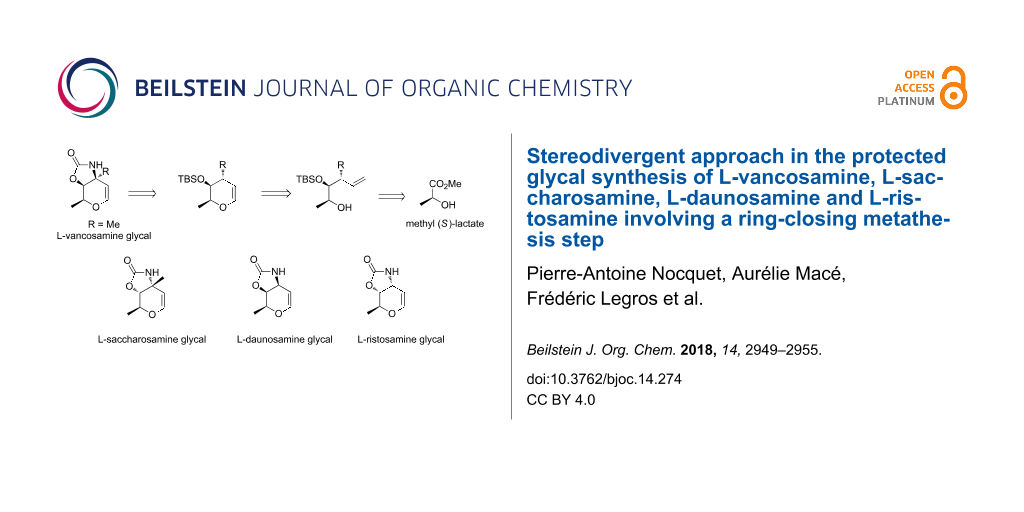
Abstract
In this paper, a new access to several chiral 3-aminoglycals as potential precursors for glycosylated natural products is reported from a common starting material, (−)-methyl-L-lactate. The stereodivergent strategy is based on the implementation of a ring-closing metathesis of vinyl ethers as key step of reaction sequences developed.
Graphical Abstract
Introduction
Several classes of medicinally useful molecules with antibiotic and anticancer activity contain in their structures 3-amino-2-deoxy sugars [1]. For instance, N,N-dimethyl-L-vancosamine is an essential component of pluramycin antibiotics such as kidamycin and pluramycin A via a C-glycosidic linkage (Figure 1).
Figure 1: N,N-Dimethyl-L-vancosamine as substructure of kidamycin and pluramycin.
Figure 1: N,N-Dimethyl-L-vancosamine as substructure of kidamycin and pluramycin.
For constructing aryl C-glycoside bonds, glycal derivatives are versatile synthetic intermediates (Figure 2). Indeed, they can be converted into glycosyl donors but can also be considered as potential coupling partners or nucleophilic moieties via the formation of transient metalated species [2]. As example concerning their use in pluramycins' syntheses, an approach to the synthesis of pluraflavin A was developed based on a Stille coupling to install the C-linked sugar residue [3]. Moreover, the addition of lithiated glycals to quinone derivatives followed by a rearrangement was also studied for the synthesis of kidamycin according to a “reverse polarity” strategy [4,5].
Figure 2: Glycals as relevant scaffolds for constructing aryl C-glycosidic linkage.
Figure 2: Glycals as relevant scaffolds for constructing aryl C-glycosidic linkage.
Considering that the glycal scaffolds are versatile building blocks with multiple applications in the field of natural product synthesis [6], the development of new asymmetric synthetic sequences with stereochemical diversity is still of high interest. Different approaches have been reported for the asymmetric synthesis of protected 3-aminoglycals from non-carbohydrate precursors. Most of them used a common methodology for the construction of the pyranosyl glycal ring which is based on a cycloisomerization reaction of chiral homopropargylic alcohols [7-10]. In some cases, the strategy used for the preparation of the corresponding alkynyl alcohols requires the handling of toxic tin reagents [8,9].
During these last years, ring-closing metathesis (RCM) of vinyl ethers have proved to be an efficient method for the preparation of chiral glycal scaffolds [11-18] as demonstrated in some total syntheses of marine polycyclic ethers [19-21]. However, to the best of our knowledge, this methodology was never evaluated for the synthesis of this kind of nitrogen-containing substrates. Taking into account our interest about the development of new synthetic approaches to pluramycins [22,23], we speculated that the cyclic vinyl ether derivative I, with the prerequisite configuration of all stereogenic centers of the carbamate-protected glycal of L-vancosamine 1, could be obtained from the alcohol derivative II using an O-vinylation–ring-closing metathesis sequence (Figure 3). Afterwards, the introduction of nitrogen in the convenient position (C3) could be performed by a stereopecific nitrene insertion reaction catalyzed by rhodium(II) complexes [24,25].
Figure 3: Strategy including a ring-closing metathesis of vinyl ethers as key step for the preparation of several carbamate-protected 3-aminoglycals.
Figure 3: Strategy including a ring-closing metathesis of vinyl ethers as key step for the preparation of sev...
Herein, we describe our outcomes related to the implementation of this strategy for the synthesis of L-vancosamine derivative 1, as well as its diastereoisomer, the carbamate-protected 3-aminoglycal of L-saccharosamine 2, employing the (S)-(−)-methyl lactate as common starting material. The efficiency and generality of this methodology was also demonstrated by a new synthesis of C-3 unbranched amino glycals, L-daunosamine 3 and L-ristosamine 4 derivatives, from the same source of chirality.
Results and Discussion
Synthesis of vancosamine and saccharosamine glycals. The chiral (−)-lactic methyl ester was identified as the privileged starting material considering that the Evans aldol reaction via boron enolates [26-28] with an appropriately O-protected aldehyde should afford the desired aldol adduct with a syn relative configuration between the two newly created chiral centers [29,30]. Moreover, the boron-mediated stereoselective aldol reaction is all the more interesting for our synthetic plan as stereochemical diversity can be generated depending on the absolute configuration of the chiral auxiliary used. The aldehyde 5 was first prepared according to a described procedure in two steps from methyl L-lactate (Scheme 1) [31]. The reaction with (R)- or (S)-oxazolidinones 6 led to the formation of 2,3-syn aldol products 7 in good yields with a very high level of diastereoselection (>20:1 for both).
Scheme 1: Evans aldol reaction for the preparation of diastereomeric compounds 13a and 13b.
Scheme 1: Evans aldol reaction for the preparation of diastereomeric compounds 13a and 13b.
After protection of the free hydroxy group, the reduction of the N-acyl oxazolidinones 8 into primary alcohols 9 was accomplished by LiBH4 in presence of water or LiAlH4 [32]. Whatever the conditions used for this step, moderate yields were obtained for the desired products due to the formation of substantial amounts of ring-opened byproducts 10 resulting from the hydride addition to the carbonyl group of the oxazolidinone ring [33,34]. The alcohols 9 were then subjected to a Swern oxidation followed by a Wittig reaction to generate the corresponding alkenes 13a,b in 90% and 63% yield, respectively, over two steps. Alternatively, we envisioned that, from the same α-substituted chiral aldehyde 5, compound 13b could be obtained in a more straightforward manner employing a strategy based on a diastereoselective allylboration reaction (Scheme 2) [35]. Indeed, the reaction of achiral pinacol (Z)-crotylboronate with 5 under neat conditions at room temperature gave a good level of diastereoselectivity for the hitherto unreported 3,4-syn-2,3-anti product 12b [36-39].
Scheme 2: Alternative preparation of 13b based on a diastereoselective allylboration.
Scheme 2: Alternative preparation of 13b based on a diastereoselective allylboration.
The syn relationship between C3 and C4 is controlled by the (Z)-geometry of the crotylboronate, while the 2,3-anti relationship can be rationalized by invoking Cornforth-like transition states [40-43]. Eventually, silylation of the homoallylic alcohol 12b afforded the expected compound 13b in 68% overall yield from 5 after purification, compared to 29% using a strategy based on an Evans’ aldol reaction.
Mildly oxidizing conditions using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) were used for the removal of the p-methoxybenzyl (PMB) group to provide alcohols 14 (Scheme 3). Several palladium(II) catalysts have been tested for the conversion of alcohols to vinyl ethers 15 [13,44-46]. We found that the best yields were obtained using Pd(TFA)2 and n-butyl vinyl ether as solvent in the presence of bathophenanthroline as ligand. In the case of Pd(OAc)2, the reaction was slower with moderate yields.
Scheme 3: O-Vinylation-ring-closing metathesis sequence for access to 3-amino glycals.
Scheme 3: O-Vinylation-ring-closing metathesis sequence for access to 3-amino glycals.
The ring-closing metathesis reaction was performed with Hoveyda–Grubbs second-generation (HG-II) catalyst to deliver the corresponding dihydropyrans 16 in excellent yields given that this kind of reaction can be sensitive to the substitution pattern contained in the substrate [11]. After silyl deprotection, the key C–H amination precursors 17a,b for the synthesis of the carbamate-protected glycal of L-vancosamine 1 and L-saccharosamine 2 were prepared in two steps by treatment of alcohols with the trichloroacetyl isocyanate reagent (TCA-NCO) followed by basic hydrolysis. The spectroscopic properties of carbamates 17 were identical to those reported in the literature [7,8]. Although the intramolecular C–H amination of compounds 17 under the Du Bois conditions [24] was already described in the literature [8,9], the reaction was nevertheless achieved with carbamate 17a in order to check the reproducibility of the final step. As expected, L-vancosamine glycal 1 was obtained in similar yield than one reported [8,47].
Synthesis of daunosamine and ristosamine glycals. As previously, the chiral pool material 5 was used for this unbranched glycal synthesis (Scheme 4). The first step was the chelation-controlled addition of allylmagnesium bromide to 5 to provide the syn diastereomer 18 in high stereoselectivity (93:7). After silylation of the free hydroxy group, the cleavage of the PMB ether with DDQ led to alcohol 20 in 77% yield for the two steps. Ring-closing metathesis of diene 21, obtained by O-vinylation of 20, gave the dihydropyran 22 in 53% overall yield for two steps. The silyl group of compound 22 was cleaved using tetrabutylammonium fluoride (TBAF) in THF to form alcohol 23 which was used directly in the next step [48].
Scheme 4: Synthesis of key intermediate 23 for the C-3 unbranched amino glycals preparation.
Scheme 4: Synthesis of key intermediate 23 for the C-3 unbranched amino glycals preparation.
At this stage, we envisioned that 23 could be also a key intermediate to access the ristosamine derivative by reversing the configuration of the stereogenic center bearing the hydroxy group (Scheme 5). With this in mind, the secondary alcohol 23 was engaged in a Mitsunobu reaction using p-nitrobenzoic acid as nucleophile to afford the expected compound 25. Hydrolysis of the ester was achieved using potassium carbonate in methanol to afford the epimeric product 26.
Scheme 5: Access to diastereoisomeric compounds 3 and 4 from 23.
Scheme 5: Access to diastereoisomeric compounds 3 and 4 from 23.
Both diastereomers 23 and 26 were converted to the corresponding known carbamates using a two step sequence. Reaction with TCA-NCO followed by a basic hydrolysis provided the desired compounds 24 and 27 in good yields and in full agreement with all reported spectroscopic data [9]. As an example, the expected protected glycal of L-daunosamine 3 [9,47] was obtained by regioselective rhodium nitrene insertion thus demonstrating the usefulness of this strategy for the synthesis of such compounds.
Conclusion
We developed an alternative route to 3-aminoglycals through ring-closing metathesis of vinyl ethers as key step in the synthesis and using a common noncarbohydrate starting material. The approach was first validated for the synthesis of protected L-vancosamine glycal and extended afterwards to prepare a diastereomeric compound as well as other unbranched C-3 aminoglycals. The use of these synthons in the synthesis of glycosylated antibiotics as kidamycin is underway in our laboratory.
Supporting Information
Supporting Information contains detailed experimental procedures with full characterization of all compounds and NMR spectra.
| Supporting Information File 1: Experimental part and NMR spectra of all compounds. | ||
| Format: PDF | Size: 2.7 MB | Download |
References
-
Hauser, F. M.; Ellenberger, S. R. Chem. Rev. 1986, 86, 35–67. doi:10.1021/cr00071a003
Return to citation in text: [1] -
Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Chem. Rev. 2018, 118, 1495–1598. doi:10.1021/acs.chemrev.7b00380
Return to citation in text: [1] -
Hartung, J.; Wright, B. J. D.; Danishefsky, S. J. Chem. – Eur. J. 2014, 20, 8731–8736. doi:10.1002/chem.201402254
Return to citation in text: [1] -
Parker, K. A. Pure Appl. Chem. 1994, 66, 2135–2138. doi:10.1351/pac199466102135
Return to citation in text: [1] -
Parker, K. A.; Koh, Y.-h. J. Am. Chem. Soc. 1994, 116, 11149–11150. doi:10.1021/ja00103a037
Return to citation in text: [1] -
Danishefsky, S. J.; Bilodeau, M. T. Angew. Chem., Int. Ed. Engl. 1996, 35, 1380–1419. doi:10.1002/anie.199613801
Return to citation in text: [1] -
Cutchins, W. W.; McDonald, F. E. Org. Lett. 2002, 4, 749–752. doi:10.1021/ol017195f
Return to citation in text: [1] [2] -
Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p
Return to citation in text: [1] [2] [3] [4] [5] -
Parker, K. A.; Chang, W. Org. Lett. 2005, 7, 1785–1788. doi:10.1021/ol050356l
Return to citation in text: [1] [2] [3] [4] [5] -
Fei, Z.; McDonald, F. E. Org. Lett. 2007, 9, 3547–3550. doi:10.1021/ol7014219
Return to citation in text: [1] -
Sturino, C. F.; Wong, J. C. Y. Tetrahedron Lett. 1998, 39, 9623–9626. doi:10.1016/s0040-4039(98)02205-9
Return to citation in text: [1] [2] -
Gurjar, M. K.; Krishna, L. M.; Reddy, B. S.; Chorghade, M. S. Synthesis 2000, 557–560. doi:10.1055/s-2000-6376
Return to citation in text: [1] -
Peczuh, M. W.; Snyder, N. L. Tetrahedron Lett. 2003, 44, 4057–4061. doi:10.1016/s0040-4039(03)00849-9
Return to citation in text: [1] [2] -
Postema, M. H. D.; Piper, J. L.; Liu, L.; Shen, J.; Faust, M.; Andreana, P. J. Org. Chem. 2003, 68, 4748–4754. doi:10.1021/jo030039x
Return to citation in text: [1] -
Adam, J.-M.; de Fays, L.; Laguerre, M.; Ghosez, L. Tetrahedron 2004, 60, 7325–7344. doi:10.1016/j.tet.2004.05.058
Return to citation in text: [1] -
Sharma, H.; Santra, S.; Debnath, J.; Antonio, T.; Reith, M.; Dutta, A. Bioorg. Med. Chem. 2014, 22, 311–324. doi:10.1016/j.bmc.2013.11.017
Return to citation in text: [1] -
Sutton, A. E.; Seigal, B. A.; Finnegan, D. F.; Snapper, M. L. J. Am. Chem. Soc. 2002, 124, 13390–13391. doi:10.1021/ja028044q
Return to citation in text: [1] -
Schmidt, B.; Biernat, A. Chem. – Eur. J. 2008, 14, 6135–6141. doi:10.1002/chem.200800567
Return to citation in text: [1] -
Majumder, U.; Cox, J. M.; Johnson, H. W. B.; Rainier, J. D. Chem. – Eur. J. 2006, 12, 1736–1746. doi:10.1002/chem.200500993
Return to citation in text: [1] -
Osei Akoto, C.; Rainier, J. D. Angew. Chem., Int. Ed. 2008, 47, 8055–8058. doi:10.1002/anie.200803791
Return to citation in text: [1] -
Clark, J. S.; Romiti, F.; Sieng, B.; Paterson, L. C.; Stewart, A.; Chaudhury, S.; Thomas, L. H. Org. Lett. 2015, 17, 4694–4697. doi:10.1021/acs.orglett.5b02093
Return to citation in text: [1] -
Mabit, T.; Siard, A.; Pantin, M.; Zon, D.; Foulgoc, L.; Sissouma, D.; Guingant, A.; Mathé-Allainmat, M.; Lebreton, J.; Carreaux, F.; Dujardin, G.; Collet, S. J. Org. Chem. 2017, 82, 5710–5719. doi:10.1021/acs.joc.7b00544
Return to citation in text: [1] -
Mabit, T.; Siard, A.; Legros, F.; Guillarme, S.; Martel, A.; Lebreton, J.; Carreaux, F.; Dujardin, G.; Collet, S. Chem. – Eur. J. 2018, 24, 14069–14074. doi:10.1002/chem.201803674
Return to citation in text: [1] -
Espino, C. G.; Du Bois, J. Angew. Chem., Int. Ed. 2001, 40, 598–600. doi:10.1002/1521-3773(20010202)40:3<598::aid-anie598>3.0.co;2-9
Return to citation in text: [1] [2] -
Du Bois, J. Org. Process Res. Dev. 2011, 15, 758–762. doi:10.1021/op200046v
Return to citation in text: [1] -
Evans, D. A.; Vogel, E.; Nelson, J. V. J. Am. Chem. Soc. 1979, 101, 6120–6123. doi:10.1021/ja00514a045
Return to citation in text: [1] -
Evans, D. A.; Nelson, J. V.; Vogel, E.; Taber, T. R. J. Am. Chem. Soc. 1981, 103, 3099–3111. doi:10.1021/ja00401a031
Return to citation in text: [1] -
Mukaiyama, T.; Inoue, T. Chem. Lett. 1976, 559–562. doi:10.1246/cl.1976.559
Return to citation in text: [1] -
Cowden, C. J.; Paterson, I. Org. React. 1997, 1–200. doi:10.1002/0471264180.or051.01
Return to citation in text: [1] -
Zhang, Z.; Collum, D. B. J. Org. Chem. 2017, 82, 7595–7601. doi:10.1021/acs.joc.7b01365
See for a mechanistic study of the Evans aldol reaction.
Return to citation in text: [1] -
Roush, W. R.; Bennett, C. E.; Roberts, S. E. J. Org. Chem. 2001, 66, 6389–6393. doi:10.1021/jo015756a
Return to citation in text: [1] -
Heravi, M. M.; Zadsirjan, V.; Farajpour, B. RSC Adv. 2016, 6, 30498–30551. doi:10.1039/c6ra00653a
Return to citation in text: [1] -
Penning, T. D.; Djuric, S. W.; Haack, R. A.; Kalish, V. J.; Miyashiro, J. M.; Rowell, B. W.; Yu, S. S. Synth. Commun. 1990, 20, 307–312. doi:10.1080/00397919008052299
Return to citation in text: [1] -
Compound 10b was not isolated.
Return to citation in text: [1] -
Lachance, H.; Hall, D. G. Allylboration of carbonyl compounds. In Organic reactions; Denmark, S. E., Ed.; John Wiley & sons: Hoboken, New Jersey, 2008; Vol. 73. doi:10.1002/0471264180.or073.01
Return to citation in text: [1] -
Hoffmann, R. W.; Weidmann, U. Chem. Ber. 1985, 118, 3966–3979. doi:10.1002/cber.19851181010
Return to citation in text: [1] -
Brinkmann, H.; Hoffmann, R. W. Chem. Ber. 1990, 123, 2395–2401. doi:10.1002/cber.19901231223
Return to citation in text: [1] -
Wuts, P. G. M.; Bigelow, S. S. J. Org. Chem. 1988, 53, 5023–5034. doi:10.1021/jo00256a023
Return to citation in text: [1] -
Roush, W. R.; Adam, M. A.; Walts, A. E.; Harris, D. J. J. Am. Chem. Soc. 1986, 108, 3422–3434. doi:10.1021/ja00272a043
Return to citation in text: [1] -
Cornforth, J. W.; Cornforth, R. H.; Mathew, K. K. J. Chem. Soc. 1959, 112–127. doi:10.1039/jr9590000112
Return to citation in text: [1] -
Roush, W. R. In Houben-Weyl, Stereoselective Synthesis; Helmchen, G.; Hoffmann, R. W.; Mulzer, J.; Schaumann, E., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 1995; Vol. E21b, pp 1410–1486.
Return to citation in text: [1] -
Cee, V. J.; Cramer, C. J.; Evans, D. A. J. Am. Chem. Soc. 2006, 128, 2920–2930. doi:10.1021/ja0555670
Return to citation in text: [1] -
Díaz-Oltra, S.; Carda, M.; Murga, J.; Falomir, E.; Marco, J. A. Chem. – Eur. J. 2008, 14, 9240–9254. doi:10.1002/chem.200800956
Return to citation in text: [1] -
Weintraub, P. M.; King, C.-H. R. J. Org. Chem. 1997, 62, 1560–1562. doi:10.1021/jo961182b
Return to citation in text: [1] -
Handerson, S.; Schlaf, M. Org. Lett. 2002, 4, 407–409. doi:10.1021/ol017104e
Return to citation in text: [1] -
Dechert-Schmitt, A.-M.; Cabral, S.; Kung, D. W. Synlett 2016, 27, 2611–2615. doi:10.1055/s-0036-1588615
Return to citation in text: [1] -
A very clean carbamate product is required to obtain a good conversion into oxazolidinone.
Return to citation in text: [1] [2] -
A partial loss of product can occur during evaporation under reduced pressure due to its low boiling point.
Return to citation in text: [1]
| 9. | Parker, K. A.; Chang, W. Org. Lett. 2005, 7, 1785–1788. doi:10.1021/ol050356l |
| 47. | A very clean carbamate product is required to obtain a good conversion into oxazolidinone. |
| 1. | Hauser, F. M.; Ellenberger, S. R. Chem. Rev. 1986, 86, 35–67. doi:10.1021/cr00071a003 |
| 6. | Danishefsky, S. J.; Bilodeau, M. T. Angew. Chem., Int. Ed. Engl. 1996, 35, 1380–1419. doi:10.1002/anie.199613801 |
| 32. | Heravi, M. M.; Zadsirjan, V.; Farajpour, B. RSC Adv. 2016, 6, 30498–30551. doi:10.1039/c6ra00653a |
| 4. | Parker, K. A. Pure Appl. Chem. 1994, 66, 2135–2138. doi:10.1351/pac199466102135 |
| 5. | Parker, K. A.; Koh, Y.-h. J. Am. Chem. Soc. 1994, 116, 11149–11150. doi:10.1021/ja00103a037 |
| 33. | Penning, T. D.; Djuric, S. W.; Haack, R. A.; Kalish, V. J.; Miyashiro, J. M.; Rowell, B. W.; Yu, S. S. Synth. Commun. 1990, 20, 307–312. doi:10.1080/00397919008052299 |
| 34. | Compound 10b was not isolated. |
| 3. | Hartung, J.; Wright, B. J. D.; Danishefsky, S. J. Chem. – Eur. J. 2014, 20, 8731–8736. doi:10.1002/chem.201402254 |
| 29. | Cowden, C. J.; Paterson, I. Org. React. 1997, 1–200. doi:10.1002/0471264180.or051.01 |
| 30. |
Zhang, Z.; Collum, D. B. J. Org. Chem. 2017, 82, 7595–7601. doi:10.1021/acs.joc.7b01365
See for a mechanistic study of the Evans aldol reaction. |
| 2. | Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Chem. Rev. 2018, 118, 1495–1598. doi:10.1021/acs.chemrev.7b00380 |
| 31. | Roush, W. R.; Bennett, C. E.; Roberts, S. E. J. Org. Chem. 2001, 66, 6389–6393. doi:10.1021/jo015756a |
| 19. | Majumder, U.; Cox, J. M.; Johnson, H. W. B.; Rainier, J. D. Chem. – Eur. J. 2006, 12, 1736–1746. doi:10.1002/chem.200500993 |
| 20. | Osei Akoto, C.; Rainier, J. D. Angew. Chem., Int. Ed. 2008, 47, 8055–8058. doi:10.1002/anie.200803791 |
| 21. | Clark, J. S.; Romiti, F.; Sieng, B.; Paterson, L. C.; Stewart, A.; Chaudhury, S.; Thomas, L. H. Org. Lett. 2015, 17, 4694–4697. doi:10.1021/acs.orglett.5b02093 |
| 24. | Espino, C. G.; Du Bois, J. Angew. Chem., Int. Ed. 2001, 40, 598–600. doi:10.1002/1521-3773(20010202)40:3<598::aid-anie598>3.0.co;2-9 |
| 25. | Du Bois, J. Org. Process Res. Dev. 2011, 15, 758–762. doi:10.1021/op200046v |
| 11. | Sturino, C. F.; Wong, J. C. Y. Tetrahedron Lett. 1998, 39, 9623–9626. doi:10.1016/s0040-4039(98)02205-9 |
| 12. | Gurjar, M. K.; Krishna, L. M.; Reddy, B. S.; Chorghade, M. S. Synthesis 2000, 557–560. doi:10.1055/s-2000-6376 |
| 13. | Peczuh, M. W.; Snyder, N. L. Tetrahedron Lett. 2003, 44, 4057–4061. doi:10.1016/s0040-4039(03)00849-9 |
| 14. | Postema, M. H. D.; Piper, J. L.; Liu, L.; Shen, J.; Faust, M.; Andreana, P. J. Org. Chem. 2003, 68, 4748–4754. doi:10.1021/jo030039x |
| 15. | Adam, J.-M.; de Fays, L.; Laguerre, M.; Ghosez, L. Tetrahedron 2004, 60, 7325–7344. doi:10.1016/j.tet.2004.05.058 |
| 16. | Sharma, H.; Santra, S.; Debnath, J.; Antonio, T.; Reith, M.; Dutta, A. Bioorg. Med. Chem. 2014, 22, 311–324. doi:10.1016/j.bmc.2013.11.017 |
| 17. | Sutton, A. E.; Seigal, B. A.; Finnegan, D. F.; Snapper, M. L. J. Am. Chem. Soc. 2002, 124, 13390–13391. doi:10.1021/ja028044q |
| 18. | Schmidt, B.; Biernat, A. Chem. – Eur. J. 2008, 14, 6135–6141. doi:10.1002/chem.200800567 |
| 26. | Evans, D. A.; Vogel, E.; Nelson, J. V. J. Am. Chem. Soc. 1979, 101, 6120–6123. doi:10.1021/ja00514a045 |
| 27. | Evans, D. A.; Nelson, J. V.; Vogel, E.; Taber, T. R. J. Am. Chem. Soc. 1981, 103, 3099–3111. doi:10.1021/ja00401a031 |
| 28. | Mukaiyama, T.; Inoue, T. Chem. Lett. 1976, 559–562. doi:10.1246/cl.1976.559 |
| 8. | Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p |
| 9. | Parker, K. A.; Chang, W. Org. Lett. 2005, 7, 1785–1788. doi:10.1021/ol050356l |
| 7. | Cutchins, W. W.; McDonald, F. E. Org. Lett. 2002, 4, 749–752. doi:10.1021/ol017195f |
| 8. | Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p |
| 9. | Parker, K. A.; Chang, W. Org. Lett. 2005, 7, 1785–1788. doi:10.1021/ol050356l |
| 10. | Fei, Z.; McDonald, F. E. Org. Lett. 2007, 9, 3547–3550. doi:10.1021/ol7014219 |
| 22. | Mabit, T.; Siard, A.; Pantin, M.; Zon, D.; Foulgoc, L.; Sissouma, D.; Guingant, A.; Mathé-Allainmat, M.; Lebreton, J.; Carreaux, F.; Dujardin, G.; Collet, S. J. Org. Chem. 2017, 82, 5710–5719. doi:10.1021/acs.joc.7b00544 |
| 23. | Mabit, T.; Siard, A.; Legros, F.; Guillarme, S.; Martel, A.; Lebreton, J.; Carreaux, F.; Dujardin, G.; Collet, S. Chem. – Eur. J. 2018, 24, 14069–14074. doi:10.1002/chem.201803674 |
| 40. | Cornforth, J. W.; Cornforth, R. H.; Mathew, K. K. J. Chem. Soc. 1959, 112–127. doi:10.1039/jr9590000112 |
| 41. | Roush, W. R. In Houben-Weyl, Stereoselective Synthesis; Helmchen, G.; Hoffmann, R. W.; Mulzer, J.; Schaumann, E., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 1995; Vol. E21b, pp 1410–1486. |
| 42. | Cee, V. J.; Cramer, C. J.; Evans, D. A. J. Am. Chem. Soc. 2006, 128, 2920–2930. doi:10.1021/ja0555670 |
| 43. | Díaz-Oltra, S.; Carda, M.; Murga, J.; Falomir, E.; Marco, J. A. Chem. – Eur. J. 2008, 14, 9240–9254. doi:10.1002/chem.200800956 |
| 35. | Lachance, H.; Hall, D. G. Allylboration of carbonyl compounds. In Organic reactions; Denmark, S. E., Ed.; John Wiley & sons: Hoboken, New Jersey, 2008; Vol. 73. doi:10.1002/0471264180.or073.01 |
| 36. | Hoffmann, R. W.; Weidmann, U. Chem. Ber. 1985, 118, 3966–3979. doi:10.1002/cber.19851181010 |
| 37. | Brinkmann, H.; Hoffmann, R. W. Chem. Ber. 1990, 123, 2395–2401. doi:10.1002/cber.19901231223 |
| 38. | Wuts, P. G. M.; Bigelow, S. S. J. Org. Chem. 1988, 53, 5023–5034. doi:10.1021/jo00256a023 |
| 39. | Roush, W. R.; Adam, M. A.; Walts, A. E.; Harris, D. J. J. Am. Chem. Soc. 1986, 108, 3422–3434. doi:10.1021/ja00272a043 |
| 48. | A partial loss of product can occur during evaporation under reduced pressure due to its low boiling point. |
| 8. | Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p |
| 9. | Parker, K. A.; Chang, W. Org. Lett. 2005, 7, 1785–1788. doi:10.1021/ol050356l |
| 8. | Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p |
| 47. | A very clean carbamate product is required to obtain a good conversion into oxazolidinone. |
| 7. | Cutchins, W. W.; McDonald, F. E. Org. Lett. 2002, 4, 749–752. doi:10.1021/ol017195f |
| 8. | Parker, K. A.; Chang, W. Org. Lett. 2003, 5, 3891–3893. doi:10.1021/ol035479p |
| 24. | Espino, C. G.; Du Bois, J. Angew. Chem., Int. Ed. 2001, 40, 598–600. doi:10.1002/1521-3773(20010202)40:3<598::aid-anie598>3.0.co;2-9 |
| 13. | Peczuh, M. W.; Snyder, N. L. Tetrahedron Lett. 2003, 44, 4057–4061. doi:10.1016/s0040-4039(03)00849-9 |
| 44. | Weintraub, P. M.; King, C.-H. R. J. Org. Chem. 1997, 62, 1560–1562. doi:10.1021/jo961182b |
| 45. | Handerson, S.; Schlaf, M. Org. Lett. 2002, 4, 407–409. doi:10.1021/ol017104e |
| 46. | Dechert-Schmitt, A.-M.; Cabral, S.; Kung, D. W. Synlett 2016, 27, 2611–2615. doi:10.1055/s-0036-1588615 |
| 11. | Sturino, C. F.; Wong, J. C. Y. Tetrahedron Lett. 1998, 39, 9623–9626. doi:10.1016/s0040-4039(98)02205-9 |
© 2018 Nocquet et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)