Abstract
Highly efficient catalytic asymmetric [3 + 2] cycloadditions using a chiral copper amide are reported. Compared with the chiral CuOTf/Et3N system, the CuHMDS system showed higher reactivity, and the desired reactions proceeded in high yields and high selectivities with catalyst loadings as low as 0.01 mol %.
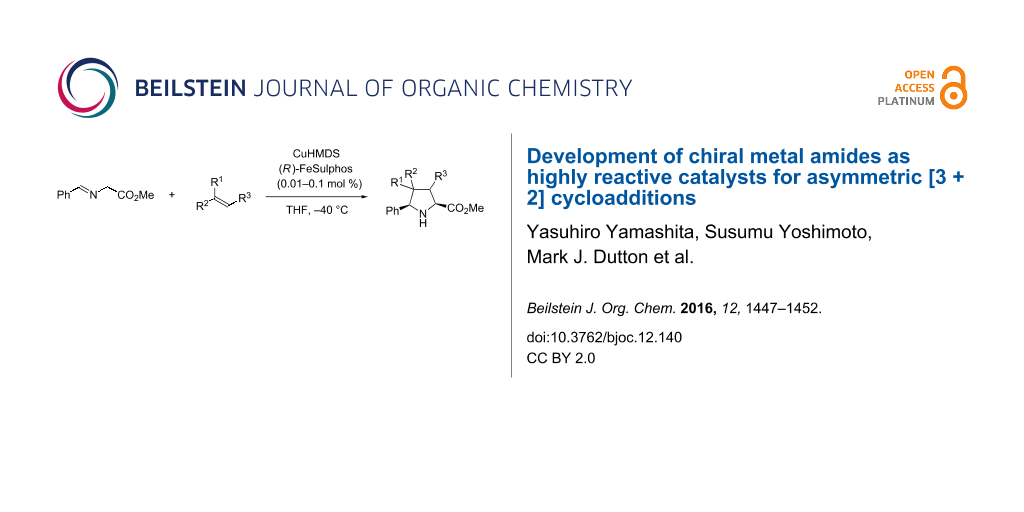
Graphical Abstract
Findings
Catalytic asymmetric synthesis is an ideal method to prepare optically active compounds [1]. In this context, catalytic asymmetric carbon–carbon bond-forming reactions that can be used for the efficient construction of fundamental frameworks of complex chiral molecules such as biologically active compounds are particularly important. Chiral Lewis acid/Brønsted base-catalyzed carbon–carbon bond-forming reactions are one of the most efficient methods from the viewpoint of atom economy because only proton transfer occurs between starting materials and target products [2]. Several kinds of chiral Lewis acid/Brønsted base-catalyst systems have been developed; however, decreasing the catalyst loading is sometimes problematic either because of the low reactivity of catalysts or because the catalyst activity can be reduced through Lewis acid–Lewis base interaction between catalysts and the formed products (product inhibition). To overcome such issues, the design and development of more reactive catalysts is required.
Our group has focused on the development of metal amides as highly reactive Lewis acid/base catalysts in carbon–carbon bond-forming reactions [3]. Recently, we have developed asymmetric [3 + 2] cycloadditions [4-8] and asymmetric Mannich-type reactions [9] by using chiral silver or copper amides as catalysts. In these reactions, it has been revealed that the metal amides have higher activity than typical silver or copper acid/base catalysts, and that less reactive substrates react smoothly to afford the desired products in high yields with high stereoselectivities. Based on these results, it was considered that metal amide catalysts might also achieve high catalyst turnover. Here, we report chiral copper amide-catalyzed asymmetric [3 + 2] cycloadditions of Schiff bases of glycine ester that proceed with low catalyst loadings (ca. 0.01 mol %).
Catalytic asymmetric [3 + 2] cycloadditions of Schiff bases of α-amino esters to olefins are useful for synthesizing optically active pyrrolidine derivatives [10-12], and many highly stereoselective reactions have been reported; for example, Co [13], Cu [14-23], Ag [24-32], Zn [33,34], Ni [35,36], and Ca [37-39] catalyst systems, and organocatalysts [40-45] have been successfully employed. In most cases, however, relatively high catalyst loadings (0.5–25 mol %) are required to achieve high yield and selectivities [15,45]. First, we investigated the catalytic asymmetric [3 + 2] cycloaddition of Schiff base 1a, prepared from glycine methyl ester and benzaldehyde, with N-phenylmaleimide (2a) in the presence of CuN(SiMe3)2 (CuHMDS) and the FeSulphos ligand, with the latter being related to the system reported by Carretero et al. [15]. The reaction produced 3aa smoothly with 3 mol % catalyst loading at −40 °C, and high endo selectivity and high enantioselectivity were obtained (Table 1, entry 1). On the other hand, application of CuOTf, FeSulphos, and Et3N gave only 47% yield of the product (Table 1, entry 2). This result indicated that the CuHMDS catalyst had higher catalyst activity than CuOTf with the additional amine base. The copper amide catalyst also showed high reactivity and selectivity with 1 mol % catalyst loading (Table 1, entry 3), and similar results were obtained in other solvents such as Et2O and toluene, although the reactivity and enantioselectivity both decreased slightly in dichloromethane (DCM, Table 1, entries 4–6). It was found that the use of the chiral CuHMDS catalyst also afforded the product with high enantioselectivity at lower catalyst loadings of 0.1 mol % (Table 1, entry 7). The effect of the amide part of the structure was then examined. Copper dialkylamides were not as reactive as CuHMDS, and lower yields were obtained (Table 1, entries 8 and 9). Interestingly, mesitylcopper also worked in a similar fashion, and good yields and high selectivities were obtained (Table 1, entry 10). This result indicated that the reaction proceeded through a product base mechanism [46-48]; however, the reactivity was lower than that of the CuHMDS system. Decreasing the catalyst loading further revealed that the reaction proceeded with 0.01 mol % loading of chiral CuHMDS catalyst without significant loss of selectivity (Table 1, entry 11).
Table 1: Chiral copper amide-catalyzed asymmetric [3 + 2] cycloadditionsa.
|
|
|||||||
| Entry | Cu catalyst | Solvent | X | Time (h) | Yield (%) | endo/exo | ee (%, endo) |
|---|---|---|---|---|---|---|---|
| 1 | CuHMDS | THF | 3 | 6 | 99 | >99:1 | 99 |
| 2b | CuOTf + Et3N | THF | 3 | 6 | 47 | 98:2 | 99 |
| 3c | CuHMDS | THF | 1 | 6 | 98 | >99:1 | >99 |
| 4c | CuHMDS | Et2O | 1 | 6 | 91 | 99:1 | 98 |
| 5c | CuHMDS | toluene | 1 | 6 | 95 | 99:1 | 99 |
| 6c | CuHMDS | DCM | 1 | 6 | 67 | 99:1 | 93 |
| 7d | CuHMDS | THF | 0.1 | 18 | 94 | >99:1 | 96 |
| 8d | CuNMe2e | THF | 0.1 | 18 | 53 | >99:1 | 94 |
| 9d | CuTMPe | THF | 0.1 | 18 | 46 | >99:1 | 98 |
| 10d | Cu(mesityl) | THF | 0.1 | 18 | 86 | >99:1 | 97 |
| 11f | CuHMDS | THF | 0.01 | 48 | 94 | >99:1 | 95 |
aThe [3 + 2] cycloaddition reaction of 0.5 M 1a (0.30 mmol) with 2a (1.1 equivalents, 0.33 mmol) were conducted at −40 °C in the presence of the copper amide prepared from CuOTf·0.5toluene complex/KHMDS/FeSulphos (1.1:1.0:1.1) in situ unless otherwise noted. bCuOTf·0.5toluene complex (0.0090 mmol) and Et3N (0.0090 mmol) were used. cThe reaction was conducted with 1a (1.0 mmol). dThe reaction was conducted with 1a (10 mmol). eThe copper amides were prepared in situ by mixing CuOTf·0.5toluene complex, FeSulphos and LiNMe2 or lithium 2,2,6,6,-tetramethylpiperidide (LiTMP). fThe reaction was conducted with 1.25 M 1a (50 mmol).
We then examined the substrate scope of the [3 + 2] cycloaddition with respect to the Schiff base (Table 2). The Schiff bases prepared from tolualdehydes were successfully employed in the reaction with 2a, and high reactivities and enantioselectivities were observed by using 0.1 mol % catalyst loading (Table 2, entries 1–4). The Schiff base from p-methoxybenzaldehyde was a good substrate (Table 2, entry 5) and reacted even in the presence of 0.01 mol % catalyst loading, albeit with a slight decrease in the enantioselectivity (Table 2, entry 6). The use of Schiff bases bearing either electron-donating or electron-withdrawing substituents were also suitable, and high yields and enantioselectivities were obtained with both 0.1 and 0.01 mol % catalyst loading (Table 2, entries 5–9). Sterically hindered substrates were also viable, and high enantioselectivities were obtained with 0.01 mol % catalyst loading (Table 2, entries 10–13).
Table 2: Scope of the reaction with respect to Schiff basesa.
|
|
|||||||
| Entry | Ar | 1 | FeSulphos (mol %) | 3 | Yield (%) | Endo/exo | ee (%, endo) |
|---|---|---|---|---|---|---|---|
| 1 | p-MeC6H4 | 1b | 0.1 | 3ba | 92 | >99:1 | >99 |
| 2 | p-MeC6H4 | 1b | 0.01 | 3ba | 91 | >99:1 | 85 |
| 3 | m-MeC6H4 | 1c | 0.1 | 3ca | 93 | >99:1 | >99 |
| 4 | o-MeC6H4 | 1d | 0.1 | 3da | 92 | 97:3 | >99 |
| 5 | p-MeOC6H4 | 1e | 0.1 | 3ea | 91 | 97:3 | 99 |
| 6 | p-MeOC6H4 | 1e | 0.01 | 3ea | 91 | >99:1 | 93 |
| 7 | p-ClC6H4 | 1f | 0.1 | 3fa | 96 | 98:2 | 92 |
| 8 | p-FC6H4 | 1g | 0.1 | 3ga | 96 | >99:1 | 99 |
| 9 | p-FC6H4 | 1g | 0.01 | 3ga | 96 | >99:1 | 98 |
| 10 | 2-naphthyl | 1h | 0.1 | 3ha | 94 | >99:1 | 99 |
| 11 | 2-naphthyl | 1h | 0.01 | 3ha | 87 | >99:1 | 97 |
| 12 | 1-naphthyl | 1i | 0.1 | 3ia | 76 | >99:1 | 99 |
| 13 | 1-naphthyl | 1i | 0.01 | 3ia | 91 | >99:1 | 98 |
aReaction conditions: For 0.1 mol % catalyst loading: the [3 + 2] cycloaddition reactions of 0.5 M 1 (10 mmol) with 2a (11 mmol) were conducted at −40 °C for 18 h by using the chiral copper amide prepared from CuOTf·0.5toluene complex (0.011 mmol), KHMDS (0.010 mmol), and FeSulphos (0.011 mmol) in situ. For 0.01 mol % catalyst loading: the [3 + 2] cycloaddition reactions of 1.25 M 1 (50 mmol) with 2a (55 mmol) were conducted at −40 °C for 48 h by using the chiral copper amide prepared from CuOTf·0.5toluene complex (0.0055 mmol), KHMDS (0.0050 mmol), and FeSulphos (0.0055 mmol) in situ.
Other electrophiles were also successfully employed with 0.1 mol % catalyst loading (Scheme 1). N-Methylmaleimide reacted with 1a in high yield with high diastereo- and enantioselectivities. The reaction with methyl acrylate also proceeded in high yield with high enantioselectivity; however, in this case the exo/endo selectivity was moderate. Methyl vinyl ketone and methyl methacrylate reacted with 1a to afford the desired [3 + 2] adducts in high yields with high selectivities. Notably, the chiral CuHMDS catalyst worked well with catalyst loadings of both 0.1 and 0.01 mol %.
Scheme 1: Scope of the reaction with other electrophiles. The [3 + 2] cycloaddition reaction of 0.5 M 1a (10 mmol) with 2 (11 mmol) was conducted at −40 °C for 18 h by using the chiral copper amide prepared from CuOTf·0.5toluene complex (0.011 mmol), KHMDS (0.010 mmol), and FeSulphos (0.011 mmol) in situ.
Scheme 1: Scope of the reaction with other electrophiles. The [3 + 2] cycloaddition reaction of 0.5 M 1a (10 ...
A proposed catalytic cycle is shown in Figure 1. Thus, the chiral CuHMDS deprotonates Schiff base 1a to generate the corresponding chiral Cu enolate B through the efficient formation of pseudo-intramolecular transition state A. Intermediate B reacted with maleimide 2a to form Cu-pyrrolidine intermediate C. H-HMDS then reacted with the latter to regenerate the chiral CuHMDS and release the product to complete the catalytic cycle. The result obtained by using a mesitylcopper catalyst suggests that the reaction could also proceed through a product base mechanism in which the Cu-pyrrolidine intermediate C deprotonates the Schiff base 1a directly; however, the higher reactivity observed upon catalysis by CuHMDS and the basicity of the intermediate indicates that the proposed cycle is reasonable when CuHMDS is used as catalyst. The high catalyst turnover may be due to the stronger Brønsted basicity of CuHMDS, which enables rapid deprotonation of the Schiff base.
Conclusion
In conclusion, highly efficient asymmetric [3 + 2] cycloadditions catalyzed by chiral CuHMDS have been described. Compared with catalysis by using the CuOTf/Et3N system, the Cu amide system showed higher reactivity, and the reactions proceeded with high enantioselectivities even with 0.01 mol % catalyst loading. Further investigations that are focused on the application of metal amide catalysts in other reactions are ongoing.
Experimental
A general experimental procedure for conducting catalytic asymmetric [3 + 2] cycloaddition reactions with 0.01 mol % catalyst loading is described. Under an Ar atmosphere, a solution of the preformed chiral CuHMDS catalyst [prepared from KHMDS (1.0 mg, 0.0050 mmol), CuOTf·0.5toluene (1.3 mg, 0.0055 mmol) and FeSulphos (2.3 mg, 0.0050 mmol) in anhydrous THF (5 mL) with heating at 40 °C for 1 h] was transferred into a well-dried 50 mL single-necked flask attached to a three-way cock (sealed with grease). The solution was cooled at −40 °C, and a mixture of 1 (50 mmol) and 2a (55 mmol) in anhydrous THF (35 mL) was added by using a cannula. The whole was stirred for 48 h at −40 °C, then the reaction was quenched by the addition of H2O, and the mixture was extracted with dichloromethane. The organic layers were combined and dried over anhydrous Na2SO4. The selectivities were determined by 1H NMR analysis and HPLC analysis after purification of a small amount of the separated crude solution. After filtration and concentration under reduced pressure, the crude product obtained was purified by recrystallization and column chromatography to determine the isolated yield of the desired product. Obtained compounds were characterized by 1H and 13C NMR and by HPLC analyses using HPLC with chiral columns. The physical data for the products were consistent with reported values [49-54].
Acknowledgements
This work was partially supported by a Grant-in-Aid for Science Research from the Japan Society for the Promotion of Science (JSPS), Global COE Program, the University of Tokyo, MEXT, Japan, and the Japan Science and Technology Agency (JST). S. Y. thanks the MERIT program, University of Tokyo, for financial support.
References
-
Ojima, I., Ed. Catalytic Asymmetric Synthesis, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2010. doi:10.1002/9780470584248
Return to citation in text: [1] -
Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2011, 50, 4760–4772. doi:10.1002/anie.201100918
Return to citation in text: [1] -
Yamashita, Y.; Kobayashi, S. Chem. – Eur. J. 2013, 19, 9420–9427. doi:10.1002/chem.201300908
Return to citation in text: [1] -
Yamashita, Y.; Guo, X.-X.; Takashita, R.; Kobayashi, S. J. Am. Chem. Soc. 2010, 132, 3262–3263. doi:10.1021/ja100101n
Return to citation in text: [1] -
Yamashita, Y.; Imaizumi, T.; Guo, X.-X.; Kobayashi, S. Chem. – Asian J. 2011, 6, 2550–2559. doi:10.1002/asia.201100246
Return to citation in text: [1] -
Yamashita, Y.; Imaizumi, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2011, 50, 4893–4896. doi:10.1002/anie.201008272
Return to citation in text: [1] -
Imaizumi, T.; Yamashita, Y.; Kobayashi, S. J. Am. Chem. Soc. 2012, 134, 20049–20052. doi:10.1021/ja311150n
Return to citation in text: [1] -
Yamashita, Y.; Nam, L. C.; Dutton, M. J.; Yoshimoto, S.; Kobayashi, S. Chem. Commun. 2015, 51, 17064–17067. doi:10.1039/C5CC07066J
Return to citation in text: [1] -
Yamashita, Y.; Yoshimoto, S.; Masuda, K.; Kobayashi, S. Asian J. Org. Chem. 2012, 1, 327–330. doi:10.1002/ajoc.201200092
Return to citation in text: [1] -
Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784–6794. doi:10.1039/c1cc10779h
Return to citation in text: [1] -
Adrio, J.; Carretero, J. C. Chem. Commun. 2014, 50, 12434–12446. doi:10.1039/C4CC04381B
Return to citation in text: [1] -
Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182
Return to citation in text: [1] -
Allway, P.; Grigg, R. Tetrahedron Lett. 1991, 32, 5817–5820. doi:10.1016/S0040-4039(00)93563-9
Return to citation in text: [1] -
Oderaotoshi, Y.; Cheng, W.; Fujitomi, S.; Kasano, Y.; Minakata, S.; Komatsu, M. Org. Lett. 2003, 5, 5043–5046. doi:10.1021/ol036076s
Return to citation in text: [1] -
Cabrera, S.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394–16395. doi:10.1021/ja0552186
Return to citation in text: [1] [2] [3] -
Gao, W.; Zhang, X.; Raghunath, M. Org. Lett. 2005, 7, 4241–4244. doi:10.1021/ol0516925
Return to citation in text: [1] -
Yan, X.-X.; Peng, Q.; Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. Angew. Chem., Int. Ed. 2006, 45, 1979–1983. doi:10.1002/anie.200503672
Return to citation in text: [1] -
Cabrera, S.; Arrayás, R. G.; Martín-Matute, B.; Cossío, F. P.; Carretero, J. C. Tetrahedron 2007, 63, 6587–6602. doi:10.1016/j.tet.2007.03.130
Return to citation in text: [1] -
Shi, M.; Shi, J.-W. Tetrahedron: Asymmetry 2007, 18, 645–650. doi:10.1016/j.tetasy.2007.02.027
Return to citation in text: [1] -
Fukuzawa, S.-i.; Oki, H. Org. Lett. 2008, 10, 1747–1750. doi:10.1021/ol8003996
Return to citation in text: [1] -
Wang, C.-J.; Liang, G.; Xue, Z.-Y.; Gao, F. J. Am. Chem. Soc. 2008, 130, 17250–17251. doi:10.1021/ja807669q
Return to citation in text: [1] -
Nájera, C.; de Garcia Retamosa, M.; Sansano, J. M. Angew. Chem., Int. Ed. 2008, 47, 6055–6058. doi:10.1002/anie.200801690
Return to citation in text: [1] -
Hernández-Toribio, J.; Arrayás, R. G.; Martín-Matute, B.; Carretero, J. C. Org. Lett. 2009, 11, 393–396. doi:10.1021/ol802664m
Return to citation in text: [1] -
Grigg, R. Tetrahedron: Asymmetry 1995, 6, 2475–2486. doi:10.1016/0957-4166(95)00323-H
Return to citation in text: [1] -
Longmire, J. M.; Wang, B.; Zhang, X. J. Am. Chem. Soc. 2002, 124, 13400–13401. doi:10.1021/ja025969x
Return to citation in text: [1] -
Chen, C.; Li, X.; Schreiber, S. L. J. Am. Chem. Soc. 2003, 125, 10174–10175. doi:10.1021/ja036558z
Return to citation in text: [1] -
Knöpfel, T. F.; Aschwanden, P.; Ichikawa, T.; Watanabe, T.; Carreira, E. M. Angew. Chem., Int. Ed. 2004, 43, 5971–5973. doi:10.1002/anie.200461286
Return to citation in text: [1] -
Alemparte, C.; Blay, G.; Jørgensen, K. A. Org. Lett. 2005, 7, 4569–4572. doi:10.1021/ol0514653
Return to citation in text: [1] -
Zeng, W.; Zhou, Y.-G. Org. Lett. 2005, 7, 5055–5058. doi:10.1021/ol0520370
Return to citation in text: [1] -
Stohler, R.; Wahl, F.; Pfaltz, A. Synthesis 2005, 1431–1436. doi:10.1055/s-2005-865313
Return to citation in text: [1] -
Zeng, W.; Chen, G.-Y.; Zhou, Y.-G.; Li, Y.-X. J. Am. Chem. Soc. 2007, 129, 750–751. doi:10.1021/ja067346f
Return to citation in text: [1] -
Kim, H. Y.; Shih, H.-J.; Knabe, W.-E.; Oh, K. Angew. Chem., Int. Ed. 2009, 48, 7420–7423. doi:10.1002/anie.200903479
Return to citation in text: [1] -
Gothelf, A. S.; Gothelf, K. V.; Hazell, R. G.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2002, 41, 4236–4238. doi:10.1002/1521-3773(20021115)41:22<4236::AID-ANIE4236>3.0.CO;2-W
Return to citation in text: [1] -
Dogan, Ö.; Koyuncu, H.; Garner, P.; Bulut, A.; Youngs, W. J.; Panzner, M. Org. Lett. 2006, 8, 4687–4690. doi:10.1021/ol061521f
Return to citation in text: [1] -
Shi, J.-W.; Zhao, M.-X.; Lei, Z.-Y.; Shi, M. J. Org. Chem. 2008, 73, 305–308. doi:10.1021/jo701561d
Return to citation in text: [1] -
Awata, A.; Arai, T. Chem. – Eur. J. 2012, 18, 8278–8282. doi:10.1002/chem.201201249
Return to citation in text: [1] -
Saito, S.; Tsubogo, T.; Kobayashi, S. J. Am. Chem. Soc. 2007, 129, 5364–5365. doi:10.1021/ja0709730
Return to citation in text: [1] -
Tsubogo, T.; Saito, S.; Seki, K.; Yamashita, Y.; Kobayashi, S. J. Am. Chem. Soc. 2008, 130, 13321–13332. doi:10.1021/ja8032058
Return to citation in text: [1] -
Hut'ka, M.; Tsubogo, T.; Kobayashi, S. Adv. Synth. Catal. 2013, 355, 1561–1569. doi:10.1002/adsc.201300171
Return to citation in text: [1] -
Vicario, J. L.; Reboredo, S.; Badía, D.; Carrillo, L. Angew. Chem., Int. Ed. 2007, 46, 5168–5170. doi:10.1002/anie.200700988
Return to citation in text: [1] -
Ibrahem, I.; Rios, R.; Vesely, J.; Córdova, A. Tetrahedron Lett. 2007, 48, 6252–6257. doi:10.1016/j.tetlet.2007.07.031
Return to citation in text: [1] -
Chen, X.-H.; Zhang, W.-Q.; Gong, L.-Z. J. Am. Chem. Soc. 2008, 130, 5652–5653. doi:10.1021/ja801034e
Return to citation in text: [1] -
Guo, C.; Xue, M.-X.; Zhu, M.-K.; Gong, L.-Z. Angew. Chem., Int. Ed. 2008, 47, 3414–3417. doi:10.1002/anie.200800003
Return to citation in text: [1] -
Liu, Y.-K.; Liu, H.; Du, W.; Yue, L.; Chen, Y.-C. Chem. – Eur. J. 2008, 14, 9873–9877. doi:10.1002/chem.200801410
Return to citation in text: [1] -
Tian, L.; Xu, G.-Q.; Li, Y.-H.; Liang, Y.-M.; Xu, P.-F. Chem. Commun. 2014, 50, 2428–2430. doi:10.1039/c3cc49504c
Return to citation in text: [1] [2] -
Poisson, T.; Gembus, V.; Oudeyer, S.; Marsais, F.; Levacher, V. J. Org. Chem. 2009, 74, 3516–3519. doi:10.1021/jo802763b
Return to citation in text: [1] -
Yazaki, R.; Kumagai, N.; Shibasaki, M. Chem. – Asian J. 2011, 6, 1778–1790. doi:10.1002/asia.201100050
Return to citation in text: [1] -
Yamashita, Y.; Suzuki, H.; Kobayashi, S. Org. Biomol. Chem. 2012, 10, 5750–5752. doi:10.1039/c2ob25522g
Return to citation in text: [1] -
Products 3aa, 3da, 3ea, 3ga, 3ha, 3ab, 3ac were reported in ref. [15].
Return to citation in text: [1] -
Products 3ba and 3fa were reported in ref. [19].
Return to citation in text: [1] -
Product 3ad was reported in ref. [23].
See also Tsuge, O.; Kanemasa, S.; Yoshioka, M. J. Org. Chem. 1998, 53, 1384–1391. doi:10.1021/jo00242a008
Return to citation in text: [1] -
Product 3ae was reported in ref. [32].
Return to citation in text: [1] -
Product 3ia was reported in ref. [35].
Return to citation in text: [1] -
Bai, J.-F.; Wang, L.-L.; Peng, L.; Guo, Y.-L.; Ming, J.-N.; Wang, F.-Y.; Xu, X.-Y.; Wang, L.-X. Eur. J. Org. Chem. 2011, 4472–4478. doi:10.1002/ejoc.201100205
See for product 3ca.
Return to citation in text: [1]
| 19. | Shi, M.; Shi, J.-W. Tetrahedron: Asymmetry 2007, 18, 645–650. doi:10.1016/j.tetasy.2007.02.027 |
| 49. | Products 3aa, 3da, 3ea, 3ga, 3ha, 3ab, 3ac were reported in ref. [15]. |
| 50. | Products 3ba and 3fa were reported in ref. [19]. |
| 51. |
Product 3ad was reported in ref. [23].
See also Tsuge, O.; Kanemasa, S.; Yoshioka, M. J. Org. Chem. 1998, 53, 1384–1391. doi:10.1021/jo00242a008 |
| 52. | Product 3ae was reported in ref. [32]. |
| 53. | Product 3ia was reported in ref. [35]. |
| 54. |
Bai, J.-F.; Wang, L.-L.; Peng, L.; Guo, Y.-L.; Ming, J.-N.; Wang, F.-Y.; Xu, X.-Y.; Wang, L.-X. Eur. J. Org. Chem. 2011, 4472–4478. doi:10.1002/ejoc.201100205
See for product 3ca. |
| 15. | Cabrera, S.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394–16395. doi:10.1021/ja0552186 |
| 1. | Ojima, I., Ed. Catalytic Asymmetric Synthesis, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2010. doi:10.1002/9780470584248 |
| 9. | Yamashita, Y.; Yoshimoto, S.; Masuda, K.; Kobayashi, S. Asian J. Org. Chem. 2012, 1, 327–330. doi:10.1002/ajoc.201200092 |
| 15. | Cabrera, S.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394–16395. doi:10.1021/ja0552186 |
| 4. | Yamashita, Y.; Guo, X.-X.; Takashita, R.; Kobayashi, S. J. Am. Chem. Soc. 2010, 132, 3262–3263. doi:10.1021/ja100101n |
| 5. | Yamashita, Y.; Imaizumi, T.; Guo, X.-X.; Kobayashi, S. Chem. – Asian J. 2011, 6, 2550–2559. doi:10.1002/asia.201100246 |
| 6. | Yamashita, Y.; Imaizumi, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2011, 50, 4893–4896. doi:10.1002/anie.201008272 |
| 7. | Imaizumi, T.; Yamashita, Y.; Kobayashi, S. J. Am. Chem. Soc. 2012, 134, 20049–20052. doi:10.1021/ja311150n |
| 8. | Yamashita, Y.; Nam, L. C.; Dutton, M. J.; Yoshimoto, S.; Kobayashi, S. Chem. Commun. 2015, 51, 17064–17067. doi:10.1039/C5CC07066J |
| 46. | Poisson, T.; Gembus, V.; Oudeyer, S.; Marsais, F.; Levacher, V. J. Org. Chem. 2009, 74, 3516–3519. doi:10.1021/jo802763b |
| 47. | Yazaki, R.; Kumagai, N.; Shibasaki, M. Chem. – Asian J. 2011, 6, 1778–1790. doi:10.1002/asia.201100050 |
| 48. | Yamashita, Y.; Suzuki, H.; Kobayashi, S. Org. Biomol. Chem. 2012, 10, 5750–5752. doi:10.1039/c2ob25522g |
| 3. | Yamashita, Y.; Kobayashi, S. Chem. – Eur. J. 2013, 19, 9420–9427. doi:10.1002/chem.201300908 |
| 40. | Vicario, J. L.; Reboredo, S.; Badía, D.; Carrillo, L. Angew. Chem., Int. Ed. 2007, 46, 5168–5170. doi:10.1002/anie.200700988 |
| 41. | Ibrahem, I.; Rios, R.; Vesely, J.; Córdova, A. Tetrahedron Lett. 2007, 48, 6252–6257. doi:10.1016/j.tetlet.2007.07.031 |
| 42. | Chen, X.-H.; Zhang, W.-Q.; Gong, L.-Z. J. Am. Chem. Soc. 2008, 130, 5652–5653. doi:10.1021/ja801034e |
| 43. | Guo, C.; Xue, M.-X.; Zhu, M.-K.; Gong, L.-Z. Angew. Chem., Int. Ed. 2008, 47, 3414–3417. doi:10.1002/anie.200800003 |
| 44. | Liu, Y.-K.; Liu, H.; Du, W.; Yue, L.; Chen, Y.-C. Chem. – Eur. J. 2008, 14, 9873–9877. doi:10.1002/chem.200801410 |
| 45. | Tian, L.; Xu, G.-Q.; Li, Y.-H.; Liang, Y.-M.; Xu, P.-F. Chem. Commun. 2014, 50, 2428–2430. doi:10.1039/c3cc49504c |
| 2. | Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2011, 50, 4760–4772. doi:10.1002/anie.201100918 |
| 15. | Cabrera, S.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394–16395. doi:10.1021/ja0552186 |
| 45. | Tian, L.; Xu, G.-Q.; Li, Y.-H.; Liang, Y.-M.; Xu, P.-F. Chem. Commun. 2014, 50, 2428–2430. doi:10.1039/c3cc49504c |
| 24. | Grigg, R. Tetrahedron: Asymmetry 1995, 6, 2475–2486. doi:10.1016/0957-4166(95)00323-H |
| 25. | Longmire, J. M.; Wang, B.; Zhang, X. J. Am. Chem. Soc. 2002, 124, 13400–13401. doi:10.1021/ja025969x |
| 26. | Chen, C.; Li, X.; Schreiber, S. L. J. Am. Chem. Soc. 2003, 125, 10174–10175. doi:10.1021/ja036558z |
| 27. | Knöpfel, T. F.; Aschwanden, P.; Ichikawa, T.; Watanabe, T.; Carreira, E. M. Angew. Chem., Int. Ed. 2004, 43, 5971–5973. doi:10.1002/anie.200461286 |
| 28. | Alemparte, C.; Blay, G.; Jørgensen, K. A. Org. Lett. 2005, 7, 4569–4572. doi:10.1021/ol0514653 |
| 29. | Zeng, W.; Zhou, Y.-G. Org. Lett. 2005, 7, 5055–5058. doi:10.1021/ol0520370 |
| 30. | Stohler, R.; Wahl, F.; Pfaltz, A. Synthesis 2005, 1431–1436. doi:10.1055/s-2005-865313 |
| 31. | Zeng, W.; Chen, G.-Y.; Zhou, Y.-G.; Li, Y.-X. J. Am. Chem. Soc. 2007, 129, 750–751. doi:10.1021/ja067346f |
| 32. | Kim, H. Y.; Shih, H.-J.; Knabe, W.-E.; Oh, K. Angew. Chem., Int. Ed. 2009, 48, 7420–7423. doi:10.1002/anie.200903479 |
| 35. | Shi, J.-W.; Zhao, M.-X.; Lei, Z.-Y.; Shi, M. J. Org. Chem. 2008, 73, 305–308. doi:10.1021/jo701561d |
| 36. | Awata, A.; Arai, T. Chem. – Eur. J. 2012, 18, 8278–8282. doi:10.1002/chem.201201249 |
| 35. | Shi, J.-W.; Zhao, M.-X.; Lei, Z.-Y.; Shi, M. J. Org. Chem. 2008, 73, 305–308. doi:10.1021/jo701561d |
| 14. | Oderaotoshi, Y.; Cheng, W.; Fujitomi, S.; Kasano, Y.; Minakata, S.; Komatsu, M. Org. Lett. 2003, 5, 5043–5046. doi:10.1021/ol036076s |
| 15. | Cabrera, S.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394–16395. doi:10.1021/ja0552186 |
| 16. | Gao, W.; Zhang, X.; Raghunath, M. Org. Lett. 2005, 7, 4241–4244. doi:10.1021/ol0516925 |
| 17. | Yan, X.-X.; Peng, Q.; Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. Angew. Chem., Int. Ed. 2006, 45, 1979–1983. doi:10.1002/anie.200503672 |
| 18. | Cabrera, S.; Arrayás, R. G.; Martín-Matute, B.; Cossío, F. P.; Carretero, J. C. Tetrahedron 2007, 63, 6587–6602. doi:10.1016/j.tet.2007.03.130 |
| 19. | Shi, M.; Shi, J.-W. Tetrahedron: Asymmetry 2007, 18, 645–650. doi:10.1016/j.tetasy.2007.02.027 |
| 20. | Fukuzawa, S.-i.; Oki, H. Org. Lett. 2008, 10, 1747–1750. doi:10.1021/ol8003996 |
| 21. | Wang, C.-J.; Liang, G.; Xue, Z.-Y.; Gao, F. J. Am. Chem. Soc. 2008, 130, 17250–17251. doi:10.1021/ja807669q |
| 22. | Nájera, C.; de Garcia Retamosa, M.; Sansano, J. M. Angew. Chem., Int. Ed. 2008, 47, 6055–6058. doi:10.1002/anie.200801690 |
| 23. | Hernández-Toribio, J.; Arrayás, R. G.; Martín-Matute, B.; Carretero, J. C. Org. Lett. 2009, 11, 393–396. doi:10.1021/ol802664m |
| 37. | Saito, S.; Tsubogo, T.; Kobayashi, S. J. Am. Chem. Soc. 2007, 129, 5364–5365. doi:10.1021/ja0709730 |
| 38. | Tsubogo, T.; Saito, S.; Seki, K.; Yamashita, Y.; Kobayashi, S. J. Am. Chem. Soc. 2008, 130, 13321–13332. doi:10.1021/ja8032058 |
| 39. | Hut'ka, M.; Tsubogo, T.; Kobayashi, S. Adv. Synth. Catal. 2013, 355, 1561–1569. doi:10.1002/adsc.201300171 |
| 13. | Allway, P.; Grigg, R. Tetrahedron Lett. 1991, 32, 5817–5820. doi:10.1016/S0040-4039(00)93563-9 |
| 23. | Hernández-Toribio, J.; Arrayás, R. G.; Martín-Matute, B.; Carretero, J. C. Org. Lett. 2009, 11, 393–396. doi:10.1021/ol802664m |
| 10. | Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784–6794. doi:10.1039/c1cc10779h |
| 11. | Adrio, J.; Carretero, J. C. Chem. Commun. 2014, 50, 12434–12446. doi:10.1039/C4CC04381B |
| 12. | Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182 |
| 33. | Gothelf, A. S.; Gothelf, K. V.; Hazell, R. G.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2002, 41, 4236–4238. doi:10.1002/1521-3773(20021115)41:22<4236::AID-ANIE4236>3.0.CO;2-W |
| 34. | Dogan, Ö.; Koyuncu, H.; Garner, P.; Bulut, A.; Youngs, W. J.; Panzner, M. Org. Lett. 2006, 8, 4687–4690. doi:10.1021/ol061521f |
| 32. | Kim, H. Y.; Shih, H.-J.; Knabe, W.-E.; Oh, K. Angew. Chem., Int. Ed. 2009, 48, 7420–7423. doi:10.1002/anie.200903479 |
© 2016 Yamashita et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)