrer, A.; Wallis, J. D.; Dunitz, J. D.; Hilti, B.; Mayer, C. W.; Bürkle, M.; Pfeiffer, J. Helv. Chim. Acta 1987, 70, 942–953. doi:10.1002/hlca.19870700405 |
| 13. | Wallis, J. D.; Dunitz, J. D. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1988, 44, 1037–1039. doi:10.1107/S0108270188001593 |
| 14. | Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y. Bull. Chem. Soc. Jpn. 1993, 66, 513–522. doi:10.1246/bcsj.66.513 |
| 15. | Avarvari, N.; Wallis, J. D. J. Mater. Chem. 2009, 19, 4061–4076. doi:10.1039/B820598A |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
| 9. | Pop, F.; Auban-Senzier, P.; Frackowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J. D.; Canadell, E.; Avarvari, N. J. Am. Chem. Soc. 2013, 135, 17176–17186. doi:10.1021/ja408350r |
| 10. | Pop, F.; Auban-Senzier, P.; Canadell, E.; Rikken, G. L. J. A.; Avarvari, N. Nat. Commun. 2014, 5, 3757. doi:10.1038/ncomms4757 |
| 16. | Zambounis, J. S.; Mayer, C. W.; Hauenstein, K.; Hilti, B.; Hofherr, W.; Pfeiffer, J.; Bürkle, M.; Rihs, G. Adv. Mater. 1992, 4, 33–35. doi:10.1002/adma.19920040106 |
| 17. | Krivickas, S. J.; Ichikawa, A.; Takahashi, K.; Tajima, H.; Wallis, J. D.; Mori, H. Synth. Met. 2011, 161, 1563–1565. doi:10.1016/j.synthmet.2011.05.019 |
| 22. | Krivickas, S. J.; Hashimoto, C.; Takahashi, K.; Wallis, J. D.; Mori, H. Phys. Status Solidi C 2012, 9, 1146–1148. doi:10.1002/pssc.201100728 |
© 2015 Krivickas et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)