Abstract
Our undergraduate research group has long focused on the preparation and investigation of electron-deficient analogs of the perimidinespirohexadienone (PSHD) family of photochromic molecular switches for potential application as "photochromic photooxidants" for gating sensitivity to photoinduced charge transfer. We previously reported the photochemistry of two closely related and more reducible quinazolinespirohexadienones (QSHDs), wherein the naphthalene of the PSHD is replaced with a quinoline. In the present work, we report our investigation of the electrochemistry of these asymmetric QSHDs. In addition to the short wavelength and photochromic long-wavelength isomers, we have found that a second, distinct long-wavelength isomer is produced electrochemically. This different long-wavelength isomer arises from a difference in the regiochemistry of spirocyclic ring-opening. The structures of both long-wavelength isomers were ascertained by cyclic voltammetry and 1H NMR analyses, in concert with computational modeling. These results are compared to those for the symmetric parent PSHD, which due to symmetry possesses only a single possible regioisomer upon either electrochemical or photochemical ring-opening. Density functional theory calculations of bond lengths, bond orders, and molecular orbitals allow the rationalization of this differential photochromic vs electrochromic behavior of the QSHDs.
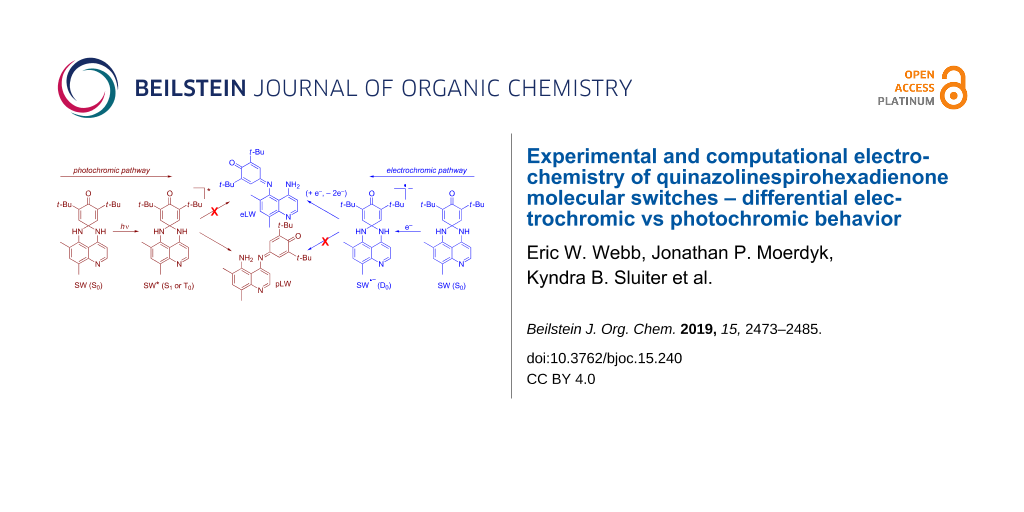
Graphical Abstract
Introduction
Photochromic molecular switches, in which light initiates reversible coloration of a short-wavelength isomer (SW) to a long-wavelength isomer (LW), which fades back to SW either thermally or photochemically, have become ubiquitous in a wide range of applications [1-5]. Typically, organic photochromism results from a spirocyclic ring-opening or other isomerization which results in increased conjugation. Electrochromism is also of increasing materials relevance, e.g., for self-dimming automotive mirror and aircraft window darkening applications [6-8]. In electrochromic applications, the color change is generally due to a change in the oxidation state of a conjugated system. This change in the oxidation state is most often concomitant with conformational and orbital occupancy changes, rather than any σ-bond-forming or breaking processes. The viologens are perhaps the most ubiquitous example of small molecule organic electrochromism [6,7,9].
One example that combines photochromic and electrochromic behavior (the latter of an unusual sort) is the class of perimidinespirohexadienones 1 (PSHDs) whose synthesis, electrochemistry and UV–vis spectroscopy were reported by Minkin and co-workers [10] for 1a (Scheme 1). Electrochemically, they report observing a single, two-electron reduction peak and two distinct one-electron oxidation peaks upon scanning using cyclic voltammetry. They therefore proposed [10] that the electrolysis of 1a proceeds by an “ECE” (electrochemical-chemical-electrochemical) mechanism (1a → 1a•− → 2a•− → 2a2− → 2a•− → 2a) in which the dienone portion of the molecule accepted the first electron, followed by a radical anion rearrangement to the long-wavelength isomer, whose radical anion is so much easier to reduce that it immediately accepts a second electron at this potential; on the oxidative return wave the subsequent oxidations of the LW dianion to its radical anion and then its neutral state are observed. Thus, in this unusual system, electrochromism proceeds by the same sort of spirocyclic ring-opening as the photochromic rearrangement but occurs from the radical anion rather than a photoexcited state. The reduction of the molecular switch necessary for electrochromism is in a sense catalytic: the rearranged product is reoxidized to a neutral LW isomer, which reverts thermally to SW upon standing, just as it does when the LW is generated photochemically.
The PSHD system was of interest to us as a potential photochromic photooxidant that would add an additional level of gating to photoinduced charge transfer (PICT) initiated processes (Figure 1). PSHDs were promising for this, as their photochromic reversion of LW back to SW proceeds purely thermally, leaving the long-wavelength absorption available for bimolecular PICT. Moreover, the LW is sufficiently more reducible that, even accounting for its lower excitation energy, the LW excited state, LW*, is more reducible than SW*, making LW the more potent photooxidant of the two. However, for practical use as photooxidants, the difference in the reduction potential between LW and SW would need to be increased further, and LW would need to be more reducible to be of use in photooxidation of relevant substrates (e.g., Dewar benzenes, quadricyclanes, or bishomocubanes as quantum amplified isomerization substrates [11-15], or vinylcarbazole or alkoxystyrene derivatives for radical cation cylcloaddition and polymerization reactions [16-20]). We thus proposed the replacement of the naphthalene in 1a with a more electron-deficient quinoline ring. Due to the saturated spirocyclic carbon insulating the dienone electrophore from the quinoline moiety in the SW form, we expected minimal change in the SW reduction potential relative to the PSHDs, but a significant difference for the completely conjugated LW isomer(s). Previously we reported the detailed synthesis of two novel quinazolinespirohexadienone (QSHD) photochromes 3a,b (Scheme 2) and their photochemical properties as well as a proof of structure for the photochemically generated long-wavelength isomer (pLW) 4a,b (not 5a,b) [21]. Herein, we report the electrochemistry of these QSHDs.
![[1860-5397-15-240-1]](/bjoc/content/figures/1860-5397-15-240-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Proposed gating of sensitivity to photoinduced charge transfer by a photochromic photooxidant in which only LW is a competent photooxidant of the donor of interest.
Figure 1: Proposed gating of sensitivity to photoinduced charge transfer by a photochromic photooxidant in wh...
Results and Discussion
Electrochemical analysis
When we replicated cyclic voltammetry (CV) experiments on PSHDs, we observed similar voltammograms for both 1a and 1b (Figure 2), consistent with the two-electron reduction and two one-electron oxidation processes reported by Minkin for 1a [10]. As expected, a growth of two reversible one-electron reduction–oxidation peaks was observed upon multiple scans, representing the reduction and oxidation of the electrogenerated long-wavelength form 1b generated in situ. As expected, either photolysis or multiple CV scans led to the same LW reduction and oxidation peaks (within the error bars indicated in Table 1). We also report an identical Eored of 2a as Minkin [10], though a 140 mV difference was found in the Eored of 1a, which can be attributed at least in part to our use of half-peak potentials for all irreversible peaks, while Minkin reported peak potentials (our peak potentials were within 60 mV of Minkin’s value). We found the parent compound 1b, whose electrochemistry was not previously reported by Minkin, to be 50 mV less reducible than 1a (−1.68 V vs −1.63 V for 1a), in qualitative agreement with our computational predictions [22]. Similarly, 2b differed from 2a by only 20 mV (Table 1) where computations predicted a minimal difference. The electrogeneration of 2b through repeated potential step bulk electrolysis of 1b in acetonitrile-d3 yielded a sufficient quantity of LW to obtain a 1H NMR spectrum, which revealed identical chemical shifts as those for the photogenerated 2b. First and second reduction potentials were also the same, within error limits, for photogenerated and electrogenerated 2b, as would be expected. This is consistent with the excellent overlap of all four voltammograms in Figure 2.
Figure 2: Cyclic voltammograms of a) 1b before irradiation or electrolysis (solid blue), b) 1b/2b after 25 scans (dotted red), c) 1b/2b upon photolysis of 1b (dashed green) scanned over a narrower potential window so as not to reduce 1b, and d) 1b/2b scanning the full potential window after photolysis of 1b (dashed orange).
Figure 2: Cyclic voltammograms of a) 1b before irradiation or electrolysis (solid blue), b) 1b/2b after 25 sc...
Table 1: Experimental and computational Eored of 1a,b and 3a,b and their LW isomers, reported in V vs SCE.
| Electrochemical | Photochemical | |||||
| Compd. | Type | Eored (SW)a | Eored (eLW·−) | Eored (eLW) | Eored (pLW·−) | Eored (pLW) |
| 1a | literatureb | −1.77 | −1.325 | −0.85 | ||
| exptl.c | −1.628 ± 0.031 | −1.220 ± 0.049 | −0.857 ± 0.010 | |||
| predictedd | −1.56 | −0.89 | −0.89 | |||
| 1b | exptlc | −1.681 ± 0.013 | −1.202 ± 0.032 | −0.871 ± 0.013 | −1.170 ± 0.060 | −0.865 ± 0.005 |
| predictedd | −1.70 | −0.90 | −0.90 | |||
| 3a | exptlc | −1.632 ± 0.030 | −1.26 ± 0.11 | −0.866 ± 0.009 | −1.029 ± 0.035 | −0.730 ± 0.005 |
| predictedd | −1.61 | −0.88 | −0.72 | |||
| 3b | exptlc | −1.631 ± 0.017 | −1.069ad ± 0.029 | −0.843 ± 0.012 | −1.008 ± 0.024 | −0.729 ± 0.004 |
| predictedd | −1.63 | −0.86 | −0.72 | |||
aIrreversible peak. Eored is reported as the half-peak potential Ep/2red (except in literature value for 1a). bReference [10]. Irreversible SW peak reported as peak potential (Epred), not half-peak potential (Ep/2red). cExperimental values in CH3CN containing 0.1 M Bu4NPF6, standardized vs ferrocene/ferrocenium and corrected to vs SCE as in reference [23]; error bars = one standard deviation from the mean of at least 7 replicates. dComputational Eored predicted using correlation 6 in reference [22] based on the energies of the corresponding ground-state and one-electron-reduced species computed using B3LYP/6-31G(d) with implicit acetonitrile solvent using the CPCM solvent model with UAKS radii, on geometries optimized in the gas-phase; predictions of second reduction potentials are not possible by this method.
The ECE mechanism reported by Minkin for the electrochromism was further supported by bulk electrochemical experiments. With repetitive conditioning and scanning under argon-deaerated conditions, the initially yellow 1b solution turned to an orange-red color, which we hypothesized to be the LW dianion, 2b2−. Upon exposure to air this solution immediately turned green, the known color of the LW isomer 2b. The addition of benzoquinone (a chemical oxidant) to the electrochemically reduced solution under argon gave similar results, consistent with our hypothesis. When the yellow solution remained open to the atmosphere during electrolysis, the initially yellow solution turned green at first (presumably while air oxidation of 2b2− to 2b could keep up with electrochemical reduction). With further electrolysis even these solutions turned to the orange-red color observed for the deaerated solutions. The solutions did become green upon prolonged exposure to air. This behavior seems indicative of insufficient transport of air into the cell through the small vents in the cell cap to replace the oxygen being consumed during repeated electrolytic scans. Similar results were observed for solutions of 3b suggesting a similar ECE mechanism to that of 1a is likely also occurring for 3b.
Cyclic voltammetric analysis of quinazolinespirohexadienone (QSHD) 3b (Figure 3) was qualitatively similar to the parent PSHDs 1a,b as expected based on structural similarity and computationally calculated molecular orbital diagrams (Figure 6 and Supporting Information File 1). Surprisingly, the Eored of the electrochemically generated LW form of 3a was more negative by 10 mV than that of 2a, even with the more electron-deficient quinoline ring. Presumably this is because the 5-position on the benzene ring of the quinoline, is the least withdrawing point of attachment, and the inductive withdrawing properties of the quinoline nitrogen are far enough removed from the electrophore to not cause any appreciable change in reduction potential. The other qualitative difference for 3b was the presence of two LW reduction peaks (LW → LW•− → LW2−) on the first scan (Figure 3a, dotted red). However, the observation of the two LW reduction peaks was consistent with UV–vis spectroscopy that indicated a significant amount of a LW isomer upon solvation, and repetitive electrochemical scans exhibited the anticipated growth of the LW reduction–oxidation peaks as more of the LW isomer was generated electrochemically (Figure 3b, solid blue).
Figure 3: Cyclic voltammograms of a) 3b (with trace 5b) before irradiation or electrolysis (solid blue), b) 3b + 5b after 25 scans (dotted red) showing the growth of the two 5b reduction peaks, c) 3b (with trace 5b) after photolyzing but without reducing 3b in the electrolysis (dashed green) which shows the two initial eLW 5b and two new pLW 4b reversible redox waves observed, and d) 3b + 4b + 5b observed by scanning the full potential window after the photolysis of 3b (dashed orange).
Figure 3: Cyclic voltammograms of a) 3b (with trace 5b) before irradiation or electrolysis (solid blue), b) 3b...
Most surprisingly, when 3b was photolyzed to form 4b in solution under similar conditions to our previous report [21] and then analyzed electrochemically (Figure 3c and d, dashed green and orange), four reduction (and oxidation) peaks in the region of the LW isomer were observed. Two reduction–oxidation couples matched the potential of the electrogenerated LW form observed earlier while the other two peaks were shifted more positive by 60–110 mV indicating the presence of a third electroactive species in addition to 3b and photogenerated 4b. Given the asymmetric nature of 3b, two distinct options for spirocyclic ring-opening exist, leading to 4b or 5b. Thus, we postulated that the electrogenerated LW form was in fact 5b.
Having found two distinct LW forms depending on generation by photolysis or electrolysis of SW 3b, we turned our attention to whether a similar phenomenon was observed for 3a. Indeed, different redox peaks were observed in the same general LW region for the CV of photolyzed versus electrogenerated LW forms of 3a, consistent with the electrogenerated formation of 5a compared against the known formation of 4a via photolysis. The voltammograms of 3a (Figure 4) did however differ from both 3b and 1b. A (presumably two-electron) reduction peak was observed for 3a but only one return oxidation wave was observed. Upon repeated scanning the two one-electron reductions of 5a → 5a•− → 5a2− were observed, but still only a single (likely two-electron) return oxidation peak was observed, possibly indicating a large overpotential for the oxidation of 5a2−. It is possible that 3a undergoes only a one-electron reduction and rearrangement of 3a → 3a•−→ 5a•− (without further reduction to 5a2−) and subsequently only one oxidation to 5a. But this would make the second one-electron reduction (thought to be 5a•− → 5a2−) observed on repeated cycling unexplainable. A more likely explanation is that the two-electron ECE reduction of 3a still occurs to give 5a2− but that the slight electron-donating nature of the additional methyl group destabilizes 5a•− enough to require a substantial overpotential for reoxidation of 5a2− to 5a•−, such that it occurs at the same potential as oxidation of 5a•− back to neutral 5a. This could occur either sequentially at the same potential or in a single two-electron process. The latter explanation seems qualitatively in better agreement with the observed voltammogram in Figure 4.
Figure 4: Cyclic voltammograms of a) 3a before irradiation or electrolysis (solid blue), b) 3a + 5a after 25 scans (dotted red) showing the growth of the two eLW 5a reduction peaks, c) 3a after photolyzing but without reducing 3a in the electrolysis (dashed green) which shows the two different reversible redox waves observed for pLW 4a, and d) 3a + 4a + 5a scanning the full potential window after the photolysis (dashed orange).
Figure 4: Cyclic voltammograms of a) 3a before irradiation or electrolysis (solid blue), b) 3a + 5a after 25 ...
In terms of achieving more potent photooxidants through the exchange of the naphthalene ring of the PSHDs for the more electron-deficient quinoline in the QSHDs, we had expected the LW isomers to become more easily reducible, with minimal change in reduction potential for the SW isomers. Indeed Eored of the SW isomers 1b, 3a, and 3b were the same within the error, and only 50 mV more reducible than 1a. Comparing the Eored of pLW isomers (4a,b relative to 2a,b), a roughly 140 mV difference is observed, with 4 being more reducible than 2 as expected. However, the difference in reduction potential between PSHD and QSHD for eLW was much less. For 5b Eored was 28 mV more positive than 2b indicating that 5b is a slightly better oxidant than the parent PSHD. But Eored of eLW 5a was surprisingly shifted 10 mV more negative than PSHD LW 2a, meaning it was actually harder to oxidize. Ultimately Eored of 2a, 2b, 5a, and 5b are essentially the same within error limits. Apparently, the quinoline is not nearly as electron withdrawing when linked through the benzo ring as when it is linked through the heteroaromatic ring.
Spectroscopic analysis
The potential for two distinct products from electrolysis or photolysis as indicated through electrochemical analysis was further supported through NMR. Previous work [21] had conclusively shown through 1H NOE NMR spectroscopy that the LW isomers resulting from photolysis of 3a and 3b were 4a and 4b, which open toward the more electron-deficient heteroaromatic ring of the quinoline and away from the R group. Unfortunately, efforts to obtain a sufficient quantity of the electrogenerated LW form of 3a (i.e., 4a or 5a) for definitive 1H NMR spectra or NOE experiments were not successful However, both UV–vis and electrochemical measurements indicated small amounts of a long wavelength form present in the dark immediately upon solvation (i.e., a thermal or solvatochromic LW form), particularly in 3a. The presence of a LW isomer prior to irradiation or electrolysis was also consistent with the 1H NMR spectrum of a sample of 3a which, while known to be pure in the solid state [21]), in solution showed the expected 3a chemical shifts but also smaller peaks (ca. 20% relative to 3a) with similar splitting and chemical shifts as those for photogenerated 4a (to which we initially erroneously attributed them [21]). However, the frequencies for this solvatochromic LW were slightly but distinctly different from either 3a or photogenerated 4a (Figure 5a, e.g., consider protons n, g, and v). Signals for 4a began to grow in with even very brief photolysis (Figure 5b), intentionally taken to low conversion (ca. 8% relative to 3a) to enable comparison to the solvatachromic LW (ca. 20% relative to 3a). Moreover, as the oxidation and reduction peaks associated with eLW 5a (Figure 3b, dotted red) grow with increasing numbers of CV scans and match those present initially in solution in small amounts thermally (Figure 3a, solid blue), we conclude the electrogenerated and thermal LW forms are the same isomer 5a, while it is the pLW isomer 4a that grows in upon photolysis (Figure 3c, dashed green; Figure 5b). Furthermore, modest NOE enhancements can be observed even on the small amounts of 5a present in these solutions. These NOEs, while weak, did aid in assigning the peaks as labelled in Figure 5b and demonstrate that the blue-labelled eLW peaks in Figure 5 are indeed on the same molecule, and that this is distinct from pLW 4a as studied previously by NOE [21]. Thus, NMR, while not conclusive on its own, was able to provide additional support for our structural assignment.
![[1860-5397-15-240-5]](/bjoc/content/figures/1860-5397-15-240-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: 1H NMR distinction between SW 3a, thermal/eLW 5a, and pLW 4a, in acetone-d6, as observed a) before and b) after photolysis.
Figure 5: 1H NMR distinction between SW 3a, thermal/eLW 5a, and pLW 4a, in acetone-d6, as observed a) before ...
UV–vis spectra taken of eLW solutions generated by bulk electrolysis in acetonitrile and acetonitrile-d3 were compared against photoirradiated (pLW) solutions. No difference was observed in the UV–vis spectrum of the LW isomer prepared from either photolysis or electrolysis of solutions of 1b (as expected due to symmetry). The λmax for the 2b photogenerated solution was at 574 nm in acetonitrile and acetonitrile-d3. The electrogenerated 2b λmax at 573 nm in both solvents differed by only 1 nm, within error limits of our instrumentation. For 4a/5a the λmax in acetonitrile-d3 was 539 nm for the photolyzed solution (4a) versus 529 nm for the electrolyzed (5a). Similarly, for 4b and 5b, the photolyzed λmax in acetonitrile was 558 nm versus 549 nm for the electrogenerated and was 564 nm versus the electrogenerated 550 nm in acetonitrile-d3. The differences in the UV–vis spectrum of about 10 nm between eLW and pLW isomers of QSHDs 3 indicated a similar length for the conjugated system but a significant enough difference to indicate different species. This is consistent with the formation of two constitutional LW isomers that are structurally similar yet distinct, as the difference in absorbance would be expected to be noticeable but not large. Observation of no difference in the UV–vis spectrum of the LW isomer prepared from either photolysis or electrolysis of solutions of 1b (able to only form one LW isomer, 2b) supports the formation of two different LW isomers for 3a,b rather than attributing the small spectral changes to the presence of electrolyte in the electrolyzed solutions, to an interaction with air, or to a side reaction in solution. The shorter wavelength for electrogenerated 5a,b may result from slightly less planarity and decreased conjugation versus the photogenerated 4a,b. The additional ortho-methyl group in 5b may exacerbate this sterically, and/or could contribute an inductively donating effect.
Finally, the thermal reversion of eLW 5a,b was visually and spectroscopically obvious to have begun within a few minutes, and to be complete within 12–18 hours. This is similar to what we previously reported for pLW 4a,b [21]. This comparatively slow and purely thermal reversion is consistent with the need for a thermodynamically unfavorable intramolecular (or solvent or adventitious acid/base-mediated) proton transfer to begin the reversion mechanism, as Minkin has described [10]. Interestingly, reversion of either LW species to SW is observed immediately upon removal of solvent, which played a role in complicating our analysis, as the LW species cannot be isolated as solids.
Computational analysis
As shown above, computationally predicted reduction potentials [7] were in very good agreement with those determined experimentally (Table 1). The mean absolute deviation between computational prediction and experimental measurement of Eored for all SW and LW structures in Table 1 is just 21 mV (27 mV root mean squared deviation), not far from the 14 mV mean standard deviation in the experimental measurements. This demonstrates that the computational correlation employed is useful in structural assignment and accurate within a standard of error of the experimental reduction potentials of these spirohexadienones’ different constitutional SW and LW isomers.
Density functional theory (DFT) calculated molecular orbitals (MOs, e.g., Figure 6 and Supporting Information File 1) indicate that the LUMO (MO 106 for 3a) lies exclusively on the dienone moiety both in 1b and in the quinazolinespirohexadienone photochromes 3a,b. Thus, the dienone moiety is likely the electrophore in the SW in all PSHD and QSHD switches, just as Minkin [10] specifically asserted for 1a. These diagrams also suggested to us that QSHDs 3 might also undergo an ECE mechanism similar to PSHDs 1, as we have now confirmed experimentally.
![[1860-5397-15-240-6]](/bjoc/content/figures/1860-5397-15-240-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: HOMO (MO 105, red and blue) and LUMO (MO 106, green and yellow) computed for 3a in its ground (S0) state. Similar results were observed for the frontier orbitals of 1b and 3b (see Supporting Information File 1).
Figure 6: HOMO (MO 105, red and blue) and LUMO (MO 106, green and yellow) computed for 3a in its ground (S0) ...
Having conclusively demonstrated the differential regiospecificity of photochromic vs electrochromic ring-opening of the QSHDs (Scheme 3) by experiment, we next sought to understand why SW would open differently upon excitation to SW* vs reduction to SW•−. We turned to computation (of 3a, 4a, and 5a) for an explanation. Due to large differences in the methods for calculating excited and ground-states, the triplet T0 state was used instead of the singlet S1 state as the photochemical intermediate (SW*) for computational purposes. Since T0 is the lowest energy triplet state, it is amenable to a ground-state computation. While the real SW* photochemical intermediate may likely be the first singlet excited state (S1), T0 and S1 possess equivalent orbital occupancy and differ only in their spin state. Our rationale for using T0 rather than S1 is that ignoring electron-exchange interaction introduces substantially less error than using different computational methods when comparing orbitals of SW* to those of SW and SW•−. Using S1 for SW* would require unbalanced ground and excited-state calculations, e.g., time-independent and time-dependent density functional theories, or single-reference and multi-reference methods. However, using T0 for SW* is a straightforward ground-state calculation, as are the calculations of D0 (the radical anion) and S0.
Scheme 3: Proposed mechanism for differential formation of pLW (4) and eLW (5) from SW (3).
Scheme 3: Proposed mechanism for differential formation of pLW (4) and eLW (5) from SW (3).
Clearly, as shown in Figure 7, the difference in reduction vs excitation is the occupancy in what was formerly the HOMO of SW. Because S0 is the common precursor to both T0 and D0, analysis of the S0 MOs was used to explain differences in the electron distributions that lead to two different products upon either excitation to form SW* (yielding pLW 4) or reduction to form SW•− (yielding eLW 5). This approach of using the S0 MOs was validated computationally, as we found there is little change in the character and relative energies of the MOs among S0, T0, and D0. The calculated isosurface generated for the S0 highest occupied molecular orbital (HOMO), MO 105, and lowest unoccupied molecular orbital (LUMO), MO 106, of 3a are displayed in Figure 6. MO 105 allows for a direct comparison of the T0 and D0 electron distributions, as these two states differ only in the population of this orbital. There is more contribution from this MO to the N–C bond (hereafter designated the benzoN–C bond, cf. Scheme 3) of the nitrogen on the benzo ring of the quinoline moiety than the N–C bond of the nitrogen off the heterocyclic ring of the quinoline moiety (hereafter referred to as the heteroN–C bond, cf. Scheme 3). This asymmetrical MO contribution is consistent with experimental observations. The absence of an electron from this orbital 105 in the case of T0 results in the preferential weakening of the benzoN–C bond over the heteroN–C bond. The formation of pLW from SW (T0) results from the breaking of this weaker bond. More electron density in the benzoN–C bond in orbital 105 might possibly suggest a stronger bond when the orbital is fully filled (as in D0), and could suggest that the heteroN–C bond might be the more likely to rupture upon reduction to SW•−.
Figure 7: Frontier orbital occupancies of relevant electronic states of 3a. Note: the photochemical excited state may be T0 (as pictured) or perhaps more likely S1 (inverted spin in molecular orbital 106 relative to T0), but T0 is more computationally tractable and is equivalent to S1 for orbital occupancy arguments.
Figure 7: Frontier orbital occupancies of relevant electronic states of 3a. Note: the photochemical excited s...
It is common to think of thermal ring-opening as looking more like that of the biradical (T0 or S1) than of the radical anion (D0), so we might have presumed the structure of any thermal or solvatochromic LW isomer that occurred to have been that of pLW (4). However, the same rationalization that more electron density in the benzoN–C bond in orbital 105 might possibly suggest a stronger bond relative to the heteroN–C bond when the orbital is fully filled in S0 is how we rationalize our observation that it is eLW (5b) that occurs in the only case where we see a purely thermal or solvatochromic cleavage. In turn we can then rationalize on the basis of sterics (R = Me vs H) that it is logical that this thermal or solvatochromic rupture might only be observable in 3b (R = H) but not 3a (R = Me). This is in good agreement with our experimental observations.
Our rationalization based on molecular orbitals was confirmed by looking at both bond lengths and bond orders of the two relevant N–C bonds in SW•− and SW*. Bond order calculations provide a more quantitative explanation for the experimental observation. Because bond stability correlates to bond order, it is expected that the N–C bond which breaks upon forming the LW product would possess a smaller bond order. The BO calculations for intermediate states T0 and D0 indeed support this prediction. Table 2 summarizes the results of BO calculations for both bonds in these states. Since geometry plays a critical role in the stability of bonds, only BO calculations done from optimized geometries for each state are displayed. As shown in Table 2, heteroN–C has a greater bond order than benzoN–C in T0, while the relative magnitude of these bond orders is reversed in D0. This is consistent with the formation of eLW 5a from 3a•− D0 (breaking the heteroN–C bond) and pLW 4a from SW* 3a*, modelled as T0 (breaking the benzoN–C bond) as shown in the proposed mechanism in Scheme 3. Finally, bond lengths were derived from geometry optimizations for D0 and T0 with and without solvent and are also included in Table 2. There is an obvious inverse relationship between calculated bond orders and bond lengths. For D0, a longer bond length is observed for heteroN–C while a longer bond length is observed for benzoN–C in T0. These results, regardless of solvent or gas-phase considerations, are consistent with the discussion above. In the cases of both D0 and T0, the longer bond is the one broken in the isomerization of SW 3a to eLW 5a and pLW 4a, respectively. Similar results are also observed for S0. Though the differences are much more modest in S0, the heteroN–C bond is computed to be both longer and weaker, as in D0, and thus it makes sense that any thermal or solvatochromic LW is the eLW isomer.
Table 2: Computed bond lengths and bond orders of the relevant N–C bonds for photochromic (T0) and electrochromic (D0) ring-opening of 3a. Bolded (longer and weaker) bond is the one that is cleaved in each case.
| State | Bond | Bond order | Bond length (Å) | Consider for |
| S0 | benzoN–C | 0.9425 | 1.478 | thermal LW/ |
| S0 | heteroN–C | 0.9346 | 1.478 | solvatochromism |
| D0 | benzoN–C | 0.8879 | 1.508 | electrochromism |
| D0 | heteroN–C | 0.8738 | 1.513 | |
| T0 | benzoN–C | 0.8143 | 1.546 | photochromism |
| T0 | heteroN–C | 0.8834 | 1.503 | |
Conclusion
While moving from PSHD (7) to QSHD (3) increased ΔEored between SW and pLW isomers, and therefore capacity to gate photoinduced charge transfer, by about 130 mV, the excited state reduction potential E*red of pLW 4 remains < +1.0 V vs SCE, insufficient to oxidize most donor molecules of relevance to materials applications. If our photochromic photooxidants are to be effective in real systems, the pLW will need to be made more electron deficient. Our computational predictions of the reduction potential [22], unfortunately not completed until after preparing the present system, were our first insight into the fact that electrochemical ring-opening was not yielding the structure we had previously proven for the photochromic pLW. We have thus demonstrated these computation’s practical utility in a real-world experimental situation, as well as their suitably high degree of accuracy. We are therefore hopeful they can help guide our search for practical photochromic photooxidants, with a more reducible pLW*.
For now, our team of undergraduate researchers has conclusively demonstrated differential regiospecificity in the photochromic vs electrochromic spirocyclic ring-opening of these QSHD molecular switches to two different LW isomers. This may provide an interesting structural framework for molecular logic or other applications that complements the recently reviewed photoelectrochromic properties observed in a range of photochromic families upon the electrochemical oxidation of either their SW or pLW isomers [24]. We have also demonstrated that any modest amount of thermal (or solvatochromic) coloration of the QSHDs is due to small amounts of the electrochromic eLW isomer 5, rather than the photochromic pLW (4) as we had previously surmised [21]. Finally, we have been able to rationalize these results computationally on the basis of bond lengths, bond orders, and molecular orbital occupancy.
Experimental
Materials
Acetonitrile was of the highest HPLC grade (used as received or dispensed through a nitrogen-purged MBraun MBS-SPS 07-299 solvent purification system) or highest anhydrous grade (used as received). Acetonitrile-d3 and acetone-d6 were used as received in 1 g ampules (Cambridge Isotope Labs). Ferrocene, tetrabutylammonioum hexafluorophosphate (TBAH), and silver nitrate were of electrochemical grade and used as received. Photochromes 1a,b were prepared according to the literature [10]. Photochromes 3a,b were prepared as we previously reported [21].
Instrumentation
Photochemical irradiations and UV–vis spectroscopy were performed as previously described [21] on 3 mL or 4 mL argon-purged acetonitrile solutions of 0.1 M TBAH supporting electrolyte and 1–5 mM analyte, sufficient to attain signals much greater than background over the full potential window considered.
Cyclic voltammetry was performed on a CHI Model 604a electrochemical analyzer or a BAS epsilon e2 electrochemical analyzer. (The equivalent BAS or CHI cells and electrodes could be used on either potentiostat interchangeably, with or without the BAS C3 cell stand.) Solutions were placed in a glass cell and bubbled with argon to deaerate for 3–5 minutes. Scans were taken under an argon blanket using a glassy carbon working electrode, a platinum wire counter electrode, and a separately sparged Ag/AgNO3 (10 mM) nonaqueous reference electrode isolated from the working compartment with a Vycor frit. In some cases, a bare Ag wire was used as a pseudo-reference instead. The working, reference, and counter-electrodes were arranged in a triangle through a Teflon cell cap. Rather than relying on either the Ag/AgNO3 reference or Ag wire pseudo-reference, the voltammetry was corrected to a ferrocene external reference. Ferrocene solutions were cyclically scanned before and after each analyte experiment, beginning in the positive (oxidative) direction over an 800 mV window roughly centered on the reduction of Fc/Fc+ redox couple at a scan rate of 0.1–0.5 V/s, 10−4 A sensitivity, and 1 mV sample interval. Internal resistance (iR) compensation was manually set to 95–99.5% of the measured resistance so that the peak separation for the reversible oxidation–reduction of ferrocene had a peak separation of ca. 60 mV and good peak shape without entering oscillation. Typical iR compensation in acetonitrile ranged from 150–230 Ω. Photochromic solutions were cyclically scanned beginning in the negative (reductive) direction at 0.1–0.5 V/s with 1 mV sample interval over an appropriate range of potentials and current sensitivity to observe the redox couples of either SW and LW or just LW isomers as desired, without reaching the solvent breakdown limit. Reduction potentials were taken as the half-peak potential of irreversible peaks or the midpoint of reversible peaks, standardized versus ferrocene (measured before and after each analyte sample), and corrected to versus SCE by adding 0.38 V [23]. At least seven replicates of each data point were obtained, with the mean value reported with error bars indicating the standard deviation from the mean among all replicates.
Potential step bulk electrolyses were performed on 3 mL argon-purged acetonitrile or acetonitrile-d3 solutions of 2 mM analyte and 0.1 M TBAH. The solution was placed in a glass cell prepared by cutting the top off a 7 mL scintillation vial and fireglazing the edges. The outer rubber of a 19/22 septum was removed with a razor blade and used to cap the vial. The rubber had holes drilled for a reference and auxiliary electrode and a slit cut for a 7 × 70 mm platinum mesh working electrode so that the surface of the mesh faced the working and auxiliary electrodes. Teflon tubing was inserted through a pinhole in the septum for an argon purge and vent. The setup used a CHI112 nonaqueous Ag/AgNO3 reference electrode. The auxiliary electrode was formed by removing the silver wire from a CHI112 nonaqueous reference electrode and replacing it with a rolled 7 × 70 mm piece of platinum mesh. The tube was filled with supporting electrolyte. Electrolyses were run on 2 to 3 mL samples, placing the platinum mesh electrode ca. 20 mm into the solution. To avoid the buildup of charge while maximizing current and efficiency, solutions were set to precondition before scanning the potential window with multiple repetitions run. Solutions of 1b were preconditioned at −2.5 V for 5 seconds and 0 V for 6 seconds and 16–20 repetitions run. Solutions of 3a,b were preconditioned at −2.2 V for 5 seconds and 0 V for 6 seconds with 16 repetitions. The electrolyzed solutions were opened to air or had a chemical oxidant (benzoquinone) added to complete the reduction of the dianion to the LW isomer.
NMR spectroscopy was performed on samples 5 mm NMR tubes (Wilmad) made of clear quartz (photolyzed samples) or amber pyrex (dark samples) on a Varian Mercury or Bruker AvanceIII 400 MHz NMR. 1H NMR experiments on 5a were performed on an argon-purged, ca. 24 mM solution in acetone-d6. 1D NOE spectra were collected using 400 manually interleaved scans with a 4 s relaxation delay, targeting a single peak per experiment, in a manner similar to that previously reported [21] for photogenerated 4a,b.
Computational modeling
Predicted ground-state reduction potentials were computed based on the energy difference of the ground-state molecule (S0) and its one electron-reduced (D0) radical anion and our published correlation of this energy difference with experimental reduction potentials (vs SCE in acetonitrile) [22]. Gas-phase geometries were optimized at the B3LYP/6-31G(d) level of theory. Single point energies were computed at the same level of theory with implicit acetonitrile solvent implemented using the Conductor-like Polarizable Continuum Model with UAKS radii. These computations were performed using the Gaussian 03 software package [25], implemented through the WebMO [26] graphical user interface on the Curie cluster [27] in the Hope College Computational Science & Modeling Laboratory on a single node (a single 2.60 GHz AMD Opteron-252 processor with 8 GB RAM and 250 GB HD).
Calculations of bond length, bond order, and molecular orbitals to rationalize the observed differential photochromic and electrochromic ring-opening of 1 to 2 and 3, respectively, were performed on the Midwest Undergraduate Computation Chemistry Consortium (MU3C) cluster [28,29]. Computations were performed on a single node (dual Intel X5650 CPU, with 6 cores running at 2.66 GHz) using the Gaussian 09 [30] software package implemented through the WebMO [26] graphical user interface. Restricted open-shell Hartree-Fock (ROHF) theory [31] with the Becke 3, Lee, Yang, and Parr (B3LYP) hybrid functional [32-34] was used for geometry optimizations, molecular orbitals, and bond orders calculations with the 6-31G(d) basis set [35] for open-shell species (T0 and D0), while conventional B3LYP (with standard Hartree-Fock theory) was used for closed-shell S0 calculations. The concerted use of ROHF and B3LYP provides a restricted open-shell B3LYP (DFT) method, which was particularly important to obtaining good bond orders for open-shell (T0 and D0) species. Bond orders were calculated using the Natural Bond Order (NBO) 3.1 package [36] contained within Gaussian 09. The geometry was first optimized for the S0 state in the gas phase. Based on this starting point, geometries for S0, T0, and the one-electron reduced D0 states were then (re)optimized with an implemented acetonitrile conductor-like polarizable continuum model (CPCM) as the self-consistent reaction field (SCRF) [37]. It is from these implicit solvent-optimized geometries that reported bond lengths were derived. Molecular orbital and bond order calculations were subsequently performed on these geometries.
Acknowledgements
This work was funded by a Camille & Henry Dreyfus Foundation Start-up Award (SU-04-040), a Cottrell College Science Award from Research Corporation (CC6653), and a Faculty Early Career Development Grant (CHE-0952768) from the US National Science Foundation (NSF). This work was also funded in part by a grant to Hope College from the Howard Hughes Medical Institute through the Undergraduate Science Education Program, which provided support for the Curie cluster [27] in the Hope College Computational Science & Modeling (CSM) Laboratory and to ALS (Computational Science & Modeling Scholars Program) and JPM (HHMI Research Scholars Program). Additional computations were conducted on the MU3C [28,29] cluster, supported by Major Research Instrumentation grants (CHE-0520704 and CHE-1039925) from the NSF, also housed in the Hope College CSM Laboratory. Support from CSM Laboratory director Prof. Brent P. Krueger and former staff member Mr. Paul Van Allsburg is also gratefully acknowledged. ALS acknowledges additional support from a Jean Dreyfus Boissevain Scholarship awarded to the Hope College Chemistry Department by the Camille & Henry Dreyfus Foundation. EJL (home institution College of the Canyons, Santa Clarita, CA) acknowledges support by a Research Experiences for Undergraduates Site award (CHE-0851194) to the Hope College Chemistry Department from the NSF.
References
-
Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermochromic Compounds; Plenum Press: New York, NY, USA, 1999; Vol. 1. doi:10.1007/b114211
Return to citation in text: [1] -
Duerr, H.; Bouas-Laurent, H., Eds. Photochromism: Molecules and Systems, revised ed.; Elsevier: Amsterdam, 2003.
Return to citation in text: [1] -
Desvergne, J.-P.; Pozzo, J.-L., Eds. Proceedings of the fourth International Symposium on Photochromism Photoswitchable Molecular Systems and Devices. Mol. Cryst. Liq. Cryst. 2005, 430–431, 1–586. doi:10.1080/15421400590946073
Return to citation in text: [1] -
Branda, N.; Tian, H., Eds. Organic Photoswitchable Multifunctional Molecules and Materials. Dyes Pigm. 2011, 89, 193–336. doi:10.1016/j.dyepig.2010.12.002
Return to citation in text: [1] -
Favaro, G.; Irie, M., Eds. Special issue on Photochromism 1 and 2. J. Photochem. Photobiol., C 2011, 12, 71–236. doi:10.1016/j.jphotochemrev.2011.08.004
Return to citation in text: [1] -
Monk, P. M. S.; Mortimer, R. J.; Rosseinsky, D. R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. doi:10.1017/cbo9780511550959
and references therein.
Return to citation in text: [1] [2] -
Baumann, K. L.; Lin, R.; Giri, P.; Franz, S. Protic-Soluble Organic Electrochromic Compounds. U.S. Pat. Appl. US2017/0146880 A1, May 25, 2017.
Return to citation in text: [1] [2] [3] -
Jarosz, T.; Gebka, K.; Stolarczyk, A.; Domagala, W. Polymers (Basel, Switz.) 2019, 11, 273. doi:10.3390/polym11020273
and references therein.
Return to citation in text: [1] -
Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. J. Mater. Chem. C 2019, 7, 4622–4637. doi:10.1039/c9tc00416e
Return to citation in text: [1] -
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein.
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Gillmore, J. G.; Neiser, J. D.; McManus, K. A.; Roh, Y.; Dombrowski, G. W.; Brown, T. G.; Dinnocenzo, J. P.; Farid, S.; Robello, D. R. Macromolecules 2005, 38, 7684–7694. doi:10.1021/ma050348k
Return to citation in text: [1] -
Kavarnos, G. J.; Turro, N. J. Chem. Rev. 1986, 86, 401–449. doi:10.1021/cr00072a005
and references therein.
Return to citation in text: [1] -
Borsub, N.; Kutal, C. J. Am. Chem. Soc. 1984, 106, 4826–4828. doi:10.1021/ja00329a030
Return to citation in text: [1] -
Evans, T. R.; Wake, R. W.; Sifain, M. M. Tetrahedron Lett. 1973, 14, 701–704. doi:10.1016/s0040-4039(00)72438-5
Return to citation in text: [1] -
Hasegawa, E.; Okada, K.; Ikeda, H.; Yamashita, Y.; Mukai, T. J. Org. Chem. 1991, 56, 2170–2178. doi:10.1021/jo00006a039
Return to citation in text: [1] -
Bauld, N. L. Hole and electron transfer catalyzed pericyclic reactions. In Advances in Electron Transfer Chemistry; Mariano, P. S., Ed.; JAI Press: Greenwich, CT, 1992; pp 1–66.
and references therein.
Return to citation in text: [1] -
Bauld, N. L.; Gao, D.; Aplin, J. T. J. Phys. Org. Chem. 1999, 12, 808–818. doi:10.1002/(sici)1099-1395(199911)12:11<808::aid-poc207>3.0.co;2-m
Return to citation in text: [1] -
Bauld, N. L.; Gao, D. Polym. Int. 2000, 49, 253–259. doi:10.1002/(sici)1097-0126(200003)49:3<253::aid-pi352>3.0.co;2-p
Return to citation in text: [1] -
Bauld, N. L.; Roh, Y. Tetrahedron Lett. 2001, 42, 1437–1439. doi:10.1016/s0040-4039(00)02301-7
Return to citation in text: [1] -
Roh, Y.; Gao, D.; Bauld, N. L. Adv. Synth. Catal. 2001, 343, 481–489. doi:10.1002/1615-4169(200107)343:5<481::aid-adsc481>3.0.co;2-s
Return to citation in text: [1] -
Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] -
Lynch, E. J.; Speelman, A. L.; Curry, B. A.; Murillo, C. S.; Gillmore, J. G. J. Org. Chem. 2012, 77, 6423–6430. doi:10.1021/jo300853k
Return to citation in text: [1] [2] [3] [4] -
Pavlishchuk, V. V.; Addison, A. W. Inorg. Chim. Acta 2000, 298, 97–102. doi:10.1016/s0020-1693(99)00407-7
Return to citation in text: [1] [2] -
Barachevsky, V. A.; Butenko, V. G. Russ. J. Gen. Chem. 2018, 88, 2747–2772. doi:10.1134/s1070363218120459
Return to citation in text: [1] -
Gaussian 03, Revision D.01; Gaussian, Inc.: Wallingford, CT, 2004.
Return to citation in text: [1] -
Schmidt, J. R.; Polik, W. F. WebMO Pro version 9.1, WebMO Enterprise versions 10.1, 12.1, 14.0; WebMO LLC: Holland, MI, 2014; available from http://www.webmo.net (accessed June 2019).
Return to citation in text: [1] [2] -
Curie Cluster. http://curie.chem.hope.edu/ (accessed June 30, 2019).
Return to citation in text: [1] [2] -
MU3C – Midwest Undergraduate Computational Chemistry Consortium. http://mu3c.chem.hope.edu/ (accessed June 30, 2019).
Return to citation in text: [1] [2] -
Kuwata, K. T.; Kohen, D.; Krueger, B. P.; Polik, W. F. Counc. Undergrad. Res. Quart. 2012, 32 (4), 9–14.
Return to citation in text: [1] [2] -
Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, 2009.
Return to citation in text: [1] -
Roothaan, C. C. J. Rev. Mod. Phys. 1960, 32, 179–185. doi:10.1103/revmodphys.32.179
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1996, 104, 1040–1046. doi:10.1063/1.470829
Return to citation in text: [1] -
Becke, A. D. Phys. Rev. A 1988, 38, 3098–3100. doi:10.1103/physreva.38.3098
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785
Return to citation in text: [1] -
Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213–222. doi:10.1007/bf00533485
Return to citation in text: [1] -
Glendening, E. D.; Reed, A. E.; Carpenter, J. E.; Weinhold, F. NBO, Version 3.1.
Return to citation in text: [1] -
Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999–3094. doi:10.1021/cr9904009
Return to citation in text: [1]
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 1. | Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermochromic Compounds; Plenum Press: New York, NY, USA, 1999; Vol. 1. doi:10.1007/b114211 |
| 2. | Duerr, H.; Bouas-Laurent, H., Eds. Photochromism: Molecules and Systems, revised ed.; Elsevier: Amsterdam, 2003. |
| 3. | Desvergne, J.-P.; Pozzo, J.-L., Eds. Proceedings of the fourth International Symposium on Photochromism Photoswitchable Molecular Systems and Devices. Mol. Cryst. Liq. Cryst. 2005, 430–431, 1–586. doi:10.1080/15421400590946073 |
| 4. | Branda, N.; Tian, H., Eds. Organic Photoswitchable Multifunctional Molecules and Materials. Dyes Pigm. 2011, 89, 193–336. doi:10.1016/j.dyepig.2010.12.002 |
| 5. | Favaro, G.; Irie, M., Eds. Special issue on Photochromism 1 and 2. J. Photochem. Photobiol., C 2011, 12, 71–236. doi:10.1016/j.jphotochemrev.2011.08.004 |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 23. | Pavlishchuk, V. V.; Addison, A. W. Inorg. Chim. Acta 2000, 298, 97–102. doi:10.1016/s0020-1693(99)00407-7 |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 22. | Lynch, E. J.; Speelman, A. L.; Curry, B. A.; Murillo, C. S.; Gillmore, J. G. J. Org. Chem. 2012, 77, 6423–6430. doi:10.1021/jo300853k |
| 28. | MU3C – Midwest Undergraduate Computational Chemistry Consortium. http://mu3c.chem.hope.edu/ (accessed June 30, 2019). |
| 29. | Kuwata, K. T.; Kohen, D.; Krueger, B. P.; Polik, W. F. Counc. Undergrad. Res. Quart. 2012, 32 (4), 9–14. |
| 6. |
Monk, P. M. S.; Mortimer, R. J.; Rosseinsky, D. R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. doi:10.1017/cbo9780511550959
and references therein. |
| 7. | Baumann, K. L.; Lin, R.; Giri, P.; Franz, S. Protic-Soluble Organic Electrochromic Compounds. U.S. Pat. Appl. US2017/0146880 A1, May 25, 2017. |
| 9. | Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. J. Mater. Chem. C 2019, 7, 4622–4637. doi:10.1039/c9tc00416e |
| 22. | Lynch, E. J.; Speelman, A. L.; Curry, B. A.; Murillo, C. S.; Gillmore, J. G. J. Org. Chem. 2012, 77, 6423–6430. doi:10.1021/jo300853k |
| 6. |
Monk, P. M. S.; Mortimer, R. J.; Rosseinsky, D. R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. doi:10.1017/cbo9780511550959
and references therein. |
| 7. | Baumann, K. L.; Lin, R.; Giri, P.; Franz, S. Protic-Soluble Organic Electrochromic Compounds. U.S. Pat. Appl. US2017/0146880 A1, May 25, 2017. |
| 8. |
Jarosz, T.; Gebka, K.; Stolarczyk, A.; Domagala, W. Polymers (Basel, Switz.) 2019, 11, 273. doi:10.3390/polym11020273
and references therein. |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 26. | Schmidt, J. R.; Polik, W. F. WebMO Pro version 9.1, WebMO Enterprise versions 10.1, 12.1, 14.0; WebMO LLC: Holland, MI, 2014; available from http://www.webmo.net (accessed June 2019). |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 16. |
Bauld, N. L. Hole and electron transfer catalyzed pericyclic reactions. In Advances in Electron Transfer Chemistry; Mariano, P. S., Ed.; JAI Press: Greenwich, CT, 1992; pp 1–66.
and references therein. |
| 17. | Bauld, N. L.; Gao, D.; Aplin, J. T. J. Phys. Org. Chem. 1999, 12, 808–818. doi:10.1002/(sici)1099-1395(199911)12:11<808::aid-poc207>3.0.co;2-m |
| 18. | Bauld, N. L.; Gao, D. Polym. Int. 2000, 49, 253–259. doi:10.1002/(sici)1097-0126(200003)49:3<253::aid-pi352>3.0.co;2-p |
| 19. | Bauld, N. L.; Roh, Y. Tetrahedron Lett. 2001, 42, 1437–1439. doi:10.1016/s0040-4039(00)02301-7 |
| 20. | Roh, Y.; Gao, D.; Bauld, N. L. Adv. Synth. Catal. 2001, 343, 481–489. doi:10.1002/1615-4169(200107)343:5<481::aid-adsc481>3.0.co;2-s |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 22. | Lynch, E. J.; Speelman, A. L.; Curry, B. A.; Murillo, C. S.; Gillmore, J. G. J. Org. Chem. 2012, 77, 6423–6430. doi:10.1021/jo300853k |
| 11. | Gillmore, J. G.; Neiser, J. D.; McManus, K. A.; Roh, Y.; Dombrowski, G. W.; Brown, T. G.; Dinnocenzo, J. P.; Farid, S.; Robello, D. R. Macromolecules 2005, 38, 7684–7694. doi:10.1021/ma050348k |
| 12. |
Kavarnos, G. J.; Turro, N. J. Chem. Rev. 1986, 86, 401–449. doi:10.1021/cr00072a005
and references therein. |
| 13. | Borsub, N.; Kutal, C. J. Am. Chem. Soc. 1984, 106, 4826–4828. doi:10.1021/ja00329a030 |
| 14. | Evans, T. R.; Wake, R. W.; Sifain, M. M. Tetrahedron Lett. 1973, 14, 701–704. doi:10.1016/s0040-4039(00)72438-5 |
| 15. | Hasegawa, E.; Okada, K.; Ikeda, H.; Yamashita, Y.; Mukai, T. J. Org. Chem. 1991, 56, 2170–2178. doi:10.1021/jo00006a039 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 23. | Pavlishchuk, V. V.; Addison, A. W. Inorg. Chim. Acta 2000, 298, 97–102. doi:10.1016/s0020-1693(99)00407-7 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 26. | Schmidt, J. R.; Polik, W. F. WebMO Pro version 9.1, WebMO Enterprise versions 10.1, 12.1, 14.0; WebMO LLC: Holland, MI, 2014; available from http://www.webmo.net (accessed June 2019). |
| 31. | Roothaan, C. C. J. Rev. Mod. Phys. 1960, 32, 179–185. doi:10.1103/revmodphys.32.179 |
| 22. | Lynch, E. J.; Speelman, A. L.; Curry, B. A.; Murillo, C. S.; Gillmore, J. G. J. Org. Chem. 2012, 77, 6423–6430. doi:10.1021/jo300853k |
| 24. | Barachevsky, V. A.; Butenko, V. G. Russ. J. Gen. Chem. 2018, 88, 2747–2772. doi:10.1134/s1070363218120459 |
| 7. | Baumann, K. L.; Lin, R.; Giri, P.; Franz, S. Protic-Soluble Organic Electrochromic Compounds. U.S. Pat. Appl. US2017/0146880 A1, May 25, 2017. |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 28. | MU3C – Midwest Undergraduate Computational Chemistry Consortium. http://mu3c.chem.hope.edu/ (accessed June 30, 2019). |
| 29. | Kuwata, K. T.; Kohen, D.; Krueger, B. P.; Polik, W. F. Counc. Undergrad. Res. Quart. 2012, 32 (4), 9–14. |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 36. | Glendening, E. D.; Reed, A. E.; Carpenter, J. E.; Weinhold, F. NBO, Version 3.1. |
| 10. |
Minkin, V. I.; Komissarov, V. N.; Kharlanov, V. A. Perimidinespirocyclohexadienones. In Organic Photochromic and Thermochromic Compounds; Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, NY, USA, 1999; Vol. 1, pp 315–340. doi:10.1007/0-306-46911-1_9
and references therein. |
| 37. | Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999–3094. doi:10.1021/cr9904009 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 32. | Becke, A. D. J. Chem. Phys. 1996, 104, 1040–1046. doi:10.1063/1.470829 |
| 33. | Becke, A. D. Phys. Rev. A 1988, 38, 3098–3100. doi:10.1103/physreva.38.3098 |
| 34. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 21. | Moerdyk, J. P.; Speelman, A. L.; Kuper, K. E., III; Heiberger, B. R.; Ter Louw, R. P.; Zeller, D. J.; Radler, A. J.; Gillmore, J. G. J. Photochem. Photobiol., A 2009, 205, 84–92. doi:10.1016/j.jphotochem.2009.04.011 |
| 35. | Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213–222. doi:10.1007/bf00533485 |
© 2019 Webb et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)