Abstract
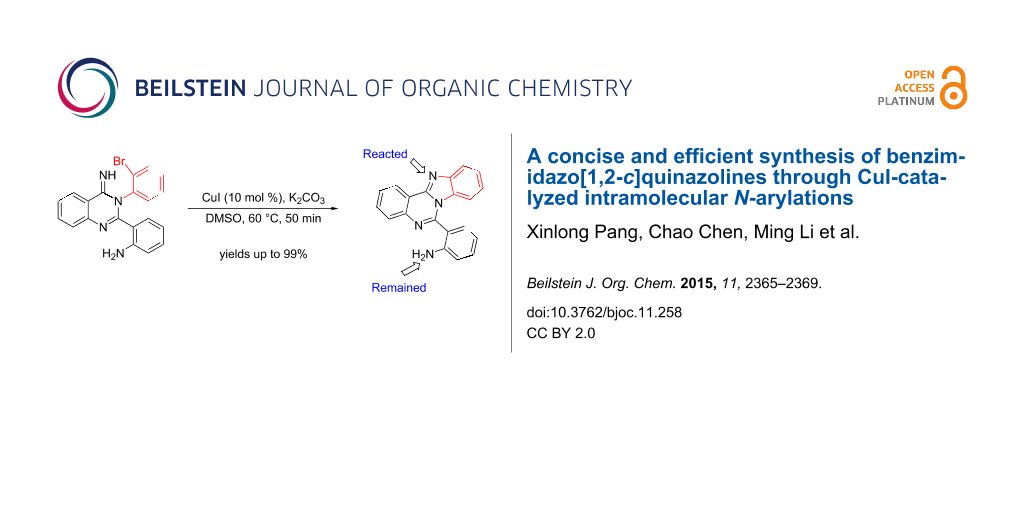
A series of functionalized benzimidazo[1,2-c]quinazoline derivatives was obtained in excellent yields under mild conditions through a CuI-catalyzed Ullmann N-arylation starting from easily available starting materials.
Graphical Abstract
Introduction
Nitrogen-containing heterocycles are ubiquitous backbones in natural products, medicine and organic materials. In addition, they are also important ligands for catalytic reactions. Recently, the conjugation of different types of azaheterocycles in the same molecule has received considerable attention since the resulting ring-fused molecules often show unique organic optoelectronic properties and bioactive activities [1,2]. Among them, benzimidazo[1,2-c]quinazolines were intensively investigated and promising biological activities were observed, such as anticancer, antiviral, antimicrobial, anti-inflammatory and anticonvulsant [3-5]. Indeed, some of them are already used as antimicrobial agents and lipid peroxidation inhibitors [6]. Consequently, the development of an efficient way to prepare various benzimidazo[1,2-c]quinazoline derivatives is highly desired. Although some methods for the synthesis of benzimidazo[1,2-c]quinazoline derivatives have been reported quite recently [7-12], they often require complicated starting materials that are not readily available and need harsh conditions. Herein we report a CuI-catalyzed concise and efficient method for the synthesis of benzimidazo[1,2-c]quinazoline derivatives through the intramolecular N-arylation reaction of bromo-substituted quinazolin-4(3H)-imines that are easily prepared from o-cyanoaniline (1) and diaryliodonium salts 2 based on our previously published method [13,14] (Scheme 1).
Scheme 1: CuI-catalyzed synthesis of benzimidazo[1,2-c]quinazolines 4 by intramolecular N-arylation of bromo-substituted quinazolin-4(3H)-imine derivatives 3.
Scheme 1: CuI-catalyzed synthesis of benzimidazo[1,2-c]quinazolines 4 by intramolecular N-arylation of bromo-...
Results and Discussion
During the study of the synthesis of various carbocycles or heterocycles with copper catalysts [13-17], we found an interesting tandem reaction of o-cyanoanilines 1 and diaryliodonium salts 2 to produce quinazolin-4(3H)-imine derivatives 3 with Cu(OTf)2 as the catalyst [13]. Encouraged by this finding, we initially attempted the reaction of o-cyanoaniline (1a) with di-(o-bromophenyl)iodonium salt 2. The reaction of 2 equiv of o-cyanoaniline (1a) with 2 in DCE at 110 °C for 6 h in the presence of 20 mol % Cu(OTf)2 bromo-substituted quinazolin-4(3H)-imine derivative 3a in 82% isolated yield. The subsequent treatment of 3a with CuI (0.1 equiv) and K2CO3 (1 equiv) in DMSO at room temperature for 50 min led to benzimidazo[1,2-c]quinazoline derivative 4a in 37% yield (Table 1, entry 1). To optimize the yield of the desired product 4a different conditions were screened. When the reaction temperature was increased to 60 °C, compound 4a was formed in 98% yield (96% isolated, Table 1, entry 3). On the other hand, the replacement of DMSO by other solvents led to lower yields of 4a even at elevated temperatures (Table 1, entries 5–9). Other copper salts such as Cu(OTf)2, CuBr or CuCl were also able to catalyze the reaction, but they were not as efficient as CuI as the catalyst (Table 1, entries 5–9). It is worth mentioning that the imino group (sp2) other than the amino group (sp3) in 3a reacted through the Cu-catalyzed Ullmann reaction [18-25].
Table 1: Optimization of reaction conditions for the synthesis of benzimidazo[1,2-c]quinazoline 4a from quinazolin-4(3H)-imine derivative 3a.
|
|
||||
| Entry | Cu salt | Temperature (°C) | Solvent | Yield (%)a |
|---|---|---|---|---|
| 1 | CuI | rt | DMSO | 37 |
| 2 | CuI | 40 | DMSO | 72 |
| 3 | CuI | 60 | DMSO | 98 (96b) |
| 4 | CuI | 110 | DMSO | 98 |
| 5 | CuI | 110 | DCE | 51 |
| 6 | CuI | 110 | CH3CN | 31 |
| 7 | CuI | 110 | DCM | 43 |
| 8 | CuI | 110 | toluene | 89 |
| 9 | CuI | 110 | DCE | 51 |
| 10 | Cu(OTf)2 | 60 | DMSO | 82 |
| 11 | CuBr | 60 | DMSO | 86 |
| 12 | CuCl | 60 | DMSO | 91 |
aEstimated crude yield by NMR with trichloroethylene as internal standard. bIsolated yield.
Inspired by the successful cyclization of quinazolin-4(3H)-imine 3a, further imines were prepared and subjected to the cyclization conditions. Notably, in this protocol, after work-up, the desired bromo-substituted quinazolin-4(3H)-imine derivatives 3 were directly employed in the next step reaction without the need for chromatographic purification and the results are summarized in Table 2. Quinazolin-4(3H)-imines 3 having methyl, fluoro or chloro substituents all worked well in the reaction and provided the corresponding quinazolines 4 in high yields (Table 2, entries 2, 3 and 6). In addition changing the position of the fluoro substituent did not affect the yield of the products (Table 2, entries 3–5).
Table 2: CuI-catalyzed synthesis of benzimidazo[1,2-c]quinazolines 4 from bromo-substituted quinazolin-4(3H)-imines 3.
| Entry | Bromo-substituted quinazolin-4(3H)-imine 3 | Benzimidazo[1,2-c]quinazoline 4 | Yielda |
|---|---|---|---|
| 1 |
3a |
4a |
96% |
| 2 |
3b |
4b |
95% |
| 3 |
3c |
4c |
95% |
| 4 |
3d |
4d |
94% |
| 5 |
3e |
4e |
93% |
| 6 |
3f |
4f |
96% |
aIsolated yield.
To further expand the scope of the protocol, we attempted the synthesis of imine 3g starting from two different nitriles. The reaction of o-cyanoaniline (1a), benzonitrile (1g) and di-(o-bromophenyl)iodonium salt 2 in the presence of Cu(OTf)2 gave the desired imine 3g together with imine 3a. After isolation of 3g it was further treated with 10 mol % of CuI in DMSO for 50 min to give product 4g in quantitative yield (Scheme 2).
Scheme 2: Cu-catalyzed reaction of o-cyanoaniline (1a), benzonitrile (1g) and di-(o-bromophenyl)iodonium salt 2 producing imine 3g and its subsequent cyclization in the presence of CuI.
Scheme 2: Cu-catalyzed reaction of o-cyanoaniline (1a), benzonitrile (1g) and di-(o-bromophenyl)iodonium salt ...
It is worth mentioning that during the course of our study, we observed that products 4 were not stable to acid. For example, treatment of 4c with aqueous HCl solution led to ring-opening product 5 (Scheme 3). The structure of 5 was confirmed by X-ray diffraction analysis (Figure 1), clearly showing the cleavage of the quinazoline ring rather than the imidazole ring [26].
Scheme 3: Acid-promoted ring-opening reaction from quinazoline 4c to 5.
Scheme 3: Acid-promoted ring-opening reaction from quinazoline 4c to 5.
![[1860-5397-11-258-1]](/bjoc/content/figures/1860-5397-11-258-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: ORTEP drawing of 5, [C20H16F2N4O]·2Cl·H2O with 35% probability ellipsoids, showing the atomic numbering scheme.
Figure 1: ORTEP drawing of 5, [C20H16F2N4O]·2Cl·H2O with 35% probability ellipsoids, showing the atomic numbe...
Conclusion
We have demonstrated a CuI-catalyzed pathway to produce functionalized benzimidazo[1,2-c]quinazoline derivatives from bromo-substituted quinazolin-4(3H)-imines through a selective intramolecular N-arylation reaction. The bromo-substituted quinazolin-4(3H)-imines are easily synthesized from readily available o-cyanoanilines and di-(o-bromophenyl)iodonium salt. The extension of the reaction and the investigation of the biological activity of the new products are currently under progress in our laboratory.
Supporting Information
| Supporting Information File 1: Full experimental procedures, characterization data, and NMR charts for compounds 3a–g and 4a–g. | ||
| Format: PDF | Size: 1.4 MB | Download |
References
-
Michael, J. P. Nat. Prod. Rep. 2008, 25, 166–187. doi:10.1039/b612168n
Return to citation in text: [1] -
Beniddir, M. A.; Le Borgne, E.; Iorga, B. I.; Loaëc, N.; Lozach, O.; Meijer, L.; Awang, K.; Litaudon, M. J. Nat. Prod. 2014, 77, 1117–1122. doi:10.1021/np400856h
Return to citation in text: [1] -
Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930. doi:10.1021/cr020033s
Return to citation in text: [1] -
Sang, P.; Xie, Y.; Zou, J.; Zhang, Y. Org. Lett. 2012, 14, 3894–3897. doi:10.1021/ol3016435
Return to citation in text: [1] -
Khajavi, M. S.; Rad-moghadam, K.; Hazarkhani, H. Synth. Commun. 1999, 29, 2617–2624. doi:10.1080/00397919908086422
Return to citation in text: [1] -
Insuasty, B. A.; Torres, H.; Quiroga, J.; Abonia, R.; Rodriguez, R.; Nogueras, M.; Sánchez, A.; Saitz, C.; Alvarez, S. L.; Zacchino, S. A. J. Chil. Chem. Soc. 2006, 51, 927–932. doi:10.4067/S0717-97072006000200018
Return to citation in text: [1] -
Xu, S.; Lu, J.; Fu, H. Chem. Commun. 2011, 47, 5596–5598. doi:10.1039/c1cc10383k
Return to citation in text: [1] -
Liu, Q.; Yang, H.; Jiang, Y.; Zhao, Y.; Fu, H. RSC Adv. 2013, 3, 15636–15644. doi:10.1039/c3ra41644e
Return to citation in text: [1] -
Lamazzi, C.; Léonce, S.; Pfeiffer, B.; Renard, P.; Guillaumet, G.; Rees, C. W.; Besson, T. Bioorg. Med. Chem. Lett. 2000, 10, 2183–2185. doi:10.1016/S0960-894X(00)00427-3
Return to citation in text: [1] -
Rohini, R.; Shanker, K.; Reddy, P. M.; Ho, Y.-P.; Ravinder, V. Eur. J. Med. Chem. 2009, 44, 3330–3339. doi:10.1016/j.ejmech.2009.03.022
Return to citation in text: [1] -
Frère, S.; Thiéry, V.; Bailly, C.; Besson, T. Tetrahedron 2003, 59, 773–779. doi:10.1016/S0040-4020(02)01593-4
Return to citation in text: [1] -
Molina, P.; Alajarin, M.; Vidal, A. Tetrahedron Lett. 1988, 29, 3849–3852. doi:10.1016/S0040-4039(00)82131-0
Return to citation in text: [1] -
Pang, X.; Chen, C.; Su, X.; Li, M.; Wen, L. Org. Lett. 2014, 16, 6228–6231. doi:10.1021/ol503156g
Return to citation in text: [1] [2] [3] -
Pang, X.; Lou, Z.; Li, M.; Wen, L.; Chen, C. Eur. J. Org. Chem. 2015, 3361–3369. doi:10.1002/ejoc.201500161
Return to citation in text: [1] [2] -
Wang, Y.; Chen, C.; Peng, J.; Li, M. Angew. Chem., Int. Ed. 2013, 52, 5323–5327. doi:10.1002/anie.201300586
Return to citation in text: [1] -
Su, X.; Chen, C.; Wang, Y.; Chen, J.; Lou, Z.; Li, M. Chem. Commun. 2013, 49, 6752–6754. doi:10.1039/C3CC43216E
Return to citation in text: [1] -
Wang, Y.; Chen, C.; Zhang, S.; Lou, Z.; Su, X.; Wen, L. Org. Lett. 2013, 15, 4794–4797. doi:10.1021/ol402164s
Return to citation in text: [1] -
Zhou, F.; Guo, J.; Liu, J.; Ding, K.; Yu, S.; Cai, Q. J. Am. Chem. Soc. 2012, 134, 14326–14329. doi:10.1021/ja306631z
Return to citation in text: [1] -
Jones, G. O.; Liu, P.; Houk, K. N.; Buchwald, S. L. J. Am. Chem. Soc. 2010, 132, 6205–6213. doi:10.1021/ja100739h
Return to citation in text: [1] -
Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r
Return to citation in text: [1] -
Corbet, J.-P.; Mignani, G. Chem. Rev. 2006, 106, 2651–2710. doi:10.1021/cr0505268
Return to citation in text: [1] -
Wen, L.-R.; Yuan, W.-K.; Li, M. J. Org. Chem. 2015, 80, 4942–4949. doi:10.1021/acs.joc.5b00288
Return to citation in text: [1] -
Huang, X.; Yang, H.; Fu, H.; Qiao, R.; Zhao, Y. Synthesis 2009, 2679–2688. doi:10.1055/s-0029-1216871
Return to citation in text: [1] -
Wan, J.-P.; Wang, H.; Liu, Y.; Ding, H. Org. Lett. 2014, 16, 5160–5163. doi:10.1021/ol502506g
Return to citation in text: [1] -
Liu, Y.; Wan, J.-P. Chem. – Asian J. 2012, 7, 1488–1501. doi:10.1002/asia.201101063
Return to citation in text: [1] -
CCDC number: 1421043. The single crystal suitable for X-ray diffraction analysis was obtained by slow evaporation from a methanol/cyclohexane solution.
Return to citation in text: [1]
| 1. | Michael, J. P. Nat. Prod. Rep. 2008, 25, 166–187. doi:10.1039/b612168n |
| 2. | Beniddir, M. A.; Le Borgne, E.; Iorga, B. I.; Loaëc, N.; Lozach, O.; Meijer, L.; Awang, K.; Litaudon, M. J. Nat. Prod. 2014, 77, 1117–1122. doi:10.1021/np400856h |
| 13. | Pang, X.; Chen, C.; Su, X.; Li, M.; Wen, L. Org. Lett. 2014, 16, 6228–6231. doi:10.1021/ol503156g |
| 14. | Pang, X.; Lou, Z.; Li, M.; Wen, L.; Chen, C. Eur. J. Org. Chem. 2015, 3361–3369. doi:10.1002/ejoc.201500161 |
| 7. | Xu, S.; Lu, J.; Fu, H. Chem. Commun. 2011, 47, 5596–5598. doi:10.1039/c1cc10383k |
| 8. | Liu, Q.; Yang, H.; Jiang, Y.; Zhao, Y.; Fu, H. RSC Adv. 2013, 3, 15636–15644. doi:10.1039/c3ra41644e |
| 9. | Lamazzi, C.; Léonce, S.; Pfeiffer, B.; Renard, P.; Guillaumet, G.; Rees, C. W.; Besson, T. Bioorg. Med. Chem. Lett. 2000, 10, 2183–2185. doi:10.1016/S0960-894X(00)00427-3 |
| 10. | Rohini, R.; Shanker, K.; Reddy, P. M.; Ho, Y.-P.; Ravinder, V. Eur. J. Med. Chem. 2009, 44, 3330–3339. doi:10.1016/j.ejmech.2009.03.022 |
| 11. | Frère, S.; Thiéry, V.; Bailly, C.; Besson, T. Tetrahedron 2003, 59, 773–779. doi:10.1016/S0040-4020(02)01593-4 |
| 12. | Molina, P.; Alajarin, M.; Vidal, A. Tetrahedron Lett. 1988, 29, 3849–3852. doi:10.1016/S0040-4039(00)82131-0 |
| 6. | Insuasty, B. A.; Torres, H.; Quiroga, J.; Abonia, R.; Rodriguez, R.; Nogueras, M.; Sánchez, A.; Saitz, C.; Alvarez, S. L.; Zacchino, S. A. J. Chil. Chem. Soc. 2006, 51, 927–932. doi:10.4067/S0717-97072006000200018 |
| 3. | Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930. doi:10.1021/cr020033s |
| 4. | Sang, P.; Xie, Y.; Zou, J.; Zhang, Y. Org. Lett. 2012, 14, 3894–3897. doi:10.1021/ol3016435 |
| 5. | Khajavi, M. S.; Rad-moghadam, K.; Hazarkhani, H. Synth. Commun. 1999, 29, 2617–2624. doi:10.1080/00397919908086422 |
| 26. | CCDC number: 1421043. The single crystal suitable for X-ray diffraction analysis was obtained by slow evaporation from a methanol/cyclohexane solution. |
| 18. | Zhou, F.; Guo, J.; Liu, J.; Ding, K.; Yu, S.; Cai, Q. J. Am. Chem. Soc. 2012, 134, 14326–14329. doi:10.1021/ja306631z |
| 19. | Jones, G. O.; Liu, P.; Houk, K. N.; Buchwald, S. L. J. Am. Chem. Soc. 2010, 132, 6205–6213. doi:10.1021/ja100739h |
| 20. | Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r |
| 21. | Corbet, J.-P.; Mignani, G. Chem. Rev. 2006, 106, 2651–2710. doi:10.1021/cr0505268 |
| 22. | Wen, L.-R.; Yuan, W.-K.; Li, M. J. Org. Chem. 2015, 80, 4942–4949. doi:10.1021/acs.joc.5b00288 |
| 23. | Huang, X.; Yang, H.; Fu, H.; Qiao, R.; Zhao, Y. Synthesis 2009, 2679–2688. doi:10.1055/s-0029-1216871 |
| 24. | Wan, J.-P.; Wang, H.; Liu, Y.; Ding, H. Org. Lett. 2014, 16, 5160–5163. doi:10.1021/ol502506g |
| 25. | Liu, Y.; Wan, J.-P. Chem. – Asian J. 2012, 7, 1488–1501. doi:10.1002/asia.201101063 |
| 13. | Pang, X.; Chen, C.; Su, X.; Li, M.; Wen, L. Org. Lett. 2014, 16, 6228–6231. doi:10.1021/ol503156g |
| 13. | Pang, X.; Chen, C.; Su, X.; Li, M.; Wen, L. Org. Lett. 2014, 16, 6228–6231. doi:10.1021/ol503156g |
| 14. | Pang, X.; Lou, Z.; Li, M.; Wen, L.; Chen, C. Eur. J. Org. Chem. 2015, 3361–3369. doi:10.1002/ejoc.201500161 |
| 15. | Wang, Y.; Chen, C.; Peng, J.; Li, M. Angew. Chem., Int. Ed. 2013, 52, 5323–5327. doi:10.1002/anie.201300586 |
| 16. | Su, X.; Chen, C.; Wang, Y.; Chen, J.; Lou, Z.; Li, M. Chem. Commun. 2013, 49, 6752–6754. doi:10.1039/C3CC43216E |
| 17. | Wang, Y.; Chen, C.; Zhang, S.; Lou, Z.; Su, X.; Wen, L. Org. Lett. 2013, 15, 4794–4797. doi:10.1021/ol402164s |
© 2015 Pang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)