Abstract
A novel series of 2’-oxa-3’-aza-4’a-carbanucleosides, featured with a triazole linker at the 5’-position, has been developed by exploiting a click chemistry reaction of 5’-azido-2’-oxa-3’-aza-4’a-carbanucleosides with substituted alkynes. Biological tests indicate an antitumor activity for the synthesized compounds: most of them inhibit cell proliferation of Vero, BS-C-1, HEp-2, MDCK, and HFF cells with a CC50 in the range of 5.0–40 μM. The synthesized compounds do not show any antiviral activity.
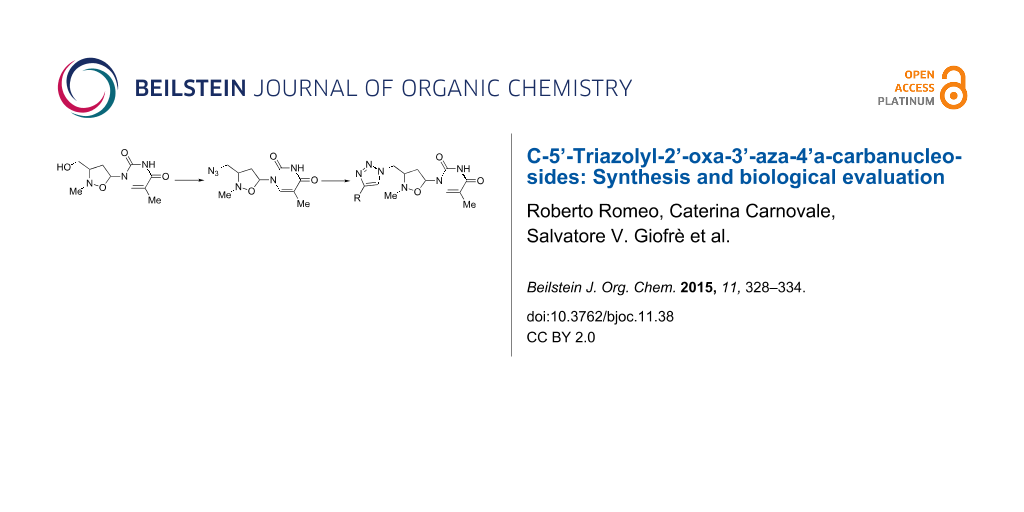
Graphical Abstract
Introduction
Synthetic modified nucleosides are of great interest as potential new lead structures in particular as antiviral or anticancer agents [1-8]. As analogues these compounds can interfere in nucleic acid synthesis or block nucleosides- and/or nucleotide-dependent biological processes by mimicking natural nucleosides and serving as inhibitors or building units [9-12]. Many structural variations of the natural nucleosides have been exploited. In general, the performed modifications included the replacement of the furanose moiety by other carbon or heterocyclic systems [13,14] or even acyclic fragments [15,16], the substitution of pyrimidine or purine natural nucleobases with unnaturally-substituted heteroaromatics or homoaromatic systems, or the modification of the phosphate P(O)–O–C bond with the non–hydrolyzable phosphonate P(O)–C linkage [17,18].
In this context, nucleoside analogues, where different carbon or heterocyclic systems replace the furanose ring, have been reported as anticancer or antiviral agents [19,20]. In particular, 2’-oxa-3’-aza-4’a-carbanucleosides 1–4, characterized by the presence of an isoxazolidine ring, represent a scaffold of modified dideoxynucleosides endowed with interesting physiological features (Figure 1) [21-27].
Figure 1: 2’-Oxa-3’-aza-modified nucleosides and 2’-oxa-3’-aza-modified nucleotides.
Figure 1: 2’-Oxa-3’-aza-modified nucleosides and 2’-oxa-3’-aza-modified nucleotides.
2’-Oxa-3’-aza-4’a-carbanucleosides 1–4 can be considered as mimics of natural nucleosides and act as terminators of the viral DNA chain. Their antiviral activity is linked to the competitive reversible inhibition of the reverse transcriptase. Furthermore, as antimetabolites, they can interact with intracellular targets to induce cytotoxicity [28-32].
Several functionalities have been inserted as linkers on the 2’-oxa-3’-aza-4’a-carbanucleoside skeleton in order to confer novel mechanisms of action for nucleoside mimics: in this context, the 1,2,3-triazole unit assumes particular interest according to its easily access and the well-known biological activity of many derivatives. In these last years, in fact, triazoles have gained considerable attention in medicinal chemistry, bioconjugation, drug-delivery, and materials science [33-38]. Moreover, the 1,2,3-triazole motif is exceedingly stable to basic or acidic hydrolysis and interacts strongly with biological targets through hydrogen bonding to nitrogen atoms as well as through dipole–dipole and π-stacking interactions [39].
Recently, a synthetic approach towards 3-hydroxymethyl-5-(1H-1,2,3-triazol)-isoxazolidines 5 has been described [40]: the obtained compounds inhibit the growth of anaplastic and follicular human thyroid cancer cell lines, with IC50 values in the range of 3.87–8.76 μM. In the same context, novel 1,2,3-triazole-appended 2’-oxa-3’-azanucleoside analogs 6 were developed [41]: Some of these compounds show a good anticancer activity against the anaplastic (8305C) and the follicular (FTC-133) human thyroid cancer cell lines, and especially on the U87MG human primary glioblastoma cell line (Figure 2).
Figure 2: Triazolyl-2’-oxa-3’-aza-4’a-carbanucleosides.
Figure 2: Triazolyl-2’-oxa-3’-aza-4’a-carbanucleosides.
Accordingly, considering that the incorporation of the triazole moiety can lead to interesting biological properties, we report in this paper the preparation of a small library of nucleoside analogues 7 (Figure 2), where the furanose ring is substituted by an isoxazolidine system and a triazole unit replaces the phosphodiester linker at 5’ position of the 2’-oxa-3’-aza-4’a-carbanucleoside. However, in order to maintain the six-bond periodicity of the oligonucleotides and thus the flexibility of the oligonucleotide chain the methylene bridge at the pseudo-5’-position was retained. The obtained compounds have shown to be endowed with an interesting antitumor activity: most of them inhibit cell proliferation of Vero, BS-C-1, HEp-2, MDCK, and HFF cells by 50% (CC50) at concentrations in the range of 5.0–40.0 μM. No antiviral activity against both RNA and DNA viruses was observed.
Results and Discussion
Chemistry
The synthetic route to 5’-triazolyl-2’-oxa-3’-aza-4’a-carbanucleosides 13 and 14 is described in Scheme 1 (and Table 1). (3′RS,5′SR)-2′-N-methyl-3′-hydroxymethyl-1′,2′-isoxazolidin-5′-ylthymine 8, obtained as the main compound, in a two-step process, by 1,3-dipolar cycloaddition of vinyl acetate to C-[(tert-butyldiphenylsilyl)oxy]-N-methylnitrone, followed by Hilbert–Jones nucleosidation using silylated thymine and TBAF [42-44], was converted into the corresponding iodo-derivative 10 by sequential tosylation and iodination.
Scheme 1: Synthesis of triazolyl isoxazolidinyl-nucleosides 13 and 14. Reagents and conditions: a) Tosyl chloride, TEA, CH2Cl2, rt, 24 h; b) NaI, acetone, reflux, 72 h; c) NaN3, CH3CN/H2O (1:10) in the presence of NH4Cl, 50 °C for 48 h; d) substituted alkynes, 17a–g, CuSO4·5H2O, sodium ascorbate, TEA, rt, 5 h.
Scheme 1: Synthesis of triazolyl isoxazolidinyl-nucleosides 13 and 14. Reagents and conditions: a) Tosyl chlo...
Table 1: C-5’-Triazolyl-2’-oxo-3’-aza-4’a-carbanucleosides 13a–g and 14a–g produced via click chemistry.
| Alkyne | R1 | Product | Yielda | Product | Yielda |
|---|---|---|---|---|---|
| 17a | –CH2CH2OH | 13a | 88 | 14a | 79 |
| 17b | –CH2OH | 13b | 84 | 14b | 81 |
| 17c | –CH2CH2CH3 | 13c | 80 | 14c | 83 |
| 17d |
|
13d | 78 | 14d | 82 |
| 17e |
|
13e | 78 | 14e | 82 |
| 17f |
|
13f | 85 | 14f | 84 |
| 17g |
|
13g | 89 | 14g | 85 |
aIsolated yield by flash chromatography.
The subsequent reaction of 10 with sodium azide, performed at 50 °C in CH3CN/H2O (1:10) in the presence of NH4Cl for 48 h afforded two azides, 11 and 12, epimeric at C-5, in a relative ratio 2:1 with a global yield of 85%. Two azides were separated by flash chromatography (CH2Cl2/MeOH 98:2 as eluent). Compound 12 originates from 11: its formation can be rationalized by considering that the acidic medium of the reaction, linked to the presence of NH4Cl, promotes an equilibrium process which starts from 11 and leads to a mixture of α- and β-anomers, via the intermediate oxonium ion 15 (path a) or 16 (path b) (Figure 3). As reported in similar systems [45], in the equilibrium mixture the β-anomer 11, thermodynamically more stable, predominates.
The structure of the obtained compounds was determined by spectroscopic data and MS analysis: the main product of the reaction was the cis derivative. NOE measurements confirm the assigned stereochemistry. For compound 11, the cis isomer, irradiation of the H-5 resonance at 5.99 ppm (as doublet of doublets) induced a positive NOE effect on H-3 resonance at 3.85–4.00 ppm (as a multiplet) and on H-4b proton (2.34–2.42 ppm, multiplet) (Scheme 1). Accordingly, in the trans derivative 12, on irradiating H-5 resonance (6.14 ppm; doublet of doublets), a positive NOE effect was detected only for the H-4a proton that resonates at 2.18 ppm as a doublet of doublet of doublets.
5’-Azido-2’-oxa-3’-aza-4’a-carbanucleosides 11 and 12 were independently engaged in a CuI-catalyzed Huisgen [3 + 2] cycloaddition reaction with a series of substituted alkynes 17, according to the procedure described by Sharpless [46] (Scheme 1 and Table 1). The click chemistry process, carried out with equimolar amounts of the respective dipolarophiles, afforded in all the cases the corresponding C-5’-triazolyl-2’-oxa-3’-aza-4’a-carbanucleosides 13 and 14 in good yields (79–89%). According to other copper-catalyzed azide–alkyne cycloadditions, no traces of 1,5-regioisomers were observed [47,48].
The structure of the obtained compounds was assessed according to 1H NMR, 13C NMR and MS data. In particular, the 1H NMR spectra of 5-methyl-1-[(3RS,5SR)-2-methyl-3-(1H-1,2,3-triazol-1-ylmethyl)isoxazolidin-5-yl]pyrimidine-2,4(1H,3H)diones 13 and 5-methyl-1-[(3RS,5RS)-2-methyl-3-(1H-1,2,3-triazol-1-ylmethyl)isoxazolidin-5-yl]pyrimidine-2,4(1H,3H)diones 14 show, besides the resonances of the protons of the isoxazolidine unit, diagnostic resonances at 7.25–7.75 ppm, as a singlet, for the proton of the triazole system, and at 4.50–5.10 and 4.25–4.75 ppm, respectively in 13 and 14, as a doublet of doublets, for the methylene group at C-4’ position.
Biological tests
The antiproliferative effect of the obtained derivatives was tested on a panel of cell lines: african green monkey kidney cells (Vero and BS-C-1), human epidermoid carcinoma larynx cells (HEp-2), Madin–Darby canine kidney (MDCK), and human foreskin fibroblast cells (HFF). In these assays the cells were in the logarithmic phase of growth.
Inhibition of cell proliferation, with a CC50 ranging from 5 to 40 µM (Table 2), has been observed for all the new synthesized compounds. In particular, compound 14d showed a high level of inhibitory activity with CC50 values of 5 μM for all the utilized cell lines, while compounds 13c, 13e, 13d, 14c, 14e, 14f and 14g show the same CC50 values only for HFF cells.
Table 2: Biological activity of C-5’-triazolyl-2’-oxa-3’-aza-4’a-carbanucleosides 13a–g and 14a–g.
| CC50 μMa | |||||
|---|---|---|---|---|---|
| Compound | VERO | HEp2 | MDCK | HFF | BS-C-1 |
| 13a | 10 | 40 | 10 | 10 | 10 |
| 14a | 20 | 40 | 20 | 20 | 20 |
| 13b | 40 | 40 | 30 | 30 | 30 |
| 14b | 20 | 40 | 20 | 20 | 10 |
| 13c | 20 | 40 | 20 | 5 | 20 |
| 14c | 20 | 40 | 20 | 5 | 20 |
| 13d | 20 | 20 | 20 | 5 | 20 |
| 14d | 5 | 5 | 5 | 5 | 5 |
| 13e | 20 | 40 | 20 | 5 | 20 |
| 14e | 20 | 40 | 20 | 5 | 20 |
| 13f | 10 | 40 | 10 | 40 | 10 |
| 14f | 20 | 40 | 20 | 5 | 20 |
| 13g | 10 | 20 | 10 | 5 | 10 |
| 14g | 10 | 20 | 10 | 5 | 10 |
aCC50: Concentration which inhibited cell growth by 50% as compared with control cultures. Values are mean ± 0.5 S.D. (estimated maximal standard deviation) of three separate assays.
Noteworthy, the relative cis, trans configuration of 13 and 14 does not seem to affect the biological effect. The cytostatic activity of the compounds was particularly exploited against HFF cell proliferation.
According to our initial hypothesis, the presence of the triazole linker at C-5’ position in the 2’-oxa-3’-aza-4’a-carbanucleoside skeleton induces a different biological effect with respect to 2’-oxa-3’-aza-4’a-carbanucleosides devoid of the triazole unit, such as compounds 2 and 8, which are endowed with antiviral activity, but do not show any cytotoxicity
The ability of compounds 13a–g and 14a–g to interfere with the replication of different DNA and RNA viruses was also evaluated, by using the subsequent cell-virus tests: (a) Vero cell for poliovirus 1, human echovirus 9, herpes simplex type 1 (HSV-1); (b) HEp-2 cell for Coxsackievirus B1, adenovirus type 2; (c) human foreskin fibroblast cells (HFF) for cytomegalovirus (CMV); (d) BS-C-1 cell (African green monkey kidney) for varicella-zoster virus (VZV); (e) Madin–Darby canine kidney (MDCK) for influenza virus A/Puerto Rico/8/34 H1N1 (PR8). Acyclovir was used as the reference compound. For the synthesized compounds, no inhibitory activity against any virus was detected until 250 μM.
Biological assays
Cells. Biological assays have been performed on African green monkey kidney cells (Vero and BS-C-1), human epithelial type 2 cells (HEp-2), human foreskin fibroblast cells (HFF), Madin-Darby canine kidney (MDCK). All cell lines were obtained from the American Type Culture Collection. The cell cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2 and grown in D-MEM (Dulbecco's modified Eagle’s Minimum Essential medium) supplemented with 10% FCS (fetal calf serum, 2 mM/L glutamine, 0.1% sodium bicarbonate, 200 μg/mL of streptomycin and 200 units/mL of penicillin G. The maintenance medium (DMEM with 2% heat inactivated FCS) was used to culture the viruses.
Cell viability. The cytotoxicity of the tested compounds was evaluated by measuring the effect created on cell morphology and/or cell growth (cytostatic activity). Cell monolayers were prepared in 24-well tissue culture plates and exposed to various concentrations of the compounds. Cytotoxicity was recorded as morphological variations (such as rounding up, shrinking and detachment) at 24, 48, 72 and 96 h, using light microscopy. Cytotoxicity was expressed as the minimum cytotoxic concentration (MCC) that caused a microscopically detectable variation of cell morphology. The extent of cytostatic activity was measured as inhibition of cell growth using the MTT method, as previously described [49,50]. The 50% cytotoxic dose (CC50) is the compound concentration required to reduce cell proliferation by 50% relative to the absorbance of the untreated control. CC50 values were estimated from graphic plots of the percentage of control as a function of the concentration of the test compounds.
Test compounds. Compounds 13 and 14 were dissolved in DMSO and diluted in maintenance medium to achieve the final required concentration. The final dilution of test compounds contained a maximum concentration of 0.01% DMSO, which had no effect on the viability of the cell lines. Stock solutions of acycloguanosine (Sigma, USA) were prepared in distilled water, filtered through 0. 2 μm filter and stored at 4 °C until use.
Viruses. In the antiviral assays the following viruses were used: Poliovirus 1 (Sabin strain: VR-1562), Human echovirus 9 (VR-1050), Herpes simplex type 1 (HSV-1: VR-260), Coxsackievirus B1 (VR-28), adenovirus type 2 (VR-1080), Cytomegalovirus (CMV: VR-538), varicella-zoster virus (VZV: VR-1367), influenza virus A/Puerto Rico/8/34 H1N1 (PR8). Viruses were obtained from the American Type Culture Collection. The tests on the antiviral activity were carried out by the 50% plaque reduction assay or by 50% virus-induced cytopathogenicity, as previously described [51]. The concentration of the compound that inhibit the formation of viral plaques or virus-induced cytopathogenicity by 50% is expressed as EC50.
Conclusion
In summary, starting from 5’-azido-2’-oxa-3’-azanucleosides, a new series of C-5’-triazolyl-2’-oxo-3’-aza-4’a-carbanucleosides has been synthesized by using a CuI-catalyzed Huisgen [3 + 2] cycloaddition with substituted alkynes. The biological assays indicate that these compounds inhibit the cell proliferation of Vero, BS-C-1, HEp-2, MDCK, and HFF cells by 50% (CC50) at concentrations in the range of 5.0–40.0 μM. No antiviral activity at subtoxic concentrations was observed.
Supporting Information
| Supporting Information File 1: Preparation and analytical data of compounds 9–14. Copies of 1H and 13C NMR spectra of all new compounds. | ||
| Format: PDF | Size: 1.8 MB | Download |
References
-
Mehellou, Y.; De Clercq, E. J. Med. Chem. 2010, 53, 521–538. doi:10.1021/jm900492g
Return to citation in text: [1] -
Štambaský, J.; Hocek, M.; Kočovský, P. Chem. Rev. 2009, 109, 6729–6764. doi:10.1021/cr9002165
Return to citation in text: [1] -
Galmarini, C. M.; Popowycz, F.; Joseph, B. Curr. Med. Chem. 2008, 15, 1072–1082. doi:10.2174/092986708784221449
Return to citation in text: [1] -
Balestrieri, E.; Matteucci, C.; Ascolani, A.; Piperno, A.; Romeo, R.; Romeo, G.; Chiacchio, U.; Mastino, A.; Macchi, B. Antimicrob. Agents Chemother. 2008, 52, 54–64. doi:10.1128/AAC.00470-07
Return to citation in text: [1] -
De Clercq, E. Nat. Rev. Microbiol. 2004, 2, 704–720. doi:10.1038/nrmicro975
Return to citation in text: [1] -
Galmarini, C. M.; Mackey, J. R.; Dumontet, C. Lancet Oncol. 2002, 3, 415–424. doi:10.1016/S1470-2045(02)00788-X
Return to citation in text: [1] -
Pathak, T. Chem. Rev. 2002, 102, 1623–1668. doi:10.1021/cr0104532
Return to citation in text: [1] -
Ferrero, M.; Gotor, V. Chem. Rev. 2000, 100, 4319–4348. doi:10.1021/cr000446y
Return to citation in text: [1] -
Saag, M. S. Top. Antivir. Med. 2012, 20, 162–167.
Return to citation in text: [1] -
Bonate, P. L.; Arthaud, L.; Cantrell, W. R.; Stephenson, K.; Secrist, J. A.; Weitman, S. Nat. Rev. Drug Discovery 2006, 5, 855–863. doi:10.1038/nrd2055
Return to citation in text: [1] -
Hatse, S.; De Clercq, E.; Balzarini, J. Biochem. Pharmacol. 1999, 58, 539–555. doi:10.1016/S0006-2952(99)00035-0
Return to citation in text: [1] -
Lauria, F.; Benfenati, D.; Raspadori, D.; Rondelli, D.; Zinzani, P. L.; Tura, S. Leuk. Lymphoma 1993, 11, 399–404. doi:10.3109/10428199309067932
Return to citation in text: [1] -
Romeo, G.; Chiacchio, U.; Corsaro, A.; Merino, P. Chem. Rev. 2010, 110, 3337–3370. doi:10.1021/cr800464r
Return to citation in text: [1] -
Merino, P. Curr. Med. Chem. 2006, 13, 539–545. doi:10.2174/092986706776055779
Return to citation in text: [1] -
Hirota, K.; Monguchi, Y.; Sajiki, H. Synthesis of Purine Acyclonucleosides via Ribofuranose-Ring Cleavage of Purine Nucleosides by Diisobutylaluminum Hydride. In Recent Advances in Nucleosides: Chemistry and Chemotherapy; Chu, C. K., Ed.; Elsevier: Amsterdam, 2002; pp 57–70. doi:10.1016/B978-044450951-2/50003-5
Return to citation in text: [1] -
Littler, E.; Zhou, E. E. In Comprehensive Medicinal Chemistry II; Taylor, J. B.; Triggle, D. J., Eds.; Elsevier, 2006; Vol. 7, pp 295–327.
Return to citation in text: [1] -
Sharma, P. L.; Nurpeisov, V.; Hernandez-Santiago, B.; Beltran, T.; Schinazi, R. F. Curr. Top. Med. Chem. 2004, 4, 895–919. doi:10.2174/1568026043388484
Return to citation in text: [1] -
Bortolini, O.; Mulani, I.; De Nino, A.; Maiuolo, L.; Nardi, M.; Russo, B.; Avnet, S. Tetrahedron 2011, 67, 5635–5641. doi:10.1016/j.tet.2011.05.098
Return to citation in text: [1] -
Piperno, A.; Chiacchio, M. A.; Iannazzo, D.; Romeo, R. Curr. Med. Chem. 2006, 13, 3675–3695. doi:10.2174/092986706779026110
Return to citation in text: [1] -
Maiuolo, L.; Bortolini, O.; De Nino, A.; Russo, B.; Gavioli, R.; Sforza, F. Aust. J. Chem. 2014, 67, 670–674. doi:10.1071/CH13511
Return to citation in text: [1] -
Merino, P.; Tejero, T.; Unzurrunzaga, F. J.; Franco, S.; Chiacchio, U.; Saita, M. G.; Iannazzo, D.; Piperno, A.; Romeo, G. Tetrahedron: Asymmetry 2005, 16, 3865–3876. doi:10.1016/j.tetasy.2005.11.004
Return to citation in text: [1] -
Chiacchio, U.; Genovese, F.; Iannazzo, D.; Librando, V.; Merino, P.; Rescifina, A.; Romeo, R.; Procopio, A.; Romeo, G. Tetrahedron 2004, 60, 441–448. doi:10.1016/j.tet.2003.11.007
Return to citation in text: [1] -
Chiacchio, U.; Corsaro, A.; Pistarà, V.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Grassi, G. Eur. J. Org. Chem. 2002, 1206–1212. doi:10.1002/1099-0690(200204)2002:7<1206::AID-EJOC1206>3.0.CO;2-0
Return to citation in text: [1] -
Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Procopio, A.; Rescifina, A.; Romeo, G.; Romeo, R. Eur. J. Org. Chem. 2001, 1893–1898. doi:10.1002/1099-0690(200105)2001:10<1893::AID-EJOC1893>3.0.CO;2-K
Return to citation in text: [1] -
Romeo, R.; Carnovale, C.; Giofrè, S. V.; Monciino, G.; Chiacchio, M. A.; Sanfilippo, C.; Macchi, B. Molecules 2014, 19, 14406–14416. doi:10.3390/molecules190914406
Return to citation in text: [1] -
Romeo, R.; Navarra, M.; Giofrè, S. V.; Carnovale, C.; Cirmi, S.; Lanza, G.; Chiacchio, M. A. Bioorg. Med. Chem. 2014, 22, 3379–3385. doi:10.1016/j.bmc.2014.04.047
Return to citation in text: [1] -
Romeo, R.; Giofrè, S. V.; Garozzo, A.; Bisignano, B.; Corsaro, A.; Chiacchio, M. A. Bioorg. Med. Chem. 2013, 21, 5688–5693. doi:10.1016/j.bmc.2013.07.031
Return to citation in text: [1] -
Romeo, R.; Carnovale, C.; Giofrè, S. V.; Romeo, G.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Pistarà, V.; Chiacchio, U. Bioorg. Med. Chem. 2012, 20, 3652–3657. doi:10.1016/j.bmc.2012.03.047
Return to citation in text: [1] -
Piperno, A.; Giofrè, S. V.; Iannazzo, D.; Romeo, R.; Romeo, G.; Chiacchio, U.; Rescifina, A.; Piotrowska, D. G. J. Org. Chem. 2010, 75, 2798–2805. doi:10.1021/jo902485m
Return to citation in text: [1] -
Chiacchio, U.; Borrello, L.; Iannazzo, D.; Merino, P.; Piperno, A.; Rescifina, A.; Richichi, B.; Romeo, G. Tetrahedron: Asymmetry 2003, 14, 2419–2425. doi:10.1016/S0957-4166(03)00525-1
Return to citation in text: [1] -
Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Rescifina, A.; Romeo, R.; Romeo, G. Tetrahedron Lett. 2001, 42, 1777–1780. doi:10.1016/S0040-4039(00)02325-X
Return to citation in text: [1] -
Romeo, R.; Giofrè, S. V.; Iaria, D.; Sciortino, M. T.; Ronsisvalle, S.; Chiacchio, M. A.; Scala, A. Eur. J. Org. Chem. 2011, 5690–5695. doi:10.1002/ejoc.201100767
Return to citation in text: [1] -
Singhal, N.; Sharma, P. K.; Kumar, N.; Duhe, R. Chem. Biol. Interface 2011, 1, 338–348.
Return to citation in text: [1] -
Singh, R. J.; Singh, D. K. E-J. Chem. 2009, 6, 796–800. doi:10.1155/2009/419214
Return to citation in text: [1] -
Moorhouse, A. D.; Moses, J. E. ChemMedChem 2008, 3, 715–723. doi:10.1002/cmdc.200700334
Return to citation in text: [1] -
Lutz, J.-F. Angew. Chem., Int. Ed. 2007, 46, 1018–1025. doi:10.1002/anie.200604050
Return to citation in text: [1] -
Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a
Return to citation in text: [1] -
Tome, A. C. In Product class 13: 1,2,3-triazoles; Stor, R.; Gilchrist, T., Eds.; Science of Synthesis, Vol. 13; Thieme: New York, 2004; pp 415–601.
Return to citation in text: [1] -
Rowan, A. S.; Nicely, N. I.; Cochrane, N.; Wlassoff, W. A.; Claiborne, A.; Hamilton, C. J. Org. Biomol. Chem. 2009, 7, 4029–4036. doi:10.1039/b913066g
Return to citation in text: [1] -
Romeo, R.; Giofrè, S. V.; Carnovale, C.; Campisi, A.; Parenti, R.; Bandini, L.; Chiacchio, M. A. Bioorg. Med. Chem. 2013, 21, 7929–7937. doi:10.1016/j.bmc.2013.10.001
Return to citation in text: [1] -
Romeo, R.; Giofrè, S. V.; Carnovale, C.; Chiacchio, M. A.; Campisi, A.; Mancuso, R.; Cirmi, S.; Navarra, A. Eur. J. Org. Chem. 2014, 5442–5447. doi:10.1002/ejoc.201402106
Return to citation in text: [1] -
Carnovale, C.; Iannazzo, D.; Nicolosi, G.; Piperno, A.; Sanfilippo, C. Tetrahedron: Asymmetry 2009, 20, 425–429. doi:10.1016/j.tetasy.2009.02.026
Return to citation in text: [1] -
Chiacchio, U.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, R.; Borrello, L.; Sciortino, M. T.; Balestrieri, E.; Macchi, B.; Mastino, A.; Romeo, G. J. Med. Chem. 2007, 50, 3747–3750. doi:10.1021/jm070285r
Return to citation in text: [1] -
Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R. Tetrahedron: Asymmetry 2002, 58, 581–587. doi:10.1016/S0040-4020(01)01161-9
Return to citation in text: [1] -
Ward, D. I.; Jeffs, S. M.; Coe, P. L.; Walker, R. T. Tetrahedron Lett. 1993, 34, 6779–6782. doi:10.1016/S0040-4039(00)61700-8
Return to citation in text: [1] -
Kolb, H. C.; Finn, M. C.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Return to citation in text: [1] -
Spiteri, C.; Moses, J. E. Angew. Chem., Int. Ed. 2010, 49, 31–33. doi:10.1002/anie.200905322
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Denizot, F.; Lang, R. J. Immunol. Methods 1986, 89, 271–277.
Return to citation in text: [1] -
Cutrì, C. C. C.; Garozzo, A.; Siracusa, M. A.; Sarvà, M. C.; Tempera, G.; Geremia, E.; Pinizzotto, M. R.; Guerrera, F. Bioorg. Med. Chem. 1998, 6, 2271–2280. doi:10.1016/S0968-0896(98)80007-2
Return to citation in text: [1] -
Garozzo, A.; Cutrì, C. C. C.; Castro, A.; Tempera, G.; Guerrera, F.; Sarvà, M. C.; Geremia, E. Antiviral Res. 2000, 45, 199–210. doi:10.1016/S0166-3542(00)00072-3
Return to citation in text: [1]
| 49. | Denizot, F.; Lang, R. J. Immunol. Methods 1986, 89, 271–277. |
| 50. | Cutrì, C. C. C.; Garozzo, A.; Siracusa, M. A.; Sarvà, M. C.; Tempera, G.; Geremia, E.; Pinizzotto, M. R.; Guerrera, F. Bioorg. Med. Chem. 1998, 6, 2271–2280. doi:10.1016/S0968-0896(98)80007-2 |
| 51. | Garozzo, A.; Cutrì, C. C. C.; Castro, A.; Tempera, G.; Guerrera, F.; Sarvà, M. C.; Geremia, E. Antiviral Res. 2000, 45, 199–210. doi:10.1016/S0166-3542(00)00072-3 |
| 1. | Mehellou, Y.; De Clercq, E. J. Med. Chem. 2010, 53, 521–538. doi:10.1021/jm900492g |
| 2. | Štambaský, J.; Hocek, M.; Kočovský, P. Chem. Rev. 2009, 109, 6729–6764. doi:10.1021/cr9002165 |
| 3. | Galmarini, C. M.; Popowycz, F.; Joseph, B. Curr. Med. Chem. 2008, 15, 1072–1082. doi:10.2174/092986708784221449 |
| 4. | Balestrieri, E.; Matteucci, C.; Ascolani, A.; Piperno, A.; Romeo, R.; Romeo, G.; Chiacchio, U.; Mastino, A.; Macchi, B. Antimicrob. Agents Chemother. 2008, 52, 54–64. doi:10.1128/AAC.00470-07 |
| 5. | De Clercq, E. Nat. Rev. Microbiol. 2004, 2, 704–720. doi:10.1038/nrmicro975 |
| 6. | Galmarini, C. M.; Mackey, J. R.; Dumontet, C. Lancet Oncol. 2002, 3, 415–424. doi:10.1016/S1470-2045(02)00788-X |
| 7. | Pathak, T. Chem. Rev. 2002, 102, 1623–1668. doi:10.1021/cr0104532 |
| 8. | Ferrero, M.; Gotor, V. Chem. Rev. 2000, 100, 4319–4348. doi:10.1021/cr000446y |
| 17. | Sharma, P. L.; Nurpeisov, V.; Hernandez-Santiago, B.; Beltran, T.; Schinazi, R. F. Curr. Top. Med. Chem. 2004, 4, 895–919. doi:10.2174/1568026043388484 |
| 18. | Bortolini, O.; Mulani, I.; De Nino, A.; Maiuolo, L.; Nardi, M.; Russo, B.; Avnet, S. Tetrahedron 2011, 67, 5635–5641. doi:10.1016/j.tet.2011.05.098 |
| 46. | Kolb, H. C.; Finn, M. C.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 |
| 15. | Hirota, K.; Monguchi, Y.; Sajiki, H. Synthesis of Purine Acyclonucleosides via Ribofuranose-Ring Cleavage of Purine Nucleosides by Diisobutylaluminum Hydride. In Recent Advances in Nucleosides: Chemistry and Chemotherapy; Chu, C. K., Ed.; Elsevier: Amsterdam, 2002; pp 57–70. doi:10.1016/B978-044450951-2/50003-5 |
| 16. | Littler, E.; Zhou, E. E. In Comprehensive Medicinal Chemistry II; Taylor, J. B.; Triggle, D. J., Eds.; Elsevier, 2006; Vol. 7, pp 295–327. |
| 47. | Spiteri, C.; Moses, J. E. Angew. Chem., Int. Ed. 2010, 49, 31–33. doi:10.1002/anie.200905322 |
| 48. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 13. | Romeo, G.; Chiacchio, U.; Corsaro, A.; Merino, P. Chem. Rev. 2010, 110, 3337–3370. doi:10.1021/cr800464r |
| 14. | Merino, P. Curr. Med. Chem. 2006, 13, 539–545. doi:10.2174/092986706776055779 |
| 42. | Carnovale, C.; Iannazzo, D.; Nicolosi, G.; Piperno, A.; Sanfilippo, C. Tetrahedron: Asymmetry 2009, 20, 425–429. doi:10.1016/j.tetasy.2009.02.026 |
| 43. | Chiacchio, U.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, R.; Borrello, L.; Sciortino, M. T.; Balestrieri, E.; Macchi, B.; Mastino, A.; Romeo, G. J. Med. Chem. 2007, 50, 3747–3750. doi:10.1021/jm070285r |
| 44. | Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R. Tetrahedron: Asymmetry 2002, 58, 581–587. doi:10.1016/S0040-4020(01)01161-9 |
| 9. | Saag, M. S. Top. Antivir. Med. 2012, 20, 162–167. |
| 10. | Bonate, P. L.; Arthaud, L.; Cantrell, W. R.; Stephenson, K.; Secrist, J. A.; Weitman, S. Nat. Rev. Drug Discovery 2006, 5, 855–863. doi:10.1038/nrd2055 |
| 11. | Hatse, S.; De Clercq, E.; Balzarini, J. Biochem. Pharmacol. 1999, 58, 539–555. doi:10.1016/S0006-2952(99)00035-0 |
| 12. | Lauria, F.; Benfenati, D.; Raspadori, D.; Rondelli, D.; Zinzani, P. L.; Tura, S. Leuk. Lymphoma 1993, 11, 399–404. doi:10.3109/10428199309067932 |
| 45. | Ward, D. I.; Jeffs, S. M.; Coe, P. L.; Walker, R. T. Tetrahedron Lett. 1993, 34, 6779–6782. doi:10.1016/S0040-4039(00)61700-8 |
| 33. | Singhal, N.; Sharma, P. K.; Kumar, N.; Duhe, R. Chem. Biol. Interface 2011, 1, 338–348. |
| 34. | Singh, R. J.; Singh, D. K. E-J. Chem. 2009, 6, 796–800. doi:10.1155/2009/419214 |
| 35. | Moorhouse, A. D.; Moses, J. E. ChemMedChem 2008, 3, 715–723. doi:10.1002/cmdc.200700334 |
| 36. | Lutz, J.-F. Angew. Chem., Int. Ed. 2007, 46, 1018–1025. doi:10.1002/anie.200604050 |
| 37. | Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a |
| 38. | Tome, A. C. In Product class 13: 1,2,3-triazoles; Stor, R.; Gilchrist, T., Eds.; Science of Synthesis, Vol. 13; Thieme: New York, 2004; pp 415–601. |
| 40. | Romeo, R.; Giofrè, S. V.; Carnovale, C.; Campisi, A.; Parenti, R.; Bandini, L.; Chiacchio, M. A. Bioorg. Med. Chem. 2013, 21, 7929–7937. doi:10.1016/j.bmc.2013.10.001 |
| 28. | Romeo, R.; Carnovale, C.; Giofrè, S. V.; Romeo, G.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Pistarà, V.; Chiacchio, U. Bioorg. Med. Chem. 2012, 20, 3652–3657. doi:10.1016/j.bmc.2012.03.047 |
| 29. | Piperno, A.; Giofrè, S. V.; Iannazzo, D.; Romeo, R.; Romeo, G.; Chiacchio, U.; Rescifina, A.; Piotrowska, D. G. J. Org. Chem. 2010, 75, 2798–2805. doi:10.1021/jo902485m |
| 30. | Chiacchio, U.; Borrello, L.; Iannazzo, D.; Merino, P.; Piperno, A.; Rescifina, A.; Richichi, B.; Romeo, G. Tetrahedron: Asymmetry 2003, 14, 2419–2425. doi:10.1016/S0957-4166(03)00525-1 |
| 31. | Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Rescifina, A.; Romeo, R.; Romeo, G. Tetrahedron Lett. 2001, 42, 1777–1780. doi:10.1016/S0040-4039(00)02325-X |
| 32. | Romeo, R.; Giofrè, S. V.; Iaria, D.; Sciortino, M. T.; Ronsisvalle, S.; Chiacchio, M. A.; Scala, A. Eur. J. Org. Chem. 2011, 5690–5695. doi:10.1002/ejoc.201100767 |
| 41. | Romeo, R.; Giofrè, S. V.; Carnovale, C.; Chiacchio, M. A.; Campisi, A.; Mancuso, R.; Cirmi, S.; Navarra, A. Eur. J. Org. Chem. 2014, 5442–5447. doi:10.1002/ejoc.201402106 |
| 21. | Merino, P.; Tejero, T.; Unzurrunzaga, F. J.; Franco, S.; Chiacchio, U.; Saita, M. G.; Iannazzo, D.; Piperno, A.; Romeo, G. Tetrahedron: Asymmetry 2005, 16, 3865–3876. doi:10.1016/j.tetasy.2005.11.004 |
| 22. | Chiacchio, U.; Genovese, F.; Iannazzo, D.; Librando, V.; Merino, P.; Rescifina, A.; Romeo, R.; Procopio, A.; Romeo, G. Tetrahedron 2004, 60, 441–448. doi:10.1016/j.tet.2003.11.007 |
| 23. | Chiacchio, U.; Corsaro, A.; Pistarà, V.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Grassi, G. Eur. J. Org. Chem. 2002, 1206–1212. doi:10.1002/1099-0690(200204)2002:7<1206::AID-EJOC1206>3.0.CO;2-0 |
| 24. | Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Procopio, A.; Rescifina, A.; Romeo, G.; Romeo, R. Eur. J. Org. Chem. 2001, 1893–1898. doi:10.1002/1099-0690(200105)2001:10<1893::AID-EJOC1893>3.0.CO;2-K |
| 25. | Romeo, R.; Carnovale, C.; Giofrè, S. V.; Monciino, G.; Chiacchio, M. A.; Sanfilippo, C.; Macchi, B. Molecules 2014, 19, 14406–14416. doi:10.3390/molecules190914406 |
| 26. | Romeo, R.; Navarra, M.; Giofrè, S. V.; Carnovale, C.; Cirmi, S.; Lanza, G.; Chiacchio, M. A. Bioorg. Med. Chem. 2014, 22, 3379–3385. doi:10.1016/j.bmc.2014.04.047 |
| 27. | Romeo, R.; Giofrè, S. V.; Garozzo, A.; Bisignano, B.; Corsaro, A.; Chiacchio, M. A. Bioorg. Med. Chem. 2013, 21, 5688–5693. doi:10.1016/j.bmc.2013.07.031 |
| 19. | Piperno, A.; Chiacchio, M. A.; Iannazzo, D.; Romeo, R. Curr. Med. Chem. 2006, 13, 3675–3695. doi:10.2174/092986706779026110 |
| 20. | Maiuolo, L.; Bortolini, O.; De Nino, A.; Russo, B.; Gavioli, R.; Sforza, F. Aust. J. Chem. 2014, 67, 670–674. doi:10.1071/CH13511 |
| 39. | Rowan, A. S.; Nicely, N. I.; Cochrane, N.; Wlassoff, W. A.; Claiborne, A.; Hamilton, C. J. Org. Biomol. Chem. 2009, 7, 4029–4036. doi:10.1039/b913066g |
© 2015 Romeo et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)