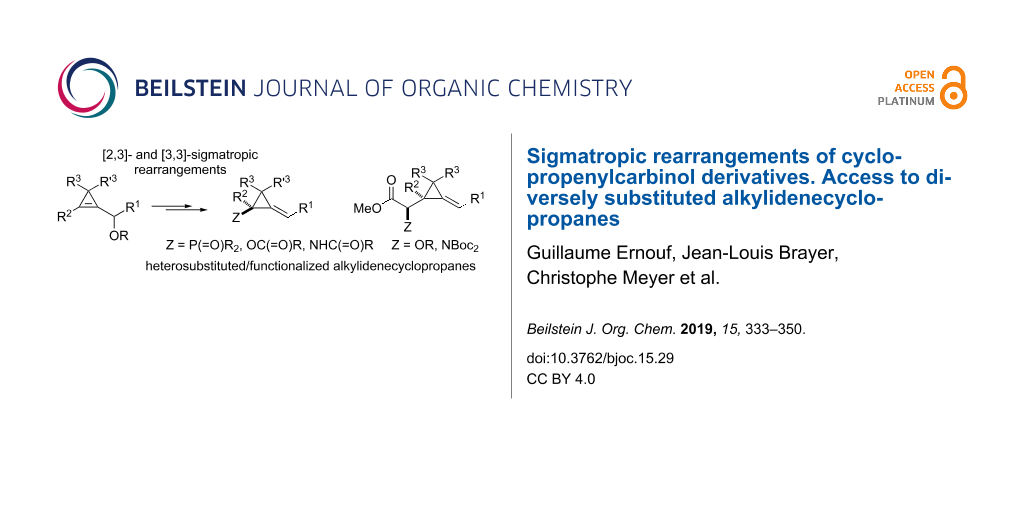
Abstract
Cyclopropenes constitute useful precursors of other classes of compounds incorporating a three-membered ring. Although the transformation of substituted cyclopropenes into alkylidenecyclopropanes can be accomplished through different strategies, this review is focusing specifically on the use of [2,3]- and [3,3]-sigmatropic rearrangements involving cyclopropenylcarbinol derivatives as substrates. These sigmatropic rearrangements, which have been developed in recent years, allow a remarkably efficient and stereoselective access to a wide variety of heterosubstituted and/or functionalized alkylidenecyclopropanes which would not be readily accessible by other strategies. The different [2,3]- and [3,3]-sigmatropic rearrangements of cyclopropenylcarbinol derivatives disclosed to date, as well as the analysis of their substrate scope and some applications of the products arising from those reactions, are presented in this review.
Graphical Abstract
Introduction
Among the ever expanding diversity of chemical transformations involving cyclopropenes, which are largely dominated by ring-cleavage processes to access functionalized acyclic compounds or to construct new carbocycles or heterocycles, those reactions that preserve the three-membered ring and enable access to diversely substituted cyclopropanes or alkylidenecyclopropanes are also synthetically useful [1-6]. The importance of this latter class of transformations is obviously related to the widespread occurrence of cyclopropanes in natural and/or bioactive compounds [7,8] and the great interest of the cyclopropyl core in new drugs development [9]. Alkylidenecyclopropanes also constitute another important class of strained carbocycles displaying a versatile chemistry owing to their multiple reactive sites (the exocyclic olefin and the proximal and distal bonds on the ring) [10-15]. Although the synthesis of alkylidenecyclopropanes can be achieved by many different routes, controlling the configuration of the exocyclic olefin as well as that of stereocenters on and adjacent to the three-membered ring remains a challenging task [15]. In this context, cyclopropenes can serve as useful precursors of substituted and functionalized alkylidenecyclopropanes. The transformation of cyclopropenes into alkylidenecyclopropanes has been achieved through different strategies (Scheme 1). The first one relies on the isomerization of the olefin in alkylcyclopropenes A from the endocyclic to the exocyclic position (Scheme 1, reaction 1) [16-18]. Owing to the relief of ring-strain, the formation of the alkylidenecyclopropane B is generally thermodynamically favored [19,20]. However, in the particular case of gem-difluorocyclopropenes A’ (R3 = R'3 = F) which possess a cyclopropenium (aromatic) character, the position of the equilibrium depends on the substituent at C1. Whereas conjugation with the phenyl group (R = Ph) provides the driving force for the base-promoted isomerization of 1-benzyl-3,3-difluorocyclopropene (A’, R = Ph) into the corresponding benzylidene(gem-difluoro-cyclopropane) (B’) [18], methylene(gem-difluorocyclopropane) (B’’, R = H) is isomerized into 1-methyl-3,3-difluorocyclopropene (A”) [21] (Scheme 1, reaction 1). Another approach relies on the reaction of cyclopropenylmethyl organometallic species C with electrophiles through an SE2’ process leading to substituted alkylidenecyclopropanes D (Scheme 1, reaction 2). Examples of those transformations include the carboxylation of a (trimethylsilylmethyl)cyclopropene in the presence of a fluoride promoter [22], and also the addition of electrophiles to (lithiomethyl)cyclopropenes generated by lithiation of the corresponding methylcyclopropenylsulfone [23] or -sulfoxide [24]. More recently, the addition of cyclopropenylmethylboronates to aldehydes was also reported [25]. A complementary strategy involves the addition of nucleophiles, in particular organometallic reagents, to cyclopropenylcarbinols or their derivatives E, which leads to alkylidenecyclopropanes F through a formal SN2’ process (Scheme 1, reaction 3) [23,26-33]. Thus, methylenecyclopropanes have been prepared by diastereoselective addition of Grignard reagents to cyclopropenylmethyl ethers, possessing a hydroxymethyl directing substituent at C3, in the absence or in the presence of a catalyst (copper or iron salt) [28-30]. Another representative transformation is the copper-catalyzed addition of Grignard reagents to secondary unprotected cyclopropenylcarbinols which proceeds with high levels of chirality transfer to afford alkylidenecyclopropanes possessing a quaternary stereocenter at C2 [31,33]. In this review, we shall exclusively focus on alternative strategies that rely either on a [2,3]-sigmatropic rearrangement (Scheme 1, reaction 4) or a [3,3]-sigmatropic rearrangement of cyclopropenylcarbinol derivatives (Scheme 1, reaction 5). These transformations have emerged as useful tools over the past few years to access hetero-substituted and/or functionalized alkylidenecyclopropanes.
Scheme 1: Representative strategies for the formation of alkylidenecyclopropanes from cyclopropenes and scope of the review.
Scheme 1: Representative strategies for the formation of alkylidenecyclopropanes from cyclopropenes and scope...
Review
[2,3]-Sigmatropic rearrangements involving cyclopropenylcarbinol derivatives
Following their report on the synthesis of chiral alkylidenecyclopropanes by copper-catalyzed addition of Grignard reagents to enantiomerically enriched cyclopropenylcarbinols [31], Marek et al. investigated other classes of transformations involving those latter strained analogs of allylic alcohols as substrates. In 2007, the [2,3]-sigmatropic rearrangement of cyclopropenylcarbinyl phosphinites was reported as a route to chiral phosphines possessing an alkylidenecyclopropane backbone [34]. The starting cyclopropenylcarbinols were readily prepared by addition of the corresponding cyclopropenyl organolithium reagents, generated in situ by treatment of 1,1,2-tribromocyclopropane with n-butyllithium (2 equiv) [35], to various aldehydes and ketones. Marek et al. observed that the treatment of cyclopropenylcarbinols 1a–h with chlorodiphenylphosphine in the presence of triethylamine (THF, rt) resulted in a very rapid formation of (alkylidenecyclopropyl)diphenylphosphine oxides 3a–h (85–94%), resulting from an efficient [2,3]-sigmatropic rearrangement of the in situ-generated phosphinites 2a–h. Primary or tertiary cyclopropenylcarbinols reacted equally well, as shown with the formation of phosphine oxides 3a (94%), 3b (93%) and 3c (87%). The [2,3]-sigmatropic rearrangement of phosphinites 2d–h derived from secondary cyclopropenylcarbinols led to the corresponding phosphine oxides 3d–h (85–93%) as a 80:20 mixture of E/Z geometric isomers, regardless of the substituent of the alcohol (at C4) and of the cyclopropene (at C2, Scheme 2) [33,34].
Scheme 2: [2,3]-Sigmatropic rearrangement of phosphinites 2a–h.
Scheme 2: [2,3]-Sigmatropic rearrangement of phosphinites 2a–h.
The [2,3]-sigmatropic rearrangement of an optically enriched phosphinite 2f, prepared from the corresponding secondary cyclopropenylcarbinol (S)-1f (ee = 99%), which in turn is readily available by applying the Sharpless kinetic resolution procedure to (±)-1f [31], was also investigated. The resulting geometric isomers (Z)-3f and (E)-3f, which were separated by flash chromatography, were found to possess optical purities identical to that of the parent substrate (S)-1f (ee = 99%) thereby confirming that complete chirality transfer occurred (from C4 to C2) during the [2,3]-sigmatropic rearrangement [33,34]. It is also worth mentioning that the absolute configuration of (Z)-3f and (E)-3f, which is opposite at C2, was assigned by comparison of their computed and experimentally observed CD spectra [33,34]. To tentatively explain the observed stereochemical outcome in the absence of additional knowledge on the transition state of the rearrangement [36], two reactive conformers G and G’ were considered which would lead to five-membered ring transition states in which the aryl group occupies a preferential pseudo-equatorial or a less favorable pseudo-axial orientation, respectively (Scheme 3) [33,34].
Scheme 3: [2,3]-Sigmatropic rearrangement of a phosphinite derived from enantioenriched cyclopropenylcarbinol (S)-1f.
Scheme 3: [2,3]-Sigmatropic rearrangement of a phosphinite derived from enantioenriched cyclopropenylcarbinol...
The authors also showed that phosphine oxide (E)-3f could be reduced to the corresponding phosphine 4 (94%) by treatment with trichlorosilane, without affecting the (arylmethylene)cyclopropane moiety (Scheme 4).
Scheme 4: Selective reduction of phosphine oxide (E)-3f.
Scheme 4: Selective reduction of phosphine oxide (E)-3f.
The great efficiency of the [2,3]-sigmatropic rearrangement of phosphinites 2a–h lacking substituents at C3 is in striking contrast with the reactivity of phosphinites possessing a geminal disubstitution at C3. In 2007, Rubin et al. reported their results on the [2,3]-sigmatropic rearrangement of cyclopropenylmethyl phosphinites derived from primary cyclopropenylcarbinols [37]. As illustrated in the case of 5a, the substrates were prepared from the tert-butyldimethylsilyl (TBS) ether of propargyl alcohol by a rhodium-catalyzed cyclopropanation with an aryldiazoacetate followed by reduction of the ester moiety and protecting group manipulation. Phosphinite 6a, generated from alcohol 5a under standard conditions, did not undergo a [2,3]-sigmatropic rearrangement into the corresponding diastereomeric phosphine oxides 7a/7’a, even upon prolonged heating (toluene, 110 °C), and underwent slow decomposition instead (Scheme 5).
Scheme 5: Attempted thermal [2,3]-sigmatropic rearrangement of phosphinite 6a.
Scheme 5: Attempted thermal [2,3]-sigmatropic rearrangement of phosphinite 6a.
This observation was in agreement with DFT calculations which indicated that the rearrangement of cyclopropenylmethyl phosphinite I, although thermodynamically favored, displays a high activation barrier compared to that of the acyclic allyl analog H. An even higher activation barrier was calculated in the case of the 3-methyl and 3-phenyl-substituted cyclopropenes, I’ and I’’, respectively, which indicates that the concerted [2,3]-sigmatropic rearrangement would require high temperatures incompatible with such thermally labile strained substrates (Scheme 6) [37].
Scheme 6: Computed activation barriers and free enthalpies.
Scheme 6: Computed activation barriers and free enthalpies.
Interestingly, the authors detected traces of methylenecyclopropanes 7a/7’a when phosphinylation of alcohol 5a was conducted at room temperature for several hours which led them to consider that the amine could play a role in promoting the [2,3]-sigmatropic rearrangement. After a screening of different tertiary amines, Rubin et al. found that DBU could be used as a base in the phosphinylation reaction but also as an efficient catalyst for the subsequent [2,3]-sigmatropic rearrangement of phosphinite 6a which afforded a 73:27 mixture of the diastereomeric phosphine oxides 7a/7’a (86%). The major diastereomer 7a corresponds to a sigmatropic rearrangement occurring on the most hindered face (cis to the aromatic group) of the cyclopropene which was somewhat surprising. Substitution at the para-position of the aromatic group at C3 significantly affected the diasteomeric ratio with an increase observed with the mesomeric donor methoxy group in favor of diastereomer 7b (7b/7’b = 78:22) compared to 7a/7’a, and a drop of diastereoselectivity when a fluorine atom (7c/7’c = 60:40) or a hydrogen atom (7d/7’d = 52:48) were present. An inversion of the face selectivity was detected in favor of diastereomer 7’e (7e/7’e = 43:57) arising from the rearrangement of phosphinite 6e possessing a p-trifluoromethylphenyl substituent. Replacement of the acetal protecting group of the hydroxymethyl substituent at C3 (R3 = CH2OMOM = CH2OCH2OMe) by an acetate (R3 = CH2OAc) did not affect the results, as illustrated in the case of 7f/7’f, but the presence of an ester moiety (R3 = CO2Me) led to the rearranged phosphine oxides 7g/7’g in rather low yield (47%) although the diasteromeric ratio remains similar to that observed for 7a/7’a. Other substituents were tolerated on the phosphorus atom including an isopropyl or a cyclohexyl group and the corresponding phosphine oxides 7h/7’h and 7i/7’i were isolated in good yields. Increasing the steric hindrance around the phosphorus atom resulted in a higher diastereoselectivity. However, the sigmatropic rearrangement of the highly hindered di(tert-butyl)phosphinite 6j and tetra(isopropyl) phosphorodiamidite 6k did not occur (Scheme 7) [37].
Scheme 7: [2,3]-Sigmatropic rearrangement of phosphinites 6a–j.
Scheme 7: [2,3]-Sigmatropic rearrangement of phosphinites 6a–j.
The mechanism proposed by Rubin et al. involves a reversible addition of the Lewis base (DBU) on the cyclopropene double bond at C2 leading to zwitterronic intermediates 8 and 8’. This would result in an increase of conformational flexibility thereby facilitating the nucleophilic displacement of the ammonium by the phosphinite through transition states TS1 and TS2 (SN2-type process), respectively. Oxaphospholanium zwitterions 9 and 9’ would then be obtained and would eventually produce the diastereomeric phosphine oxides 7 and 7’. Computational studies indicated that the facial selectivity of the initial attack of the Lewis base (DBU) was not responsible for the observed diastereocontrol because of the low difference between the activation barriers of the reactions leading to 8 and 8’, regardless of the aromatic substituent. Since 8 and 8’ were in rapid equilibrium with phosphinite 6, the diastereoselectivity should depend on the relative stabilities of the transition states TS1 and TS’1. An electron-donating group at the para-position of the aromatic ring could contribute to the stabilization of TS1, in which the Ar–C3–C2–P dihedral angle is close to 0°, by considering the mesomeric form TS2. The observed dependence of the diastereoselectivity on the σ+ Hammett constant of the para substituents further supported the proposed mechanism (Scheme 8) [37].
Scheme 8: Proposed mechanism for the Lewis base-catalyzed rearrangement of phosphinites 6.
Scheme 8: Proposed mechanism for the Lewis base-catalyzed rearrangement of phosphinites 6.
To date and to the best of our knowledge, reports on [2,3]-sigmatropic rearrangements of cyclopropenylcarbinol derivatives appear to be limited to the synthesis of alkylidenecyclopropanes incorporating a phosphorus atom. Cyclopropenylcarbinol derivatives can also lead to other heterosubstituted alkylidenecyclopropanes by using [3,3]-sigmatropic rearrangements.
[3,3]-Sigmatropic rearrangements involving cyclopropenylcarbinol derivatives
Access to heterosubstituted alkylidenecyclopropanes
The interest of secondary cyclopropenylcarbinol derivatives in [3,3]-sigmatropic rearrangements was first highlighted by Marek et al. who investigated the transposition of cyclopropenylcarbinyl esters [33,34]. The [3,3]-sigmatropic rearrangement of acetate 10a took place during filtration on silica gel and afforded alkylidene(acetoxycyclopropane) 11a in 90% yield. The ease with which the rearrangement of 10a occurred was attributed to the relief of ring strain but also to the favorable conjugation of the olefin with the two phenyl groups (R1 = R’1 = Ph). Alkylidenecyclopropane 11a could also be obtained in similar yields (92% or 87%, respectively) by heating acetate 10a in dichloromethane at reflux or by treatment with dry Amberlyst® 15 (a sulfonic acid resin) [33,34]. The rearrangement of the tertiary acetates 10b (R1 = R’1 = Me) and 10c (R1 = Ph, R’1 = Me) could also be achieved by filtration through silica gel and led to 11b (91%) and 11c (83%). The latter non-symmetrical tetrasubstituted alkene 11c was obtained as a 67:33 mixture of geometric isomers (Scheme 9) [33,34].
Scheme 9: [3,3]-Sigmatropic rearrangement of tertiary cyclopropenylcarbinyl acetates 10a–c.
Scheme 9: [3,3]-Sigmatropic rearrangement of tertiary cyclopropenylcarbinyl acetates 10a–c.
The rearrangement of secondary cyclopropenylcarbinyl acetates 10d–g could be achieved in the presence of Amberlyst® 15 and led exclusively to the (E)-alkylidene(acetoxycyclopropanes) 11d–g (E/Z > 99:1) in good yields (70–77%). The acetate could also be replaced by a benzoate as illustrated with the formation of alkylidenecyclopropane 11h (60%) from substrate 10h. The authors mentioned that the sigmatropic rearrangement did not proceed under such mild conditions for substrates possessing an alkyl group instead of an aryl group at C4 but no additional details were provided. The high diastereoselectivity was explained by considering a six-membered chair-like cyclic transition state model TS3 in which the substituent at the α position of the ester (C4) preferentially occupies a pseudo-equatorial position. Although a cationic mechanism could have also been envisioned under the acidic conditions used, the optically enriched acetates 10d and 10e (ee > 98%) led to the corresponding alkylidenecyclopropanes 11d and 11e with complete chirality transfer (ee > 98%) at C2, thereby probing the concerted suprafacial nature of the rearrangement (Scheme 10) [33,34]. The acidic promotor may be simply assisting the dissociation of the C4–O bond in the transition state TS3 whilst an aromatic group (R1 = Ar) would contribute to the stabilization of a developing positive charge at C4.
Scheme 10: [3,3]-Sigmatropic rearrangement of secondary cyclopropenylcarbinyl esters 10d–h.
Scheme 10: [3,3]-Sigmatropic rearrangement of secondary cyclopropenylcarbinyl esters 10d–h.
The [3,3]-sigmatropic rearrangement of cyclopropenylcarbinyl acetates provides a straightforward and stereoselective entry to alkylidene(acyloxycyclopropanes). Only a few compounds of this family had been previously generated by photochemical reactions (from 4-isopropylidene-3,3-dimethyl-1-thietan-2-thione [38] or from a 4-alkylidene-Δ1-pyrazoline [39]) or by pyrolysis of the sodium salt of 3-propionyloxytetramethylcyclobutanone tosyl hydrazone [40]. It is also worth mentioning that completely divergent reactivities have also been reported for cyclopropenylcarbinyl esters in the presence of transition metal catalysts [41,42].
Alkylidene(aminocyclopropane) derivatives constitute another interesting class of heterosubstituted alkylidenecyclopropanes which have been previously synthesized by a Curtius rearrangement of acyl azides derived from alkylidenecyclopropane carboxylic acids [43] or by elimination reactions applied to suitably substituted aminocyclopropane derivatives [44-46].
In 2014, Hyland et al. disclosed the Overman rearrangement [47] of cyclopropenylcarbinyl trichloroacetimidates [48]. The optimal conditions for the generation of imidates 12a–i involved treatment of secondary cyclopropenylcarbinols with trichloroacetonitrile in the presence of a catalytic amount of DBU (15 mol %) in CH2Cl2 (−78 °C to −10 °C, 2–3 h) [48,49]. The crude imidates 12a–i were then directly engaged in the [3,3]-sigmatropic rearrangement step which was triggered by heating in the presence of K2CO3 in CH2Cl2 (30 °C, 40 h). These latter conditions, which were optimized for imidate 12a, enabled the formation of p-bromobenzylidene[(N-trichloroacetylamino)cyclopropane] 13a as a single (E)-isomer in 63% overall yield (two steps from the corresponding alcohol). Compound 13a was obtained in lower yield in the absence of a base (21%) or when DMF was used as the solvent (53%) though a considerable rate acceleration (22 h instead of 40 h) was observed compared to CH2Cl2. In the presence of PdCl2(MeCN)2 (5 mol %), only traces of 13a were detected and significant decomposition of 12a took place. As in the case of the [3,3]-sigmatropic rearrangement of cyclopropenylcarbinyl acetates, the observed stereoselectivity was explained by invoking a chair-like transition state model TS4 in which the aryl group preferentially occupies a pseudo-equatorial orientation (Scheme 11) [48]. Although the presence of a halogen atom was tolerated, as illustrated with the formation of the benzylidenecyclopropanes 13a (63%) and 13h (48%), higher yields were obtained in the case of imidates 13b–d, possessing an electro-neutral or an electron-rich aromatic group, which afforded compounds 13b (83%), 13c (98%) and 13d (77%), substituted by a phenyl, a p-tolyl or a p-anisyl group, respectively. The rearrangement of imidate 12f possessing a m-anisyl substituent afforded benzylidene cyclopropane 13f in a lower yield (47%) compared to 13d (77%). The rearrangement of imidate 12i possessing an electron-rich N-tosylpyrrol-2-yl heteroaromatic group, afforded alkylidenecyclopropane 13i in nearly quantitative yield. Conversely, no rearrangement took place in the case of imidates 12e and 12g in which the aromatic group was substituted by a strongly electron-withdrawing nitro group at the para- or the meta-position, respectively. All these observations point toward the development of a positive charge at the C4 carbon atom (adjacent to the R1 substituent) in the transition state TS4, as was also suggested previously in the [3,3]-sigmatropic rearrangement of cyclopropenylcarbinyl acetates. Alkylidenecyclopropane 13j could not be synthesized because trichloroacetimidate 12j was not obtained by treatment of the corresponding cyclopropenylcarbinol substituted by an n-undecyl group with trichloroacetonitrile, even under forcing conditions. The authors tentatively suggested this may be due to the sterically hindered n-undecyl chain although this issue was not fully investigated (Scheme 11) [48].
Scheme 11: [3,3]-Sigmatropic rearrangement of trichoroacetimidates 12a–i.
Scheme 11: [3,3]-Sigmatropic rearrangement of trichoroacetimidates 12a–i.
While attempts to access the free alkylidene(aminocyclopropanes) from the corresponding trichloroacetamides proved unsuccessful by hydrolysis (1 M aqueous HCl or KOH, EtOH) or reduction (DIBAL-H or NaBH4), Hyland et al. showed that the treatment of (arylmethylene)cyclopropane 13f with Cs2CO3 in anhydrous DMF, followed by the addition of an excess of pyrrolidine, produced urea 14f (24%) [48,49]. The moderate yield of 14f was attributed to the instability of the in situ-generated isocyanate 15f under the reaction conditions [50]. When trichloroacetamide 13f was treated with an excess of pyrrolidine and Cs2CO3 in bench grade (undried) DMF, the reaction followed a different pathway and delivered α-oxoacetamide 16f (58%) instead of urea 14f [48,49]. This type of transformation had already been reported [51] and interpreted by a Favorskii-type mechanism, presumably involving the formation of the gem-dichloro-α-lactam intermediate 17f which would undergo ring opening by nucleophilic addition of pyrrolidine followed by hydrolysis of the resulting α,α-dichloro-α-aminoacetamide 18f (Scheme 12).
Scheme 12: Reaction of trichloroacetamide 13f with pyrrolidine.
Scheme 12: Reaction of trichloroacetamide 13f with pyrrolidine.
To access aminocyclopropanes, the hydrogenation of (arylmethylene)cyclopropane 13f was achieved in the presence of Pd/C as a catalyst. Concomitant hydrogenolysis of two carbon–chlorine bonds also took place under these conditions and a 71:29 diastereomeric mixture of the monochloracetamides 19f/19’f was obtained (41%). The rather small difference of steric hindrance between the methyl and the N-acylamino group explained the modest face selectivity of hydrogen addition which preferentially occurred on the face of the olefin opposite to the N-chloroacetylamino substituent (Scheme 13) [48].
Scheme 13: Catalytic hydrogenation of (arylmethylene)cyclopropropane 13f.
Scheme 13: Catalytic hydrogenation of (arylmethylene)cyclopropropane 13f.
3,3-Disubstituted cyclopropenylcarbinols could not be used as substrates in the Overman rearrangement. This limitation of the substrate scope is due to the instability of the corresponding trichloroacetimidates. Thus cyclopropenylcarbinols 20a–c possessing gem-dimethyl substitution at C3 were converted to imidates 21a–c but upon treatment with silica gel (CH2Cl2, −10 °C), those latter compounds were converted into α-allenic tertiary alcohols 22a–c (30–61%) The formation of alcohols 22a–c was explained by a mechanism involving ionization of the C4–O bond in imidates 21a–c, followed by ring opening of the alkylidenecyclopropyl cationic intermediates 23a–c [52] and addition of water to the resulting α-allenic carbocations 24a–c (Scheme 14) [48].
Scheme 14: Instability of trichloroacetimidates 21a–c derived from cyclopropenylcarbinols 20a–c.
Scheme 14: Instability of trichloroacetimidates 21a–c derived from cyclopropenylcarbinols 20a–c.
As a complementary strategy, our group examined the [3,3]-sigmatropic rearrangement of cyanates derived from cyclopropenylcarbinols [53]. The allyl cyanate to isocyanate rearrangement displays many interesting features such as the possibility to generate the reactive species by dehydration of carbamates under mild conditions and the ultimate formation of isocyanates which can be derivatized in situ [54]. The conditions were optimized with alcohol 25 substituted by a 2-phenylethyl group at the oxygen-bearing carbon atom (C4) and possessing gem-disubstitution at C3 on the three-membered ring. Alcohol 25 was readily converted to carbamate 26 by reaction with trichloroacetyl isocyanate followed by cleavage of the trichloroacetyl group by alkaline hydrolysis. Dehydration of carbamate 26 was achieved by treatment with trifluoroacetic anhydride in the presence of triethylamine under mild conditions (CH2Cl2, −78 °C) [55] and the in situ-generated cyanate 27 underwent a sigmatropic rearrangement into the corresponding isocyanate 28. The formation of this reactive isocyanate intermediate was ascertained by the addition of morpholine which enabled the isolation of urea 29 in good yield (78%). It is worth noting that alkylidenecyclopropane 29 was formed with high diastereoselectivity (E/Z ≥ 95:5) at low temperature (−78 °C) but a slight erosion of diastereoselectivity was observed (E/Z = 88:12) when the same sequence was performed at 0 °C. The stereochemical outcome was in agreement with a six-membered transition state model TS5 in which the three atoms of the cyanate (O=C=N) moiety would be arranged in an almost linear fashion (an angle of 173° was calculated in the allyl cyanate to isocyanate transition state) [56] and the substituent at C4 would preferentially occupy a pseudo-equatorial orientation. Additionally, the same sequence applied to the enantioenriched alcohol (R)-25 (ee = 88%) delivered urea (−)-29 with essentially the same optical purity (ee = 86%), thereby indicating that chirality transfer (from C4 to C2) occurred during the sigmatropic rearrangement of cyanate 27 into isocyanate 28 (Scheme 15) [53].
Scheme 15: [3,3]-Sigmatropic rearrangement of cyanate 27 generated from cyclopropenylcarbinyl carbamate 26.
Scheme 15: [3,3]-Sigmatropic rearrangement of cyanate 27 generated from cyclopropenylcarbinyl carbamate 26.
All attempts to isolate isocyanate 28 were unsuccessful but derivatization of this latter reactive intermediate could be achieved in situ by addition of a broad range of nucleophiles, which were either used as co-solvents or added in excess. Thus, reaction with pyrrolidine, imidazole, methanol, allyl alcohol, benzyl alcohol and 9-fluorenemethanol (FmOH) provided the corresponding urea 30, N-carbamoyl imidazole 31 and carbamates 32–35, respectively, in good yields (69–80%). The reaction of isocyanate 28 with tert-butanol was sluggish even by heating at 40 °C but could be accelerated by addition of Ti(OiPr)4 (10 mol %) to deliver the corresponding N-Boc- carbamate 36 (81%). The condensation of isocyanate 28 with N-Boc-glycine in the presence of DMAP (Goldschmidt–Wick coupling) [57] provided amide 37 in 70% yield (Scheme 16) [53].
Scheme 16: Synthesis of alkylidene(aminocyclopropane) derivatives 30–37 from carbamate 26.
Scheme 16: Synthesis of alkylidene(aminocyclopropane) derivatives 30–37 from carbamate 26.
The examination of the substrate scope indicated that a broad range of alkyl chains, possibly incorporating heteroatoms, were compatible with the dehydration–[3,3]-sigmatropic sequence, as illustrated with the isolation of compounds 38–40 (72–79%) after nucleophilic trapping of the generated isocyanate intermediates with allyl alcohol. Benzylidenecyclopropane 41 was also obtained in good yield (70%) but the efficiency of the sigmatropic rearrangement dropped for carbamates in which the aromatic group at C4 is substituted by an electron-withdrawing group at the para-position. Indeed, N-Alloc (arylmethylene)(aminocyclopropanes) 42 and 43, substituted by a p-fluorophenyl and a p-(trifluoromethyl)phenyl group, respectively, were isolated in moderate yield (53%). Moreover, (p-nitrophenylmethylene)cyclopropane 44 could not be obtained under these conditions [53]. These results indicate that the [3,3]-sigmatropic rearrangement of cyclopropenylcarbinyl cyanates, as previously reported for their allylic counterparts [56], does not involve a synchronous process because dissociation of the C4–O bond is more advanced in the transition state TS5 than the formation of the C2–N bond (Scheme 15). The rearrangement of cyclopropenylcarbinyl cyanates accommodates various substituents at C3, as well as the presence of a substituent at C2 or even a fully substituted cyclopropene ring, as shown with the successful formation of alkylidenecyclopropanes 45–48 (58–86%, Scheme 17) [53].
Scheme 17: Scope of the dehydration–[3,3]-sigmatropic rearrangement sequence of cyclopropenylcarbinyl carbamates.
Scheme 17: Scope of the dehydration–[3,3]-sigmatropic rearrangement sequence of cyclopropenylcarbinyl carbamat...
Interestingly, alkylidene(isocyanatocyclopropanes) arising from the [3,3]-sigmatropic rearrangement of cyclopropenylcarbinyl cyanates could also be derivatized into trifluoroacetamides. This transformation was discovered fortuitously when carbamate 49 was treated with an excess of trifluoroacetic anhydride (2 equiv) in the presence of Et3N (3 equiv) to achieve the dehydration–sigmatropic rearrangement sequence. Trifluoroacetamide 50 (67%) was the product directly formed under these conditions and the Lewis basic character of the pyridine ring was suspected to be responsible for the observed reactivity (Scheme 18).
Scheme 18: Formation of trifluoroacetamide 50 from carbamate 49.
Scheme 18: Formation of trifluoroacetamide 50 from carbamate 49.
With the aim of achieving the same derivatization in the case of other substrates devoid of a pyridine ring, several 3,3-dimethylcyclopropenylcarbinyl carbamates were engaged in the dehydration–[3,3]-sigmatropic rearrangement sequence under the previously used conditions but trifluoroacetic anhydride (1.5 equiv) and pyridine (1.5 equiv) were then subsequently added to the reaction mixture. Under these conditions, the corresponding trifluoroacetamides 51–54 could be effectively isolated in good yields (73–85%). The addition of pyridine to the isocyanates J arising from the [3,3]-sigmatropic rearrangement would probably generate the zwitterionic intermediates K which would then react with trifluoroacetic anhydride to produce N,O-bis(trifluoroacetyl)carbamates L. Trifluoroacetamides 51–54 would be generated from adducts L after hydrolysis of the reaction mixture (Scheme 19) [53].
Scheme 19: Formation of alkylidene[(N-trifluoroacetylamino)cyclopropanes] 51–54.
Scheme 19: Formation of alkylidene[(N-trifluoroacetylamino)cyclopropanes] 51–54.
To control the diastereoselectivity of the hydrogenation of alkylidene[(N-acylamino)cyclopropanes] possessing a single substituent at C2, it is possible to rely either on the steric hindrance or on the coordinating ability of the amide group. Thus, the hydrogenation of trifluoroacetamide 51 catalyzed by Pd/C afforded N-trifluoroacetylaminocyclopropane 55 as the major diastereomer (55/55’ = 92:8) because of the preferential addition of hydrogen on the less hindered face of the trisubstituted alkene opposite to the trifluoroacetamide moiety. A reversal of face selectivity can be observed by performing a directed iridium(I)-catalyzed hydrogenation in the presence of Crabtree’s catalyst [58] which afforded aminocyclopropane 55’ as the major diastereomer (55’/55 = 90:10, Scheme 20) [53].
Scheme 20: Diastereoselective hydrogenation of alkylidenecyclopropane 51.
Scheme 20: Diastereoselective hydrogenation of alkylidenecyclopropane 51.
The potential of [3,3]-sigmatropic rearrangements involving cyclopropenylcarbinol derivatives is not restricted to the synthesis of heterosubstituted alkylidenecyclopropanes and was also exploited to access functionalized alkylidenecyclopropanes, with creation of a new carbon–carbon bond on the three-membered ring with the control of two contiguous stereocenters.
Ireland–Claisen rearrangement of cyclopropenylcarbinyl esters
The Ireland–Claisen rearrangement of silyl ketene acetals generated from allylic (or propargylic) esters is arguably one of the most useful variant of the Claisen rearrangement that has found countless applications in organic synthesis [59]. The feasibility of the Ireland–Claisen rearrangement of cyclopropenylcarbinyl esters was investigated in the case of glycolates 56a–l which were readily prepared by coupling of the corresponding cyclopropenylcarbinols with (4-methoxybenzyloxy)acetic acid. Enolization of glycolates 56a–l was carried out by treatment with Me3SiCl (4 equiv) followed by addition of KHMDS (usually 4 equiv) in THF at −78 °C. The resulting silyl ketene acetals of (Z)-configuration 57a–l, arising from O-silylation of the corresponding chelated potassium enolates [60], underwent an efficient [3,3]-sigmatropic rearrangement upon warming to room temperature. After an acidic work-up and treatment of the crude carboxylic acids with trimethylsilyldiazomethane, the resulting α-alkoxy methyl esters 58a–l, incorporating an alkylidenecyclopropane moiety, were obtained as single detectable diastereomers [61]. As in the previously discussed [3,3]-sigmatropic rearrangements, the observed stereochemical outcome was in agreement with a six-membered chair-like transition state model TS6 in which the substituent at the α-position of the oxygen atom (C4) preferentially occupies a pseudo-equatorial position. The scope of the reaction is rather broad as the substituent at C4 can be an alkyl chain, possibly incorporating a protected alcohol, as illustrated with the formation of alkylidenecyclopropanes 58a (86%), 58b (60%) and 58c (84%). It is worth mentioning that despite the use of a strong base (KHMDS) and the acidity of the “vinylic” protons of cyclopropenes which is comparable to that of a terminal alkyne [62], cyclopropenylcarbinyl glycolates devoid of substituents at C2 were viable substrates. The sequence allowed access to benzylidenecyclopropane 58d (93%) and to (arylmethylene)cyclopropane 58e in excellent yield (90%), despite the presence of the electron-withdrawing trifluoromethyl substituent at the para-position of the aromatic ring. Some heteroaromatic groups were also tolerated at C4, as shown with the synthesis of (heteroarylmethylene) cyclopropanes 58f–h (60–72%). The gem-dimethyl substitution at C3 which was common to the previous cyclopropenylcarbinyl glycolates 56a–h, could be suppressed and the corresponding alkylidenecyclopropane 58i was produced in excellent yield (94%). More sterically hindered substituents were tolerated at C3, as illustrated with the isolation of the spirocyclic compounds 58j (60%) and 58k (77%), and alkylidenecyclopropane 58l possessing a fully substituted three-membered ring was also formed in excellent yield (96%). That the Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates proceeded with chirality transfer was also verified in the case of alkylidenecyclopropanes 58a and 58i which were obtained with optical purities (ee = 87% and ee = 97%, respectively) identical to those of the corresponding enantioenriched precursors (R)-56a and (R)-56i (Scheme 21) [61].
Scheme 21: Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates 56a–l.
Scheme 21: Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates 56a–l.
The addition of a cyclopropenyllithium to an aldehyde is arguably the most widely used method to access cyclopropenylcarbinols but Gevorgyan et al. disclosed an interesting organocatalytic route to cyclopropenylcarbinols possessing gem-diester substitution at C3 [63]. As illustrated with the preparation of alcohol 60, the strategy relies on a sila-Morita–Baylis–Hillman reaction between cyclopropenylsilane 59 and 3-phenylpropanal catalyzed by electron-rich tris(2,4,6-trimethoxyphenyl)phosphine (TTMPP) [63]. After desilylation, cyclopropenylcarbinol 60 was converted into glycolate 61 under standard conditions and the latter ester was engaged in the Ireland–Claisen rearrangement. Because the gem-diester substitution at C3 increased the acidity of the proton at C2 in substrate 61 [64], silylation of that position took place under the reaction conditions prior to the Ireland–Claisen rearrangement which eventually produced alkylidenecyclopropane 62 (56%) with high diastereoselectivity. The trimethylsilyl substituent at C2 could then be easily removed by treatment of 62 with tetrabutylammonium fluoride under buffered conditions (AcOH, THF, 0 °C) to afford alkylidenecyclopropane 63 (92%, Scheme 22) [61].
Scheme 22: Synthesis and Ireland–Claisen rearrangement of glycolate 61 possessing gem-diester substitution at C3.
Scheme 22: Synthesis and Ireland–Claisen rearrangement of glycolate 61 possessing gem-diester substitution at ...
The Ireland–Claisen rearrangement was then extended to a challenging class of cyclopropylcarbinyl glycolates possessing gem-difluoro substitution at C3 [65]. Gem-difluorocyclopropenes are accessible by difluorocyclopropenation of alkynes with difluorocarbene but these compounds display poor stability in most cases and readily undergo hydrolysis into cyclopropenones which possess an aromatic character [66,67]. Gem-difluorocyclopropenylcarbinyl glycolates 65a–n were prepared by slow addition of an excess of trimethylsilyl fluorosulfonyldifluoroacetate (TFDA) [68] to a solution of propargyl glycolates 64a–n containing NaF in diglyme at 120 °C. Difluorocyclopropene 65a could be purified by flash chromatography on silica gel and was isolated in 86% yield but this compound rapidly underwent decomposition upon storage. The instability of glycolates 65a–n was a critical issue which was solved by carrying those intermediate compounds directly in the sigmatropic rearrangement. Byproducts arising from the difluorocyclopropenation reaction (CO2, SO2 and Me3SiF) were simply removed by argon sparging of the reaction mixture and the Ireland–Claisen rearrangement was then triggered by addition of Me3SiCl (4 equiv) and KHMDS (4 equiv), (THF, −78 °C to rt). Subsequent hydrolysis and treatment with trimethylsilyldiazomethane afforded the corresponding α-alkoxy methyl esters 66a–h, and 66k–n possessing a 3,3-difluoroalkylidenecyclopropane scaffold. This two-step difluorocyclopropenation–Ireland–Claisen rearrangement sequence was applied to propargyl glycolates 64a–e possessing a phenyl, a p-methoxyphenyl, a p-bromophenyl, an o-chlorophenyl or a 1-naphthyl substituent at the acetylenic position, as illustrated with the formation of compounds 66a–e (63–76%, two steps from the corresponding propargylic glycolates). Not surprisingly, chirality transfer (from C4 to C2) also occurred in the Ireland–Claisen rearrangement, as demonstrated by the formation of (−)-66b (ee = 95%) from optically enriched (S)-64b (ee = 96%). Heteroaromatic groups (indol-3-yl and 3-thienyl) were tolerated at the acetylenic position and the corresponding glycolates 64f and 64g led to compounds 66f and 66g in 70% yield. A p-acetylphenyl group was compatible as shown with the isolation of alkylidenecyclopropane 66h (65%) but it should be noted that the electron-withdrawing methyl ketone was converted to a trimethylsilyl enol ether upon treatment with KHMDS/Me3SiCl. By contrast, an electron-withdrawing p-nitrophenyl group was not tolerated because the intermediate cyclopropene 65i underwent decomposition under the reaction conditions of the Ireland–Claisen rearrangement, presumably because of competitive deprotonation at C4. A phenyl substituent was incompatible at C4 as the corresponding substrate 65j decomposed upon treatment with KHMDS/Me3SiCl. This was explained by a competitive abstraction of the hydrogen at C4 by the base thereby resulting in side reactions. However, various alkyl substituents could be present at the propargylic position in glycolates 64k–n which afforded the corresponding rearranged compounds 66k–n in moderate yields (40–61%, Scheme 23) [65].
Scheme 23: Synthesis of alkylidene(gem-difluorocyclopropanes) 66a–h, and 66k–n from propargyl glycolates 64a–n.
Scheme 23: Synthesis of alkylidene(gem-difluorocyclopropanes) 66a–h, and 66k–n from propargyl glycolates 64a–n....
With the goal of accessing α-amino acid derivatives incorporating an alkylidenecyclopropane, the Ireland–Claisen rearrangement of N,N-diBoc glycinates 67a and 67b was explored. The reaction conditions were essentially the same as those described previously with glycolates 56a–l except that LiHMDS was used as the base in the enolization step [69]. The (Z)-silyl ketene acetals 68a and 68b were generated, in agreement with previous results disclosed by Carbery et al. with allylic N,N-diBoc glycinates [69], and underwent a Ireland–Claisen rearrangement to afford N,N-diBoc α-amino esters 69a (78%) and 69b (91%) in good yields and with high diastereoselectivity [61]. Although cleavage of the two Boc groups could not be achieved cleanly upon exposure of 69b to a large excess of trifluoroacetic acid, this operation could be accomplished in a sequential manner by addition of trifluoroacetic acid (2 equiv, CH2Cl2, 0 °C) and then by treatment of the resulting N-Boc carbamate 70 (97%) with trimethylsilyl triflate in the presence of 2,6-lutidine to generate α-amino ester 71 (99%, Scheme 24) [61].
Scheme 24: Ireland–Claisen rearrangement of N,N-diBoc glycinates 67a and 67b.
Scheme 24: Ireland–Claisen rearrangement of N,N-diBoc glycinates 67a and 67b.
Alkylidenecyclopropanes resulting from the Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates and glycinates can serve as useful precursors of other classes of functionalized cyclopropanes. As shown previously with alkylidene(aminocyclopropane) derivatives, diastereoselective hydrogenation reactions of alkylidenecyclopropanes possessing a single substituent at C2 can be carried out with complementary face selectivities, depending on the conditions and substrates. Thus, the hydrogenation of 58a catalyzed by Rh/C occurred on the less hindered face of the alkene and gave rise to cyclopropyl α-alkoxy ester 72 as a single detectable diastereomer. When Pd/C was used as the catalyst, cleavage of the PMB group took place concomitantly and the α-hydroxy ester 73 arising from addition of hydrogen on the less-hindered face of the olefin was obtained predominantly (73/73’ = 90:10) albeit with lower diastereocontrol compared to the protected alcohol 58a. Cleavage of the PMB ether in 58a was achieved purposely with DDQ so that a hydroxy-directed hydrogenation of the resulting α-hydroxy ester 74 could be carried out in the presence of Crabtree’s catalyst [58], thereby allowing access to cyclopropylcarbinol 73’ with high diastereoselectivity (73’/73 = 97:3, Scheme 25).
Scheme 25: Diastereoselective hydrogenation of alkylidenecyclopropanes 58a and 74.
Scheme 25: Diastereoselective hydrogenation of alkylidenecyclopropanes 58a and 74.
By taking advantage of the directing effect of a hydroxy group, diastereoselective hydrogenations of alkylidenecyclopropanes possessing two substituents at C2 could be achieved. As illustrated for alkylidene(gem-difluorocyclopropane) 66a, cleavage of the PMB group and subsequent hydrogenation of the resulting α-hydroxy ester 75 (75%) in the presence of Crabtree’s catalyst delivered the gem-difluorocyclopropane 76 (91%) as a single diastereomer. The reduction of ester 76 with LiAlH4 and oxidative cleavage of the resulting 1,2-diol with NaIO4 delivered the highly substituted gem-difluorocyclopropanecarboxaldehyde 77 (72%) possessing a quaternary stereocenter (Scheme 26) [65].
Scheme 26: Synthesis of functionalized gem-difluorocyclopropanes 76 and 77 from alkylidenecyclopropane 66a.
Scheme 26: Synthesis of functionalized gem-difluorocyclopropanes 76 and 77 from alkylidenecyclopropane 66a.
Other examples of post-functionalization involve iodolactonization reactions which were applied to α-hydroxy esters 74 and 75 using N-iodosuccinimide (MeCN/H2O, 50 °C) [70], or to the N-benzylamine generated from α-amino ester 71 (by reductive animation with benzaldehyde) in the presence of I2 and K2CO3 (MeCN, rt) [71]. These iodocyclizations led to the oxabicyclic compounds 78 (98%) and 79 (99%), and to the azabicyclic product 80 (45%), respectively, with high diastereoselectivities (Scheme 27) [61,65].
Scheme 27: Access to oxa- and azabicyclic compounds 78–80.
Scheme 27: Access to oxa- and azabicyclic compounds 78–80.
Conclusion
In recent years, [2,3]- and [3,3]-sigmatropic rearrangements of cyclopropenylcarbinol derivatives have emerged as useful tools for the stereoselective synthesis of a wide variety of alkylidenecyclopropanes, substituted by heteroatoms (P, O, N, F) and/or incorporating valuable functional groups (α-alkoxy or α-amino acid derivatives) which are potentially useful for further functionalization. The reactivity of heterosubstituted/functionalized alkylidenecyclopropanes arising from those sigmatropic rearrangements, which are not easily accessible by other strategies, has only been sparingly investigated to date but the results summarized in this short review, in conjunction with the very rich chemistry of alkylidenecyclopropanes, may stimulate further investigations in this particular area.
References
-
Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117–3179. doi:10.1021/cr050988l
Return to citation in text: [1] -
Marek, I.; Simaan, S.; Masarwa, A. Angew. Chem., Int. Ed. 2007, 46, 7364–7376. doi:10.1002/anie.200604774
Return to citation in text: [1] -
Zhu, Z.-B.; Wei, Y.; Shi, M. Chem. Soc. Rev. 2011, 40, 5534–5563. doi:10.1039/c1cs15074j
Return to citation in text: [1] -
Miege, F.; Meyer, C.; Cossy, J. Beilstein J. Org. Chem. 2011, 7, 717–734. doi:10.3762/bjoc.7.82
Return to citation in text: [1] -
Vicente, R. Synthesis 2016, 48, 2343–2360. doi:10.1055/s-0035-1561644
Return to citation in text: [1] -
Dian, L.; Marek, I. Chem. Rev. 2018, 118, 8415–8434. doi:10.1021/acs.chemrev.8b00304
Return to citation in text: [1] -
Salaün, J. Cyclopropane Derivatives and their Diverse Biological Activities. In Small Ring Compounds in Organic Synthesis VI. Topics in Current Chemistry; Meijere, A., Ed.; Springer: Berlin, Heidelberg, 2000; Vol. 207, pp 1–67. doi:10.1007/3-540-48255-5_1
Return to citation in text: [1] -
Donaldson, W. A. Tetrahedron 2001, 57, 8589–8627. doi:10.1016/s0040-4020(01)00777-3
Return to citation in text: [1] -
Talele, T. T. J. Med. Chem. 2016, 59, 8712–8756. doi:10.1021/acs.jmedchem.6b00472
Return to citation in text: [1] -
Lautens, M.; Klute, W.; Tam, W. Chem. Rev. 1996, 96, 49–92. doi:10.1021/cr950016l
Return to citation in text: [1] -
Brandi, A.; Goti, A. Chem. Rev. 1998, 98, 589–636. doi:10.1021/cr940341t
Return to citation in text: [1] -
Nakamura, I.; Yamamoto, Y. Adv. Synth. Catal. 2002, 344, 111–129. doi:10.1002/1615-4169(200202)344:2<111::aid-adsc111>3.0.co;2-0
Return to citation in text: [1] -
Brandi, A.; Cicchi, S.; Cordero, F. M.; Goti, A. Chem. Rev. 2014, 114, 7317–7420. doi:10.1021/cr400686j
Return to citation in text: [1] -
Pellissier, H. Tetrahedron 2014, 70, 4991–5031. doi:10.1016/j.tet.2014.04.057
Return to citation in text: [1] -
Audran, G.; Pellissier, H. Adv. Synth. Catal. 2010, 352, 575–608. doi:10.1002/adsc.200900872
Return to citation in text: [1] [2] -
Köster, R.; Arora, S.; Binger, P. Angew. Chem., Int. Ed. Engl. 1969, 8, 205–206. doi:10.1002/anie.196902052
Return to citation in text: [1] -
Vincens, M.; Dumont, C.; Vidal, M. Tetrahedron 1981, 37, 2683–2694. doi:10.1016/s0040-4020(01)98975-6
Return to citation in text: [1] -
Sang, R.; Yang, H.-B.; Shi, M. Tetrahedron Lett. 2013, 54, 3591–3594. doi:10.1016/j.tetlet.2013.04.076
Return to citation in text: [1] [2] -
Wiberg, K. B.; Fenoglio, R. A. J. Am. Chem. Soc. 1968, 90, 3395–3397. doi:10.1021/ja01015a018
Return to citation in text: [1] -
Bach, R. D.; Dmitrenko, O. J. Am. Chem. Soc. 2004, 126, 4444–4452. doi:10.1021/ja036309a
Return to citation in text: [1] -
Shibuya, A.; Okada, M.; Nakamura, Y.; Kibashi, M.; Horikawa, H.; Taguchi, T. Tetrahedron 1999, 55, 10325–10340. doi:10.1016/s0040-4020(99)00588-8
Return to citation in text: [1] -
Weatherhead-Kloster, R. A.; Corey, E. J. Org. Lett. 2006, 8, 171–174. doi:10.1021/ol052752+
Return to citation in text: [1] -
Padwa, A.; Wannamaker, M. W. Tetrahedron 1991, 47, 6139–6156. doi:10.1016/s0040-4020(01)86547-9
Return to citation in text: [1] [2] -
Zohar, E.; Stanger, A.; Marek, I. Synlett 2005, 2239–2241. doi:10.1055/s-2005-872247
Return to citation in text: [1] -
Baumann, A. N.; Music, A.; Karaghiosoff, K.; Didier, D. Chem. Commun. 2016, 52, 2529–2532. doi:10.1039/c5cc09904h
Return to citation in text: [1] -
Babin, D.; Pilorge, F.; Delbarre, L. M.; Demoute, J. P. Tetrahedron 1995, 51, 9603–9610. doi:10.1016/0040-4020(95)00535-g
Return to citation in text: [1] -
Nüske, H.; Bräse, S.; de Meijere, A. Synlett 2000, 1467–1469. doi:10.1055/s-2000-7627
See for the addition of malonates to π-allyl-palladium complexes derived cyclopropenylmethyl acetates leading to the cyclopropene isomer except in the case of one tertiary acetate.
Return to citation in text: [1] -
Yang, Z.; Xie, X.; Fox, J. M. Angew. Chem., Int. Ed. 2006, 45, 3960–3962. doi:10.1002/anie.200600531
Return to citation in text: [1] [2] -
Xie, X.; Yang, Z.; Fox, J. M. J. Org. Chem. 2010, 75, 3847–3850. doi:10.1021/jo1002938
Return to citation in text: [1] [2] -
Xie, X.; Fox, J. M. Synthesis 2013, 45, 1807–1814. doi:10.1055/s-0033-1338876
Return to citation in text: [1] [2] -
Simaan, S.; Masarwa, A.; Bertus, P.; Marek, I. Angew. Chem., Int. Ed. 2006, 45, 3963–3965. doi:10.1002/anie.200600556
Return to citation in text: [1] [2] [3] [4] -
Simaan, S.; Marek, I. Chem. Commun. 2009, 292–294. doi:10.1039/b817710d
See for the copper-catalyzed reduction of secondary cyclopropenylcarbinols which has also been reported.
Return to citation in text: [1] -
Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] -
Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Baird, M. S.; Hussain, H. H.; Nethercott, W. J. Chem. Soc., Perkin Trans. 1 1986, 1845–1853. doi:10.1039/p19860001845
Return to citation in text: [1] -
Liron, F.; Knochel, P. Chem. Commun. 2004, 304–305. doi:10.1039/b313979d
See for the stereoselective [2,3]-sigmatropic rearrangement of acyclic allylic phosphinites.
Return to citation in text: [1] -
Rubina, M.; Woodward, E. W.; Rubin, M. Org. Lett. 2007, 9, 5501–5504. doi:10.1021/ol702473s
Return to citation in text: [1] [2] [3] [4] -
Muthuramu, K.; Ramamurthy, V. J. Chem. Soc., Chem. Commun. 1980, 243–244. doi:10.1039/c39800000243
Return to citation in text: [1] -
Andrews, S. D.; Day, A. C. Chem. Commun. 1966, 667–669. doi:10.1039/c19660000667
Return to citation in text: [1] -
Maier, G.; Straßer, M. Tetrahedron Lett. 1966, 7, 6453–6456. doi:10.1016/s0040-4039(00)76125-9
Return to citation in text: [1] -
Seraya, E.; Slack, E.; Ariafard, A.; Yates, B. F.; Hyland, C. J. T. Org. Lett. 2010, 12, 4768–4771. doi:10.1021/ol101862u
Return to citation in text: [1] -
Archambeau, A.; Nguyen, D.-V.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2016, 22, 6100–6110. doi:10.1002/chem.201505063
Return to citation in text: [1] -
Obame, G.; Brémond, P.; Pannecouque, C.; Audran, G. Synthesis 2013, 45, 2612–2618. doi:10.1055/s-0033-1339311
Return to citation in text: [1] -
Li, K.; Du, W.; Que, N. L. S.; Liu, H.-w. J. Am. Chem. Soc. 1996, 118, 8763–8764. doi:10.1021/ja960822p
Return to citation in text: [1] -
Zhao, Z.; Liu, H.-w. J. Org. Chem. 2002, 67, 2509–2514. doi:10.1021/jo010994r
Return to citation in text: [1] -
Kawanaka, Y.; Kobayashi, K.; Kusuda, S.; Tatsumi, T.; Murota, M.; Nishiyama, T.; Hisaichi, K.; Fujii, A.; Hirai, K.; Naka, M.; Komeno, M.; Odagaki, Y.; Nakai, H.; Toda, M. Bioorg. Med. Chem. 2003, 11, 1723–1743. doi:10.1016/s0968-0896(03)00034-8
Return to citation in text: [1] -
Overman, L. E.; Carpenter, N. E. Org. React. 2005, 66, 1–107. doi:10.1002/0471264180.or066.01
Return to citation in text: [1] -
Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
The reaction conditions are those indicated accurately in the experimental section.
Return to citation in text: [1] [2] [3] -
Nishikawa, T.; Urabe, D.; Tomita, M.; Tsujimoto, T.; Iwabuchi, T.; Isobe, M. Org. Lett. 2006, 8, 3263–3265. doi:10.1021/ol061123c
Return to citation in text: [1] -
Shinkevich, E.; Deblander, J.; Matthijs, S.; Jacobs, J.; De Kimpe, N.; Tehrani, K. A. Org. Biomol. Chem. 2011, 9, 538–548. doi:10.1039/c0ob00391c
Return to citation in text: [1] -
Gallego, G.; Ariafard, A.; Tran, K.; Sandoval, D.; Choi, L.; Chen, Y.-H.; Yates, B. F.; Tao, F.-M.; Hyland, C. J. T. Org. Biomol. Chem. 2011, 9, 3359–3363. doi:10.1039/c0ob01046d
Return to citation in text: [1] -
Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
See for a review. Nocquet, P.-A.; Henrion, S.; Macé, A.; Carboni, B.; Villalgordo, J. M.; Carreaux, F. Eur. J. Org. Chem. 2017, 1295–1307. doi:10.1002/ejoc.201601316
Return to citation in text: [1] -
Roy, S.; Spino, C. Org. Lett. 2006, 8, 939–942. doi:10.1021/ol053061g
Return to citation in text: [1] -
Henrion, S.; Carboni, B.; Cossío, F. P.; Roisnel, T.; Villalgordo, J. M.; Carreaux, F. J. Org. Chem. 2016, 81, 4633–4644. doi:10.1021/acs.joc.6b00505
Return to citation in text: [1] [2] -
Schuemacher, A. C.; Hoffmann, R. W. Synthesis 2001, 243–246. doi:10.1055/s-2001-10813
Return to citation in text: [1] -
Crabtree, R. H.; Davis, M. W. J. Org. Chem. 1986, 51, 2655–2661. doi:10.1021/jo00364a007
Return to citation in text: [1] [2] -
Nubbenmeyer, U.; Hiersemann, M., Eds. The Claisen Rearrangement. Methods and Applications; Wiley-VCH: Weinheim, Germany, 2007.
Return to citation in text: [1] -
Gould, T. J.; Balestra, M.; Wittman, M. D.; Gary, J. A.; Rossano, L. T.; Kallmerten, J. J. Org. Chem. 1987, 52, 3889–3901. doi:10.1021/jo00226a032
Return to citation in text: [1] -
Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Fattahi, A.; McCarthy, R. E.; Ahmad, M. R.; Kass, S. R. J. Am. Chem. Soc. 2003, 125, 11746–11750. doi:10.1021/ja035725s
Return to citation in text: [1] -
Chuprakov, S.; Malyshev, D. A.; Trofimov, A.; Gevorgyan, V. J. Am. Chem. Soc. 2007, 129, 14868–14869. doi:10.1021/ja077437s
Return to citation in text: [1] [2] -
Zrinski, I.; Eckert-Maksić, M. Synth. Commun. 2003, 33, 4071–4077. doi:10.1081/scc-120026348
Return to citation in text: [1] -
Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965–3975. doi:10.1021/acs.joc.7b00197
Return to citation in text: [1] [2] [3] [4] -
Bessard, Y.; Schlosser, M. Tetrahedron 1991, 47, 7323–7328. doi:10.1016/s0040-4020(01)89734-9
Return to citation in text: [1] -
Cheng, Z.-L.; Chen, Q.-Y. Chin. J. Chem. 2006, 24, 1219–1224. doi:10.1002/cjoc.200690227
Return to citation in text: [1] -
Tian, F.; Kruger, V.; Bautista, O.; Duan, J.-X.; Li, A.-R.; Dolbier, W. R.; Chen, Q.-Y. Org. Lett. 2000, 2, 563–564. doi:10.1021/ol0055622
Return to citation in text: [1] -
Tellam, J. P.; Carbery, D. R. J. Org. Chem. 2010, 75, 7809–7821. doi:10.1021/jo1017124
Return to citation in text: [1] [2] -
Wang, B.-Y.; Huang, J.-W.; Liu, L.-P.; Shi, M. Synlett 2005, 421–424. doi:10.1055/s-2004-837218
Return to citation in text: [1] -
Fu, W.; Huang, X. Tetrahedron Lett. 2008, 49, 562–565. doi:10.1016/j.tetlet.2007.11.068
Return to citation in text: [1]
| 37. | Rubina, M.; Woodward, E. W.; Rubin, M. Org. Lett. 2007, 9, 5501–5504. doi:10.1021/ol702473s |
| 37. | Rubina, M.; Woodward, E. W.; Rubin, M. Org. Lett. 2007, 9, 5501–5504. doi:10.1021/ol702473s |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 65. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965–3975. doi:10.1021/acs.joc.7b00197 |
| 63. | Chuprakov, S.; Malyshev, D. A.; Trofimov, A.; Gevorgyan, V. J. Am. Chem. Soc. 2007, 129, 14868–14869. doi:10.1021/ja077437s |
| 64. | Zrinski, I.; Eckert-Maksić, M. Synth. Commun. 2003, 33, 4071–4077. doi:10.1081/scc-120026348 |
| 63. | Chuprakov, S.; Malyshev, D. A.; Trofimov, A.; Gevorgyan, V. J. Am. Chem. Soc. 2007, 129, 14868–14869. doi:10.1021/ja077437s |
| 41. | Seraya, E.; Slack, E.; Ariafard, A.; Yates, B. F.; Hyland, C. J. T. Org. Lett. 2010, 12, 4768–4771. doi:10.1021/ol101862u |
| 42. | Archambeau, A.; Nguyen, D.-V.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2016, 22, 6100–6110. doi:10.1002/chem.201505063 |
| 43. | Obame, G.; Brémond, P.; Pannecouque, C.; Audran, G. Synthesis 2013, 45, 2612–2618. doi:10.1055/s-0033-1339311 |
| 39. | Andrews, S. D.; Day, A. C. Chem. Commun. 1966, 667–669. doi:10.1039/c19660000667 |
| 69. | Tellam, J. P.; Carbery, D. R. J. Org. Chem. 2010, 75, 7809–7821. doi:10.1021/jo1017124 |
| 40. | Maier, G.; Straßer, M. Tetrahedron Lett. 1966, 7, 6453–6456. doi:10.1016/s0040-4039(00)76125-9 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 65. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965–3975. doi:10.1021/acs.joc.7b00197 |
| 38. | Muthuramu, K.; Ramamurthy, V. J. Chem. Soc., Chem. Commun. 1980, 243–244. doi:10.1039/c39800000243 |
| 69. | Tellam, J. P.; Carbery, D. R. J. Org. Chem. 2010, 75, 7809–7821. doi:10.1021/jo1017124 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 66. | Bessard, Y.; Schlosser, M. Tetrahedron 1991, 47, 7323–7328. doi:10.1016/s0040-4020(01)89734-9 |
| 67. | Cheng, Z.-L.; Chen, Q.-Y. Chin. J. Chem. 2006, 24, 1219–1224. doi:10.1002/cjoc.200690227 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 68. | Tian, F.; Kruger, V.; Bautista, O.; Duan, J.-X.; Li, A.-R.; Dolbier, W. R.; Chen, Q.-Y. Org. Lett. 2000, 2, 563–564. doi:10.1021/ol0055622 |
| 44. | Li, K.; Du, W.; Que, N. L. S.; Liu, H.-w. J. Am. Chem. Soc. 1996, 118, 8763–8764. doi:10.1021/ja960822p |
| 45. | Zhao, Z.; Liu, H.-w. J. Org. Chem. 2002, 67, 2509–2514. doi:10.1021/jo010994r |
| 46. | Kawanaka, Y.; Kobayashi, K.; Kusuda, S.; Tatsumi, T.; Murota, M.; Nishiyama, T.; Hisaichi, K.; Fujii, A.; Hirai, K.; Naka, M.; Komeno, M.; Odagaki, Y.; Nakai, H.; Toda, M. Bioorg. Med. Chem. 2003, 11, 1723–1743. doi:10.1016/s0968-0896(03)00034-8 |
| 47. | Overman, L. E.; Carpenter, N. E. Org. React. 2005, 66, 1–107. doi:10.1002/0471264180.or066.01 |
| 70. | Wang, B.-Y.; Huang, J.-W.; Liu, L.-P.; Shi, M. Synlett 2005, 421–424. doi:10.1055/s-2004-837218 |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 71. | Fu, W.; Huang, X. Tetrahedron Lett. 2008, 49, 562–565. doi:10.1016/j.tetlet.2007.11.068 |
| 58. | Crabtree, R. H.; Davis, M. W. J. Org. Chem. 1986, 51, 2655–2661. doi:10.1021/jo00364a007 |
| 65. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965–3975. doi:10.1021/acs.joc.7b00197 |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 51. | Shinkevich, E.; Deblander, J.; Matthijs, S.; Jacobs, J.; De Kimpe, N.; Tehrani, K. A. Org. Biomol. Chem. 2011, 9, 538–548. doi:10.1039/c0ob00391c |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 50. | Nishikawa, T.; Urabe, D.; Tomita, M.; Tsujimoto, T.; Iwabuchi, T.; Isobe, M. Org. Lett. 2006, 8, 3263–3265. doi:10.1021/ol061123c |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 49. | The reaction conditions are those indicated accurately in the experimental section. |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 49. | The reaction conditions are those indicated accurately in the experimental section. |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 49. | The reaction conditions are those indicated accurately in the experimental section. |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 65. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965–3975. doi:10.1021/acs.joc.7b00197 |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 48. | Howard, J. K.; Amin, C.; Lainhart, B.; Smith, J. A.; Rimington, J.; Hyland, C. J. T. J. Org. Chem. 2014, 79, 8462–8468. doi:10.1021/jo501423u |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 52. | Gallego, G.; Ariafard, A.; Tran, K.; Sandoval, D.; Choi, L.; Chen, Y.-H.; Yates, B. F.; Tao, F.-M.; Hyland, C. J. T. Org. Biomol. Chem. 2011, 9, 3359–3363. doi:10.1039/c0ob01046d |
| 1. | Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117–3179. doi:10.1021/cr050988l |
| 2. | Marek, I.; Simaan, S.; Masarwa, A. Angew. Chem., Int. Ed. 2007, 46, 7364–7376. doi:10.1002/anie.200604774 |
| 3. | Zhu, Z.-B.; Wei, Y.; Shi, M. Chem. Soc. Rev. 2011, 40, 5534–5563. doi:10.1039/c1cs15074j |
| 4. | Miege, F.; Meyer, C.; Cossy, J. Beilstein J. Org. Chem. 2011, 7, 717–734. doi:10.3762/bjoc.7.82 |
| 5. | Vicente, R. Synthesis 2016, 48, 2343–2360. doi:10.1055/s-0035-1561644 |
| 6. | Dian, L.; Marek, I. Chem. Rev. 2018, 118, 8415–8434. doi:10.1021/acs.chemrev.8b00304 |
| 15. | Audran, G.; Pellissier, H. Adv. Synth. Catal. 2010, 352, 575–608. doi:10.1002/adsc.200900872 |
| 28. | Yang, Z.; Xie, X.; Fox, J. M. Angew. Chem., Int. Ed. 2006, 45, 3960–3962. doi:10.1002/anie.200600531 |
| 29. | Xie, X.; Yang, Z.; Fox, J. M. J. Org. Chem. 2010, 75, 3847–3850. doi:10.1021/jo1002938 |
| 30. | Xie, X.; Fox, J. M. Synthesis 2013, 45, 1807–1814. doi:10.1055/s-0033-1338876 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 10. | Lautens, M.; Klute, W.; Tam, W. Chem. Rev. 1996, 96, 49–92. doi:10.1021/cr950016l |
| 11. | Brandi, A.; Goti, A. Chem. Rev. 1998, 98, 589–636. doi:10.1021/cr940341t |
| 12. | Nakamura, I.; Yamamoto, Y. Adv. Synth. Catal. 2002, 344, 111–129. doi:10.1002/1615-4169(200202)344:2<111::aid-adsc111>3.0.co;2-0 |
| 13. | Brandi, A.; Cicchi, S.; Cordero, F. M.; Goti, A. Chem. Rev. 2014, 114, 7317–7420. doi:10.1021/cr400686j |
| 14. | Pellissier, H. Tetrahedron 2014, 70, 4991–5031. doi:10.1016/j.tet.2014.04.057 |
| 15. | Audran, G.; Pellissier, H. Adv. Synth. Catal. 2010, 352, 575–608. doi:10.1002/adsc.200900872 |
| 31. | Simaan, S.; Masarwa, A.; Bertus, P.; Marek, I. Angew. Chem., Int. Ed. 2006, 45, 3963–3965. doi:10.1002/anie.200600556 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 9. | Talele, T. T. J. Med. Chem. 2016, 59, 8712–8756. doi:10.1021/acs.jmedchem.6b00472 |
| 25. | Baumann, A. N.; Music, A.; Karaghiosoff, K.; Didier, D. Chem. Commun. 2016, 52, 2529–2532. doi:10.1039/c5cc09904h |
| 57. | Schuemacher, A. C.; Hoffmann, R. W. Synthesis 2001, 243–246. doi:10.1055/s-2001-10813 |
| 7. | Salaün, J. Cyclopropane Derivatives and their Diverse Biological Activities. In Small Ring Compounds in Organic Synthesis VI. Topics in Current Chemistry; Meijere, A., Ed.; Springer: Berlin, Heidelberg, 2000; Vol. 207, pp 1–67. doi:10.1007/3-540-48255-5_1 |
| 8. | Donaldson, W. A. Tetrahedron 2001, 57, 8589–8627. doi:10.1016/s0040-4020(01)00777-3 |
| 23. | Padwa, A.; Wannamaker, M. W. Tetrahedron 1991, 47, 6139–6156. doi:10.1016/s0040-4020(01)86547-9 |
| 26. | Babin, D.; Pilorge, F.; Delbarre, L. M.; Demoute, J. P. Tetrahedron 1995, 51, 9603–9610. doi:10.1016/0040-4020(95)00535-g |
| 27. |
Nüske, H.; Bräse, S.; de Meijere, A. Synlett 2000, 1467–1469. doi:10.1055/s-2000-7627
See for the addition of malonates to π-allyl-palladium complexes derived cyclopropenylmethyl acetates leading to the cyclopropene isomer except in the case of one tertiary acetate. |
| 28. | Yang, Z.; Xie, X.; Fox, J. M. Angew. Chem., Int. Ed. 2006, 45, 3960–3962. doi:10.1002/anie.200600531 |
| 29. | Xie, X.; Yang, Z.; Fox, J. M. J. Org. Chem. 2010, 75, 3847–3850. doi:10.1021/jo1002938 |
| 30. | Xie, X.; Fox, J. M. Synthesis 2013, 45, 1807–1814. doi:10.1055/s-0033-1338876 |
| 31. | Simaan, S.; Masarwa, A.; Bertus, P.; Marek, I. Angew. Chem., Int. Ed. 2006, 45, 3963–3965. doi:10.1002/anie.200600556 |
| 32. |
Simaan, S.; Marek, I. Chem. Commun. 2009, 292–294. doi:10.1039/b817710d
See for the copper-catalyzed reduction of secondary cyclopropenylcarbinols which has also been reported. |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 21. | Shibuya, A.; Okada, M.; Nakamura, Y.; Kibashi, M.; Horikawa, H.; Taguchi, T. Tetrahedron 1999, 55, 10325–10340. doi:10.1016/s0040-4020(99)00588-8 |
| 23. | Padwa, A.; Wannamaker, M. W. Tetrahedron 1991, 47, 6139–6156. doi:10.1016/s0040-4020(01)86547-9 |
| 56. | Henrion, S.; Carboni, B.; Cossío, F. P.; Roisnel, T.; Villalgordo, J. M.; Carreaux, F. J. Org. Chem. 2016, 81, 4633–4644. doi:10.1021/acs.joc.6b00505 |
| 18. | Sang, R.; Yang, H.-B.; Shi, M. Tetrahedron Lett. 2013, 54, 3591–3594. doi:10.1016/j.tetlet.2013.04.076 |
| 24. | Zohar, E.; Stanger, A.; Marek, I. Synlett 2005, 2239–2241. doi:10.1055/s-2005-872247 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 19. | Wiberg, K. B.; Fenoglio, R. A. J. Am. Chem. Soc. 1968, 90, 3395–3397. doi:10.1021/ja01015a018 |
| 20. | Bach, R. D.; Dmitrenko, O. J. Am. Chem. Soc. 2004, 126, 4444–4452. doi:10.1021/ja036309a |
| 54. | See for a review. Nocquet, P.-A.; Henrion, S.; Macé, A.; Carboni, B.; Villalgordo, J. M.; Carreaux, F. Eur. J. Org. Chem. 2017, 1295–1307. doi:10.1002/ejoc.201601316 |
| 16. | Köster, R.; Arora, S.; Binger, P. Angew. Chem., Int. Ed. Engl. 1969, 8, 205–206. doi:10.1002/anie.196902052 |
| 17. | Vincens, M.; Dumont, C.; Vidal, M. Tetrahedron 1981, 37, 2683–2694. doi:10.1016/s0040-4020(01)98975-6 |
| 18. | Sang, R.; Yang, H.-B.; Shi, M. Tetrahedron Lett. 2013, 54, 3591–3594. doi:10.1016/j.tetlet.2013.04.076 |
| 22. | Weatherhead-Kloster, R. A.; Corey, E. J. Org. Lett. 2006, 8, 171–174. doi:10.1021/ol052752+ |
| 35. | Baird, M. S.; Hussain, H. H.; Nethercott, W. J. Chem. Soc., Perkin Trans. 1 1986, 1845–1853. doi:10.1039/p19860001845 |
| 31. | Simaan, S.; Masarwa, A.; Bertus, P.; Marek, I. Angew. Chem., Int. Ed. 2006, 45, 3963–3965. doi:10.1002/anie.200600556 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 58. | Crabtree, R. H.; Davis, M. W. J. Org. Chem. 1986, 51, 2655–2661. doi:10.1021/jo00364a007 |
| 56. | Henrion, S.; Carboni, B.; Cossío, F. P.; Roisnel, T.; Villalgordo, J. M.; Carreaux, F. J. Org. Chem. 2016, 81, 4633–4644. doi:10.1021/acs.joc.6b00505 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 37. | Rubina, M.; Woodward, E. W.; Rubin, M. Org. Lett. 2007, 9, 5501–5504. doi:10.1021/ol702473s |
| 37. | Rubina, M.; Woodward, E. W.; Rubin, M. Org. Lett. 2007, 9, 5501–5504. doi:10.1021/ol702473s |
| 36. |
Liron, F.; Knochel, P. Chem. Commun. 2004, 304–305. doi:10.1039/b313979d
See for the stereoselective [2,3]-sigmatropic rearrangement of acyclic allylic phosphinites. |
| 62. | Fattahi, A.; McCarthy, R. E.; Ahmad, M. R.; Kass, S. R. J. Am. Chem. Soc. 2003, 125, 11746–11750. doi:10.1021/ja035725s |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 60. | Gould, T. J.; Balestra, M.; Wittman, M. D.; Gary, J. A.; Rossano, L. T.; Kallmerten, J. J. Org. Chem. 1987, 52, 3889–3901. doi:10.1021/jo00226a032 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 61. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 3786–3789. doi:10.1021/acs.orglett.5b01759 |
| 33. | Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem. – Eur. J. 2009, 15, 8449–8464. doi:10.1002/chem.200901074 |
| 34. | Masarwa, A.; Stanger, A.; Marek, I. Angew. Chem., Int. Ed. 2007, 46, 8039–8042. doi:10.1002/anie.200702713 |
| 53. | Ernouf, G.; Brayer, J.-L.; Folléas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. Chem. – Eur. J. 2018, 24, 15104–15111. doi:10.1002/chem.201803231 |
| 31. | Simaan, S.; Masarwa, A.; Bertus, P.; Marek, I. Angew. Chem., Int. Ed. 2006, 45, 3963–3965. doi:10.1002/anie.200600556 |
| 59. | Nubbenmeyer, U.; Hiersemann, M., Eds. The Claisen Rearrangement. Methods and Applications; Wiley-VCH: Weinheim, Germany, 2007. |
© 2019 Ernouf et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)