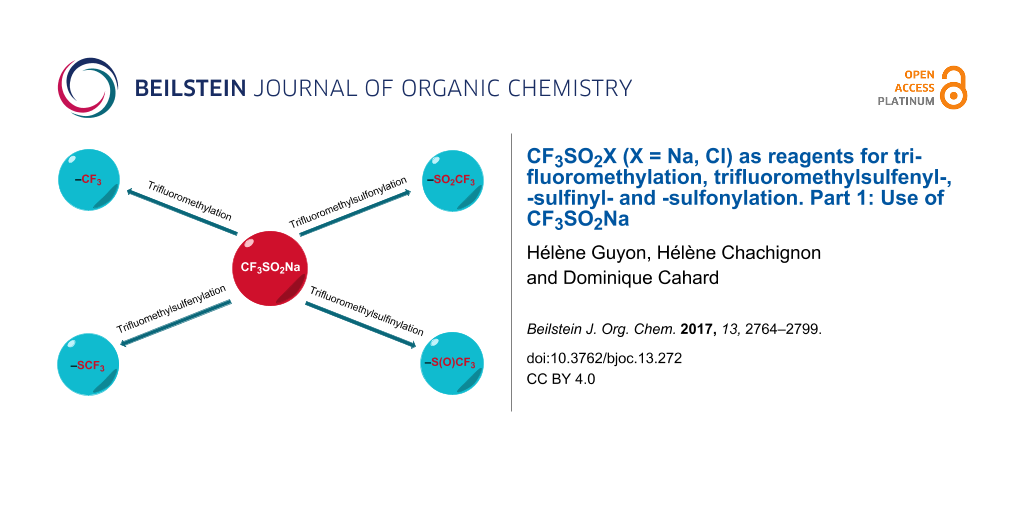
Abstract
Sodium trifluoromethanesulfinate, CF3SO2Na, and trifluoromethanesulfonyl chloride, CF3SO2Cl, are two popular reagents that are widely used for the direct trifluoromethylation of a large range of substrates. Further, these two reagents are employed for the direct trifluoromethylsulfenylation and trifluoromethylsulfinylation, the introduction of the SCF3 and the S(O)CF3 group, respectively. In addition to the aforementioned reactions, the versatility of these two reagents is presented in other reactions such as sulfonylation and chlorination. This first part is dedicated to sodium trifluoromethanesulfinate.
Graphical Abstract
Introduction
In organofluorine chemistry, the CF3 group occupies a place of choice as privileged structural motif in the development of multifaceted catalysts and ligands for organic synthesis as well as in the design of pharmaceuticals, agrochemicals and specialty materials [1-4]. The trifluoromethyl group is most of the time linked to a carbon atom but can also be encountered with chalcogens (S, Se, O) and nitrogen. The CF3S motif, which was till recently considered as an emerging substituent, is henceforth a tamed substituent as a result of abundant literature describing methods to prepare CF3S-featuring molecules [5]. Its oxidised congeners CF3S(O) and CF3SO2 are also well-developed as structural units in biologically active compounds, catalysts for synthesis, and functional materials. The chemistry of CF3Se derivatives is less developed but basically shares the same synthetic approaches with CF3S analogues [6,7]. The CF3O group is also of high interest but difficulties still exist to easily introduce this motif directly onto organic molecules [8]. The reasons behind such massive interest for the CF3 group are due to the specific physical and chemical properties of the compounds that contain it. The CF3 group has a large van der Waals volume somewhere between those of the iPr and the t-Bu groups [9]. Its electronegativity is comparable to that of oxygen (4.0 versus 3.5 on the Pauling scale) and its hydrophobicity is large (0 for H, 0.51 for Me, and 1.07 for CF3) [10]. In this respect, judicious installation of CF3 group(s) in catalysts or ligands is an effective tool to tune their reactivity and selectivity in synthesis. As a pharmacophore, CF3 substantially improves the catabolic stability, lipophilicity, and transport rate. In association with chalcogens (OCF3, SCF3, SeCF3), the CF3 group imparts enhanced lipophilicity of aromatic compounds in comparison with aryl–CF3 analogues (Hansch’s hydrophobic parameter π(SCF3) = 1.44; π(OCF3) = 1.04 versus π(CF3) = 0.88). Trifluoromethyl sources are manifold displaying nucleophilic, electrophilic or radical reactivities [11]. The most popular nucleophilic trifluoromethylating reagent is certainly the trifluoromethyltrimethylsilane, CF3SiMe3, known as the Ruppert–Prakash reagent discovered in 1984 by Ruppert and applied for trifluoromethylation in 1989 by Prakash and Olah. More recently, renewed investigation on the use of fluoroform, CF3H, as an ideal source of trifluoromethide offered new horizons for atom-economical, low-cost trifluoromethylation reactions. With regard to electrophilic CF3 donors, S-(trifluoromethyl)sulfonium salts developed by Yagupolskii and Umemoto and hypervalent iodine(III)-CF3 reagents developed by Togni are widely employed in a variety of trifluoromethylation reactions [12]. Traditional radical CF3 sources include the gaseous trifluoroiodomethane CF3I and trifluorobromomethane CF3Br. More conveniently, the liquid trifluoromethanesulfonyl chloride CF3SO2Cl and the solid sodium trifluoromethanesulfinate CF3SO2Na that are commercially available at reasonable prices, are easy-to-handle sources of trifluoromethyl radicals. Remarkably, CF3SO2Na and CF3SO2Cl are multi-purpose reagents since they not only act as CF3 donors by extrusion of SO2, but also, under certain reaction conditions, the sulfur atom is retained for trifluoromethylsulfenylation (also named trifluoromethylthiolation), trifluoromethylsulfinylation, or trifluoromethylsulfonylation reactions. Typically, CF3SO2Na reacts under oxidative conditions whereas CF3SO2Cl requires reductive conditions. The advent of a new dynamism and the versatility of these two reagents have been recently demonstrated through several creative research articles; hence, this review aims to collect the recent progresses in the diverse uses of CF3SO2Na and CF3SO2Cl reagents. A special emphasis is placed on mechanistic studies. The review is divided in Part 1 and Part 2 that are published back-to-back. The literature is comprehensively covered through July 2017.
Review
Sodium trifluoromethanesulfinate (alternate names: sodium triflinate, trifluoromethanesulfinic acid sodium salt, Langlois reagent), CAS No. 2926-29-6, MW 156.06, is a stable white solid (mp 350 °C) soluble in water and slightly soluble in acetonitrile, methanol and acetone [13]. Although this reagent was prepared in 1955 by Haszeldine [14] and in 1976 by Roesky [15], it is only in 1991 that Langlois reported the trifluoromethylation of aromatic compounds under oxidative conditions [16]. Since then, the use of CF3SO2Na has grown considerably for the creation of Csp3–CF3, Csp2–CF3 and Csp–CF3 bonds [17-19]. This reagent is also conveniently used in trifluoromethylsulfinylation and trifluoromethylsulfonylation reactions. More recently, from 2015, CF3SO2Na has found a new application as source of SCF3 for direct trifluoromethylsulfenylation.
1 Trifluoromethylation
Csp3–CF3 bond-forming reactions
Synthesis of α-trifluoromethyl ketones from alkenes: After their original reports on the trifluoromethylation of aromatics (see later in the text, Scheme 34) [16] and disulfides (Scheme 69) [20], Langlois and co-workers demonstrated that enol acetates 1a–c were converted into the corresponding α-trifluoromethyl ketones upon treatment with CF3SO2Na with tert-butyl hydroperoxide (TBHP) and a catalytic amount of copper(II) triflate (Scheme 1) [21]. The scope was rather narrow and yields were moderate to poor. In particular, enol acetate 1b, prepared from symmetrical undecan-6-one, gave a mixture of the desired α-CF3 ketone and two isomeric enol acetates 1b'. In 2014, Li, Duan and co-workers applied the conditions described by Langlois to a series of enol acetates 3 derived from aryl and heteroaryl ketones featuring a single enolizable position (Scheme 2) [22]. Notably, it was found that Cu(II) and Cu(I) salts gave similar yields. A radical trifluoromethylation was suggested for this transformation. The CF3•, which was generated by reaction of tert-butyl hydroperoxide with CF3SO2Na in the presence of copper(I), reacted at the more electron-rich carbon atom of the C=C double bond to give the radical species 5 that was oxidised by copper(II) into the corresponding cationic intermediate 6 via a single electron transfer (SET). Finally, the acetyl cation was eliminated to provide the α-CF3 carbonyl compound 4 (Scheme 3).
Scheme 1: Trifluoromethylation of enol acetates by Langlois.
Scheme 1: Trifluoromethylation of enol acetates by Langlois.
Scheme 2: Trifluoromethylation of (het)aryl enol acetates.
Scheme 2: Trifluoromethylation of (het)aryl enol acetates.
![[1860-5397-13-272-i3]](/bjoc/content/inline/1860-5397-13-272-i3.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 3: Mechanism for the trifluoromethylation of enol acetates.
Scheme 3: Mechanism for the trifluoromethylation of enol acetates.
Unactivated olefins are widely available substrates prone to be transformed into α-trifluoromethyl carbonyl compounds under oxidative trifluoromethylation as first reported by Maiti and co-workers in 2013. The reaction is operationally simple, conducted under air at room temperature and presents a predictable reactivity pattern as well as a wide functional group tolerance. The substrate scope was evaluated on 33 styrenes, β-substituted styrenes, heteroaromatic olefins and vinyl cycloalkanes (Scheme 4). The trifluoromethyl radical was generated from CF3SO2Na by means of an oxidative system comprising catalytic amounts of silver(I) nitrate and potassium persulfate K2S2O8. Both atmospheric oxygen and K2S2O8 can be the source of the oxygen atom of the ketone moiety. A series of experiments that include the formation of TEMPO–CF3 (TEMPO: 2,2,6,6-tetramethylpiperidine 1-oxyl), the detection of Ag(0) by X-ray photoelectron spectroscopy, the retardation of the reaction in absence of air and an 18O-labeling reaction led the authors to propose the mechanism described in Scheme 4 [23].
![[1860-5397-13-272-i4]](/bjoc/content/inline/1860-5397-13-272-i4.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 4: Oxidative trifluoromethylation of unactivated olefins and mechanistic pathway.
Scheme 4: Oxidative trifluoromethylation of unactivated olefins and mechanistic pathway.
Because some limitations appeared with heterocycles such as quinolone, indole, pyrimidine, thiophene, etc. the Maiti group reported an alternative approach toward α-trifluoromethyl ketones starting from (hetero)arylacetylenes 7 and also aliphatic terminal alkynes 8 (Scheme 5) [24]. The trifluoromethyl radical was generated from CF3SO2Na as indicated earlier, oxygen from air was the source of the oxygen atom, and N-methylpyrrolidine (NMP) acted as solvent and source of the hydrogen atom to convert the peroxo intermediate 9 to its hydroperoxo form 10. As proof of the mechanism, N-methylsuccinimide (11) was identified in all these reactions.
![[1860-5397-13-272-i5]](/bjoc/content/inline/1860-5397-13-272-i5.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 5: Oxidative trifluoromethylation of acetylenic substrates.
Scheme 5: Oxidative trifluoromethylation of acetylenic substrates.
Simultaneously to Maiti’s work, Luo and co-workers reported a metal-free protocol for the trifluoromethylation of styrenes with CF3SO2Na, tert-butyl hydroperoxide and benzoquinone (BQ) as oxidant. The reactions were run at 80 °C for 16 h to give mixtures of α-trifluoromethyl ketones 12 and the corresponding alcohols 13 (Scheme 6) [25]. The scope was limited to simple substituted styrenes and yields were only moderate although super-stoichiometric amounts of reagents were used. The ratio 12/13 ranged from 31:69 to 66:34 but a subsequent reduction or oxidation allowed to access either the alcohol or the ketone exclusively.
Scheme 6: Metal free trifluoromethylation of styrenes.
Scheme 6: Metal free trifluoromethylation of styrenes.
The use of transition metal catalysts and/or a large excess of organic oxidants can be obstacles to production. In a quest for ideal conditions, Lei and co-workers exposed heteroatom-functionalised alkenes 14 to aerobic Cvinyl–heteroatom bond oxygenation under metal-free conditions, where oxygen from air worked in concert with a catalytic amount of potassium persulfate to activate CF3SO2Na (Scheme 7) [26]. The heteroatom must be a good leaving group or part of it (X = Br, Cl, NHCOMe, N3, OP(O)(OEt)2). A mechanistic investigation demonstrated the role of oxygen: 93% isotopic purity of the ketone product was obtained when using 18O2; no reaction occurred under N2 instead of O2; K2S2O8 rather than O2 served as initiator; the radical CF3SO2• could either extrude SO2 to CF3• or react with O2 to re-initiate the radical chain process (Scheme 7).
![[1860-5397-13-272-i7]](/bjoc/content/inline/1860-5397-13-272-i7.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 7: Synthesis of α-trifluoromethylated ketones by oxytrifluoromethylation of heteroatom-functionalised alkenes.
Scheme 7: Synthesis of α-trifluoromethylated ketones by oxytrifluoromethylation of heteroatom-functionalised ...
Vinyl azides were used by Liu and co-workers as precursors of α-trifluoromethylated ketones by reaction of CF3SO2Na under photoredox catalysis. The substrate scope was broad and the reaction proceeded with high functional group tolerance; indeed, aryl-, alkyl-, hetero-functionalised terminal as well as non-terminal vinyl azides 15 were compatible with the reaction conditions. In the presence of the organic photocatalyst N-methyl-9-mesitylacridinium (17), CF3SO2Na was converted into CF3• upon visible-light irradiation. The CF3• radical reacted with the vinyl azide to give the iminyl radical 18 that was reduced by Mes-Acr• (Mes-Acr: 9-mesityl-10-methylacridinium) into the iminyl anion 19. After protonation and hydrolysis of the imine function, the α-trifluoromethylated ketones 16 were obtained in moderate to good yields (Scheme 8) [27].
![[1860-5397-13-272-i8]](/bjoc/content/inline/1860-5397-13-272-i8.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 8: Catalysed photoredox trifluoromethylation of vinyl azides.
Scheme 8: Catalysed photoredox trifluoromethylation of vinyl azides.
Alkenyl MIDA (N-methylimidodiacetic) boronates 20, as functionalised alkenes, were transformed into α-trifluoromethyl-α-boryl ketones 21 by oxidative trifluoromethylation with CF3SO2Na. In that case, 2-iodoxybenzoic acid (IBX) was used as the oxidant to generate the trifluoromethylated radical 22 and atmospheric oxygen was the oxygen source to form the ketone (Scheme 9) [28].
Scheme 9: Oxidative difunctionalisation of alkenyl MIDA boronates.
Scheme 9: Oxidative difunctionalisation of alkenyl MIDA boronates.
Synthesis of β-trifluoromethyl ketones from cyclopropanols: Moving away the CF3 group by one carbon atom further from the carbonyl function would provide the corresponding β-trifluoromethyl ketones. For this aim, distally trifluoromethyl ketones were synthesised by the ring-opening of cyclopropanol derivatives by means of per- or polyfluorinated sulfonates, including CF3SO2Na. Kananovich and co-workers demonstrated that sodium triflinate, under Langlois’ conditions, served as a precursor of trifluoromethyl copper species that were involved in the ring-opening trifluoromethylation of tertiary cyclopropanols 23 (Scheme 10) [29]. The substrate scope was broad allowing access to various β-trifluoromethyl ketones 24 featuring aryl, alkyl, functionalised alkyl, alkenyl, acetal, silylated alcohol, pyran, and piperidine functionalities (R group in 24). Mechanistic studies by 19F NMR allowed to identify CF3 complexes of copper(I) and copper(III) and the predominance of an electrophilic pathway (versus SET pathway) was suggested by regioselective trifluoromethylation of a case substrate. Although Cu(II) acetate was used, the authors mentioned that Cu(I) can be formed in situ by reduction with MeOH or the cyclopropanol. Concomitant mechanisms are depicted in Scheme 10.
![[1860-5397-13-272-i10]](/bjoc/content/inline/1860-5397-13-272-i10.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 10: Synthesis of β-trifluoromethyl ketones from cyclopropanols.
Scheme 10: Synthesis of β-trifluoromethyl ketones from cyclopropanols.
β-Trifluoromethyl ketones could also be obtained from allylic alcohols 25 by a cascade trifluoromethylation/1,2-aryl migration. Yang, Xia and co-workers employed sodium triflinate under metal-free conditions with ammonium persulfate as the oxidant that was necessary to generate the CF3 radical (Scheme 11) [30].
Scheme 11: Aryltrifluoromethylation of allylic alcohols.
Scheme 11: Aryltrifluoromethylation of allylic alcohols.
Amino- and azotrifluoromethylation of alkenes: Alkene trifluoromethylation was applied to the construction of indole, pyrazole and pyridazinone moieties via a multicomponent cascade reaction developed by Antonchick and Matcha in 2014 [31]. The method was based on the reaction between simple alkenes, sodium triflinate and diazonium salts. The CF3 radical was produced from CF3SO2Na by oxidation with H2O2 in the presence of silver nitrate. Then, CF3• was added to the terminal position of the alkene to give radical 26 that was trapped by the arenediazonium salt to form the radical cation 27, which was reduced into 28 prior to be converted into nitrogen heterocycles via [1,3]-hydride shift and cyclisation steps (Scheme 12). The reaction was regioselective and had a broad scope. This application of alkene trifluoromethylation provided a convenient entry to trifluoromethylated nitrogen heterocycles.
![[1860-5397-13-272-i12]](/bjoc/content/inline/1860-5397-13-272-i12.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 12: Cascade multicomponent synthesis of nitrogen heterocycles via azotrifluoromethylation of alkenes.
Scheme 12: Cascade multicomponent synthesis of nitrogen heterocycles via azotrifluoromethylation of alkenes.
Subsequently, a photoredox-catalysed azotrifluoromethylation of unactivated alkenes was developed by Chen, Xiao and co-workers [32]. The scope was broad for a variety of unactivated alkenes, functionalised or not, and aryldiazonium salts (Scheme 13). Interestingly, the method did not require stoichiometric amounts of oxidant and further transformation of the azotrifluoromethyl products allowed a Fisher indole synthesis. From a mechanistic point of view, the excited photocatalyst was oxidised by the aryldiazonium salt to produce [Ru(bpy)3]3+ (bpy: 2,2’-bipyridine) as the oxidant to generate the CF3 radical from CF3SO2Na with extrusion of SO2. Then, CF3• underwent a radical addition to the alkene to form the radical 29, which was trapped by the aryldiazonium salt to give the radical cation 30. Finally, 30 was reduced by [Ru(bpy)3]2+* to end up with the product 31 (Scheme 13).
![[1860-5397-13-272-i13]](/bjoc/content/inline/1860-5397-13-272-i13.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 13: Photocatalytic azotrifluoromethylation of alkenes with aryldiazonium salts and CF3SO2Na.
Scheme 13: Photocatalytic azotrifluoromethylation of alkenes with aryldiazonium salts and CF3SO2Na.
The aminotrifluoromethylation of alkenes in an intramolecular version was reported by Zhang and co-workers in 2017 (Scheme 14) [33]. Langlois’ conditions with tert-butyl hydroperoxide and a catalytic amount of copper(II) triflate were used to prepare a series of CF3-containing indoline, pyrrolidine, lactam and lactone.
Scheme 14: Copper-promoted intramolecular aminotrifluoromethylation of alkenes with CF3SO2Na.
Scheme 14: Copper-promoted intramolecular aminotrifluoromethylation of alkenes with CF3SO2Na.
Oxytrifluoromethylation of alkenes: The difunctionalisation of alkenes including a trifluoromethylation step was extended to carbon–oxygen bond formation via oxytrifluoromethylation. We have already described some examples of such a reaction leading to α-trifluoromethyl ketones (vide supra) [26]. Now synthetic routes are presented leading to vicinal trifluoromethyl alcohols. In 2013 Qing and Jiang described the oxytrifluoromethylation of alkenes with hydroxamic acids 32 and CF3SO2Na under Langlois’ conditions with the couple t-BuOOH/copper salt (Scheme 15) [34]. A competitive formation of two radicals, CF3• and the amidoxyl radical [ArN(CO2Me)O•] 33, would lead to two regioisomeric oxytrifluoromethylated products. Fortunately, this issue was solved by the primary formation of the CF3 radical and thus a regioselective addition. After optimization of the reaction conditions with styrene as model alkene, the method was applied to a wide range of alkenes featuring various functional groups. Further reduction of the N–O bond by Mo(CO)6 gave the corresponding alcohols.
Scheme 15: Oxytrifluoromethylation of alkenes with CF3SO2Na and hydroxamic acid.
Scheme 15: Oxytrifluoromethylation of alkenes with CF3SO2Na and hydroxamic acid.
A protocol free of peroxide initiator was developed by Yang, Vicic and co-workers using a manganese salt and O2 from air [35]. Styrene derivatives were transformed preferentially into hydroxytrifluoromethylated compounds 34 versus the corresponding ketones 35 in moderate to good selectivities (Scheme 16). In the case of 1,2-disubstituted alkenes, mixtures of syn- and anti-isomers were obtained. A radical pathway was supported by several observations: (i) addition of TEMPO suppressed the reaction; (ii) an induction period was observed followed by acceleration with consumption of styrene; (iii) vinyl triflone was detected indicating the formation of CF3SO2•; (iv) formation of CF3SO3– via oxidation of CF3SO2• (Scheme 16).
![[1860-5397-13-272-i16]](/bjoc/content/inline/1860-5397-13-272-i16.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 16: Manganese-catalysed oxytrifluoromethylation of styrene derivatives.
Scheme 16: Manganese-catalysed oxytrifluoromethylation of styrene derivatives.
A metal-free approach with in situ generation of the peroxide from the combination of NMP and O2 as the radical initiator was proposed by Lei and co-workers [36]. This method was based on a previous work by Maiti (see Scheme 5) [24] but did not require a metal to generate the CF3 radical. Tertiary β-trifluoromethyl alcohols 36 were obtained in good yields from a variety of di- and trisubstituted alkenes (Scheme 17). Labelling and IR experiments were conducted to investigate the reaction mechanism as well as kinetic studies that revealed the reaction rate dependence on O2 diffusion.
Scheme 17: Oxytrifluoromethylation of alkenes with NMP/O2 and CF3SO2Na.
Scheme 17: Oxytrifluoromethylation of alkenes with NMP/O2 and CF3SO2Na.
A case of intramolecular oxytrifluoromethylation of alkenes leading to oxazolines 37 was described by Fu and co-workers in 2014 [37]. In this work iodobenzene diacetate (PIDA) was used as the oxidant to generate the CF3 radical from CF3SO2Na (Scheme 18).
Scheme 18: Intramolecular oxytrifluoromethylation of alkenes.
Scheme 18: Intramolecular oxytrifluoromethylation of alkenes.
Hydrotrifluoromethylation of alkenes: Direct alkene hydrotrifluoromethylation by means of CF3SO2K under electrochemical oxidation was first reported by Tommasino and co-workers in 2002 on three alkenes but yields were below 20% due to the formation of oxidised byproducts [38]. Nicewicz and co-workers in 2013 found suitable reaction conditions for the alkene hydrotrifluoromethylation using CF3SO2Na [39]. The single electron oxidation of CF3SO2Na was performed by visible-light activated N-methyl-9-mesitylacridinium as a photoredox catalyst. Two hydrogen atom donors, 20 mol % of methyl thiosalicylate 38 for aliphatic alkenes (or 1 equiv of thiophenol 39 for styrenyl alkenes) and 2,2,2-trifluoroethanol (TFE), worked in concert for the hydrogen atom transfer with complete suppression of the oxidised trifluoromethylated byproducts. The method was regioselective for mono-, di-, and trisubstituted aliphatic alkenes and styrenyl alkenes with a broad substrate scope (Scheme 19). As an exception, 1,2-disubstituted alkenes and chalcone gave low regioselectivities with mixed Markovnikov and anti-Markovnikov products.
![[1860-5397-13-272-i19]](/bjoc/content/inline/1860-5397-13-272-i19.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 19: Hydrotrifluoromethylation of styrenyl alkenes and unactivated aliphatic alkenes.
Scheme 19: Hydrotrifluoromethylation of styrenyl alkenes and unactivated aliphatic alkenes.
The CF3 radical is electrophilic in nature and, as such, not prone to readily react with electron-deficient alkenes. Nevertheless, Lefebvre, Hoffmann and Rueping reported that N-substituted maleimides, maleic anhydride and dimethyl maleate were hydrotrifluoromethylated with CF3SO2Na in the presence of 4,4’-dimethoxybenzophenone as photosensitiser under near-UV irradiation (350 nm) and hexafluoroisopropanol (HFIP) as a proton donor (Scheme 20) [40]. The reactions were performed in batch and under continuous flow conditions with rate enhancement for the latter setup. It was proposed that the CF3 radical added onto the substrate while the ketyl radical 41 was protonated by HFIP. Then, hydrogen transfer gave the hydrotrifluoromethylated product 40 and the sensitiser was regenerated (Scheme 20). In the same paper, the authors also realised the same chemical transformation under visible light irradiation at 450 nm by means of the iridium photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 ([4,4’-bis(tert-butyl)-2,2’-bipyridine]bis[3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl]iridium(III) hexafluorophosphate), which delivered comparable and even higher yields of the products in longer reaction times but with lower catalyst loading (1 mol %).
![[1860-5397-13-272-i20]](/bjoc/content/inline/1860-5397-13-272-i20.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 20: Hydrotrifluoromethylation of electron-deficient alkenes.
Scheme 20: Hydrotrifluoromethylation of electron-deficient alkenes.
Both unactivated terminal alkenes and electron-deficient alkenes (Michael acceptors) were successfully hydrotrifluoromethylated under irradiation with 36 W blue LEDs in the presence of an iridium photoredox catalyst as reported by Zhu, Zhang and co-workers [41]. Of the photocatalysts tested, Ir[dF(CF3)ppy]2(dtbbpy)PF6 had appropriate redox potentials and gave the best results. A wide range of terminal alkenes featuring several functional groups reacted with exclusive anti-Markovnikov selectivity. Notably, styrene failed to react under these conditions. A selection of α,β-unsaturated electron-withdrawing motifs that included a sulfone, esters, an amide, and a ketone were investigated for the first time and the β-addition products were obtained regioselectively in moderate to good yields (Scheme 21). It was suggested that the methylene radical formed by addition of the CF3 radical onto the alkene was reduced by the sulfinate anion and the corresponding carbanion was protonated by methanol.
Scheme 21: Hydrotrifluoromethylation of alkenes by iridium photoredox catalysis.
Scheme 21: Hydrotrifluoromethylation of alkenes by iridium photoredox catalysis.
Halo- and pseudohalotrifluoromethylation of alkenes: The direct iodotrifluoromethylation was previously achieved by means of gaseous CF3I until Liu and co-workers reported the convenient use of CF3SO2Na and iodide pentoxide, I2O5, in combination for the iodotrifluoromethylation of alkenes and alkynes (see later in the text) [42]. After an optimisation with 4-chlorostyrene, the reaction was developed with a wide range of terminal and internal alkenes bearing diverse functional groups such as halogens, nitro, sulfonate, sulfamide, carboxylate, amide, ether, carbonyl, and hydroxy that were all well-tolerated (Scheme 22). A mixed solvent system of dichloromethane and water was used in a sealed tube at 110 °C. Mechanistic studies by electron spin resonance were carried out in which both the CF3• and the β-CF3 alkyl radical intermediate were observed by using 2-methyl-2-nitrosopropane as a radical spin trap. A single-electron oxidative free-radical process was clearly ascertained. For the iodination step, the authors proposed that the β-CF3 alkyl radical was intercepted by I2, which was formed by a multistep redox process from I2O5. In continuation of this work, the same research group described the bromotrifluoromethylation of alkenes under similar reaction conditions but using sodium bromate, NaBrO3, as a bromine source (Scheme 22) [43].
Scheme 22: Iodo- and bromotrifluoromethylation of alkenes by CF3SO2Na/I2O5 or CF3SO2Na / NaBrO3.
Scheme 22: Iodo- and bromotrifluoromethylation of alkenes by CF3SO2Na/I2O5 or CF3SO2Na / NaBrO3.
The photoredox-catalysed chloro-, bromo- and also (trifluoromethylthio)trifluoromethylation of unactivated alkenes was studied by Liu and co-workers in 2017 (Scheme 23) [44]. The Langlois reagent was combined with N-halophthalimides 42a,b or N-trifluoromethylthiosaccharin 43 in the presence of N-methyl-9-mesitylacridinium under visible light irradiation at room temperature. Terminal, internal, and gem-substituted alkenes bearing imide, ester, amide, ketone, aldehyde and electron-rich aryl functional groups were suitable substrates. Notably, diethyl 2,2-diallylmalonate as a diene gave the cyclised product resulting of a radical cascade. It has to be noticed that the reactions were conducted in the presence of 2 equivalents of trifluoroacetic or p-toluenesulfonic acid; yet, there was no mention of hydrotrifluoromethylated side-products. The mechanism was similar to previous examples to generate the β-CF3 alkyl radical intermediate 44, which was trapped by halogen atom transfer from the halogenating agent. The nitrogen-centered radical 45 oxidised Mes-Acr* by a single-electron-transfer process to restart the catalytic cycle (Scheme 23).
![[1860-5397-13-272-i23]](/bjoc/content/inline/1860-5397-13-272-i23.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 23: N-methyl-9-mesityl acridinium and visible-light-induced chloro-, bromo- and SCF3 trifluoromethylation of alkenes.
Scheme 23: N-methyl-9-mesityl acridinium and visible-light-induced chloro-, bromo- and SCF3 trifluoromethylati...
Carbotrifluoromethylation of alkenes: The strategies for carbotrifluoromethylation of alkenes with CF3SO2Na are very much based on methods described earlier in the text: (i) reactions mediated by tert-butyl hydroperoxide and a catalytic amount of copper; (ii) metal-catalysed or metal-free reactions with K2S2O8, I2O5 or a hypervalent iodine reagent, and (iii) photochemical activation. Most of the works concerned cascade intramolecular reactions in which a C–C bond is formed after the initial trifluoromethylation.
Therefore, Lipshutz and co-workers reported a copper-catalysed intramolecular carbotrifluoromethylation of N-arylacrylamides 46 with CF3SO2Na to produce oxindoles 47 [45]. Addition of the CF3 radical to such an electron-deficient alkene should be unfavourable. However, the subsequent annulation step drove the cascade process toward oxindole synthesis. The reaction utilised Langlois’ conditions with tert-butyl hydroperoxide and a catalytic amount of Cu(II), but with 10 mol % of tetramethylethylenediamine (TMEDA). Organic solvents were replaced by pure water and the aqueous medium can be recycled up to five times. The substrate scope was large when tertiary amides were used. A secondary arylamide failed to give the expected product. With a substituent at the meta-position of the aniline ring, a mixture of regioisomers was obtained. Various alkenes with substituents (R3) were investigated and the oxindoles were obtained in moderate to high yields (Scheme 24).
Scheme 24: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na / TBHP by Lipshutz.
Scheme 24: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na / TBHP by Lipshutz.
Simultaneously, Lei and co-workers published the same reaction under slightly different conditions [46]. They used a combination CF3SO2Na/TBHP in the presence of catalytic amounts of copper chloride and triphenylphosphine. Trisubstituted alkenes (R3 and R4 ≠ H) were employed as substrates and diastereoisomers were obtained. The tert-butoxyl radical was generated from TBHP and Cu(n) via a SET process, which then, it reacted with CF3SO2Na to liberate CF3•. The subsequent addition of CF3• to the β-position of the C=C bond of the acrylamide gave the intermediate 48, which underwent an intramolecular radical annulation to produce the aryl radical 49. Finally, oxidation of 49 by Cu(n + 1) and aromatisation afforded the oxindole and regenerated the copper catalyst (Scheme 25).
![[1860-5397-13-272-i25]](/bjoc/content/inline/1860-5397-13-272-i25.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 25: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/TBHP reported by Lei.
Scheme 25: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/TBHP reported by Lei.
The same indoles bearing a 2,2,2-trifluoroethyl side-chain were also obtained in reactions performed with CF3SO2Na and (NH4)2S2O8 as the oxidant in the presence of a catalytic amount of AgNO3 as reported by Tan and co-workers (Scheme 26) [47]. In the absence of AgNO3 the reaction did not work. Notably, N-alkyl and N-aryl protected substrates worked well, whereas N-acyl and N–H derivatives failed to deliver the desired products. Mechanistically, Ag(I) was initially oxidised to Ag(II) by the persulfate anion; then, CF3SO2– was oxidised to CF3SO2• that generated CF3• by release of SO2. Addition of the CF3 radical to the alkene led to the radical intermediate 50, which underwent intramolecular cyclisation into 51. The sulfate radical anion then oxidised intermediate 51 into the final oxindole (Scheme 26).
![[1860-5397-13-272-i26]](/bjoc/content/inline/1860-5397-13-272-i26.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 26: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/(NH4)2S2O8.
Scheme 26: Carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/(NH4)2S2O8.
In an independent work Wang and co-workers demonstrated that silver nitrate was not necessary for the reaction to proceed in acetonitrile and water at 80 °C (Scheme 27) [48]. Again, N-acyl and N–H derivatives failed to deliver the desired products and meta-substituted phenyl rings produced mixtures of regioisomers. Under these metal-free conditions, it was proposed that the CF3 radical was formed uniquely by reaction of CF3SO2Na with K2S2O8.
Scheme 27: Metal-free carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/K2S2O8 reported by Wang.
Scheme 27: Metal-free carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/K2S2O8 reported by Wang.
N-Arylacrylamides could also react with CF3SO2Na under metal-free conditions by replacing tert-butyl hydroperoxide or the persulfate by hypervalent iodine oxidants such as iodobenzene diacetate (PIDA, Scheme 28) [49], or iodobenzene bis(trifluoroacetate) (PIFA) [50]. Fu and co-workers proposed the reaction mechanism depicted in Scheme 28. PIDA reacted with CF3SO2Na under heating conditions to produce two radicals: CF3• along with PhI•OAc. Addition of the CF3 radical to the alkene followed by intramolecular cyclisation mediated by PhI•OAc gave the desired oxindole with release of PhI and AcOH (Scheme 28) [49].
Scheme 28: Metal-free carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/PIDA reported by Fu.
Scheme 28: Metal-free carbotrifluoromethylation of N-arylacrylamides with CF3SO2Na/PIDA reported by Fu.
For another metal-free trifluoromethylation/cyclisation of N-arylacrylamides by means of a different oxidant, Liu and co-workers reported the use of CF3SO2Na in combination with iodide pentoxide in a similar way to their iodotrifluoromethylation of alkenes (see earlier in the text) [42] (Scheme 29a) [51]. Interestingly, this cascade reaction was also applied to enynes for the synthesis of pyrrolidines 52 (Scheme 29b) [51]. A single-electron oxidative free-radical process was ascertained for the generation of CF3•. From enynes, the iodination step was realised by I2, which was formed by a multistep redox process from I2O5.
![[1860-5397-13-272-i29]](/bjoc/content/inline/1860-5397-13-272-i29.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 29: Metal-free cascade trifluoromethylation/cyclisation of N-arylmethacrylamides (a) and enynes (b) with CF3SO2Na/I2O5.
Scheme 29: Metal-free cascade trifluoromethylation/cyclisation of N-arylmethacrylamides (a) and enynes (b) wit...
The intramolecular carbotrifluoromethylations of alkenes from acrylamides and methacrylamides, so far described, provided oxindoles via a 5-exo trig cyclization. Starting from cinnamamides 53, Mai, Xiao and co-workers reported a 6-endo trig cyclisation leading to 3,4-disubstituted dihydroquinolin-2(1H)-ones 54 (Scheme 30) [52]. Ag(I) was oxidised by the persulfate anion (S2O82–) to generate the Ag(II) cation and the sulfate radical anion; then, the Ag(II) oxidised CF3SO2Na into CF3• with extrusion of SO2. The CF3 radical reacted with the C=C double bond of the cinnamamide leading to the intermediate 55 that underwent 6-endo trig cyclisation to 56 that finally aromatised to the desired product 54 trans-selectively (Scheme 30).
![[1860-5397-13-272-i30]](/bjoc/content/inline/1860-5397-13-272-i30.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 30: Trifluoromethylation/cyclisation of N-arylcinnamamides: Synthesis of 3,4-disubstituted dihydroquinolin-2(1H)-ones.
Scheme 30: Trifluoromethylation/cyclisation of N-arylcinnamamides: Synthesis of 3,4-disubstituted dihydroquino...
In 2016, Xia and co-workers described a metal-free, UV-light-mediated difunctionalisation of alkenes with CF3SO2Na for the synthesis of phenanthrene and anthrone derivatives [53]. The substrates were either α,β-unsaturated ketones 57 (Scheme 31a) or γ,δ-unsaturated ketones 58 (Scheme 31b). Benzophenone (BP) or anthracene-9,10-dione (AQ) were used as sensitizers under irradiation using a UV lamp at 280 nm. A radical pathway that involves CF3• was established after a negative reaction in the presence of TEMPO (TEMPO–CF3 was detected by GC–MS).
Scheme 31: Trifluoromethylation/cyclisation of aromatic-containing unsaturated ketones.
Scheme 31: Trifluoromethylation/cyclisation of aromatic-containing unsaturated ketones.
An example of difunctionalisation of unactivated alkenes with CF3SO2Na and an heteroaryl group in which the heteroarylation was realised by a distal heteroaryl ipso-migration was provided in 2017 by Zhu and co-workers (Scheme 32) [54]. A variety of nitrogen containing heteroaryl groups showcased the migratory aptitude selectively in the presence of an aryl or an alkyl group. The number of methylene units between the alkene and the tertiary alcohol function was studied: n = 0, 2, and 3 were suitable for generating thermodynamically favoured 3, 5, and 6-membered cyclic transition states; the reaction failed with n = 1, 4. Experimental and computational studies allowed the authors to propose the mechanism depicted in Scheme 32. First, the CF3 radical was generated from CF3SO2Na and PIFA. Then, addition of CF3• to the alkene gave the alkyl radical 59 that added to the ipso position of the heteroaryl group to form radical 60. Next, homolysis of the C–C σ-bond in 60 provided the more stable hydroxyalkyl radical 61. This radical was oxidised by PIFA to yield the cationic intermediate 62, which finally lost a proton to furnish the reaction product.
![[1860-5397-13-272-i32]](/bjoc/content/inline/1860-5397-13-272-i32.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 32: Chemo- and regioselective cascade trifluoromethylation/heteroaryl ipso-migration of unactivated alkenes.
Scheme 32: Chemo- and regioselective cascade trifluoromethylation/heteroaryl ipso-migration of unactivated alk...
1,2-Bis-trifluoromethylation of alkenes: Alkenes were efficiently and chemoselectively bis-trifluoromethylated under Langlois’ conditions with CF3SO2Na. Indeed, Qing and co-workers prepared 1,2-bis(trifluoromethylated) compounds 63 with in situ generated CF3 radicals (Scheme 33). In order to avoid the formation of dimerised side products, it was demonstrated that an increase of the CF3 radical concentration, obtained by increasing the amount of copper catalyst, was beneficial to the chemoselectivity. Both styrene derivatives and terminal unactivated alkenes were suitable substrates in this transformation but not internal alkenes [55].
Scheme 33: Copper-mediated 1,2-bis(trifluoromethylation) of alkenes.
Scheme 33: Copper-mediated 1,2-bis(trifluoromethylation) of alkenes.
Csp2–CF3 bond-forming reactions
Direct trifluoromethylation of arenes and heteroarenes: In 1991, Langlois and co-workers reported the first trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions (Scheme 34) [16]. The scope was quite narrow with electron-rich aromatics and mixtures of regioisomers were often obtained. For instance, from aniline, two isomers were obtained in 13% overall yield, and from 1,3-dimethoxybenzene, four products (regioisomers + bis-CF3 compounds) were obtained in 90% overall yield. For this transformation, a radical process was proposed: the trifluoromethyl radical CF3• was generated by reaction of tert-butyl hydroperoxide with CF3SO2Na in the presence of a copper(II) catalyst (Scheme 34).
Scheme 34: Trifluoromethylation of aromatics with CF3SO2Na reported by Langlois.
Scheme 34: Trifluoromethylation of aromatics with CF3SO2Na reported by Langlois.
Substrates with sensitive functional groups may not be tolerated under such reaction conditions and a large excess amount of peroxide was necessary to reach high yields. That is how, in 1998, Smertenko and co-workers described a milder electrochemical trifluoromethylation of a series of aromatic compounds using CF3SO2Na in acetonitrile [56]. Furthermore, the electrochemical oxidation of the trifluoromethylsulfinate anion (from CF3SO2K) generated the trifluoromethyl radical for the reaction of electron-rich aromatics and alkenes [38].
It is twenty years after Langlois’ pioneering work that the direct trifluoromethylation of heteroaromatic compounds was re-investigated by Baran and co-workers in 2011 [57]. This group reported a C−H trifluoromethylation protocol that was operationally simple, scalable, achieved at room temperature, working with a variety of electron-deficient and -rich heteroaromatic systems tolerating various functional groups such as unprotected alcohols, amines, ketones, esters, halides and nitriles. Importantly, the trifluoromethylation proceeded at the innate reactive positions of the heterocycles; however, it was noticed that the regioselectivity can be tuned simply by solvent choice. Langlois and others employed catalytic metal salts for reaction initiation but Baran’s group demonstrated that metal additives were not required for a productive reaction, only trace metals found in Langlois’ reagent could be responsible for reaction initiation. The scope was evaluated on pyridines, pyrroles, indoles, pyrimidines, pyrazines, phthalazines, quinoxalines, deazapurine, thiadiazoles, uracils, xanthenes and pyrazolino-pyrimidines (Scheme 35). The combination of previous studies with new observations allowed to propose a putative mechanism (Scheme 35) as well as unproductive pathways (formation of CF3H from CF3• by abstraction of a hydrogen atom or reaction of CF3• with isobutene generated from t-BuOOH).
![[1860-5397-13-272-i35]](/bjoc/content/inline/1860-5397-13-272-i35.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 35: Baran’s oxidative C–H trifluoromethylation of heterocycles.
Scheme 35: Baran’s oxidative C–H trifluoromethylation of heterocycles.
This oxidative trifluoromethylation method was exploited for the synthesis of modified nucleosides, in particular 8-CF3-2’-deoxyguanosine and 8-CF3-inosine in 39 and 73% yields, respectively [58].
The same copper-free method was applied for the trifluoromethylation of a variety of electron-deficient 4-substituted acetanilides or anilines (Scheme 36). In these reaction conditions, Cao’s group reported that acetanilides or anilines featuring electron-donating substituents at the para-position of the acetamino group afforded mixtures of isomeric C−H trifluoromethylation products in moderate yields. However, with substrates bearing electron-withdrawing groups, ortho-CF3 acetanilides or anilines were obtained as sole products [59].
Scheme 36: Trifluoromethylation of acetanilides and anilines.
Scheme 36: Trifluoromethylation of acetanilides and anilines.
To meet high expectations of environmentally low impact chemical reactions, Lipshutz and co-workers carried out the trifluoromethylation of heterocycles using aqueous micellar conditions based on the surfactant TPGS−750−M in water at room temperature. The trifluoromethyl radical was generated from CF3SO2Na and t-BuOOH. In comparaison to Baran’s results, in all cases, the yields were improved. Advantageously, the aqueous medium can be recycled (Scheme 37) [60].
Scheme 37: Trifluoromethylation of heterocycles in water.
Scheme 37: Trifluoromethylation of heterocycles in water.
Even though it is a proven fact after 2011 and Baran’s work that no-added metal trifluoromethylation with CF3SO2Na are highly efficient, the original Langlois’ conditions were nevertheless applied to a series of heteroarenes. Li and co-workers reported the synthesis of 3-trifluoromethylcoumarins 64 by Cu(I)-catalysed trifluoromethylation with CF3SO2Na and t-BuOOH in a continuous-flow reactor [61]. After optimisation of the reaction conditions in batch, the optimal reaction conditions were established in a continuous-flow reactor at a flow rate of 100 µL min−1 at 60 °C for 40 min. The substrate scope was evaluated on 11 coumarins and showed that both electron-rich and electron-deficient functional groups were tolerated (Scheme 38).
![[1860-5397-13-272-i38]](/bjoc/content/inline/1860-5397-13-272-i38.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 38: Trifluoromethylation of coumarins in a continuous-flow reactor.
Scheme 38: Trifluoromethylation of coumarins in a continuous-flow reactor.
Zou and co-workers applied the conditions described by Langlois or Baran (CF3SO2Na/t-BuOOH/cat. Cu(II) or CF3SO2Na/t-BuOOH, respectively) for the trifluoromethylation of coumarins but no reaction was observed. By testing other oxidants, they found that Mn(OAc)3 was a good oxidant for this reaction and allowed to carry out the trifluoromethylation exclusively at the α-position of the carbonyl group in the pyranone ring. The substrate scope was large and included 17 coumarins, 2 quinolines and 3 pyrimidinones. With coumarins bearing electron-donating groups on the phenyl ring, the 3-trifluoromethylated compounds were obtained in 50–56% yields. However, coumarins bearing electron-withdrawing groups gave yields up to 70%. As for the mechanism, the trifluoromethyl radical was generated from CF3SO2Na and Mn(OAc)3, then CF3• added regioselectively onto the coumarin to give intermediate radical 65, which was oxidised by Mn(OAc)3 to form the carbocation 66 and, after deprotonation, the trifluoromethyl compounds (Scheme 39) [62].
![[1860-5397-13-272-i39]](/bjoc/content/inline/1860-5397-13-272-i39.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 39: Oxidative trifluoromethylation of coumarins, quinolines and pyrimidinones.
Scheme 39: Oxidative trifluoromethylation of coumarins, quinolines and pyrimidinones.
The same group also reported a straightforward method for the trifluoromethylation of pyrimidinones and pyridinones under the same reaction conditions. 5-Trifluoromethylpyrimidinones and 3-trifluoromethylpyridinones were selectively obtained in moderate to good yields (Scheme 40). It was observed that the substituent R1 provided no stabilisation for the radical intermediate, so the less bulky substituents at 6-position of pyrimidinones or 4-position of pyridinones facilitated the trifluoromethyl radical attack [63].
Scheme 40: Oxidative trifluoromethylation of pyrimidinones and pyridinones.
Scheme 40: Oxidative trifluoromethylation of pyrimidinones and pyridinones.
Catalytic amounts of phosphovanadomolybdic acid, a heteropolyacid catalyst (HPA), was used by Mizuno, Yamaguchi and co-workers for the oxidative C−H trifluoromethylation of arenes and heteroarenes in the presence of CF3SO2Na and O2 as the terminal oxidant. This method allowed the trifluoromethylation of arenes bearing electron-donating as well as electron-withdrawing groups in moderate to good yields (Scheme 41) [64]. It has to be noted that bis-CF3 products as well as regioisomers were also characterised or detected in small amounts in most cases. A radical mechanism was proposed as described in Scheme 41.
![[1860-5397-13-272-i41]](/bjoc/content/inline/1860-5397-13-272-i41.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 41: Phosphovanadomolybdic acid-catalysed direct C−H trifluoromethylation.
Scheme 41: Phosphovanadomolybdic acid-catalysed direct C−H trifluoromethylation.
Imidazopyridines have demonstrated many interesting features toward biological activities and the incorporation of a trifluoromethyl group into such architectures was expected to alter their properties. Therefore, Hajra and co-workers reported a direct and regioselective method for the trifluoromethylation of imidazopyridines 67 and other imidazoheterocycles 68 [65]. The combination CF3SO2Na/t-BuOOH/cat. AgNO3 at room temperature under air was applied to 17 imidazopyridines and 3 imidazoheterocycles (Scheme 42). Good yields were obtained when the phenyl moiety was substituted by electron-donating groups. As a result of absence of reactivity in presence of the radical scavenger TEMPO, a radical pathway was proposed. Under argon atmosphere, only trace amounts of the CF3 product were obtained clearly indicating the crucial role of aerial oxygen in the catalytic cycle (see mechanism in Scheme 42).
![[1860-5397-13-272-i42]](/bjoc/content/inline/1860-5397-13-272-i42.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 42: Oxidative trifluoromethylation of imidazopyridines and imidazoheterocycles.
Scheme 42: Oxidative trifluoromethylation of imidazopyridines and imidazoheterocycles.
Simultaneously, Tang and co-workers reported a greener strategy for the trifluoromethylation of imidazoheterocycles with CF3SO2Na in a recyclable mixed medium of 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) and water [66]. The substrate scope was investigated on 11 imidazothiazoles, 13 imidazo[1,2-a]pyridines and 6 imidazoles (Scheme 43). The reaction was simple, achieved at room temperature and had a good tolerance for various functional groups. However, for the trifluoromethylation of imidazoles, it was essential to have a phenyl as a substituent in order to get good yields. A radical mechanism was proposed but the role of oxygen was not discussed.
Scheme 43: Oxidative trifluoromethylation of imidazoheterocycles and imidazoles in ionic liquid/water.
Scheme 43: Oxidative trifluoromethylation of imidazoheterocycles and imidazoles in ionic liquid/water.
Aminoquinoline derivatives are found in naturally occurring and synthetic bioactive compounds, most notable for their antimalarial activity. So, it was not surprising that trifluoromethyl analogues were prepared in particular by means of CF3SO2Na. Indeed, 5-trifluoromethyl-8-aminoquinoline derivatives 69 were regioselectively synthesised under various reaction conditions. Cai and co-workers reported the trifluoromethylation of 8-aminoquinolines selectively at position 5 by using CF3SO2Na, CuBr2 in a catalytic amount and azobisisobutyronitrile (AIBN) as an oxidant (Scheme 44) [67]. This process had a broad tolerance toward a wide range of functional groups. Aliphatic amides, aromatic amides and carboxamides with heterocyclic substituents were compatible with the reaction conditions. A series of control experiments that included the inhibition of the reaction in the presence of TEMPO, deuteration and isotope effect experiments were carried out and led the authors to propose the single-electron transfer mechanism presented in Scheme 44.
![[1860-5397-13-272-i44]](/bjoc/content/inline/1860-5397-13-272-i44.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 44: Oxidative trifluoromethylation of 8-aminoquinolines.
Scheme 44: Oxidative trifluoromethylation of 8-aminoquinolines.
Simultaneously, Shen, Zhang and co-workers reported a milder regioselective trifluoromethylation of 8-aminoquinolines using the supported catalyst CS@Cu(OAc)2 (CS = chitosan), potassium persulfate as the oxidant and CF3SO2Na as the CF3• source (Scheme 45) [68]. After optimisation of the reaction conditions, the authors studied the effect of structural variations in the substrate (R1 = aryl, heteroaryl, alkyl; R2 = H, 6-OMe, 2-Me). Aniline amides gave no conversion nor did quinoline having an ester group at the 8 position instead of the 8-amino group. The chitosan-based copper catalyst was efficiently reused in five cycles of this heterogeneous trifluoromethylation.
Scheme 45: Oxidative trifluoromethylation of various 8-aminoquinolines using the supported catalyst CS@Cu(OAc)2.
Scheme 45: Oxidative trifluoromethylation of various 8-aminoquinolines using the supported catalyst CS@Cu(OAc)2...
In 2017, Lu, Weng and co-workers reported a protocol for the para-selective trifluoromethylation of naphthylamide 70, instead of the previously studied quinolines, with CF3SO2Na, tert-butyl hydroperoxide and Cu(OAc)2·H2O as oxidant (Scheme 46) [69].
Scheme 46: Oxidative trifluoromethylation of the naphthylamide 70.
Scheme 46: Oxidative trifluoromethylation of the naphthylamide 70.
tert-Butyl hydroperoxide could be replaced by sodium persulfate as demonstrated in 2016 by Gong and co-workers who reported a direct C−H trifluoromethylation of arenes with CF3SO2Na in a mixture of water and acetonitrile (Scheme 47) [70]. Various trifluoromethylated arenes were obtained in moderate to excellent yields. However, to achieve high yields in this trifluoromethylation, one (or more) alkoxy group(s) must be present on the arenes in order to stabilise the free-radical intermediate (see mechanism in Scheme 47). Control experiments such as the trapping of the trifluoromethyl radical with the scavenger TEMPO or with benzoquinone were performed and a radical process was proposed. This mild and safe transformation had a good tolerance for various functional groups.
![[1860-5397-13-272-i47]](/bjoc/content/inline/1860-5397-13-272-i47.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 47: Oxidative trifluoromethylation of various arenes in the presence of CF3SO2Na and sodium persulfate.
Scheme 47: Oxidative trifluoromethylation of various arenes in the presence of CF3SO2Na and sodium persulfate.
In 2013, Shibata and co-workers reported a transition-metal-free oxidative trifluoromethylation of arenes with CF3SO2Na and phenyliodine bis(trifluoroacetate) (PIFA) instead of tert-butyl hydroperoxide as the oxidant in hexafluoroisopropanol (HFIP) at room temperature [71]. In order to obtain good results for this transformation, it should be noted that the electron-donating nature of the aromatic substituents was a crucial point. In the case of unsymmetrical biaryl substrates, mixtures of regioisomers at C4 and C5 were obtained (Scheme 48). In the reaction mechanism, PIFA played a dual role in the activation of the arene via a π-complex and in the generation of the CF3 radical from CF3SO2Na.
![[1860-5397-13-272-i48]](/bjoc/content/inline/1860-5397-13-272-i48.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 48: Trifluoromethylation of electron-rich arenes and unsymmetrical biaryls with CF3SO2Na in the presence of PIFA.
Scheme 48: Trifluoromethylation of electron-rich arenes and unsymmetrical biaryls with CF3SO2Na in the presenc...
More recently, Maruoka and co-workers reported the synthesis of trifluoromethylated coumarin 71 and flavone 72 with CF3SO2Na (2 equiv), the hypervalent iodine F5-PIFA (pentafluorophenyliodine bis(trifluoroacetate)) (2 equiv) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 0.6 equiv). The trifluoromethylated compounds were obtained in moderate yields (Figure 1) [50].
Figure 1: Trifluoromethylated coumarin and flavone.
Figure 1: Trifluoromethylated coumarin and flavone.
To avoid the use of transition-metal catalysts and/or an excess amount of oxidants, the Itoh group reported a simple metal-free, direct trifluoromethylation of arenes and heteroarenes using a photoredox-based process under visible-light irradiation. This method used CF3SO2Na as source of the trifluoromethyl group and a catalytic amount of anthraquinone-2-carboxylic acid (AQN-2-CO2H). The scope was achieved on arenes and heteroarenes (10 examples). Once more, electron-rich aromatic compounds were converted into the corresponding trifluoromethylated products in good yields (Scheme 49). The oxidation–reduction potentials were determined by cyclic voltammetry and a catalytic cycle was proposed in which the CF3 radical was generated from CF3SO2Na via the organocatalyst AQN-2-CO2H and visible light (Scheme 49) [72].
![[1860-5397-13-272-i49]](/bjoc/content/inline/1860-5397-13-272-i49.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 49: Metal-free trifluoromethylation catalysed by a photoredox organocatalyst.
Scheme 49: Metal-free trifluoromethylation catalysed by a photoredox organocatalyst.
Rueping’s group described three examples of prototypical (hetero)aromatic substrates that were trifluoromethylated with CF3SO2Na in the presence of 4,4’-dimethoxybenzophenone as photosensitiser under near-UV irradiation (350 nm) and HFIP as a proton donor [40]. More recently, Yuan and co-workers reported an efficient and operationally simple method for the direct trifluoromethylation of a wide variety of arenes and heteroarenes under visible-light irradiation [73]. The substrate scope was evaluated on 30 arenes and heteroarenes using 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) as photocatalyst and CF3SO2Na as the CF3 radical source. The reaction conditions tolerated a broad range of functional groups and the yields ranged from 31 to 68% (Scheme 50). During the process, the quinone was converted into hydroquinone and a regeneration process was established by passing through a cartridge of MnO2. A mechanism for this transformation was proposed as a result of electron paramagnetic resonance spectroscopy experiments (Scheme 50).
![[1860-5397-13-272-i50]](/bjoc/content/inline/1860-5397-13-272-i50.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 50: Quinone-mediated trifluoromethylation of arenes and heteroarenes.
Scheme 50: Quinone-mediated trifluoromethylation of arenes and heteroarenes.
In 2016, Li, Mi and co-workers reported a simple and clean approach for the direct trifluoromethylation of unactivated arenes and heteroarenes through a photoreduction without any metal catalyst nor oxidant. The radical initiators were as simple as acetone or diacetyl for the generation of CF3 radicals. Indeed, the authors demonstrated that photoexcited acetone was capable to trigger the trifluoromethylation reaction efficiently. So, acetone in this process was used as solvent and photosensitiser. The reactions were carried out under UV irradiation with either a 300 W xenon lamp (emission wavelengths between 200 and 1000 nm) or the photoreactor (λ = 254 nm). This photochemical process allowed the synthesis of trifluoromethylated arenes (Scheme 51), but also heteroarenes and nucleosides in good yields [74]. Further to this work, it should be noted that Davies, MacMillan and co-workers have designed an integrated small-scale photoreactor that enabled acceleration of this photocatalytic reaction [75].
![[1860-5397-13-272-i51]](/bjoc/content/inline/1860-5397-13-272-i51.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 51: Metal- and oxidant-free photochemical trifluoromethylation of arenes.
Scheme 51: Metal- and oxidant-free photochemical trifluoromethylation of arenes.
Trifluoromethylation of arenediazonium compounds: Langlois’ conditions were applied in the copper-mediated Sandmeyer-type trifluoromethylation of aryldiazonium compounds. The scope of this reaction was investigated on 12 aryldiazonium compounds. The mild reaction conditions allowed the tolerance of various groups such as ester, aryl, nitrile, amine, ketone, nitro, sulfonate and bromo (Scheme 52). In this process, the CF3 radical was stabilised by the presence of an excess amount of CuBF4(MeCN)4 and the tridentate ligand 2,2’;6’,2"-terpyridine (tpy) [76]. Notably, a change in the copper source caused the predominant trifluoromethanesulfonylation of the substrate (introduction of the SO2CF3 group, vide infra). A one-pot version starting from anilines was recently developed [77].
![[1860-5397-13-272-i52]](/bjoc/content/inline/1860-5397-13-272-i52.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 52: Copper-mediated trifluoromethylation of arenediazonium tetrafluoroborates.
Scheme 52: Copper-mediated trifluoromethylation of arenediazonium tetrafluoroborates.
Trifluoromethylation of aryl-, vinyl-, alkynylboronic acids: In 2012, Sanford and co-workers reported, for the first time, the copper-mediated radical trifluoromethylation of aryl- and heteroarylboronic acids using CF3SO2Na and TBHP as an oxidant. The substrate scope was evaluated on 24 aryl and heteroaryl boronic acids. The process was compatible with various functional groups. Arenes bearing electron-donating groups reacted in excellent yields under the CuCl-mediated conditions. However, arenes with electron-withdrawing substituents necessitated (MeCN)4CuPF6 and a base, NaHCO3, to allow the trifluoromethylation to proceed in good yields (Scheme 53) [78].
Scheme 53: Oxidative trifluoromethylation of aryl- and heteroarylboronic acids.
Scheme 53: Oxidative trifluoromethylation of aryl- and heteroarylboronic acids.
In the continuity of Sanford’s work, Beller’s group reported the synthesis of trifluoromethylated arenes from arylboronic acids as well as trifluoromethylated vinylarenes [79]. The substrate scope was realised on 17 arylboronic acids and 8 vinylboronic acids (Scheme 54). The protocol was robust and tolerated various functional groups. However, large excesses of both CF3SO2Na and TBHP were required. In this process, a ligand, 2,4,6-collidine, was used in order to increase the yield of the transformation. For the styrenylboronic acids, electron-withdrawing and electron-donating substituents on the aryl ring were compatible with the reaction conditions. Based on experimental observations, the authors proposed two possible mechanisms for this trifluoromethylation (Scheme 54). In path A, transmetallation of the boronic acid with the active Cu(II) species 73 gave the arylcopper(II) complex 74, which reacted with CF3• to afford the arylcopper(III) complex 75. Next, a reductive elimination gave the trifluoromethylated product with release of the Cu(I) complex 76 that was re-oxidised to the active copper(II) catalyst 73 to close the cycle. In path B, the copper(II) complex 73 reacted with CF3• to form the copper(III) complex 77, which after transmetallation with the boronicacid gave the same intermediate 75.
![[1860-5397-13-272-i54]](/bjoc/content/inline/1860-5397-13-272-i54.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 54: Oxidative trifluoromethylation of aryl- and vinylboronic acids.
Scheme 54: Oxidative trifluoromethylation of aryl- and vinylboronic acids.
Trifluoromethylation of potassium organotrifluoroborates: In 2013, Molander, Rombouts and co-workers simply applied the reaction conditions described by Sanford for boronic acids to a series of unsaturated potassium organotrifluoroborates (Scheme 55) [80]. In the case of aryl- and heteroaryltrifluoroborates, electron-rich substrates were efficiently trifluoromethylated although the increase of the steric hindrance caused a decrease in yields. On the other hand, the yields obtained with electron-poor potassium organotrifluoroborates were lower. In the same paper, the preparation of trifluoromethyl-substituted alkynes and alkenes from alkynyl- and alkenyltrifluoroborates was demonstrated albeit in moderate yields. The yields of the trifluoromethylation of alkenyltrifluoroborates strongly depended on the substitution of the olefin (Scheme 55).
Scheme 55: Oxidative trifluoromethylation of unsaturated potassium organotrifluoroborates.
Scheme 55: Oxidative trifluoromethylation of unsaturated potassium organotrifluoroborates.
Simultaneously, Dubbaka and co-workers reported the trifluoromethylation of aryl-, heteroaryl-, and vinyltrifluoroborates with CF3SO2Na (Scheme 56) [81]. The authors used basically the same protocol as Sanford and Molander but observed that a more diluted reaction medium gave improved reaction yields.
Scheme 56: Oxidative trifluoromethylation of (hetero)aryl- and vinyltrifluoroborates.
Scheme 56: Oxidative trifluoromethylation of (hetero)aryl- and vinyltrifluoroborates.
Trifluoromethylation of alkenes by decarboxylation: Liu and co-workers were the first to describe the copper-catalysed decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids in the presence of CF3SO2Na and TBHP [82]. Various (hetero)arenes were compatible with these reaction conditions (Scheme 57); nevertheless, cinnamic acids bearing electron-donating groups afforded the corresponding products in better yields. The stereoselectivity was moderate to high from 76:24 to 99:1. Interestingly, aryls bearing a nitro or a chloro substituent gave the corresponding α-trifluoromethyl ketones. This process was interesting for the construction of Cvinyl–CF3 bonds via a radical addition–elimination (Scheme 57).
![[1860-5397-13-272-i57]](/bjoc/content/inline/1860-5397-13-272-i57.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 57: Copper−catalysed decarboxylative trifluoromethylation of cinnamic acids.
Scheme 57: Copper−catalysed decarboxylative trifluoromethylation of cinnamic acids.
Soon after, Maiti and co-workers reported an iron-mediated trifluoromethylation of α,β-unsaturated carboxylic acids with CF3SO2Na, iron(III) chloride and potassium persulfate as oxidant [83]. The substrate scope was evaluated on 10 cinnamic acids (Scheme 58). Under these conditions, both electron-rich or electron-poor arenes gave the vinylic CF3 products in good yields and with excellent stereoselectivities. A mechanism was proposed starting from the generation of iron carboxylate and the reaction with the CF3 radical followed by extrusion of CO2 and radical coupling (Scheme 58).
![[1860-5397-13-272-i58]](/bjoc/content/inline/1860-5397-13-272-i58.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 58: Iron-mediated decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids.
Scheme 58: Iron-mediated decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids.
Two major drawbacks appeared in the above-mentioned works: halo-substituted aryl derivatives failed to give the expected products and α,β-unsaturated carboxylic acids substituted at the β-position were not studied. Accordingly, Li, Duan and co-workers reported a copper/silver-catalysed decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids with CF3SO2Na that tolerated various substrates bearing halogens as well as α,β-unsaturated carboxylic acids substituted at the β-position (Scheme 59) [84]. It was even noticed that β-methyl or β-phenyl-substituted cinnamic acids gave better yields compared to unsubstituted cinnamic acid. All products were obtained with excellent stereoselectivity. To gain insight about the mechanism, the authors used TEMPO as a radical scavenger and concluded that CF3 radical was involved in this reaction (Scheme 59).
![[1860-5397-13-272-i59]](/bjoc/content/inline/1860-5397-13-272-i59.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 59: Cu/Ag-catalysed decarboxylative trifluoromethylation of cinnamic acids.
Scheme 59: Cu/Ag-catalysed decarboxylative trifluoromethylation of cinnamic acids.
A metal-free protocol for the decarboxylative trifluoromethylation of cinnamic acids with CF3SO2Na was reported by Shang, Liu and co-workers using iodine pentoxide (I2O5) as oxidant. The substrate scope was evaluated on 9 α,β-unsaturated carboxylic acids substituted by electron-rich aryls (Scheme 60) [85]. The mild conditions gave the trifluoromethylated products in excellent yields with high stereoselectivities. Nevertheless, the reaction conditions did not allow the formation of products with electron-withdrawing substituents on the aromatic ring, such as the nitro group. The case of halogen-substituted cinnamic acids was not studied. Finally, mechanistic studies conducted by electron-spin resonance and by spin trapping technology suggested a free-radical addition– elimination process (Scheme 60).
![[1860-5397-13-272-i60]](/bjoc/content/inline/1860-5397-13-272-i60.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 60: I2O5-Promoted decarboxylative trifluoromethylation of cinnamic acids.
Scheme 60: I2O5-Promoted decarboxylative trifluoromethylation of cinnamic acids.
Trifluoromethylation of alkenes by denitration: Another route to the Cvinyl–CF3 bond is the denitrative trifluoromethylation of β-nitrostyrenes. More generally, this transformation has attracted much attention as a useful method for the construction of Cvinyl–R moieties. In 2016, Li, Duan and co-workers reported this trifluoromethylation by means of CF3SO2Na catalysed by silver nitrate in the presence of a large excess of di-tert-butyl peroxide (DTBP) as the oxidant and tetrabutylammonium iodide (TBAI) as a phase-transfer catalyst (Scheme 61) [86]. The substrate scope was evaluated on 16 β-nitrostyrenes and substrates bearing electron-donating groups as well as halides afforded the corresponding trifluoromethylstyrenes in moderate to good yields. However, the substrates with electron-withdrawing groups (CN, NO2) led to trifluoromethylated products in poor yields. This transformation was highly stereoselective, only (E)-isomers of the products were obtained. Experiments in the presence of 1,1-diphenylethylene as a radical scavenger led the authors to propose a radical process for the trifluoromethylation as described in Scheme 61.
![[1860-5397-13-272-i61]](/bjoc/content/inline/1860-5397-13-272-i61.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 61: Silver(I)-catalysed denitrative trifluoromethylation of β-nitrostyrenes.
Scheme 61: Silver(I)-catalysed denitrative trifluoromethylation of β-nitrostyrenes.
Trifluoromethylation of styrene derivatives: An obviously simple route for the synthesis of trifluoromethylated styrene derivatives is the direct C–H trifluoromethylation of alkenes. Very recently, Shen, Loh and co-workers reported a copper-catalysed direct trifluoromethylation of the vinylic Csp2–H bond of styrene derivatives with CF3SO2Na, di-tert-butyl peroxide, 10 mol % of copper(I) iodide, 1-methylimidazole (NMI) as ligand to copper and tetrabutylammonium iodide (TBAI) as additive (Scheme 62) [87]. The mild reaction conditions allowed a wide variety of functional groups to be tolerated and afforded a series of trifluoromethylated styrenes in moderate to good yields and with excellent stereoselectivity. However, aliphatic alkenes or styrenes bearing electron-withdrawing groups were not suitable for this reaction. Based on control experiments, the authors proposed a radical pathway for this reaction (Scheme 62).
![[1860-5397-13-272-i62]](/bjoc/content/inline/1860-5397-13-272-i62.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 62: Copper-catalysed direct trifluoromethylation of styrene derivatives.
Scheme 62: Copper-catalysed direct trifluoromethylation of styrene derivatives.
Trifluoromethylation of enamines: Highly functionalised alkenes represented by (Z)-methyl-3-(phenylamino)acrylates 78 were subjected to Baran’s conditions for the synthesis of β-trifluoromethylated enamines. Indeed, Jiang, Wu and co-workers reported mild, transition-metal-free conditions, insensitive to air and water, for the synthesis of a wide range of CF3-enamines using CF3SO2Na and TBHP as initiator and oxidant (Scheme 63) [88]. Moderate to good yields were obtained and only (E)-isomers were observed. The authors carried out several experiments such as the use of TEMPO or 2,6-di-tert-butyl-4-methylphenol (BHT) as scavengers in order to investigate the mechanism of this reaction (Scheme 63).
![[1860-5397-13-272-i63]](/bjoc/content/inline/1860-5397-13-272-i63.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 63: Transition-metal-free synthesis of β-trifluoromethylated enamines.
Scheme 63: Transition-metal-free synthesis of β-trifluoromethylated enamines.
Trifluoromethylation of alkynes: Together with the iodotrifluoromethylation of alkenes leading to Csp3–CF3 products (see Scheme 22), the Liu group extended the free-radical iodotrifluoromethylation to alkynes with the combination of CF3SO2Na/I2O5 and assistance of NaHCO3 (Scheme 64) [42]. The substrate scope was carried out on various aryl-substituted alkynes and one propargylic ester; (E)-CF3 alkenyl iodides were obtained stereoselectively in moderate to high yields via a free-radical process.
Scheme 64: I2O5-mediated iodotrifluoromethylation of alkynes.
Scheme 64: I2O5-mediated iodotrifluoromethylation of alkynes.
Trifluoromethylation of isonitriles: The synthesis of trifluoromethylated phenanthridines 79 from aryl isonitriles has been the recent subject of several investigations as potential structural unit in pharmaceuticals. In this context, CF3SO2Na was used by Zhang and co-workers in a silver-catalysed tandem trifluoromethylation and cyclisation of aryl isonitriles (Scheme 65) [89]. A wide variety of aryl isocyanides were transformed into the corresponding phenanthridines in moderate to good yields; some regioisomers were obtained depending on the biphenyl substituents. Here again, a radical pathway was established for this transformation (Scheme 65).
Scheme 65: Silver-catalysed tandem trifluoromethylation/cyclisation of aryl isonitriles.
Scheme 65: Silver-catalysed tandem trifluoromethylation/cyclisation of aryl isonitriles.
Simultaneously, Lu and co-workers reported a transition-metal-free synthesis of the trifluoromethylphenanthridine 79a in 58% yield using the system CF3SO2Na/K2S2O8/K2CO3 in H2O/CH3CN at 80 °C [90]. In addition, Maruoka and co-workers used the system CF3SO2Na/PIFA/AcONa in AcOEt at room temperature for 1.5 h to get 79a in 74% yield [50]. Mid 2017, Ao, Liu and co-workers exploited the reaction conditions developed previously for the photoredox trifluoromethylation of vinyl azides (see Scheme 8) in the synthesis of fluorinated phenanthridines 79. The yields were moderate to good and regioselectivity issues appeared depending on the biphenyl substituents (Scheme 66) [91].
Scheme 66: Photoredox trifluoromethylation of 2-isocyanobiphenyls.
Scheme 66: Photoredox trifluoromethylation of 2-isocyanobiphenyls.
Csp–CF3 bond-forming reactions
For the synthesis of trifluoromethylacetylenes 81, a copper-mediated trifluoromethylation of potassium alkynyltrifluoroborates 80 with CF3SO2Na was developed by Dubbaka and co-workers (Scheme 67) [92]. The scope was large including aryl, heteroaryl, alkenyl, and aliphatic alkynyltrifluoroborates and the yields were moderate. Mechanistically, the CF3 radical was generated under Langlois’ conditions by means of tert-butyl hydroperoxide and CuCl.
Scheme 67: Trifluoromethylation of potassium alkynyltrifluoroborates with CF3SO2Na.
Scheme 67: Trifluoromethylation of potassium alkynyltrifluoroborates with CF3SO2Na.
N–CF3 bond-forming reactions
C-, O-, and S-trifluoromethylated compounds are common in the field of biologically active molecules unlike the N–CF3 motif despite the huge number of nitrogen-containing pharmaceuticals. In 2017, Selander and co-workers reported a chemoselective N-trifluoromethylation of nitrosoarenes 82 in the presence of CF3SO2Na, a catalytic amount of copper(II), tert-butyl hydroperoxide as oxidant and hydroquinone as additive [93]. No reaction was observed in the absence of the copper salt. The scope was evaluated on 14 nitrosoarenes featuring electron-poor and electron-rich groups. The reaction conditions were suitable with a wide variety of functionalised nitrosoarenes and the corresponding trifluoromethylated hydroxylamines 83 were obtained in moderate to good yields (Scheme 68). The authors proposed a radical mechanism with two pathways for the generation of the CF3 radical, either by copper species or by tert-BuO• (Scheme 68). Interestingly, N–CF3 anilines were easily obtained after reduction of the N–O bond.
Scheme 68: N-trifluoromethylation of nitrosoarenes with CF3SO2Na (SQ: semiquinone).
Scheme 68: N-trifluoromethylation of nitrosoarenes with CF3SO2Na (SQ: semiquinone).
S–CF3 bond-forming reactions
Synthetic routes to trifluoromethylthiolated compounds are diverse, one is the S–CF3 bond formation (see next section for C–SCF3 bond formation). For this purpose, Langlois and co-workers used aliphatic and aromatic disulfides, RS–SR, which reacted with CF3SO2Na in the presence of an oxidant, t-BuOOH providing the best yields, to afford the trifluoromethyl thioethers (Scheme 69a) [20]. Other oxidants such as K2S2O8 and (NH4)2Ce(NO3)6 displayed lower reactivity and selectivity. Only one sulfenyl moiety of the disulfide was trifluoromethylated in this approach, the second being oxidised without trifluoromethylation. Aryl disulfides exhibited a lower reactivity and selectivity because side radical aryl C–H trifluoromethylation occurred competitively. This approach was applied to the synthesis of S-trifluoromethyl-containing amino acids 85 from dithio-amino acids 84 (Scheme 69b) [94].
Scheme 69: Trifluoromethylation of disulfides with CF3SO2Na.
Scheme 69: Trifluoromethylation of disulfides with CF3SO2Na.
Apart from disulfides, thiols were also used as substrates by Yi and co-workers to produce several trifluoromethyl thioethers and aliphatic trifluoromethylthiols. Their contribution was inspired by the previous work of Liu, using iodine pentoxide as an inexpensive inorganic oxidant [42] to generate the CF3 radical and release of iodine, which then reacted with the thiol to first form the corresponding disulfide 86 (detected as reaction intermediate) and further the sulfenyl iodide 87. The final SCF3 product was the result of the reaction of CF3• with either 86 or/and 87 (Scheme 70) [95]. The reaction performed well only at high reaction temperature (110 °C) for both thiophenols, benzylthiols and mercapto derivatives. The same research group recently reported different reaction conditions to obtain the same SCF3 products by means of CF3SO2Na in the presence of potassium persulfate and a catalytic amount of silver nitrate at 80 °C in acetonitrile and water [96].
![[1860-5397-13-272-i70]](/bjoc/content/inline/1860-5397-13-272-i70.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 70: Trifluoromethylation of thiols with CF3SO2Na/I2O5.
Scheme 70: Trifluoromethylation of thiols with CF3SO2Na/I2O5.
2 Trifluoromethylsulfenylation
In the early development of the direct electrophilic trifluoromethylthiolation, trifluoromethanethiol (CF3SH), trifluoromethanesulfenyl chloride (CF3SCl), and bis(trifluoromethyl) disulfide (CF3SSCF3) were the only reagents available, but their gaseous and toxic nature precluded a wider use. Fortunately, in the recent years, a collection of stable and easy-to-handle reagents was designed to perform efficient trifluoromethylthiolations [97]. Less sophisticated and suitable for large scale industrial use, CF3SO2Na was first used in trifluoromethylthiolation reactions in 2015 by Yi, Zhang and co-workers who reported the direct copper-catalysed trifluoromethylthiolation of indoles, pyrroles as well as enamines in the presence of (EtO)2P(O)H as reductant, CuCl and DMSO in toluene at 110 °C (Scheme 71) [98]. Unlike its use in trifluoromethylation reactions, here, no extrusion of SO2 ocurred from CF3SO2Na thus allowing the transfer of the SCF3 motif. Under these reaction conditions, indoles bearing an electron-donating group gave better yields compared to those with electron-rich groups. Using the same protocol, the trifluoromethylsulfenylated pyrroles and enamines were obtained in good to excellent yields. After mechanistic studies by 19F NMR spectroscopy, the authors proposed that bis(trifluoromethyl) disulfide, CF3SSCF3, was generated in situ (Scheme 71, path B). The route through path A and CF3SOH was also envisaged but should lead only to the formation of small amount of product.
![[1860-5397-13-272-i71]](/bjoc/content/inline/1860-5397-13-272-i71.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 71: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/(EtO)2P(O)H/CuCl/DMSO.
Scheme 71: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/(EtO)2P(O)H/CuCl/DMSO.
The same authors further demonstrated that the trifluoromethylsulfenylation can be conducted under metal-free conditions by replacing CuCl with trimethylsilyl chloride (TMSCl) to generate the cation CF3S+ in the presence of (EtO)2P(O)H [99]. Again, the electrophilic trifluoromethylsulfenylation of indoles was studied but also the trifluoromethylthiolation of electron-rich arenes (Scheme 72). Using this protocol, trifluoromethylsulfenylated compounds were obtained in moderate to excellent yields. Compared to the CuCl-mediated approach, this method directly converted CF3SOH into CF3S+ by reaction with TMSCl (Scheme 72).
Scheme 72: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/(EtO)2P(O)H/TMSCl.
Scheme 72: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/(EtO)2P(O)H/TMSCl.
Based on this above-mentioned work and simultaneously to the metal-free approach, Cai and co-workers reported the direct trifluoromethylsulfenylation of indoles, pyrroles and enamines, with CF3SO2Na, triphenylphosphine and N-chlorophthalimide without added metal and at room temperature (Scheme 73) [100]. In general, the substrates were successfully trifluoromethylsulfenylated in moderate to excellent yields by in situ generated CF3SCl as suggested by 19F NMR studies and previous literature reports.
![[1860-5397-13-272-i73]](/bjoc/content/inline/1860-5397-13-272-i73.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 73: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PPh3/N-chlorophthalimide.
Scheme 73: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PPh3/N-chlorophthalimide.
Different phosphorus reductive reagents were evaluated by Liu, Lin and co-workers. It was found that phosphorus trichloride (PCl3) allowed to react with CF3SO2Na and a series of indoles afforded the corresponding 3-CF3S derivatives selectively in good to excellent yields (Scheme 74) [101]. Unlike the work of Cai using PPh3 and N-chlorophthalimide, here, PCl3 was used both as a reducing and chlorinating reagent. 19F NMR investigation of reaction intermediates showed the signal of CF3SCl, species which was interpreted as the key intermediate in the reaction system. Noteworthy, switching from PCl3 to P(O)Cl3 allowed the synthesis of the trifluoromethylsulfinyl derivatives instead of the trifluoromethylsulfenyl ones (see next section).
Scheme 74: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PCl3.
Scheme 74: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PCl3.
At the same time, Zhao, Lu and co-workers described similar observations. The trifluoromethylsulfenylation of indoles and electron-rich aromatics was realised with CF3SO2Na in the presence of PCl3 (1.2 instead of 3 equivalents) in acetonitrile at 60 °C (Scheme 75) [102]. The authors proposed a reaction mechanism that involved the 3-trifluoromethylsulfinyl intermediate 88 (Scheme 75). Consequently, a decrease of the amount of PCl3 and the temperature was proposed to interrupt the reaction and to obtain 88 selectively (see next section).
![[1860-5397-13-272-i75]](/bjoc/content/inline/1860-5397-13-272-i75.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 75: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PCl3.
Scheme 75: Electrophilic trifluoromethylsulfenylation by means of CF3SO2Na/PCl3.
Aryl iodides served as substrates in a programmable regioselective trifluoromethylsulfenylation by direct coupling with CuSCF3 generated form CF3SO2Na in the presence of copper(I) chloride and triphenylphosphine. Indeed, Yang, Vicic and co-workers described the deoxygenative reduction of CF3SO2Na to form the ligand-free CuSCF3 intermediate that reacted with a diverse array of aryl iodides in high yields (Scheme 76) [103]. In addition, CuSCF3 was reacted with various ligands to furnish air-stable [LCu(SCF3)] complexes as trifluoromethylsulfenylating agents.
Scheme 76: Trifluoromethylsulfenylation of aryl iodides with in situ generated CuSCF3 (DMI: 1,3-dimethyl-2-imidazolidinone).
Scheme 76: Trifluoromethylsulfenylation of aryl iodides with in situ generated CuSCF3 (DMI: 1,3-dimethyl-2-imi...
3 Trifluoromethylsulfinylation
In 1999, Langlois and co-workers used the system CF3SO2Na/POCl3 as an efficient and inexpensive equivalent of the CF3S(O)+ cation for the trifluoromethylsulfinylation of various amines, alcohols as well as carbon nucleophiles (Scheme 77) [104]. In the case of primary amines, the addition of one equivalent of diisopropylethylamine to the reaction medium was necessary to trap acidic phosphoric species coming from the reaction and to reach higher product yields. N-Methylpyrrole was used as an example of C-nucleophile affording the corresponding S(O)CF3 product in 60% yield.
Scheme 77: Pioneering trifluoromethylsulfinylation of N, O, and C-nucleophiles.
Scheme 77: Pioneering trifluoromethylsulfinylation of N, O, and C-nucleophiles.
A few years later, the same group reported the synthesis of the trifluoromethanesulfinamide 89 derived from (1R,2S)-ephedrine using the process described above (Scheme 78). Compound 89 was used as an efficient trifluoromethylating agent for a wide range of non-enolisable and enolisable carbonyl compounds [105].
Scheme 78: Trifluoromethylsulfinylation of (1R,2S)-ephedrine (Im: imidazole; DIEA: N,N-diisopropylethylamine).
Scheme 78: Trifluoromethylsulfinylation of (1R,2S)-ephedrine (Im: imidazole; DIEA: N,N-diisopropylethylamine).
Other N-aryl- and N-alkyltrifluoromethanesulfinamides have been prepared from the corresponding anilines or aliphatic amines with CF3SO2Na and phosphoryl chloride for further reactions with arynes [106] or in the synthesis of trifluoromethylsulfonimidates [107].
Another direct trifluoromethylsulfinylation method was described by Wakselman’s group in 2001 with CF3SO2Na in the presence of triflic acid (Scheme 79) [108]. This method was applied to simple aryls featuring a halogen atom, a OCF3 group or an acetanilide function. In this reaction, ortho/para regioisomers were formed in ratios from 25:75 to 3:97. With regard to the mechanism, the sulfinate was protonated to generate the highly electrophilic CF3S(OH)2+ species that underwent an SEAr with arenes. A further development of the method consisted in the replacement of triflic acid with triflic anhydride in dichloromethane, and this strategy has been applied to the synthesis of trifluoromethylsulfonium salts, known as Umemoto’s reagents [109-111].
Scheme 79: Trifluoromethylsulfinylation of substituted benzenes with CF3SO2Na/CF3SO3H.
Scheme 79: Trifluoromethylsulfinylation of substituted benzenes with CF3SO2Na/CF3SO3H.
After these initial works, it is only in 2017 that new results using CF3SO2Na appeared in the field. As briefly mentioned in the preceding section, Liu, Lin and co-workers evaluated different phosphorus reductive reagents and found that PCl3 (3 equiv) afforded the corresponding 3-CF3S derivatives whereas P(O)Cl3 (1 equiv) allowed to get selectively the trifluoromethylsulfinyl derivatives 90 (Scheme 80) [101].
Scheme 80: Trifluoromethylsulfinylation of indoles with CF3SO2Na/P(O)Cl3.
Scheme 80: Trifluoromethylsulfinylation of indoles with CF3SO2Na/P(O)Cl3.
With the same objective of partial reduction of CF3SO2Na with phosphorus reagents for the selective trifluoromethylsulfinylation, Zhao, Lu and co-workers noticed that a decrease of the amount of PCl3 and a slight lowering of the temperature were beneficial to the desired trifluoromethylsulfinylation (Scheme 81) [102].
Scheme 81: Trifluoromethylsulfinylation of indoles with CF3SO2Na/PCl3.
Scheme 81: Trifluoromethylsulfinylation of indoles with CF3SO2Na/PCl3.
4 Trifluoromethylsulfonylation
The trifluoromethylsulfonyl motif (SO2CF3, Tf, triflyl) is highly electron-withdrawing (SO2CF3, σm = 0.79, σp = 0.93) and trifluoromethylsulfones (or triflones), in which the trifluoromethylsulfonyl group is attached to a carbon atom, are moderatly lipophilic (SO2CF3, π = 0.55 versus CF3, π = 0.88). Therefore, the SO2CF3 group has been frequently employed in catalysts and ligands, as subunit of bioactive compounds, or as building block for advanced functional materials. Trifluoromethylsulfones have been prepared by several approaches [5], in particular by means of CF3SO2Na as compiled hereafter.
Csp3–SO2CF3 bond-forming reactions
The trifluoromethanesulfinate, triflinate anion, possesses a low nucleophilicity thus affecting its potential in substitution reactions. Mainly primary bromides and some secondary ones (α-bromo ketones and esters) could be converted into trifluoromethylsulfones. From benzyl bromides, phase-transfer conditions [112] and/or high temperatures [113-115] were required to obtain the triflones in low to high yields (Scheme 82). Using benzyl chloride in propionitrile at reflux led to 90% yield of the corresponding triflone [115]. Methyl iodide was also used as substrate for the formation of methyl trifluoromethylsulfone [116]. The displacement of a tosyl group by CF3SO2Na in the presence of n-Bu4NI in THF worked with 60 % yield [117].
Scheme 82: Formation of triflones from benzyl bromides (DMA: dimethylacetamide).
Scheme 82: Formation of triflones from benzyl bromides (DMA: dimethylacetamide).
α-Trifluoromethylsulfonyl ketones, esters, and amides were prepared by displacement of the corresponding bromide (Scheme 83a) [112,118] or chloride (Scheme 83b,c) [118,119] under various conditions. Long reaction times and high temperatures were often required.
Scheme 83: Formation of α-trifluoromethylsulfonyl ketones, esters, and amides.
Scheme 83: Formation of α-trifluoromethylsulfonyl ketones, esters, and amides.
Allylic trifluoromethylsulfones could be prepared from aromatic allylic alcohols or esters and CF3SO2Na in the presence of p-toluenesulfonic acid at 100 °C in dioxane. The reaction was highly regioselective, worked well with primary, secondary and tertiary allylic alcohols, and tolerated a wide range of functions (Scheme 84) [120]. The reaction proceeded through an SN1-type mechanism by the formation of a cationic Π-allyl intermediate by means of p-toluenesulfonic acid.
Scheme 84: Allylic trifluoromethanesulfonylation of aromatic allylic alcohols.
Scheme 84: Allylic trifluoromethanesulfonylation of aromatic allylic alcohols.
Csp2–SO2CF3 bond-forming reactions
Aryl triflones: The synthesis of aryl trifluoromethylsulfones was investigated by Shekhar and co-workers in 2013. First, Pd- and Cu-catalysed coupling reactions with iodobenzene or arylboronic acids failed because of the poor nucleophilicity of CF3SO2Na [121]. It was next found that aryl iodonium salts reacted with CF3SO2Na in the presence of copper catalysts. In particular cuprous oxide in DMF gave the triflones in moderate to high yields from a variety of symmetrical and unsymmetrical (het)aryl iodonium salts 91 bearing various functional groups and counteranions (Scheme 85) [121]. The reaction was sulfonyl-retentive and was likely to proceed via a nonradical pathway, probably involving Cu(I)/Cu(III) intermediates.
Scheme 85: Copper-catalysed couplings of aryl iodonium salts with CF3SO2Na.
Scheme 85: Copper-catalysed couplings of aryl iodonium salts with CF3SO2Na.
Another example of trifluoromethanesulfonylation of a diaryl iodonium salt was reported by Li and co-workers as a single case, among several other nucleophiles, using 10 mol % of copper(II) triflate in dichloroethane at 80 °C [122]. The hypervalent iodine species could also be generated in situ [123].
In 2016, Shekhar’s group finally succeeded in the trifluoromethanesulfonylation of the more readily available (het)aryl triflates and some aryl chlorides using a combination of Pd2(dba)3 and the bulky RockPhos phosphine ligand in toluene and tris(3,6-dioxaheptyl)amine (TDA) as phase-transfer catalyst to facilitate the reaction with the sparingly soluble CF3SO2Na (Scheme 86) [124].
Scheme 86: Palladium-catalysed trifluoromethanesulfonylation of aryl triflates and chlorides with CF3SO2Na.
Scheme 86: Palladium-catalysed trifluoromethanesulfonylation of aryl triflates and chlorides with CF3SO2Na.
Arenediazonium tetrafluoroborates 92 were also suitable substrates for the formation of triflones. Xu, Qing and a co-worker demonstrated that CF3SO2Na produced triflones when engaged in a Cu-catalysed reaction in DMSO (Scheme 87) [76]. The reaction was sulfonyl-retentive whereas in the presence of the oxidant tert-butyl hydroperoxide the chemoselective trifluoromethylation was obtained (see Scheme 52).
Scheme 87: Copper-catalysed coupling of arenediazonium tetrafluoroborates with CF3SO2Na.
Scheme 87: Copper-catalysed coupling of arenediazonium tetrafluoroborates with CF3SO2Na.
Finally, a metal-free protocol was reported by Singh and co-workers, in which in situ generated benzyne was reacted with CF3SO2Na (Scheme 88) [125].
Scheme 88: Synthesis of phenyltriflone via coupling of benzyne with CF3SO2Na.
Scheme 88: Synthesis of phenyltriflone via coupling of benzyne with CF3SO2Na.
Vinyl triflones: The synthesis of two vinyl trifluoromethylsulfones was reported in 2004 by Ochiai and co-workers by treatment of λ3-bromane 93 with CF3SO2Na in DCM at 0 °C (Scheme 89) [126]. The reaction also produced 1-alkynyltriflones as minor products. The 1-alkynyl-λ3-bromanes acted as Michael acceptors for the sulfinate anion and underwent tandem Michael addition–carbene insertion reactions to yield the 1-trifluoromethanesulfonylcyclopentenes.
Scheme 89: Synthesis of 1-trifluoromethanesulfonylcyclopentenes from 1-alkynyl-λ3-bromanes and CF3SO2Na.
Scheme 89: Synthesis of 1-trifluoromethanesulfonylcyclopentenes from 1-alkynyl-λ3-bromanes and CF3SO2Na.
Vinyl triflones with exclusive E-selectivity could also be prepared from allylic alcohols and CF3SO2Na under metal-free conditions as reported by Xu, Ji and co-workers (Scheme 90) [127]. Under acidic conditions, the π-allylic carbocation reacted with the sulfinyl anion leading to allylsulfone 95, which underwent an electrophilic addition of PIDA and capture of the benzylic carbocation by water. Then, a concerted proton elimination and C–I bond cleavage occurred to give the triflylated allylic alcohol 94.
![[1860-5397-13-272-i90]](/bjoc/content/inline/1860-5397-13-272-i90.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 90: One-pot synthesis of functionalised vinyl triflones.
Scheme 90: One-pot synthesis of functionalised vinyl triflones.
Huang, Xu and co-workers demonstrated that styrenes, but also vinylbenzofuran and vinylthiophene, reacted with CF3SO2Na in the presence of iodine and CuCN to afford regioselectively the internal vinyl triflones 96 (Scheme 91) [128]. A cationic reaction mechanism was proposed by electrophilic addition of I2 (or the in situ generated CF3SO2I) to the olefin. The highly strained iodonium bridge subsequently reacted with the sulfinyl anion to afford the iodotriflylated product, which eliminated HI to give the vinyl triflone. The role of CuCN was not precised at this stage.
Scheme 91: Regioselective synthesis of vinyltriflones from styrenes.
Scheme 91: Regioselective synthesis of vinyltriflones from styrenes.
Csp–SO2CF3 bond-forming reactions
Apart from the side products described in Scheme 89, a variety of acetylenic triflones 98 was proposed by Zhang and co-workers. Alkynyl(phenyl) iodonium tosylates 97 reacted with CF3SO2Na in a transition metal-free protocol to furnish the acetylenic triflones under mild conditions (Scheme 92) [129]. The authors suggested that alkylidene carbene intermediates might be formed followed by a 1,2-rearrangement to yield the triflones. The reaction from ethynylbenzene and CF3SO2Na in the presence of Koser’s reagent allowed to obtain the corresponding triflone albeit in a 33% yield.
![[1860-5397-13-272-i92]](/bjoc/content/inline/1860-5397-13-272-i92.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 92: Trifluoromethanesulfonylation of alkynyl(phenyl) iodonium tosylates by CF3SO2Na.
Scheme 92: Trifluoromethanesulfonylation of alkynyl(phenyl) iodonium tosylates by CF3SO2Na.
S–SO2CF3 and Se–SO2CF3 bond-forming reactions
Langlois’ group prepared thiotrifluoromethanesulfonates 100 from sulfenyl chlorides 99 or disulfides 101 by two methods: first, sulfenyl chlorides were reacted with CF3SO2Na in DCM at room temperature [130]; second, a wider scope was obtained by reaction of disulfides with CF3SO2Na under oxidative conditions with either bromine [130] or PIFA [131] (Scheme 93a). These methodologies were extended to phenylselenyl chloride and diphenyl diselenide (Scheme 93b).
Scheme 93: Synthesis of thio- and selenotrifluoromethanesulfonates.
Scheme 93: Synthesis of thio- and selenotrifluoromethanesulfonates.
Conclusion
Sodium trifluoromethanesulfinate was used for the first time in direct radical trifluoromethylation under oxidative conditions by the Langlois group in 1991. Since that time its chemistry has evolved sporadically till 2011 and then immensely thanks to Baran’s contribution. It enjoyed a rapid growth not only as donor of the trifluoromethyl group but also as a trifluoromethylsulfenylating agent, for the transfer of the whole SCF3 motif, and as trifluoromethanesulfinylating agent for S(O)CF3 transfer. CF3SO2Na is an easy-to-handle, inexpensive reagent that has became versatile for the construction of sophisticated fluorinated products in the pharmaceutical chemistry. It is the authors’ hope that this review will contribute to stimulate further research toward new reactions and applications for this reagent that could include the formation of new bonds with other heteroatoms, novel tandem reactions, and the exploration of asymmetric reactions, so far absent with CF3SO2Na, to cite but a few examples.
Acknowledgements
Our research was supported by the Centre National de la Recherche Scientifique CNRS, Normandy University, and Labex SynOrg (ANR-11-LABX-0029). H.C. thanks the French “Ministère de la Recherche et de l’Enseignement Supèrieur” for a doctoral fellowship. H.G. thanks Normandy University for a postdoctoral grant. The French Fluorine Network (GIS Fluor) is also acknowledged.
References
-
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004.
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Harsanyi, A.; Sandford, G. Green Chem. 2015, 17, 2081–2086. doi:10.1039/c4gc02166e
Return to citation in text: [1] -
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b
Return to citation in text: [1] [2] -
Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524
Return to citation in text: [1] -
Glenadel, Q.; Ismalaj, E.; Billard, T. Eur. J. Org. Chem. 2017, 530–533. doi:10.1002/ejoc.201601526
Return to citation in text: [1] -
Besset, T.; Jubault, P.; Pannecoucke, X.; Poisson, T. Org. Chem. Front. 2016, 3, 1004–1010. doi:10.1039/c6qo00164e
Return to citation in text: [1] -
Seebach, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 1320–1367. doi:10.1002/anie.199013201
Return to citation in text: [1] -
McClinton, M. A.; McClinton, D. A. Tetrahedron 1992, 48, 6555–6666. doi:10.1016/S0040-4020(01)80011-9
Return to citation in text: [1] -
Ma, J.-A.; Cahard, D. J. Fluorine Chem. 2007, 128, 975–996. doi:10.1016/j.jfluchem.2007.04.026
Return to citation in text: [1] -
Shibata, N.; Matsnev, A.; Cahard, D. Beilstein J. Org. Chem. 2010, 6, No. 65. doi:10.3762/bjoc.6.65
Return to citation in text: [1] -
Ye, Y. e-EROS Encyclopedia of Reagents for Organic 2014, 1–4. doi:10.1002/047084289X.rn01689
Return to citation in text: [1] -
Haszeldine, R. N.; Kidd, J. M. J. Chem. Soc. 1955, 2901–2910. doi:10.1039/JR9550002901
Return to citation in text: [1] -
Roesky, H. W.; Holtschneider, G. J. Fluorine Chem. 1976, 7, 77–84. doi:10.1016/s0022-1139(00)83984-6
Return to citation in text: [1] -
Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525–7528. doi:10.1016/0040-4039(91)80524-a
Return to citation in text: [1] [2] [3] -
Zhang, C. Adv. Synth. Catal. 2014, 356, 2895–2906. doi:10.1002/adsc.201400370
Return to citation in text: [1] -
Langlois, B. R. Once Upon a Time Was the Langlois' Reagent: A “Sleeping Beauty”. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds; Groult, H.; Leroux, F.; Tressaud, A., Eds.; Elsevier, 2017; pp 125–140.
Return to citation in text: [1] -
Lefebvre, Q. Synlett 2017, 28, 19–23. doi:10.1055/s-0036-1588643
Return to citation in text: [1] -
Clavel, J.-L.; Langlois, B.; Laurent, E.; Roidot, N. Phosphorus, Sulfur Silicon Relat. Elem. 1991, 59, 169–172. doi:10.1080/10426509108045716
Return to citation in text: [1] [2] -
Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1992, 33, 1291–1294. doi:10.1016/s0040-4039(00)91604-6
Return to citation in text: [1] -
Lu, Y.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. J. Fluorine Chem. 2014, 161, 128–133. doi:10.1016/j.jfluchem.2014.01.020
Return to citation in text: [1] -
Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Angew. Chem., Int. Ed. 2013, 52, 9747–9750. doi:10.1002/anie.201303576
Return to citation in text: [1] -
Maji, A.; Hazra, A.; Maiti, D. Org. Lett. 2014, 16, 4524–4527. doi:10.1021/ol502071g
Return to citation in text: [1] [2] -
Luo, H.-Q.; Zhang, Z.-P.; Dong, W.; Luo, X.-Z. Synlett 2014, 25, 1307–1311. doi:10.1055/s-0033-1341057
Return to citation in text: [1] -
Lu, Q.; Liu, C.; Huang, Z.; Ma, Y.; Zhang, J.; Lei, A. Chem. Commun. 2014, 50, 14101–14104. doi:10.1039/c4cc06328g
Return to citation in text: [1] [2] -
Qin, H.-T.; Wu, S.-W.; Liu, J.-L.; Liu, F. Chem. Commun. 2017, 53, 1696–1699. doi:10.1039/c6cc10035j
Return to citation in text: [1] -
Lv, W.-X.; Zeng, Y.-F.; Li, Q.; Chen, Y.; Tan, D.-H.; Yang, L.; Wang, H. Angew. Chem., Int. Ed. 2016, 55, 10069–10073. doi:10.1002/anie.201604898
Return to citation in text: [1] -
Konik, Y. A.; Kudrjashova, M.; Konrad, N.; Kaabel, S.; Jarving, I.; Lopp, M.; Kananovich, D. G. Org. Biomol. Chem. 2017, 15, 4635–4643. doi:10.1039/C7OB00680B
Return to citation in text: [1] -
Huang, H.-L.; Yan, H.; Gao, G.-L.; Yang, C.; Xia, W. Asian J. Org. Chem. 2015, 4, 674–677. doi:10.1002/ajoc.201500096
Return to citation in text: [1] -
Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 11960–11964. doi:10.1002/anie.201406464
Return to citation in text: [1] -
Yu, X.-L.; Chen, J.-R.; Chen, D.-Z.; Xiao, W.-J. Chem. Commun. 2016, 52, 8275–8278. doi:10.1039/c6cc03335k
Return to citation in text: [1] -
Zhang, H.-Y.; Huo, W.; Ge, C.; Zhao, J.; Zhang, Y. Synlett 2017, 28, 962–965. doi:10.1055/s-0036-1588400
Return to citation in text: [1] -
Jiang, X.-Y.; Qing, F.-L. Angew. Chem., Int. Ed. 2013, 52, 14177–14180. doi:10.1002/anie.201307595
Return to citation in text: [1] -
Yang, Y.; Liu, Y.; Jiang, Y.; Zhang, Y.; Vicic, D. A. J. Org. Chem. 2015, 80, 6639–6648. doi:10.1021/acs.joc.5b00781
Return to citation in text: [1] -
Liu, C.; Lu, Q.; Huang, Z.; Zhang, J.; Liao, F.; Peng, P.; Lei, A. Org. Lett. 2015, 17, 6034–6037. doi:10.1021/acs.orglett.5b03035
Return to citation in text: [1] -
Yu, J.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 3669–3675. doi:10.1002/adsc.201400144
Return to citation in text: [1] -
Tommasino, J.-B.; Brondex, A.; Medebielle, M.; Thomalla, M.; Langlois, B. R.; Billard, T. Synlett 2002, 1697–1699. doi:10.1055/s-2002-34210
Return to citation in text: [1] [2] -
Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Chem. Sci. 2013, 4, 3160–3165. doi:10.1039/c3sc51209f
Return to citation in text: [1] -
Lefebvre, Q.; Hoffmann, N.; Rueping, M. Chem. Commun. 2016, 52, 2493–2496. doi:10.1039/c5cc09881e
Return to citation in text: [1] [2] -
Zhu, L.; Wang, L.-S.; Li, B.; Fu, B.; Zhang, C.-P.; Li, W. Chem. Commun. 2016, 52, 6371–6374. doi:10.1039/c6cc01944g
Return to citation in text: [1] -
Hang, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3648–3651. doi:10.1021/ol501380e
Return to citation in text: [1] [2] [3] [4] -
Liu, Z.-Q.; Liu, D. J. Org. Chem. 2017, 82, 1649–1656. doi:10.1021/acs.joc.6b02812
Return to citation in text: [1] -
Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Chem. Commun. 2017, 53, 7638–7641. doi:10.1039/c7cc01903c
Return to citation in text: [1] -
Yang, F.; Klumphu, P.; Liang, Y.-M.; Lipshutz, B. H. Chem. Commun. 2014, 50, 936–938. doi:10.1039/c3cc48131j
Return to citation in text: [1] -
Lu, Q.; Liu, C.; Peng, P.; Liu, Z.; Fu, L.; Huang, J.; Lei, A. Asian J. Org. Chem. 2014, 3, 273–276. doi:10.1002/ajoc.201300236
Return to citation in text: [1] -
Liu, J.; Zhuang, S.; Gui, Q.; Chen, X.; Yang, Z.; Tan, Z. Eur. J. Org. Chem. 2014, 3196–3202. doi:10.1002/ejoc.201400087
Return to citation in text: [1] -
Wei, W.; Wen, J.; Yang, D.; Liu, X.; Guo, M.; Dong, R.; Wang, H. J. Org. Chem. 2014, 79, 4225–4230. doi:10.1021/jo500515x
Return to citation in text: [1] -
Shi, L.; Yang, X.; Wang, Y.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 1021–1028. doi:10.1002/adsc.201300995
Return to citation in text: [1] [2] -
Sakamoto, R.; Kashiwagi, H.; Selvakumar, S.; Moteki, S. A.; Maruoka, K. Org. Biomol. Chem. 2016, 14, 6417–6421. doi:10.1039/c6ob01245k
Return to citation in text: [1] [2] [3] -
Zhang, L.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3688–3691. doi:10.1021/ol5014747
Return to citation in text: [1] [2] -
Mai, W.-P.; Wang, J.-T.; Yang, L.-R.; Yuan, J.-W.; Xiao, Y.-M.; Mao, P.; Qu, L.-B. Org. Lett. 2014, 16, 204–207. doi:10.1021/ol403196h
Return to citation in text: [1] -
Li, B.; Fan, D.; Yang, C.; Xia, W. Org. Biomol. Chem. 2016, 14, 5293–5297. doi:10.1039/c6ob00912c
Return to citation in text: [1] -
Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C. J. Am. Chem. Soc. 2017, 139, 1388–1391. doi:10.1021/jacs.6b11234
Return to citation in text: [1] -
Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2015, 17, 1906–1909. doi:10.1021/acs.orglett.5b00601
Return to citation in text: [1] -
Smertenko, E. A.; Datsenko, S. D.; Ignat'ev, N. V. Russ. J. Electrochem. 1998, 34, 46–51.
Return to citation in text: [1] -
Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. doi:10.1073/pnas.1109059108
Return to citation in text: [1] -
Musumeci, D.; Irace, C.; Santamaria, R.; Montesarchio, D. Med. Chem. Commun. 2013, 4, 1405–1410. doi:10.1039/c3md00159h
Return to citation in text: [1] -
Wu, M.; Ji, X.; Dai, W.; Cao, S. J. Org. Chem. 2014, 79, 8984–8989. doi:10.1021/jo501221h
Return to citation in text: [1] -
Fennewald, J. C.; Lipshutz, B. H. Green Chem. 2014, 16, 1097–1100. doi:10.1039/c3gc42119h
Return to citation in text: [1] -
Zhang, X.; Huang, P.; Li, Y.; Duan, C. Org. Biomol. Chem. 2015, 13, 10917–10922. doi:10.1039/c5ob01516b
Return to citation in text: [1] -
Cao, X.-H.; Pan, X.; Zhou, P.-J.; Zou, J.-P.; Asekun, O. T. Chem. Commun. 2014, 50, 3359–3362. doi:10.1039/c3cc49689a
Return to citation in text: [1] -
Zhang, P.-Z.; Li, C.-K.; Zhang, G.-Y.; Zhang, L.; Jiang, Y.-J.; Zou, J.-P. Tetrahedron 2016, 72, 3250–3255. doi:10.1016/j.tet.2016.04.048
Return to citation in text: [1] -
Li, C.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. New J. Chem. 2017, 41, 1417–1420. doi:10.1039/c6nj03654f
Return to citation in text: [1] -
Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332–1337. doi:10.1021/jo502928e
Return to citation in text: [1] -
Ji, X.-M.; Wei, L.; Chen, F.; Tang, R.-Y. RSC Adv. 2015, 5, 29766–29773. doi:10.1039/C5RA02888D
Return to citation in text: [1] -
Jin, L.-K.; Lu, G.-P.; Cai, C. Org. Chem. Front. 2016, 3, 1309–1313. doi:10.1039/c6qo00369a
Return to citation in text: [1] -
Shen, C.; Xu, J.; Ying, B.; Zhang, P. ChemCatChem 2016, 8, 3560–3564. doi:10.1002/cctc.201601068
Return to citation in text: [1] -
Li, J.-M.; Wang, Y.-H.; Yu, Y.; Wu, R.-B.; Weng, J.; Lu, G. ACS Catal. 2017, 7, 2661–2667. doi:10.1021/acscatal.6b03671
Return to citation in text: [1] -
Wang, D.; Deng, G.-J.; Chen, S.; Gong, H. Green Chem. 2016, 18, 5967–5970. doi:10.1039/c6gc02000c
Return to citation in text: [1] -
Yang, Y.-D.; Iwamoto, K.; Tokunaga, E.; Shibata, N. Chem. Commun. 2013, 49, 5510–5512. doi:10.1039/c3cc41667d
Return to citation in text: [1] -
Cui, L.; Matusaki, Y.; Tada, N.; Miura, T.; Uno, B.; Itoh, A. Adv. Synth. Catal. 2013, 355, 2203–2207. doi:10.1002/adsc.201300199
Return to citation in text: [1] -
Chang, B.; Shao, H.; Yan, P.; Qiu, W.; Weng, Z.; Yuan, R. ACS Sustainable Chem. Eng. 2017, 5, 334–341. doi:10.1021/acssuschemeng.6b01682
Return to citation in text: [1] -
Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. J. Am. Chem. Soc. 2016, 138, 5809–5812. doi:10.1021/jacs.6b02782
Return to citation in text: [1] -
Le, C. C.; Wismer, M. K.; Shi, Z.-C.; Zhang, R.; Conway, D. V.; Li, G.; Vachal, P.; Davies, I. W.; MacMillan, D. W. C. ACS Cent. Sci. 2017, 3, 647–653. doi:10.1021/acscentsci.7b00159
Return to citation in text: [1] -
Zhang, K.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2015, 80, 7658–7665. doi:10.1021/acs.joc.5b01295
Return to citation in text: [1] [2] -
Hong, J.; Wang, G.; Huo, L.; Zheng, C. Chin. J. Chem. 2017, 35, 1761–1767. doi:10.1002/cjoc.201700311
Return to citation in text: [1] -
Ye, Y.; Künzi, S. A.; Sanford, M. S. Org. Lett. 2012, 14, 4979–4981. doi:10.1021/ol3022726
Return to citation in text: [1] -
Li, Y.; Wu, L.; Neumann, H.; Beller, M. Chem. Commun. 2013, 49, 2628–2630. doi:10.1039/c2cc36554e
Return to citation in text: [1] -
Presset, M.; Oehlrich, D.; Rombouts, F.; Molander, G. A. J. Org. Chem. 2013, 78, 12837–12843. doi:10.1021/jo4023233
Return to citation in text: [1] -
Dubbaka, S. R.; Salla, M.; Bolisetti, R.; Nizalapur, S. RSC Adv. 2014, 4, 6496–6499. doi:10.1039/C3RA46837B
Return to citation in text: [1] -
Li, Z.; Cui, Z.; Liu, Z.-Q. Org. Lett. 2013, 15, 406–409. doi:10.1021/ol3034059
Return to citation in text: [1] -
Patra, T.; Deb, A.; Manna, S.; Sharma, U.; Maiti, D. Eur. J. Org. Chem. 2013, 5247–5250. doi:10.1002/ejoc.201300473
Return to citation in text: [1] -
Yin, J.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. Synthesis 2014, 46, 607–612. doi:10.1055/s-0033-1338578
Return to citation in text: [1] -
Shang, X.-J.; Li, Z.; Liu, Z.-Q. Tetrahedron Lett. 2015, 56, 233–235. doi:10.1016/j.tetlet.2014.11.076
Return to citation in text: [1] -
Huang, P.; Li, Y.; Fu, X.; Zhang, R.; Jin, K.; Wang, W.; Duan, C. Tetrahedron Lett. 2016, 57, 4705–4708. doi:10.1016/j.tetlet.2016.09.016
Return to citation in text: [1] -
Wu, L.-H.; Zhao, K.; Shen, Z.-L.; Loh, T.-P. Org. Chem. Front. 2017, 4, 1872–1875. doi:10.1039/c7qo00416h
Return to citation in text: [1] -
Jiang, H.; Huang, W.; Yu, Y.; Yi, S.; Li, J.; Wu, W. Chem. Commun. 2017, 53, 7473–7476. doi:10.1039/c7cc03125d
Return to citation in text: [1] -
Liu, Y.-R.; Tu, H.-Y.; Zhang, X.-G. Synthesis 2015, 47, 3460–3466. doi:10.1055/s-0034-1378810
Return to citation in text: [1] -
Lu, S.; Gong, Y.; Zhou, D. J. Org. Chem. 2015, 80, 9336–9341. doi:10.1021/acs.joc.5b01518
Return to citation in text: [1] -
Fang, J.; Shen, W.-G.; G.-Z., Ao; Liu, F. Org. Chem. Front. 2017, 4, 2049–2053. doi:10.1039/C7QO00473G
Return to citation in text: [1] -
Dubbaka, S. R.; Nizalapur, S.; Atthunuri, A. R.; Salla, M.; Mathew, T. Tetrahedron 2014, 70, 2118–2121. doi:10.1016/j.tet.2014.02.005
Return to citation in text: [1] -
van der Werf, A.; Hribersek, M.; Selander, N. Org. Lett. 2017, 19, 2374–2377. doi:10.1021/acs.orglett.7b00908
Return to citation in text: [1] -
Langlois, B.; Montègre, D.; Roidot, N. J. Fluorine Chem. 1994, 68, 63–66. doi:10.1016/0022-1139(93)02982-k
Return to citation in text: [1] -
Ma, J.-J.; Yi, W.-B.; Lu, G.-P.; Cai, C. Catal. Sci. Technol. 2016, 6, 417–421. doi:10.1039/c5cy01561h
Return to citation in text: [1] -
Ma, J.-j.; Liu, Q.-r.; Lu, G.-p.; Yi, W.-b. J. Fluorine Chem. 2017, 193, 113–117. doi:10.1016/j.jfluchem.2016.11.010
Return to citation in text: [1] -
Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445–454. doi:10.1002/cjoc.201500890
Return to citation in text: [1] -
Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495
Return to citation in text: [1] -
Yan, Q.; Jiang, L.; Yi, W.; Liu, Q.; Zhang, W. Adv. Synth. Catal. 2017, 359, 2471–2480. doi:10.1002/adsc.201700270
Return to citation in text: [1] -
Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a
Return to citation in text: [1] -
Sun, D.-W.; Jiang, X.; Jiang, M.; Lin, Y.; Liu, J.-T. Eur. J. Org. Chem. 2017, 3505–3511. doi:10.1002/ejoc.201700661
Return to citation in text: [1] [2] -
Zhao, X.; Wei, A.; Yang, B.; Li, T.; Li, Q.; Qiu, D.; Lu, K. J. Org. Chem. 2017, 82, 9175–9181. doi:10.1021/acs.joc.7b01226
Return to citation in text: [1] [2] -
Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790
Return to citation in text: [1] -
Billard, T.; Greiner, A.; Langlois, B. R. Tetrahedron 1999, 55, 7243–7250. doi:10.1016/s0040-4020(99)00364-6
Return to citation in text: [1] -
Roussel, S.; Billard, T.; Langlois, B. R.; Saint-Jalmes, L. Synlett 2004, 2119–2122. doi:10.1055/s-2004-831322
Return to citation in text: [1] -
Liu, Z.; Larock, R. C. J. Am. Chem. Soc. 2005, 127, 13112–13113. doi:10.1021/ja054079p
Return to citation in text: [1] -
Richards-Taylor, C. S.; Martinez-Lamenca, C.; Leenaerts, J. E.; Trabanco, A. A.; Oehlrich, D. J. Org. Chem. 2017, 82, 9898–9904. doi:10.1021/acs.joc.7b01628
Return to citation in text: [1] -
Wakselman, C.; Tordeux, M.; Freslon, C.; Saint-Jalmes, L. Synlett 2001, 550–552. doi:10.1055/s-2001-12316
Return to citation in text: [1] -
Magnier, E.; Blazejewski, J.-C.; Tordeux, M.; Wakselman, C. Angew. Chem., Int. Ed. 2006, 45, 1279–1282. doi:10.1002/anie.200503776
Return to citation in text: [1] -
Okazaki, T.; Laali, K. K.; Reddy, A. S. J. Fluorine Chem. 2014, 165, 91–95. doi:10.1016/j.jfluchem.2014.06.021
Return to citation in text: [1] -
Umemoto, T.; Zhang, B.; Zhu, T.; Zhou, X.; Zhang, P.; Hu, S.; Li, Y. J. Org. Chem. 2017, 82, 7708–7719. doi:10.1021/acs.joc.7b00669
Return to citation in text: [1] -
Eugene, F.; Langlois, B.; Laurent, E. J. Fluorine Chem. 1994, 66, 301–309. doi:10.1016/0022-1139(93)02909-x
Return to citation in text: [1] [2] -
Meshcheryakov, V. I.; Shainyan, B. A. Russ. J. Org. Chem. 2004, 40, 390–396. doi:10.1023/B:RUJO.0000034977.74026.b3
Return to citation in text: [1] -
Hasegawa, A.; Naganawa, Y.; Fushimi, M.; Ishihara, K.; Yamamoto, H. Org. Lett. 2006, 8, 3175–3178. doi:10.1021/ol060939a
Return to citation in text: [1] -
Hasegawa, A.; Ishikawa, T.; Ishihara, K.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2005, 78, 1401–1410. doi:10.1246/bcsj.78.1401
Return to citation in text: [1] [2] -
Zhu, S.; Chu, Q.; Xu, G.; Qin, C.; Xu, Y. J. Fluorine Chem. 1998, 91, 195–198. doi:10.1016/s0022-1139(98)00240-1
Return to citation in text: [1] -
Wu, W.-L.; Asberom, T.; Bara, T.; Bennett, C.; Burnett, D. A.; Clader, J.; Domalski, M.; Greenlee, W. J.; Josien, H.; McBriar, M.; Rajagopalan, M.; Vicarel, M.; Xu, R.; Hyde, L. A.; Del Vecchio, R. A.; Cohen-Williams, M. E.; Song, L.; Lee, J.; Terracina, G.; Zhang, Q.; Nomeir, A.; Parker, E. M.; Zhang, L. Bioorg. Med. Chem. Lett. 2013, 23, 844–849. doi:10.1016/j.bmcl.2012.11.047
Return to citation in text: [1] -
Kong, H. I.; Crichton, J. E.; Manthorpe, J. M. Tetrahedron Lett. 2011, 52, 3714–3717. doi:10.1016/j.tetlet.2011.05.015
Return to citation in text: [1] [2] -
Kong, H. I.; Gill, M. A.; Hrdina, A. H.; Crichton, J. E.; Manthorpe, J. M. J. Fluorine Chem. 2013, 153, 151–161. doi:10.1016/j.jfluchem.2013.03.020
Return to citation in text: [1] -
Liao, J.; Guo, W.; Zhang, Z.; Tang, X.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 1304–1309. doi:10.1021/acs.joc.5b02674
Return to citation in text: [1] -
Cullen, S. C.; Shekhar, S.; Nere, N. K. J. Org. Chem. 2013, 78, 12194–12201. doi:10.1021/jo401868x
Return to citation in text: [1] [2] -
Xie, F.; Zhang, Z.; Yu, X.; Tang, G.; Li, X. Angew. Chem., Int. Ed. 2015, 54, 7405–7409. doi:10.1002/anie.201502278
Return to citation in text: [1] -
Wang, F.; Yu, X.; Qi, Z.; Li, X. Chem. – Eur. J. 2016, 22, 511–516. doi:10.1002/chem.201504179
Return to citation in text: [1] -
Smyth, L. A.; Phillips, E. M.; Chan, V. S.; Napolitano, J. G.; Henry, R.; Shekhar, S. J. Org. Chem. 2016, 81, 1285–1294. doi:10.1021/acs.joc.5b02523
Return to citation in text: [1] -
Aithagani, S. K.; Yempalla, K. R.; Munagala, G.; Vishwakarma, R. A.; Singh, P. P. RSC Adv. 2014, 4, 50208–50211. doi:10.1039/c4ra07370c
Return to citation in text: [1] -
Ochiai, M.; Tada, N.; Nishi, Y.; Murai, K. Chem. Commun. 2004, 2894–2895. doi:10.1039/B410830B
Return to citation in text: [1] -
Chu, X.-Q.; Meng, H.; Xu, X.-P.; Ji, S.-J. Chem. – Eur. J. 2015, 21, 11359–11368. doi:10.1002/chem.201500469
Return to citation in text: [1] -
Hua, L.-N.; Li, H.; Qing, F.-L.; Huang, Y.; Xu, X.-H. Org. Biomol. Chem. 2016, 14, 8443–8447. doi:10.1039/C6OB01567K
Return to citation in text: [1] -
Han, J.-B.; Yang, L.; Chen, X.; Zha, G.-F.; Zhang, C.-P. Adv. Synth. Catal. 2016, 358, 4119–4124. doi:10.1002/adsc.201600717
Return to citation in text: [1] -
Billard, T.; Langlois, B. R.; Large, S.; Anker, D.; Roidot, N.; Roure, P. J. Org. Chem. 1996, 61, 7545–7550. doi:10.1021/jo960619c
Return to citation in text: [1] [2] -
Billard, T.; Langlois, B. R. J. Fluorine Chem. 1997, 84, 63–64. doi:10.1016/s0022-1139(97)00009-2
Return to citation in text: [1]
| 71. | Yang, Y.-D.; Iwamoto, K.; Tokunaga, E.; Shibata, N. Chem. Commun. 2013, 49, 5510–5512. doi:10.1039/c3cc41667d |
| 50. | Sakamoto, R.; Kashiwagi, H.; Selvakumar, S.; Moteki, S. A.; Maruoka, K. Org. Biomol. Chem. 2016, 14, 6417–6421. doi:10.1039/c6ob01245k |
| 69. | Li, J.-M.; Wang, Y.-H.; Yu, Y.; Wu, R.-B.; Weng, J.; Lu, G. ACS Catal. 2017, 7, 2661–2667. doi:10.1021/acscatal.6b03671 |
| 70. | Wang, D.; Deng, G.-J.; Chen, S.; Gong, H. Green Chem. 2016, 18, 5967–5970. doi:10.1039/c6gc02000c |
| 68. | Shen, C.; Xu, J.; Ying, B.; Zhang, P. ChemCatChem 2016, 8, 3560–3564. doi:10.1002/cctc.201601068 |
| 75. | Le, C. C.; Wismer, M. K.; Shi, Z.-C.; Zhang, R.; Conway, D. V.; Li, G.; Vachal, P.; Davies, I. W.; MacMillan, D. W. C. ACS Cent. Sci. 2017, 3, 647–653. doi:10.1021/acscentsci.7b00159 |
| 73. | Chang, B.; Shao, H.; Yan, P.; Qiu, W.; Weng, Z.; Yuan, R. ACS Sustainable Chem. Eng. 2017, 5, 334–341. doi:10.1021/acssuschemeng.6b01682 |
| 74. | Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. J. Am. Chem. Soc. 2016, 138, 5809–5812. doi:10.1021/jacs.6b02782 |
| 72. | Cui, L.; Matusaki, Y.; Tada, N.; Miura, T.; Uno, B.; Itoh, A. Adv. Synth. Catal. 2013, 355, 2203–2207. doi:10.1002/adsc.201300199 |
| 40. | Lefebvre, Q.; Hoffmann, N.; Rueping, M. Chem. Commun. 2016, 52, 2493–2496. doi:10.1039/c5cc09881e |
| 80. | Presset, M.; Oehlrich, D.; Rombouts, F.; Molander, G. A. J. Org. Chem. 2013, 78, 12837–12843. doi:10.1021/jo4023233 |
| 98. | Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495 |
| 81. | Dubbaka, S. R.; Salla, M.; Bolisetti, R.; Nizalapur, S. RSC Adv. 2014, 4, 6496–6499. doi:10.1039/C3RA46837B |
| 97. | Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445–454. doi:10.1002/cjoc.201500890 |
| 78. | Ye, Y.; Künzi, S. A.; Sanford, M. S. Org. Lett. 2012, 14, 4979–4981. doi:10.1021/ol3022726 |
| 100. | Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a |
| 79. | Li, Y.; Wu, L.; Neumann, H.; Beller, M. Chem. Commun. 2013, 49, 2628–2630. doi:10.1039/c2cc36554e |
| 99. | Yan, Q.; Jiang, L.; Yi, W.; Liu, Q.; Zhang, W. Adv. Synth. Catal. 2017, 359, 2471–2480. doi:10.1002/adsc.201700270 |
| 76. | Zhang, K.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2015, 80, 7658–7665. doi:10.1021/acs.joc.5b01295 |
| 42. | Hang, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3648–3651. doi:10.1021/ol501380e |
| 77. | Hong, J.; Wang, G.; Huo, L.; Zheng, C. Chin. J. Chem. 2017, 35, 1761–1767. doi:10.1002/cjoc.201700311 |
| 94. | Langlois, B.; Montègre, D.; Roidot, N. J. Fluorine Chem. 1994, 68, 63–66. doi:10.1016/0022-1139(93)02982-k |
| 96. | Ma, J.-j.; Liu, Q.-r.; Lu, G.-p.; Yi, W.-b. J. Fluorine Chem. 2017, 193, 113–117. doi:10.1016/j.jfluchem.2016.11.010 |
| 95. | Ma, J.-J.; Yi, W.-B.; Lu, G.-P.; Cai, C. Catal. Sci. Technol. 2016, 6, 417–421. doi:10.1039/c5cy01561h |
| 20. | Clavel, J.-L.; Langlois, B.; Laurent, E.; Roidot, N. Phosphorus, Sulfur Silicon Relat. Elem. 1991, 59, 169–172. doi:10.1080/10426509108045716 |
| 84. | Yin, J.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. Synthesis 2014, 46, 607–612. doi:10.1055/s-0033-1338578 |
| 85. | Shang, X.-J.; Li, Z.; Liu, Z.-Q. Tetrahedron Lett. 2015, 56, 233–235. doi:10.1016/j.tetlet.2014.11.076 |
| 82. | Li, Z.; Cui, Z.; Liu, Z.-Q. Org. Lett. 2013, 15, 406–409. doi:10.1021/ol3034059 |
| 83. | Patra, T.; Deb, A.; Manna, S.; Sharma, U.; Maiti, D. Eur. J. Org. Chem. 2013, 5247–5250. doi:10.1002/ejoc.201300473 |
| 101. | Sun, D.-W.; Jiang, X.; Jiang, M.; Lin, Y.; Liu, J.-T. Eur. J. Org. Chem. 2017, 3505–3511. doi:10.1002/ejoc.201700661 |
| 51. | Zhang, L.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3688–3691. doi:10.1021/ol5014747 |
| 109. | Magnier, E.; Blazejewski, J.-C.; Tordeux, M.; Wakselman, C. Angew. Chem., Int. Ed. 2006, 45, 1279–1282. doi:10.1002/anie.200503776 |
| 110. | Okazaki, T.; Laali, K. K.; Reddy, A. S. J. Fluorine Chem. 2014, 165, 91–95. doi:10.1016/j.jfluchem.2014.06.021 |
| 111. | Umemoto, T.; Zhang, B.; Zhu, T.; Zhou, X.; Zhang, P.; Hu, S.; Li, Y. J. Org. Chem. 2017, 82, 7708–7719. doi:10.1021/acs.joc.7b00669 |
| 52. | Mai, W.-P.; Wang, J.-T.; Yang, L.-R.; Yuan, J.-W.; Xiao, Y.-M.; Mao, P.; Qu, L.-B. Org. Lett. 2014, 16, 204–207. doi:10.1021/ol403196h |
| 108. | Wakselman, C.; Tordeux, M.; Freslon, C.; Saint-Jalmes, L. Synlett 2001, 550–552. doi:10.1055/s-2001-12316 |
| 102. | Zhao, X.; Wei, A.; Yang, B.; Li, T.; Li, Q.; Qiu, D.; Lu, K. J. Org. Chem. 2017, 82, 9175–9181. doi:10.1021/acs.joc.7b01226 |
| 51. | Zhang, L.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3688–3691. doi:10.1021/ol5014747 |
| 101. | Sun, D.-W.; Jiang, X.; Jiang, M.; Lin, Y.; Liu, J.-T. Eur. J. Org. Chem. 2017, 3505–3511. doi:10.1002/ejoc.201700661 |
| 1. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004. |
| 2. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 3. | Harsanyi, A.; Sandford, G. Green Chem. 2015, 17, 2081–2086. doi:10.1039/c4gc02166e |
| 4. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 105. | Roussel, S.; Billard, T.; Langlois, B. R.; Saint-Jalmes, L. Synlett 2004, 2119–2122. doi:10.1055/s-2004-831322 |
| 104. | Billard, T.; Greiner, A.; Langlois, B. R. Tetrahedron 1999, 55, 7243–7250. doi:10.1016/s0040-4020(99)00364-6 |
| 107. | Richards-Taylor, C. S.; Martinez-Lamenca, C.; Leenaerts, J. E.; Trabanco, A. A.; Oehlrich, D. J. Org. Chem. 2017, 82, 9898–9904. doi:10.1021/acs.joc.7b01628 |
| 106. | Liu, Z.; Larock, R. C. J. Am. Chem. Soc. 2005, 127, 13112–13113. doi:10.1021/ja054079p |
| 9. | Seebach, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 1320–1367. doi:10.1002/anie.199013201 |
| 57. | Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. doi:10.1073/pnas.1109059108 |
| 8. | Besset, T.; Jubault, P.; Pannecoucke, X.; Poisson, T. Org. Chem. Front. 2016, 3, 1004–1010. doi:10.1039/c6qo00164e |
| 6. | Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524 |
| 7. | Glenadel, Q.; Ismalaj, E.; Billard, T. Eur. J. Org. Chem. 2017, 530–533. doi:10.1002/ejoc.201601526 |
| 56. | Smertenko, E. A.; Datsenko, S. D.; Ignat'ev, N. V. Russ. J. Electrochem. 1998, 34, 46–51. |
| 103. | Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790 |
| 5. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 38. | Tommasino, J.-B.; Brondex, A.; Medebielle, M.; Thomalla, M.; Langlois, B. R.; Billard, T. Synlett 2002, 1697–1699. doi:10.1055/s-2002-34210 |
| 102. | Zhao, X.; Wei, A.; Yang, B.; Li, T.; Li, Q.; Qiu, D.; Lu, K. J. Org. Chem. 2017, 82, 9175–9181. doi:10.1021/acs.joc.7b01226 |
| 13. | Ye, Y. e-EROS Encyclopedia of Reagents for Organic 2014, 1–4. doi:10.1002/047084289X.rn01689 |
| 55. | Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2015, 17, 1906–1909. doi:10.1021/acs.orglett.5b00601 |
| 12. | Shibata, N.; Matsnev, A.; Cahard, D. Beilstein J. Org. Chem. 2010, 6, No. 65. doi:10.3762/bjoc.6.65 |
| 16. | Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525–7528. doi:10.1016/0040-4039(91)80524-a |
| 11. | Ma, J.-A.; Cahard, D. J. Fluorine Chem. 2007, 128, 975–996. doi:10.1016/j.jfluchem.2007.04.026 |
| 53. | Li, B.; Fan, D.; Yang, C.; Xia, W. Org. Biomol. Chem. 2016, 14, 5293–5297. doi:10.1039/c6ob00912c |
| 10. | McClinton, M. A.; McClinton, D. A. Tetrahedron 1992, 48, 6555–6666. doi:10.1016/S0040-4020(01)80011-9 |
| 54. | Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C. J. Am. Chem. Soc. 2017, 139, 1388–1391. doi:10.1021/jacs.6b11234 |
| 60. | Fennewald, J. C.; Lipshutz, B. H. Green Chem. 2014, 16, 1097–1100. doi:10.1039/c3gc42119h |
| 120. | Liao, J.; Guo, W.; Zhang, Z.; Tang, X.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 1304–1309. doi:10.1021/acs.joc.5b02674 |
| 61. | Zhang, X.; Huang, P.; Li, Y.; Duan, C. Org. Biomol. Chem. 2015, 13, 10917–10922. doi:10.1039/c5ob01516b |
| 118. | Kong, H. I.; Crichton, J. E.; Manthorpe, J. M. Tetrahedron Lett. 2011, 52, 3714–3717. doi:10.1016/j.tetlet.2011.05.015 |
| 119. | Kong, H. I.; Gill, M. A.; Hrdina, A. H.; Crichton, J. E.; Manthorpe, J. M. J. Fluorine Chem. 2013, 153, 151–161. doi:10.1016/j.jfluchem.2013.03.020 |
| 58. | Musumeci, D.; Irace, C.; Santamaria, R.; Montesarchio, D. Med. Chem. Commun. 2013, 4, 1405–1410. doi:10.1039/c3md00159h |
| 59. | Wu, M.; Ji, X.; Dai, W.; Cao, S. J. Org. Chem. 2014, 79, 8984–8989. doi:10.1021/jo501221h |
| 121. | Cullen, S. C.; Shekhar, S.; Nere, N. K. J. Org. Chem. 2013, 78, 12194–12201. doi:10.1021/jo401868x |
| 116. | Zhu, S.; Chu, Q.; Xu, G.; Qin, C.; Xu, Y. J. Fluorine Chem. 1998, 91, 195–198. doi:10.1016/s0022-1139(98)00240-1 |
| 115. | Hasegawa, A.; Ishikawa, T.; Ishihara, K.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2005, 78, 1401–1410. doi:10.1246/bcsj.78.1401 |
| 112. | Eugene, F.; Langlois, B.; Laurent, E. J. Fluorine Chem. 1994, 66, 301–309. doi:10.1016/0022-1139(93)02909-x |
| 118. | Kong, H. I.; Crichton, J. E.; Manthorpe, J. M. Tetrahedron Lett. 2011, 52, 3714–3717. doi:10.1016/j.tetlet.2011.05.015 |
| 117. | Wu, W.-L.; Asberom, T.; Bara, T.; Bennett, C.; Burnett, D. A.; Clader, J.; Domalski, M.; Greenlee, W. J.; Josien, H.; McBriar, M.; Rajagopalan, M.; Vicarel, M.; Xu, R.; Hyde, L. A.; Del Vecchio, R. A.; Cohen-Williams, M. E.; Song, L.; Lee, J.; Terracina, G.; Zhang, Q.; Nomeir, A.; Parker, E. M.; Zhang, L. Bioorg. Med. Chem. Lett. 2013, 23, 844–849. doi:10.1016/j.bmcl.2012.11.047 |
| 5. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 66. | Ji, X.-M.; Wei, L.; Chen, F.; Tang, R.-Y. RSC Adv. 2015, 5, 29766–29773. doi:10.1039/C5RA02888D |
| 113. | Meshcheryakov, V. I.; Shainyan, B. A. Russ. J. Org. Chem. 2004, 40, 390–396. doi:10.1023/B:RUJO.0000034977.74026.b3 |
| 114. | Hasegawa, A.; Naganawa, Y.; Fushimi, M.; Ishihara, K.; Yamamoto, H. Org. Lett. 2006, 8, 3175–3178. doi:10.1021/ol060939a |
| 115. | Hasegawa, A.; Ishikawa, T.; Ishihara, K.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2005, 78, 1401–1410. doi:10.1246/bcsj.78.1401 |
| 67. | Jin, L.-K.; Lu, G.-P.; Cai, C. Org. Chem. Front. 2016, 3, 1309–1313. doi:10.1039/c6qo00369a |
| 112. | Eugene, F.; Langlois, B.; Laurent, E. J. Fluorine Chem. 1994, 66, 301–309. doi:10.1016/0022-1139(93)02909-x |
| 64. | Li, C.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. New J. Chem. 2017, 41, 1417–1420. doi:10.1039/c6nj03654f |
| 65. | Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332–1337. doi:10.1021/jo502928e |
| 62. | Cao, X.-H.; Pan, X.; Zhou, P.-J.; Zou, J.-P.; Asekun, O. T. Chem. Commun. 2014, 50, 3359–3362. doi:10.1039/c3cc49689a |
| 63. | Zhang, P.-Z.; Li, C.-K.; Zhang, G.-Y.; Zhang, L.; Jiang, Y.-J.; Zou, J.-P. Tetrahedron 2016, 72, 3250–3255. doi:10.1016/j.tet.2016.04.048 |
| 33. | Zhang, H.-Y.; Huo, W.; Ge, C.; Zhao, J.; Zhang, Y. Synlett 2017, 28, 962–965. doi:10.1055/s-0036-1588400 |
| 26. | Lu, Q.; Liu, C.; Huang, Z.; Ma, Y.; Zhang, J.; Lei, A. Chem. Commun. 2014, 50, 14101–14104. doi:10.1039/c4cc06328g |
| 129. | Han, J.-B.; Yang, L.; Chen, X.; Zha, G.-F.; Zhang, C.-P. Adv. Synth. Catal. 2016, 358, 4119–4124. doi:10.1002/adsc.201600717 |
| 34. | Jiang, X.-Y.; Qing, F.-L. Angew. Chem., Int. Ed. 2013, 52, 14177–14180. doi:10.1002/anie.201307595 |
| 128. | Hua, L.-N.; Li, H.; Qing, F.-L.; Huang, Y.; Xu, X.-H. Org. Biomol. Chem. 2016, 14, 8443–8447. doi:10.1039/C6OB01567K |
| 125. | Aithagani, S. K.; Yempalla, K. R.; Munagala, G.; Vishwakarma, R. A.; Singh, P. P. RSC Adv. 2014, 4, 50208–50211. doi:10.1039/c4ra07370c |
| 76. | Zhang, K.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2015, 80, 7658–7665. doi:10.1021/acs.joc.5b01295 |
| 127. | Chu, X.-Q.; Meng, H.; Xu, X.-P.; Ji, S.-J. Chem. – Eur. J. 2015, 21, 11359–11368. doi:10.1002/chem.201500469 |
| 126. | Ochiai, M.; Tada, N.; Nishi, Y.; Murai, K. Chem. Commun. 2004, 2894–2895. doi:10.1039/B410830B |
| 40. | Lefebvre, Q.; Hoffmann, N.; Rueping, M. Chem. Commun. 2016, 52, 2493–2496. doi:10.1039/c5cc09881e |
| 122. | Xie, F.; Zhang, Z.; Yu, X.; Tang, G.; Li, X. Angew. Chem., Int. Ed. 2015, 54, 7405–7409. doi:10.1002/anie.201502278 |
| 41. | Zhu, L.; Wang, L.-S.; Li, B.; Fu, B.; Zhang, C.-P.; Li, W. Chem. Commun. 2016, 52, 6371–6374. doi:10.1039/c6cc01944g |
| 121. | Cullen, S. C.; Shekhar, S.; Nere, N. K. J. Org. Chem. 2013, 78, 12194–12201. doi:10.1021/jo401868x |
| 38. | Tommasino, J.-B.; Brondex, A.; Medebielle, M.; Thomalla, M.; Langlois, B. R.; Billard, T. Synlett 2002, 1697–1699. doi:10.1055/s-2002-34210 |
| 124. | Smyth, L. A.; Phillips, E. M.; Chan, V. S.; Napolitano, J. G.; Henry, R.; Shekhar, S. J. Org. Chem. 2016, 81, 1285–1294. doi:10.1021/acs.joc.5b02523 |
| 39. | Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Chem. Sci. 2013, 4, 3160–3165. doi:10.1039/c3sc51209f |
| 123. | Wang, F.; Yu, X.; Qi, Z.; Li, X. Chem. – Eur. J. 2016, 22, 511–516. doi:10.1002/chem.201504179 |
| 24. | Maji, A.; Hazra, A.; Maiti, D. Org. Lett. 2014, 16, 4524–4527. doi:10.1021/ol502071g |
| 37. | Yu, J.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 3669–3675. doi:10.1002/adsc.201400144 |
| 35. | Yang, Y.; Liu, Y.; Jiang, Y.; Zhang, Y.; Vicic, D. A. J. Org. Chem. 2015, 80, 6639–6648. doi:10.1021/acs.joc.5b00781 |
| 36. | Liu, C.; Lu, Q.; Huang, Z.; Zhang, J.; Liao, F.; Peng, P.; Lei, A. Org. Lett. 2015, 17, 6034–6037. doi:10.1021/acs.orglett.5b03035 |
| 42. | Hang, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3648–3651. doi:10.1021/ol501380e |
| 43. | Liu, Z.-Q.; Liu, D. J. Org. Chem. 2017, 82, 1649–1656. doi:10.1021/acs.joc.6b02812 |
| 44. | Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Chem. Commun. 2017, 53, 7638–7641. doi:10.1039/c7cc01903c |
| 49. | Shi, L.; Yang, X.; Wang, Y.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 1021–1028. doi:10.1002/adsc.201300995 |
| 131. | Billard, T.; Langlois, B. R. J. Fluorine Chem. 1997, 84, 63–64. doi:10.1016/s0022-1139(97)00009-2 |
| 42. | Hang, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3648–3651. doi:10.1021/ol501380e |
| 130. | Billard, T.; Langlois, B. R.; Large, S.; Anker, D.; Roidot, N.; Roure, P. J. Org. Chem. 1996, 61, 7545–7550. doi:10.1021/jo960619c |
| 49. | Shi, L.; Yang, X.; Wang, Y.; Yang, H.; Fu, H. Adv. Synth. Catal. 2014, 356, 1021–1028. doi:10.1002/adsc.201300995 |
| 50. | Sakamoto, R.; Kashiwagi, H.; Selvakumar, S.; Moteki, S. A.; Maruoka, K. Org. Biomol. Chem. 2016, 14, 6417–6421. doi:10.1039/c6ob01245k |
| 47. | Liu, J.; Zhuang, S.; Gui, Q.; Chen, X.; Yang, Z.; Tan, Z. Eur. J. Org. Chem. 2014, 3196–3202. doi:10.1002/ejoc.201400087 |
| 48. | Wei, W.; Wen, J.; Yang, D.; Liu, X.; Guo, M.; Dong, R.; Wang, H. J. Org. Chem. 2014, 79, 4225–4230. doi:10.1021/jo500515x |
| 45. | Yang, F.; Klumphu, P.; Liang, Y.-M.; Lipshutz, B. H. Chem. Commun. 2014, 50, 936–938. doi:10.1039/c3cc48131j |
| 130. | Billard, T.; Langlois, B. R.; Large, S.; Anker, D.; Roidot, N.; Roure, P. J. Org. Chem. 1996, 61, 7545–7550. doi:10.1021/jo960619c |
| 46. | Lu, Q.; Liu, C.; Peng, P.; Liu, Z.; Fu, L.; Huang, J.; Lei, A. Asian J. Org. Chem. 2014, 3, 273–276. doi:10.1002/ajoc.201300236 |
| 90. | Lu, S.; Gong, Y.; Zhou, D. J. Org. Chem. 2015, 80, 9336–9341. doi:10.1021/acs.joc.5b01518 |
| 50. | Sakamoto, R.; Kashiwagi, H.; Selvakumar, S.; Moteki, S. A.; Maruoka, K. Org. Biomol. Chem. 2016, 14, 6417–6421. doi:10.1039/c6ob01245k |
| 42. | Hang, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2014, 16, 3648–3651. doi:10.1021/ol501380e |
| 89. | Liu, Y.-R.; Tu, H.-Y.; Zhang, X.-G. Synthesis 2015, 47, 3460–3466. doi:10.1055/s-0034-1378810 |
| 87. | Wu, L.-H.; Zhao, K.; Shen, Z.-L.; Loh, T.-P. Org. Chem. Front. 2017, 4, 1872–1875. doi:10.1039/c7qo00416h |
| 88. | Jiang, H.; Huang, W.; Yu, Y.; Yi, S.; Li, J.; Wu, W. Chem. Commun. 2017, 53, 7473–7476. doi:10.1039/c7cc03125d |
| 86. | Huang, P.; Li, Y.; Fu, X.; Zhang, R.; Jin, K.; Wang, W.; Duan, C. Tetrahedron Lett. 2016, 57, 4705–4708. doi:10.1016/j.tetlet.2016.09.016 |
| 20. | Clavel, J.-L.; Langlois, B.; Laurent, E.; Roidot, N. Phosphorus, Sulfur Silicon Relat. Elem. 1991, 59, 169–172. doi:10.1080/10426509108045716 |
| 21. | Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1992, 33, 1291–1294. doi:10.1016/s0040-4039(00)91604-6 |
| 17. | Zhang, C. Adv. Synth. Catal. 2014, 356, 2895–2906. doi:10.1002/adsc.201400370 |
| 18. | Langlois, B. R. Once Upon a Time Was the Langlois' Reagent: A “Sleeping Beauty”. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds; Groult, H.; Leroux, F.; Tressaud, A., Eds.; Elsevier, 2017; pp 125–140. |
| 19. | Lefebvre, Q. Synlett 2017, 28, 19–23. doi:10.1055/s-0036-1588643 |
| 16. | Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525–7528. doi:10.1016/0040-4039(91)80524-a |
| 15. | Roesky, H. W.; Holtschneider, G. J. Fluorine Chem. 1976, 7, 77–84. doi:10.1016/s0022-1139(00)83984-6 |
| 93. | van der Werf, A.; Hribersek, M.; Selander, N. Org. Lett. 2017, 19, 2374–2377. doi:10.1021/acs.orglett.7b00908 |
| 16. | Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525–7528. doi:10.1016/0040-4039(91)80524-a |
| 91. | Fang, J.; Shen, W.-G.; G.-Z., Ao; Liu, F. Org. Chem. Front. 2017, 4, 2049–2053. doi:10.1039/C7QO00473G |
| 14. | Haszeldine, R. N.; Kidd, J. M. J. Chem. Soc. 1955, 2901–2910. doi:10.1039/JR9550002901 |
| 92. | Dubbaka, S. R.; Nizalapur, S.; Atthunuri, A. R.; Salla, M.; Mathew, T. Tetrahedron 2014, 70, 2118–2121. doi:10.1016/j.tet.2014.02.005 |
| 24. | Maji, A.; Hazra, A.; Maiti, D. Org. Lett. 2014, 16, 4524–4527. doi:10.1021/ol502071g |
| 22. | Lu, Y.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. J. Fluorine Chem. 2014, 161, 128–133. doi:10.1016/j.jfluchem.2014.01.020 |
| 23. | Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Angew. Chem., Int. Ed. 2013, 52, 9747–9750. doi:10.1002/anie.201303576 |
| 31. | Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 11960–11964. doi:10.1002/anie.201406464 |
| 32. | Yu, X.-L.; Chen, J.-R.; Chen, D.-Z.; Xiao, W.-J. Chem. Commun. 2016, 52, 8275–8278. doi:10.1039/c6cc03335k |
| 29. | Konik, Y. A.; Kudrjashova, M.; Konrad, N.; Kaabel, S.; Jarving, I.; Lopp, M.; Kananovich, D. G. Org. Biomol. Chem. 2017, 15, 4635–4643. doi:10.1039/C7OB00680B |
| 30. | Huang, H.-L.; Yan, H.; Gao, G.-L.; Yang, C.; Xia, W. Asian J. Org. Chem. 2015, 4, 674–677. doi:10.1002/ajoc.201500096 |
| 27. | Qin, H.-T.; Wu, S.-W.; Liu, J.-L.; Liu, F. Chem. Commun. 2017, 53, 1696–1699. doi:10.1039/c6cc10035j |
| 28. | Lv, W.-X.; Zeng, Y.-F.; Li, Q.; Chen, Y.; Tan, D.-H.; Yang, L.; Wang, H. Angew. Chem., Int. Ed. 2016, 55, 10069–10073. doi:10.1002/anie.201604898 |
| 25. | Luo, H.-Q.; Zhang, Z.-P.; Dong, W.; Luo, X.-Z. Synlett 2014, 25, 1307–1311. doi:10.1055/s-0033-1341057 |
| 26. | Lu, Q.; Liu, C.; Huang, Z.; Ma, Y.; Zhang, J.; Lei, A. Chem. Commun. 2014, 50, 14101–14104. doi:10.1039/c4cc06328g |
© 2017 Guyon et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)