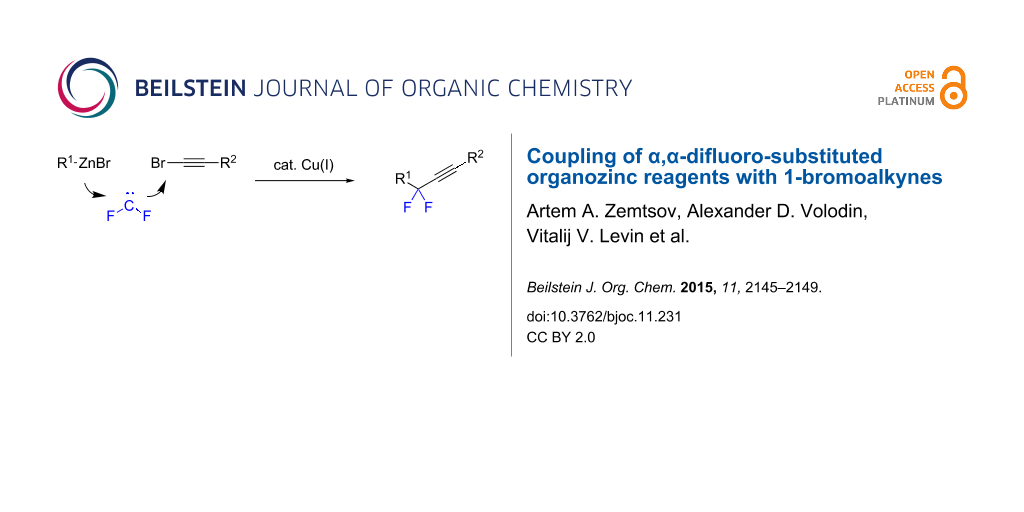
Abstract
α,α-Difluoro-substituted organozinc reagents generated from conventional organozinc compounds and difluorocarbene couple with 1-bromoalkynes affording gem-difluorinated alkynes. The cross-coupling proceeds in the presence of catalytic amounts of copper iodide in dimethylformamide under ligand-free conditions.
Graphical Abstract
Introduction
gem-Difluorinated organic compounds have attracted increasing attention nowadays due to their applicability in medicinal chemistry [1,2] and other fields. Indeed, unique stereoelectronic properties of the CF2-unit may be exploited in conformational analysis [3-5], carbohydrate and peptide research [6,7], and reaction engineering [8,9].
Typically, the difluoromethylene fragment is created by deoxyfluorination, which requires harsh or hazardous conditions [10,11]. Alternatively, functional group manipulations starting from available CF2-containing building blocks can be considered, but multistep sequences render this approach laborious [12-14]. Difluoro-substituted cyclopropanes and cyclopropenes constitute a specific class of compounds accessible by difluorocarbene addition to multiple bonds [15].
Recently, we proposed a general method for assembling gem-difluorinated structures from organozinc reagents 1, difluorocarbene, and a terminating electrophile [16-21] (Scheme 1). (Bromodifluoromethyl)trimethylsilane [16-18] or potassium bromodifluoroacetate [19] can be used as precursors of difluorocarbene. In this process, the use of C-electrophiles is particularly important since it allows for the formation of two C–C bonds within one experimental run. Previously, as C-electrophiles in this methodology, only allylic substrates [17] and nitrostryrenes (with the NO2 serving as a leaving group) [20], were employed. Herein, we report that 1-bromoalkynes, which are known to be involved in reactions with various organometallic compounds [22-27], can be used as suitable coupling partners for difluorinated organozinc compounds 2. This reaction provides straightforward access to α,α-difluorinated alkynes [13,14,28-31]. Our method is based on facile zinc/copper exchange allowing for versatile couplings described for non-fluorinated organozinc compounds [32-37].
Scheme 1: Reaction of organozinc compounds.
Scheme 1: Reaction of organozinc compounds.
Results and Discussion
Organozinc compound 2a generated from benzylzinc bromide was first evaluated in a reaction with haloalkynes derived from phenylacetylene (Table 1). First, most reactive iodo-substituted alkyne 3a-I (X = I) was evaluated in the presence of copper iodide (10 mol %). Expected product 4a was formed in 12% yield, but its yield was tripled simply by adding 2 equiv of DMF additive (Table 1, entries 1 and 2). However, in these experiments, the reaction mixtures contained about 40% of (2,2-difluoro-2-iodoethyl)benzene (PhCH2CF2I) arising from zinc/iodine exchange between 2a and the iodoalkyne. Chloroalkyne 3a-Cl was markedly less reactive, likely because of the strong carbon–chlorine bond. Fortunately, bromoalkyne 3a-Br provided the best results, with the optimal conditions involving the use of DMF as a solvent and only 5 mol % of copper iodide at 0 °C to room temperature, which afforded the coupling product in 79% isolated yield (Table 1, entry 5). The addition of various ligands, as well as the use of other copper salts, did not had a beneficial effect.
Table 1: Optimization studies.
|
|
|||||||
| Entry | X | 2a (equiv) | Conditions | Solvent | CuI (equiv) | Additive (equiv) | Yield of 4a, %a |
|---|---|---|---|---|---|---|---|
| 1 | I | 2 | −50 °C → rt; 4 h at rt | MeCN | 0.1 | – | 12 |
| 2 | I | 1.3 | −50 °C → rt; 4 h at rt | MeCN | 0.1 | DMF (2) | 35 |
| 3 | Cl | 2 | 0 °C → rt; 16 h at rt | MeCN | 0.1 | DMF (2) | 32 |
| 4 | Br | 1.5 | 0 °C → rt; 16 h at rt | MeCN | 0.1 | DMF (2) | 60 |
| 5 | Br | 1.5 | 0 °C → rt; 16 h at rt | DMF | 0.05 | – | 79b |
aDetermined by 19F NMR with internal standard. bIsolated yield.
Under the optimized conditions, a series of organozinc compounds 2 were coupled with bromoalkynes 3 (Table 2). Good yields of coupling products 4 were typically achieved. The reaction tolerates ester groups or TBS-protected hydroxy groups. Aromatic iodide also remains unaffected (Table 2, entry 2).
Table 2: Reaction of organozinc compounds 2 with bromoalkynes 3.
|
|
||||
| Entry | 2 | 3 | 4 | Yield of 4, %a |
|---|---|---|---|---|
| 1 |
2a |
3b |
4b |
84 |
| 2 | 2a |
3c |
4c |
82 |
| 3 | 2a |
3d |
4d |
70 |
| 4 | 2a |
3e |
4e |
84 |
| 5 | 2a |
3f |
4f |
67 |
| 6b | 2a |
3g |
4g |
80 |
| 7b | 2a |
3h |
4h |
75 |
| 8 |
2b |
3a-Br |
4i |
80 |
| 9 |
2e |
3a-Br |
4j |
81 |
| 10 |
2c |
3a-Br |
4k |
72 |
| 11b |
2c |
3g |
4l |
71 |
| 12b |
2d |
3g |
4m |
62 |
aIsolated yield. bThe crude product was desilylated.
As for the mechanism, we believe that the reaction starts with the zinc/copper exchange resulting in the formation of fluorinated organocopper species 5 (Scheme 2). Compound 5 interacts with bromoalkyne 3 either by oxidative addition generating copper(III) intermediate 6 or by triple bond carbometallation [38] generating copper(I) intermediate 7. Subsequent reductive elimination (from 6) or β-elimination (from 7) leads to the product and regenerates the copper(I) catalyst.
Conclusion
In summary, a method for the copper-catalyzed coupling of α,α-difluoro-substituted organozinc compounds with 1-bromoalkynes has been developed. The reaction is performed under mild conditions affording gem-difluoro-substituted alkynes in good yields.
Supporting Information
| Supporting Information File 1: Full experimental details, compound characterization, and copies of NMR spectra. | ||
| Format: PDF | Size: 2.1 MB | Download |
References
-
Ojima, I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; John Wiley & Sons: Chichester, 2009.
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons: Hoboken, New Jersey, 2008. doi:10.1002/9780470281895
Return to citation in text: [1] -
O'Hagan, D.; Wang, Y.; Skibinski, M.; Slawin, A. M. Z. Pure Appl. Chem. 2012, 84, 1587–1595. doi:10.1351/PAC-CON-11-09-26
Return to citation in text: [1] -
Wang, Y.; Callejo, R.; Slawin, A. M. Z.; O'Hagan, D. Beilstein J. Org. Chem. 2014, 10, 18–25. doi:10.3762/bjoc.10.4
Return to citation in text: [1] -
Urbina-Blanco, C. A.; Skibinski, M.; O'Hagan, D.; Nolan, S. P. Chem. Commun. 2013, 49, 7201–7203. doi:10.1039/c3cc44312d
Return to citation in text: [1] -
Leclerc, E.; Pannecoucke, X.; Ethève-Quelquejeu, M.; Sollogoub, M. Chem. Soc. Rev. 2013, 42, 4270–4283. doi:10.1039/C2CS35403A
Return to citation in text: [1] -
Kubyshkin, V. S.; Mykhailiuk, P. K.; Afonin, S.; Ulrich, A. S.; Komarov, I. V. Org. Lett. 2012, 14, 5254–5257. doi:10.1021/ol302412a
Return to citation in text: [1] -
Baskin, J. M.; Prescher, J. A.; Laughlin, S. T.; Agard, N. J.; Chang, P. V.; Miller, I. A.; Lo, A.; Codelli, J. A.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 16793–16797. doi:10.1073/pnas.0707090104
Return to citation in text: [1] -
Codelli, J. A.; Baskin, J. M.; Agard, N. J.; Bertozzi, C. R. J. Am. Chem. Soc. 2008, 130, 11486–11493. doi:10.1021/ja803086r
Return to citation in text: [1] -
Tozer, M. J.; Herpin, T. F. Tetrahedron 1996, 52, 8619–8683. doi:10.1016/0040-4020(96)00311-0
Return to citation in text: [1] -
Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75.
Return to citation in text: [1] -
Qing, F.-L.; Zheng, F. Synlett 2011, 1052–1072. doi:10.1055/s-0030-1259947
Return to citation in text: [1] -
Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2015, 21, 12836–12865. doi:10.1002/chem.201501475
Return to citation in text: [1] [2] -
Gao, B.; Ni, C.; Hu, J. Chimia 2014, 68, 414–418. doi:10.2533/chimia.2014.414
Return to citation in text: [1] [2] -
Dolbier, W. R., Jr.; Battiste, M. A. Chem. Rev. 2003, 103, 1071–1098. doi:10.1021/cr010023b
Return to citation in text: [1] -
Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2013, 15, 917–919. doi:10.1021/ol400122k
Return to citation in text: [1] [2] -
Zemtsov, A. A.; Kondratyev, N. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. J. Org. Chem. 2014, 79, 818–822. doi:10.1021/jo4024705
Return to citation in text: [1] [2] [3] -
Smirnov, V. O.; Struchkova, M. I.; Arkhipov, D. E.; Korlyukov, A. A.; Dilman, A. D. J. Org. Chem. 2014, 79, 11819–11823. doi:10.1021/jo5023537
Return to citation in text: [1] [2] -
Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 171, 97–101. doi:10.1016/j.jfluchem.2014.08.021
Return to citation in text: [1] [2] -
Kondratyev, N. S.; Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 176, 89–92. doi:10.1016/j.jfluchem.2015.06.001
Return to citation in text: [1] [2] -
Smirnov, V. O.; Maslov, A. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Russ. Chem. Bull. 2014, 63, 2564–2566. doi:10.1007/s11172-014-0778-1
Return to citation in text: [1] -
Thaler, T.; Guo, L.-N.; Mayer, P.; Knochel, P. Angew. Chem., Int. Ed. 2011, 50, 2174–2177. doi:10.1002/anie.201006879
Return to citation in text: [1] -
Corpet, M.; Bai, X.-Z.; Gosmini, C. Adv. Synth. Catal. 2014, 356, 2937–2942. doi:10.1002/adsc.201400369
Return to citation in text: [1] -
Cornelissen, L.; Lefrancq, M.; Riant, O. Org. Lett. 2014, 16, 3024–3027. doi:10.1021/ol501140p
Return to citation in text: [1] -
Wang, S.; Wang, M.; Wang, L.; Wang, B.; Li, P.; Yang, J. Tetrahedron 2011, 67, 4800–4806. doi:10.1016/j.tet.2011.05.031
Return to citation in text: [1] -
Castagnolo, D.; Botta, M. Eur. J. Org. Chem. 2010, 3224–3228. doi:10.1002/ejoc.201000393
Return to citation in text: [1] -
Brand, J. P.; Waser, J. Chem. Soc. Rev. 2012, 41, 4165–4179. doi:10.1039/c2cs35034c
Return to citation in text: [1] -
Besset, T.; Poisson, T.; Pannecoucke, X. Eur. J. Org. Chem. 2014, 7220–7225. doi:10.1002/ejoc.201402937
Return to citation in text: [1] -
Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656–2661. doi:10.1021/jo7025965
Return to citation in text: [1] -
Hammond, G. B. J. Fluorine Chem. 2006, 127, 476–488. doi:10.1016/j.jfluchem.2005.12.024
Return to citation in text: [1] -
Xu, B.; Mae, M.; Hong, J. A.; Li, Y.; Hammond, G. B. Synthesis 2006, 803–806. doi:10.1055/s-2006-926334
Return to citation in text: [1] -
Malosh, C. F.; Ready, J. M. J. Am. Chem. Soc. 2004, 126, 10240–10241. doi:10.1021/ja0467768
Return to citation in text: [1] -
Thapa, S.; Kafle, A.; Gurung, S. K.; Montoya, A.; Riedel, P.; Giri, R. Angew. Chem., Int. Ed. 2015, 54, 8236–8240. doi:10.1002/anie.201502379
Return to citation in text: [1] -
Karstens, W. F. J.; Moolenaar, M. J.; Rutjes, F. P. J. T.; Grabowska, U.; Speckamp, W. N.; Hiemstra, H. Tetrahedron Lett. 1999, 40, 8629–8632. doi:10.1016/S0040-4039(99)01808-0
Return to citation in text: [1] -
Knochel, P. Organomagnesium and Organozinc Chemistry. In Organometallics in Synthesis; Schlosser, M., Ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2013; pp 223–372. doi:10.1002/9781118484722.ch2
Return to citation in text: [1] -
Knochel, P. In Metal-Catalyzed Cross-Coupling Reactions; Diederich, F.; Stang, P. J., Eds.; Wiley-VCH: Weinheim, 1998; pp 387–419.
Return to citation in text: [1] -
Geurts, K.; Fletcher, S. P.; van Zijl, A. W.; Minnaard, A. J.; Feringa, B. L. Pure Appl. Chem. 2008, 80, 1025–1037. doi:10.1351/pac200880051025
Return to citation in text: [1] -
Cahiez, G.; Gager, O.; Buendia, J. Angew. Chem., Int. Ed. 2010, 49, 1278–1281. doi:10.1002/anie.200905816
Return to citation in text: [1]
| 1. | Ojima, I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; John Wiley & Sons: Chichester, 2009. |
| 2. | Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons: Hoboken, New Jersey, 2008. doi:10.1002/9780470281895 |
| 10. | Tozer, M. J.; Herpin, T. F. Tetrahedron 1996, 52, 8619–8683. doi:10.1016/0040-4020(96)00311-0 |
| 11. | Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75. |
| 32. | Malosh, C. F.; Ready, J. M. J. Am. Chem. Soc. 2004, 126, 10240–10241. doi:10.1021/ja0467768 |
| 33. | Thapa, S.; Kafle, A.; Gurung, S. K.; Montoya, A.; Riedel, P.; Giri, R. Angew. Chem., Int. Ed. 2015, 54, 8236–8240. doi:10.1002/anie.201502379 |
| 34. | Karstens, W. F. J.; Moolenaar, M. J.; Rutjes, F. P. J. T.; Grabowska, U.; Speckamp, W. N.; Hiemstra, H. Tetrahedron Lett. 1999, 40, 8629–8632. doi:10.1016/S0040-4039(99)01808-0 |
| 35. | Knochel, P. Organomagnesium and Organozinc Chemistry. In Organometallics in Synthesis; Schlosser, M., Ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2013; pp 223–372. doi:10.1002/9781118484722.ch2 |
| 36. | Knochel, P. In Metal-Catalyzed Cross-Coupling Reactions; Diederich, F.; Stang, P. J., Eds.; Wiley-VCH: Weinheim, 1998; pp 387–419. |
| 37. | Geurts, K.; Fletcher, S. P.; van Zijl, A. W.; Minnaard, A. J.; Feringa, B. L. Pure Appl. Chem. 2008, 80, 1025–1037. doi:10.1351/pac200880051025 |
| 8. | Baskin, J. M.; Prescher, J. A.; Laughlin, S. T.; Agard, N. J.; Chang, P. V.; Miller, I. A.; Lo, A.; Codelli, J. A.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 16793–16797. doi:10.1073/pnas.0707090104 |
| 9. | Codelli, J. A.; Baskin, J. M.; Agard, N. J.; Bertozzi, C. R. J. Am. Chem. Soc. 2008, 130, 11486–11493. doi:10.1021/ja803086r |
| 38. | Cahiez, G.; Gager, O.; Buendia, J. Angew. Chem., Int. Ed. 2010, 49, 1278–1281. doi:10.1002/anie.200905816 |
| 6. | Leclerc, E.; Pannecoucke, X.; Ethève-Quelquejeu, M.; Sollogoub, M. Chem. Soc. Rev. 2013, 42, 4270–4283. doi:10.1039/C2CS35403A |
| 7. | Kubyshkin, V. S.; Mykhailiuk, P. K.; Afonin, S.; Ulrich, A. S.; Komarov, I. V. Org. Lett. 2012, 14, 5254–5257. doi:10.1021/ol302412a |
| 22. | Thaler, T.; Guo, L.-N.; Mayer, P.; Knochel, P. Angew. Chem., Int. Ed. 2011, 50, 2174–2177. doi:10.1002/anie.201006879 |
| 23. | Corpet, M.; Bai, X.-Z.; Gosmini, C. Adv. Synth. Catal. 2014, 356, 2937–2942. doi:10.1002/adsc.201400369 |
| 24. | Cornelissen, L.; Lefrancq, M.; Riant, O. Org. Lett. 2014, 16, 3024–3027. doi:10.1021/ol501140p |
| 25. | Wang, S.; Wang, M.; Wang, L.; Wang, B.; Li, P.; Yang, J. Tetrahedron 2011, 67, 4800–4806. doi:10.1016/j.tet.2011.05.031 |
| 26. | Castagnolo, D.; Botta, M. Eur. J. Org. Chem. 2010, 3224–3228. doi:10.1002/ejoc.201000393 |
| 27. | Brand, J. P.; Waser, J. Chem. Soc. Rev. 2012, 41, 4165–4179. doi:10.1039/c2cs35034c |
| 3. | O'Hagan, D.; Wang, Y.; Skibinski, M.; Slawin, A. M. Z. Pure Appl. Chem. 2012, 84, 1587–1595. doi:10.1351/PAC-CON-11-09-26 |
| 4. | Wang, Y.; Callejo, R.; Slawin, A. M. Z.; O'Hagan, D. Beilstein J. Org. Chem. 2014, 10, 18–25. doi:10.3762/bjoc.10.4 |
| 5. | Urbina-Blanco, C. A.; Skibinski, M.; O'Hagan, D.; Nolan, S. P. Chem. Commun. 2013, 49, 7201–7203. doi:10.1039/c3cc44312d |
| 13. | Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2015, 21, 12836–12865. doi:10.1002/chem.201501475 |
| 14. | Gao, B.; Ni, C.; Hu, J. Chimia 2014, 68, 414–418. doi:10.2533/chimia.2014.414 |
| 28. | Besset, T.; Poisson, T.; Pannecoucke, X. Eur. J. Org. Chem. 2014, 7220–7225. doi:10.1002/ejoc.201402937 |
| 29. | Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656–2661. doi:10.1021/jo7025965 |
| 30. | Hammond, G. B. J. Fluorine Chem. 2006, 127, 476–488. doi:10.1016/j.jfluchem.2005.12.024 |
| 31. | Xu, B.; Mae, M.; Hong, J. A.; Li, Y.; Hammond, G. B. Synthesis 2006, 803–806. doi:10.1055/s-2006-926334 |
| 16. | Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2013, 15, 917–919. doi:10.1021/ol400122k |
| 17. | Zemtsov, A. A.; Kondratyev, N. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. J. Org. Chem. 2014, 79, 818–822. doi:10.1021/jo4024705 |
| 18. | Smirnov, V. O.; Struchkova, M. I.; Arkhipov, D. E.; Korlyukov, A. A.; Dilman, A. D. J. Org. Chem. 2014, 79, 11819–11823. doi:10.1021/jo5023537 |
| 17. | Zemtsov, A. A.; Kondratyev, N. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. J. Org. Chem. 2014, 79, 818–822. doi:10.1021/jo4024705 |
| 16. | Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2013, 15, 917–919. doi:10.1021/ol400122k |
| 17. | Zemtsov, A. A.; Kondratyev, N. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. J. Org. Chem. 2014, 79, 818–822. doi:10.1021/jo4024705 |
| 18. | Smirnov, V. O.; Struchkova, M. I.; Arkhipov, D. E.; Korlyukov, A. A.; Dilman, A. D. J. Org. Chem. 2014, 79, 11819–11823. doi:10.1021/jo5023537 |
| 19. | Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 171, 97–101. doi:10.1016/j.jfluchem.2014.08.021 |
| 20. | Kondratyev, N. S.; Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 176, 89–92. doi:10.1016/j.jfluchem.2015.06.001 |
| 21. | Smirnov, V. O.; Maslov, A. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Russ. Chem. Bull. 2014, 63, 2564–2566. doi:10.1007/s11172-014-0778-1 |
| 20. | Kondratyev, N. S.; Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 176, 89–92. doi:10.1016/j.jfluchem.2015.06.001 |
| 15. | Dolbier, W. R., Jr.; Battiste, M. A. Chem. Rev. 2003, 103, 1071–1098. doi:10.1021/cr010023b |
| 12. | Qing, F.-L.; Zheng, F. Synlett 2011, 1052–1072. doi:10.1055/s-0030-1259947 |
| 13. | Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2015, 21, 12836–12865. doi:10.1002/chem.201501475 |
| 14. | Gao, B.; Ni, C.; Hu, J. Chimia 2014, 68, 414–418. doi:10.2533/chimia.2014.414 |
| 19. | Levin, V. V.; Zemtsov, A. A.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2015, 171, 97–101. doi:10.1016/j.jfluchem.2014.08.021 |
© 2015 Zemtsov et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)