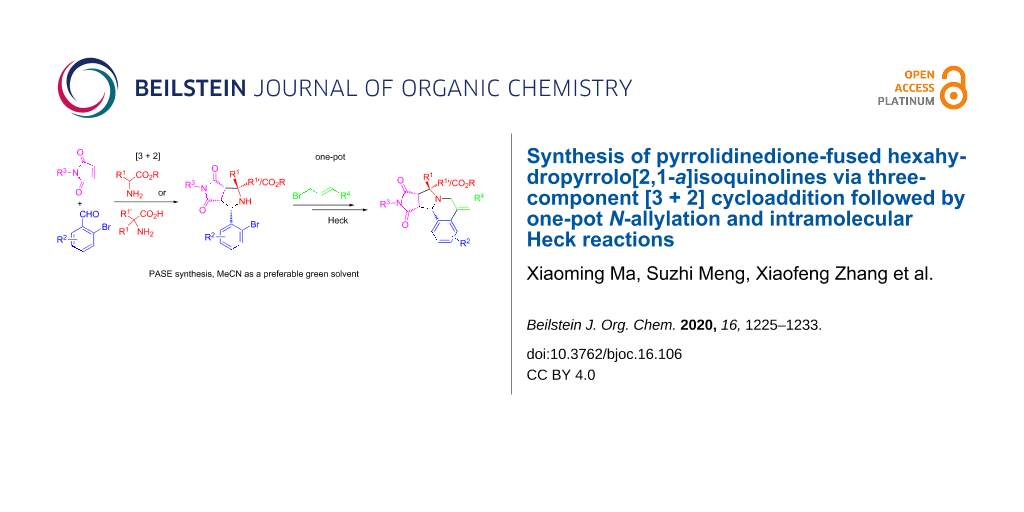
Abstract
Two kinds of [3 + 2] cycloaddition intermediates generated from the three-component reactions of 2-bromobenzaldehydes and maleimides with amino esters or amino acids were used for a one-pot N-allylation and intramolecular Heck reactions to form pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinolines. The multicomponent reaction was combined with one-pot reactions to make a synthetic method with good pot, atom and step economy. MeCN was used as a preferable green solvent for the reactions.
Graphical Abstract
Introduction
Pyrrolo[2,1-a]isoquinoline and hexahydropyrrolo[2,1-a]isoquinoline are privileged heterocyclic rings existing in many natural products and synthetic compounds possessing antitumor, antibacterial, antiviral, antioxidizing, and other biological activities (Figure 1) [1,2]. For example, the alkaloid crispine A isolated from Carduus crispus L has antitumor activity [3]. Erythrina alkaloids have curare-like neuromuscular blocking activities [4], and also antioxidant activity against DPPH free radicals [5]. Lamellarins isolated from marine invertebrates [6] are inhibitors for HIV-1 integrase and also have immuno modulatory activity [7,8]. Trolline has inhibitory activity against Gram-negative and Gram-positive bacteria [9], also as free radical scavenger in rat brain [10]. Organic chemists have been continuously interested in the development of methods for the synthesis of pyrrolo[2,1-a]isoquinolines and related ring systems [11-15], while medicinal chemists have also been interested in making related compounds for biological screening and drug development [16,17].
Figure 1: Bioactive pyrrolo[2,1-a]isoquinolines and hexahydropyrrolo[2,1-a]isoquinolines.
Figure 1: Bioactive pyrrolo[2,1-a]isoquinolines and hexahydropyrrolo[2,1-a]isoquinolines.
Multicomponent reactions (MCRs) have been developed as highly efficient tools for assembling heterocyclic scaffolds related to natural products [18-20]. Among the well-established MCRs, three-component 1,3-dipolar cycloadditions of benzaldehydes, maleimides, and amino esters have been developed for making N-containing 5-membered heterocycles (Scheme 1) [21,22]. The [3 + 2] cycloadditions of maleimides with stabilized azomethine ylides I-a generated from the condensation of aldehydes and amino esters for making pyrrolidines II-a have been well-reported [23-26], while the [3 + 2] cycloaddition of the less stable azomethine ylides I-b generated from the reaction of aldehydes and amino acids for pyrrolidines II-b was less explored [27-29].
Scheme 1: [3 + 2] Cycloaddition with amino esters or amino acids.
Scheme 1: [3 + 2] Cycloaddition with amino esters or amino acids.
In recent years, our lab has reported a series of 1,3-dipolar cycloaddition-initiated methods for the synthesis of diverse heterocycles A–J bearing fused polycyclic rings such as tetrahydroepiminobenzo[b]azocines, tetrahydropyrrolobenzodiazepinones, triazolobenzodiazepines and tetrahydrochromeno[3,4-b]pyrrolizine (Scheme 2) [30-39]. Many of these scaffolds were synthesized through the combination of MCR and one-pot synthesis. A literature search indicated that a [3 + 2] cycloaddition-initiated method has also been used for the synthesis of hexahydropyrrolo[2,1-a]isoquinolines by employing stable 1,3-dilpolar compounds generated from amino esters [15,40] or isoquinolines [41-49]. We like to report in this paper our effort on the synthesis of pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinolines via sequential 1,3-dipolar cycloaddition, N-allylation, and intramolecular Heck cyclization reactions [50-54] (Scheme 2, K). Both stabilized and non-stabilized azomethine ylides could be used for the initial [3 + 2] cycloaddition. A multicomponent reaction was combined with one-pot reactions to make it a green synthetic method with pot, atom and step economy (PASE) [55,56].
Scheme 2: Scaffolds derived from the initial [3 + 2] adducts.
Scheme 2: Scaffolds derived from the initial [3 + 2] adducts.
Results and Discussion
Following the reported procedures for amino ester- and amino acid-based [3 + 2] cycloaddition reactions, pyrrolidine adducts 5 and 6 were synthesized by a three-component reaction of 1 or 2 with 2-bromobenzaldehydes 3 and maleimides 4 (Scheme 3) [30,37]. The cycloaddition reactions were diastereoselective (>20:1 dr for adducts 5 and >6:1 dr for adducts 6). The major diastereomers of 5 and 6 were isolated for following N-allylation and intramolecular Heck reactions.
Scheme 3: [3 + 2] Cycloaddition with amino esters or amino acids. Conditions: 1:3:4 (1.2:1:1.1), Et3N (1.5 equiv), EtOH (3 mL), 110 °C for 6 h; 2:3:4 (1.2:1:1), AcOH (0.3 equiv), MeCN (3 mL), 110 °C for 6 h.
Scheme 3: [3 + 2] Cycloaddition with amino esters or amino acids. Conditions: 1:3:4 (1.2:1:1.1), Et3N (1.5 eq...
Adduct 5a generated from [3 + 2] cycloaddition was used as a model compound to develop the reaction conditions for the one-pot N-allylation and intramolecular Heck reactions (Table 1). N-Allylation of 5a with 3-bromopropene (7) for 8a was accomplished by heating the reaction mixtures in MeCN at 105 °C for 4 h. After evaporating unreacted 3-bromopropene (7) from the reaction mixture, crude product 8a was used for developing the intramolecular Heck reaction by screening Pd(II) catalysts, ligands, bases, additives, solvents, temperatures and reaction time (Table 1). The initial intramolecular Heck reactions were carried out using 10 mol % of Pd(OAc)2 or 10 mol % of PdCl2 with 20 mol % of PPh3 as a ligand and 2 equiv of K2CO3 in MeCN at 80 °C for 10 h without additive to give 6-exo-cyclized product 9a in 32% and 18% yields, respectively (Table 1, entries 1 and 2). Addition of NaOAc increased the yield of 9a to 71% (Table 1, entry 3). Other attempts to improve the Heck reaction using different ligands, such as (P(o-tol)3, PCy3 and dppm, were not successful (Table 1, entries 4–6). The reaction at 105 °C in MeCN gave 9a in 78% yield (Table 1, entry 7), while at 120 °C in DMF gave 9a in 77% yield (Table 1, entry 11). Reducing the amount of Pd(OAc)2 to 5 mol % or the reaction temperature to 40 °C gave lower product yields (Table 1, entries 8 and 10). Double the amount of Pd(OAc)2 to 20 mol% gave 9a in 79% yield, just 1% increase than that of using 10 mol % of catalyst (Table 1, entry 9). Besides K2CO3, other bases including Na2CO3, Cs2CO3 and Et3N were also used for the Heck reaction, but none of them improved the product yield (Table 1, entries 12–14). A base-free control reaction gave 9a in 10% yield (Table 1, entry 15). Thus, the optimized conditions for the Heck reaction was to use 10 mol % of Pd(OAc)2, 20 mol % of PPh3, 2 equiv of K2CO3 and 1 equiv of NaOAc in 3 mL of MeCN at 105 °C for 3 h which give 9a in 78% yield (Table 1, entry 7). It is worth noting that there was no 9ab observed as a byproduct because 6-exo cyclization is more favorable [50,51].
Table 1: Optimization of the one-pot reaction conditions.a
|
|
||||||||
| entry | Pd Cat. | ligand | base | additive | solvent | temp [°C] | time [h] | yield [%]b |
| 1 | Pd(OAc)2 | PPh3 | K2CO3 | – | MeCN | 80 | 10 | 32 |
| 2 | PdCl2 | PPh3 | K2CO3 | – | MeCN | 80 | 10 | 18 |
| 3 | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | MeCN | 80 | 6 | 71 |
| 4 | Pd(OAc)2 | P(o-tol)3 | K2CO3 | NaOAc | MeCN | 80 | 6 | 61 |
| 5 | Pd(OAc)2 | PCy3 | K2CO3 | NaOAc | MeCN | 80 | 6 | 45 |
| 6 | Pd(OAc)2 | dppm | K2CO3 | NaOAc | MeCN | 80 | 6 | 58 |
| 7 | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | MeCN | 105 | 3 | 78 |
| 8c | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | MeCN | 105 | 3 | 28 |
| 9d | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | MeCN | 105 | 3 | 79 |
| 10 | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | MeCN | 40 | 12 | 13 |
| 11 | Pd(OAc)2 | PPh3 | K2CO3 | NaOAc | DMF | 120 | 3 | 77 |
| 12 | Pd(OAc)2 | PPh3 | Na2CO3 | NaOAc | MeCN | 105 | 6 | 19 |
| 13 | Pd(OAc)2 | PPh3 | Cs2CO3 | NaOAc | MeCN | 105 | 6 | 34 |
| 14 | Pd(OAc)2 | PPh3 | Et3N | NaOAc | MeCN | 105 | 6 | 11 |
| 15 | Pd(OAc)2 | PPh3 | – | NaOAc | MeCN | 105 | 6 | 10 |
aReaction conditions: 0.5 mmol 5a in 3 mL MeCN, 7 (3 equiv), K2CO3 (2 equiv) for N-allylation; Pd catalyst (10 mol %), ligand (20 mol %), base (2 equiv) and NaOAc (1 equiv) in 3 mL solvent under nitrogen for the Heck reaction; P(o-tol)3 = tri(o-tolyl)phosphine, dppm = 1,1-bis(diphenylphosphino)methane. bIsolated yield. cPd(OAc)2 5 mol %, PPh3 10 mol %. dPd(OAc)2 20 mol %, PPh3 40 mol %.
The optimized reaction conditions were then employed for the synthesis of analogs of 9 (Scheme 4). A variety of [3 + 2] cycloaddition adducts 5 bearing different R, R1, R2 and R3 groups, derived from amino esters 1, 2-bromobenzaldehydes 3 and maleimides 4, were subjected to N-allylation followed by intramolecular Heck reaction to pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinoline compounds 9a–o in moderate to good yields as a single isomers which were confirmed by 1H NMR analysis of the crude reaction mixtures. The substitution groups R3 (Me, Et, Ph, Bn, c-C6H11) on maleimide have no significant influence on the product yields to afford 9a–f in 73–80% yields. The substituent groups R2 including electron-donating (Me, OMe, -OCH2O) or -withdrawing groups (CF3, Cl) on the benzene ring have a little effect on the yield of products 9h–l. Product 9m bearing a naphthyl group was produced in 70% yield. Product 9n containing a pyridine ring was not obtained due to the low yield at the N-allylation step. Same result happened to 9o in which hindered iBu blocked the N-allylation.
Scheme 4: Synthesis of pyrrolo[2,1-a]isoquinolines 9. Reaction conditions: 5 (0.5 mmol, 1 equiv), 7 (3 equiv) and K2CO3 (2 equiv) in MeCN (3 mL) for N-allylation; then Pd(OAc)2 (10 mol %), PPh3 (20 mol %), K2CO3 (2 equiv) and NaOAc (1 equiv) in MeCN (3 mL) under nitrogen for the Heck reaction. Isolated yield.
Scheme 4: Synthesis of pyrrolo[2,1-a]isoquinolines 9. Reaction conditions: 5 (0.5 mmol, 1 equiv), 7 (3 equiv)...
We next employed intermediated 6 prepared from the decarboxylative [3+2] cycloaddition of amino acids for one-pot N-allylation and intramolecular Heck reactions under the same optimized conditions developed in Table 1. Pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinoline 11a–i were produced in 65–78% yields also as single isomers (Scheme 5).
Scheme 5: Synthesis of pyrrolo[2,1-a]isoquinolines 11. Reaction conditions: 6 (0.5 mmol, 1 equiv), 7 (3 equiv) and K2CO3 (2 equiv) in MeCN (3 mL) for the N-allylation; then Pd(OAc)2 (10 mol %), PPh3 (20 mol %), K2CO3 (2 equiv) and NaOAc (1 equiv) in MeCN (3 mL) under nitrogen for the Heck reaction. Isolated yield.
Scheme 5: Synthesis of pyrrolo[2,1-a]isoquinolines 11. Reaction conditions: 6 (0.5 mmol, 1 equiv), 7 (3 equiv...
Allylation of [3 + 2] adducts 5 or 6 with cinnamyl bromide were also conducted and the intermediates were used for the Heck reaction for making products 12a–d (Scheme 6). Even the allylated intermediates were not terminal alkenes, the Heck reaction gave the Z-products exclusively [52].
Scheme 6: Synthesis of pyrrolo[2,1-a]isoquinolines 12. Reaction conditions: 5 or 6 (0.5 mmol, 1 equiv), cinnamyl bromide (3 equiv) and K2CO3 (2 equiv) in MeCN (3 mL) for the N-allylation; then Pd(OAc)2 (10 mol %), PPh3 (20 mol %), K2CO3 (2 equiv) and NaOAc (1 equiv) in MeCN (3 mL) under nitrogen for the Heck reaction. Isolated yield.
Scheme 6: Synthesis of pyrrolo[2,1-a]isoquinolines 12. Reaction conditions: 5 or 6 (0.5 mmol, 1 equiv), cinna...
A general mechanism for Pd-catalyzed intramolecular Heck reaction of 8a for the synthesis of pyrrolo[2,1-a]isoquinoline 9a is shown in Scheme 7. The oxidative addition of the Pd(0) species to alkene intermediate 8a leads to Pd-complex I. Intramolecular coordination of Pd-complex I with the C–C double bond forms complex II which is followed by the syn insertion of alkene to give complex III [50,51]. Subsequent β-hydride elimination of III gives complex IV which undergoes dissociation to afford product 9a. The hydridopalladium(II) halide is converted to the catalytically active Pd(0) with a base.
Scheme 7: Plausible mechanism for the synthesis of 9a.
Scheme 7: Plausible mechanism for the synthesis of 9a.
Conclusion
In summary, we have developed an efficient method through a three-component [3 + 2] cycloaddition followed by a one-pot N-allylation and an intramolecular Heck reaction for the synthesis of pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinolines. Two different kinds of [3 + 2] adducts generated from the reactions of amino esters or amino acids were used as the key intermediates for sequential transformations. A high synthetic efficiency was achieved by the combination of a three-component reaction with one-pot reactions. This synthetic sequence is a new addition of our [3 + 2] cycloaddition-initiated reactions for making diverse cyclic scaffolds.
Experimental
General procedure for the synthesis of pyrrolidine adducts 5
A solution of amino ester 1 (1.2 mmol), 2-bromobenzaldehyde 3 (1 mmol) and maleimide 4 (1.1 mmol) in EtOH (3 mL) with Et3N (1.5 mmol) was heated at 110 °C for 6 h in a sealed vial. The concentrated reaction mixture was isolated by column chromatography on silica gel to afford adduct 5 in 85–90% yield.
General procedure for the synthesis of pyrrolidine adducts 6
A solution of 2-aminoisobutyric acid (2, 1.2 mmol), 2-bromobenzaldehyde 3 (1 mmol) and maleimide 4 (1 mmol) in MeCN (3 mL) with AcOH (0.3 mmol) was heated at 110 °C for 6 h in a sealed vial. The concentrated reaction mixture was isolated by column chromatography on silica gel to afford adduct 6 in 75–85% yield.
General procedure for the synthesis of pyrrolo[2,1-a]isoquinolines 9 or 11
To a solution of pyrrolidine adduct 5 or 6 (0.5 mmol), 3-bromopropene (7, 1.5 mmol) in MeCN (3 mL) was added K2CO3 (1 mmol), the mixture was heated at 105 °C for 4 h in a sealed vial. Upon the completion of reaction as monitored by HPLC or LC–MS, the mixture was evaporated under vacuum to remove unreacted 3-bromopropene to give crude N-allylation intermediate 8 or 10. Without further purification, it was used for the Heck reaction with Pd(OAc)2 (0.05 mmol), PPh3 (0.1 mmol), K2CO3 (1 mmol) and NaOAc (0.5 mmol) in MeCN (3 mL) at 105 °C for 3 h under nitrogen atmosphere. After aqueous work up, the crude product was purified by flash chromatography to afford product 9 or 11.
General procedure for the synthesis of pyrrolo[2,1-a]isoquinolines 12
To a solution of pyrrolidine adduct 5 or 6 (0.5 mmol), cinnamyl bromide (1.5 mmol) in MeCN (3 mL) was added K2CO3 (1 mmol), the mixture was heated at 105 °C for 4 h in a sealed vial. Upon the completion of reaction as monitored by HPLC or LC–MS, the mixture was evaporated and the unreacted cinnamyl bromide was isolated to give N-allylation intermediate which was then used for the Heck reaction with Pd(OAc)2 (10 mol %), PPh3 (20 mol %), K2CO3 (2 equiv) and NaOAc (1 equiv) in MeCN (3 mL) at 105 °C for 3 h under nitrogen atmosphere. After aqueous work-up, the crude product was purified by flash chromatography to afford product 12.
Supporting Information
| Supporting Information File 1: General reaction procedures, compound characterization data, and copies of NMR spectra. | ||
| Format: PDF | Size: 6.1 MB | Download |
References
-
Pässler, U.; Knöller, H. J. The pyrrolo[2,1-a]isoquinoline alkaloids. In The Alkaloids: Chemistry and Biology; Knöller, H. J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Vol. 70, pp 79–151. doi:10.1016/b978-0-12-391426-2.00002-5
Return to citation in text: [1] -
Fan, H.; Peng, J.; Hamann, M. T.; Hu, J.-F. Chem. Rev. 2008, 108, 264–287. doi:10.1021/cr078199m
Return to citation in text: [1] -
Zhang, Q.; Tu, G.; Zhao, Y.; Cheng, T. Tetrahedron 2002, 58, 6795–6798. doi:10.1016/s0040-4020(02)00792-5
Return to citation in text: [1] -
Zhang, F.; Simpkins, N. S.; Blake, A. J. Org. Biomol. Chem. 2009, 7, 1963–1979. doi:10.1039/b900189a
Return to citation in text: [1] -
Parsons, A. F.; Palframan, M. J. Erythrina and related alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G. A., Ed.; Academic Press: London, UK, 2010; Vol. 68, pp 39–81. doi:10.1016/s1099-4831(10)06802-1
Return to citation in text: [1] -
Andersen, R. J.; Faulkner, D. J.; He, C. H.; Van Duyne, G. D.; Clardy, J. J. Am. Chem. Soc. 1985, 107, 5492–5495. doi:10.1021/ja00305a027
Return to citation in text: [1] -
Reddy, M. V. R.; Rao, M. R.; Rhodes, D.; Hansen, M. S. T.; Rubins, K.; Bushman, F. D.; Venkateswarlu, Y.; Faulkner, D. J. J. Med. Chem. 1999, 42, 1901–1907. doi:10.1021/jm9806650
Return to citation in text: [1] -
Malla Reddy, S.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A. K.; Venkateswarlu, Y. Tetrahedron 2005, 61, 9242–9247. doi:10.1016/j.tet.2005.07.067
Return to citation in text: [1] -
Wang, R.-F.; Yang, X.-W.; Ma, C. M.; Cai, S.-Q.; Li, J.-N.; Shoyama, Y. Heterocycles 2004, 63, 1443–1448. doi:10.3987/com-04-10062
Return to citation in text: [1] -
Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phytother. Res. 2009, 23, 1032–1035. doi:10.1002/ptr.2742
Return to citation in text: [1] -
Lin, W.; Ma, S. Org. Chem. Front. 2017, 4, 958–966. doi:10.1039/c7qo00062f
Return to citation in text: [1] -
Umihara, H.; Yoshino, T.; Shimokawa, J.; Kitamura, M.; Fukuyama, T. Angew. Chem., Int. Ed. 2016, 55, 6915–6918. doi:10.1002/anie.201602650
Return to citation in text: [1] -
Komatsubara, M.; Umeki, T.; Fukuda, T.; Iwao, M. J. Org. Chem. 2014, 79, 529–537. doi:10.1021/jo402181w
Return to citation in text: [1] -
Kapat, A.; Kumar, P. S.; Baskaran, S. Beilstein J. Org. Chem. 2007, 3, No. 49. doi:10.1186/1860-5397-3-49
Return to citation in text: [1] -
Dondas, H. A.; Fishwick, C. W. G.; Gai, X.; Grigg, R.; Kilner, C.; Dumrongchai, N.; Kongkathip, B.; Kongkathip, N.; Polysuk, C.; Sridharan, V. Angew. Chem., Int. Ed. 2005, 44, 7570–7574. doi:10.1002/anie.200502066
Return to citation in text: [1] [2] -
Sun, H.; Tawa, G.; Wallqvist, A. Drug Discovery Today 2012, 17, 310–324. doi:10.1016/j.drudis.2011.10.024
Return to citation in text: [1] -
Hu, Y.; Stumpfe, D.; Bajorath, J. J. Med. Chem. 2017, 60, 1238–1246. doi:10.1021/acs.jmedchem.6b01437
Return to citation in text: [1] -
Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e
Return to citation in text: [1] -
Haji, M. Beilstein J. Org. Chem. 2016, 12, 1269–1301. doi:10.3762/bjoc.12.121
Return to citation in text: [1] -
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r
Return to citation in text: [1] -
Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484–4517. doi:10.1021/cr050011g
Return to citation in text: [1] -
Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182
Return to citation in text: [1] -
Amornraksa, K.; Grigg, R.; Gunaratne, H. Q. N.; Kemp, J.; Sridharan, V. J. Chem. Soc., Perkin Trans. 1 1987, 2285–2296. doi:10.1039/p19870002285
Return to citation in text: [1] -
Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c
Return to citation in text: [1] -
Harju, K.; Yli-Kauhaluoma, J. Mol. Diversity 2005, 9, 187–207. doi:10.1007/s11030-005-1339-1
Return to citation in text: [1] -
Zhang, W.; Zhang, X.; Ma, X.; Zhang, W. Tetrahedron Lett. 2018, 59, 3845–3847. doi:10.1016/j.tetlet.2018.09.023
Return to citation in text: [1] -
Tsuge, O.; Kanemasa, S.; Ohe, M.; Takenaka, S. Bull. Chem. Soc. Jpn. 1987, 60, 4079–4089. doi:10.1246/bcsj.60.4079
Return to citation in text: [1] -
Grigg, R.; Idle, J.; McMeekin, P.; Vipond, D. J. Chem. Soc., Chem. Commun. 1987, 49–51. doi:10.1039/c39870000049
Return to citation in text: [1] -
Grigg, R.; Surendrakumar, S.; Thianpatanagul, S.; Vipond, D. J. Chem. Soc., Perkin Trans. 1 1988, 2693–2701. doi:10.1039/p19880002693
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Xie, G.; Awad, J. M.; Zhang, W. Tetrahedron Lett. 2019, 60, 151127. doi:10.1016/j.tetlet.2019.151127
Return to citation in text: [1] [2] -
Zhang, W.; Lu, Y.; Hiu-Tung Chen, C.; Zeng, L.; Kassel, D. B. J. Comb. Chem. 2006, 8, 687–695. doi:10.1021/cc060061e
Return to citation in text: [1] -
Lu, Q.; Huang, X.; Song, G.; Sun, C.-M.; Jasinski, J. P.; Keeley, A. C.; Zhang, W. ACS Comb. Sci. 2013, 15, 350–355. doi:10.1021/co400026s
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Zhang, W. Green Chem. 2019, 21, 4489–4494. doi:10.1039/c9gc01642b
Return to citation in text: [1] -
Muthengi, A.; Zhang, X.; Dhawan, G.; Zhang, W.; Corsini, F.; Zhang, W. Green Chem. 2018, 20, 3134–3139. doi:10.1039/c8gc01099d
Return to citation in text: [1] -
Zhang, X.; Qiu, W.; Ma, X.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. J. Org. Chem. 2018, 83, 13536–13542. doi:10.1021/acs.joc.8b02046
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601
Return to citation in text: [1] [2] -
Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487
Return to citation in text: [1] -
Muthengi, A.; Erickson, J.; Muriph, R. E.; Zhang, W. J. Org. Chem. 2019, 84, 5927–5935. doi:10.1021/acs.joc.9b00448
Return to citation in text: [1] -
Grigg, R.; Coulter, T. Tetrahedron Lett. 1991, 32, 1359–1362. doi:10.1016/s0040-4039(00)79667-5
Return to citation in text: [1] -
Dumitrascu, F.; Caira, M. R.; Georgescu, E.; Georgescu, F.; Draghici, C.; Popa, M. M. Heteroat. Chem. 2011, 22, 723–729. doi:10.1002/hc.20740
Return to citation in text: [1] -
Boudriga, S.; Haddad, S.; Askri, M.; Soldera, A.; Knorr, M.; Strohmann, C.; Golz, C. RSC Adv. 2019, 9, 11082–11091. doi:10.1039/c8ra09884k
Return to citation in text: [1] -
Peng, W.; Zhu, S. J. Chem. Soc., Perkin Trans. 1 2001, 3204–3210. doi:10.1039/b103586j
Return to citation in text: [1] -
An, J.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Tetrahedron Lett. 2013, 54, 3834–3837. doi:10.1016/j.tetlet.2013.05.053
Return to citation in text: [1] -
Bastrakov, M. A.; Starosotnikov, A. M. Russ. Chem. Bull. 2019, 68, 1729–1734. doi:10.1007/s11172-019-2617-x
Return to citation in text: [1] -
Shang, Y.; Wang, L.; He, X.; Zhang, M. RSC Adv. 2012, 2, 7681–7688. doi:10.1039/c2ra21116e
Return to citation in text: [1] -
Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M. M.; Dumitrescu, D. Molecules 2013, 18, 2635–2645. doi:10.3390/molecules18032635
Return to citation in text: [1] -
Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c
Return to citation in text: [1] -
Muthusaravanan, S.; Perumal, S.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2010, 51, 6439–6443. doi:10.1016/j.tetlet.2010.09.128
Return to citation in text: [1] -
Link, J. T. The intramolecular heck reaction. In Organic Reactions; Overman, L. E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; Vol. 60, pp 157–213. doi:10.1002/0471264180.or060.02
Return to citation in text: [1] [2] [3] -
Bharath Kumar Reddy, P.; Ravi, K.; Mahesh, K.; Leelavathi, P. Tetrahedron Lett. 2018, 59, 4039–4043. doi:10.1016/j.tetlet.2018.09.068
Return to citation in text: [1] [2] [3] -
Wang, G.; Liu, C.; Li, B.; Wang, Y.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Tetrahedron 2017, 73, 6372–6380. doi:10.1016/j.tet.2017.09.034
Return to citation in text: [1] [2] -
Murru, S.; McGough, B.; Srivastava, R. S. Org. Biomol. Chem. 2014, 12, 9133–9138. doi:10.1039/c4ob01614a
Return to citation in text: [1] -
Hou, C.; Chen, H.; Xu, X.; Zhu, F.; Guo, L.; Jiang, M.; Yang, C.; Deng, L. Eur. J. Org. Chem. 2015, 3040–3043. doi:10.1002/ejoc.201500180
Return to citation in text: [1] -
Zhang, W.; Yi, W.-B. Pot, atom, and Step Economy (PASE) Synthesis; Springer: Cham, Switzerland, 2019. doi:10.1007/978-3-030-22596-4_1
Return to citation in text: [1] -
Clarke, P. A.; Santos, S.; Martin, W. H. C. Green Chem. 2007, 9, 438–440. doi:10.1039/b700923b
Return to citation in text: [1]
| 55. | Zhang, W.; Yi, W.-B. Pot, atom, and Step Economy (PASE) Synthesis; Springer: Cham, Switzerland, 2019. doi:10.1007/978-3-030-22596-4_1 |
| 56. | Clarke, P. A.; Santos, S.; Martin, W. H. C. Green Chem. 2007, 9, 438–440. doi:10.1039/b700923b |
| 41. | Dumitrascu, F.; Caira, M. R.; Georgescu, E.; Georgescu, F.; Draghici, C.; Popa, M. M. Heteroat. Chem. 2011, 22, 723–729. doi:10.1002/hc.20740 |
| 42. | Boudriga, S.; Haddad, S.; Askri, M.; Soldera, A.; Knorr, M.; Strohmann, C.; Golz, C. RSC Adv. 2019, 9, 11082–11091. doi:10.1039/c8ra09884k |
| 43. | Peng, W.; Zhu, S. J. Chem. Soc., Perkin Trans. 1 2001, 3204–3210. doi:10.1039/b103586j |
| 44. | An, J.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Tetrahedron Lett. 2013, 54, 3834–3837. doi:10.1016/j.tetlet.2013.05.053 |
| 45. | Bastrakov, M. A.; Starosotnikov, A. M. Russ. Chem. Bull. 2019, 68, 1729–1734. doi:10.1007/s11172-019-2617-x |
| 46. | Shang, Y.; Wang, L.; He, X.; Zhang, M. RSC Adv. 2012, 2, 7681–7688. doi:10.1039/c2ra21116e |
| 47. | Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M. M.; Dumitrescu, D. Molecules 2013, 18, 2635–2645. doi:10.3390/molecules18032635 |
| 48. | Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c |
| 49. | Muthusaravanan, S.; Perumal, S.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2010, 51, 6439–6443. doi:10.1016/j.tetlet.2010.09.128 |
| 50. | Link, J. T. The intramolecular heck reaction. In Organic Reactions; Overman, L. E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; Vol. 60, pp 157–213. doi:10.1002/0471264180.or060.02 |
| 51. | Bharath Kumar Reddy, P.; Ravi, K.; Mahesh, K.; Leelavathi, P. Tetrahedron Lett. 2018, 59, 4039–4043. doi:10.1016/j.tetlet.2018.09.068 |
| 52. | Wang, G.; Liu, C.; Li, B.; Wang, Y.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Tetrahedron 2017, 73, 6372–6380. doi:10.1016/j.tet.2017.09.034 |
| 53. | Murru, S.; McGough, B.; Srivastava, R. S. Org. Biomol. Chem. 2014, 12, 9133–9138. doi:10.1039/c4ob01614a |
| 54. | Hou, C.; Chen, H.; Xu, X.; Zhu, F.; Guo, L.; Jiang, M.; Yang, C.; Deng, L. Eur. J. Org. Chem. 2015, 3040–3043. doi:10.1002/ejoc.201500180 |
| 1. | Pässler, U.; Knöller, H. J. The pyrrolo[2,1-a]isoquinoline alkaloids. In The Alkaloids: Chemistry and Biology; Knöller, H. J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Vol. 70, pp 79–151. doi:10.1016/b978-0-12-391426-2.00002-5 |
| 2. | Fan, H.; Peng, J.; Hamann, M. T.; Hu, J.-F. Chem. Rev. 2008, 108, 264–287. doi:10.1021/cr078199m |
| 6. | Andersen, R. J.; Faulkner, D. J.; He, C. H.; Van Duyne, G. D.; Clardy, J. J. Am. Chem. Soc. 1985, 107, 5492–5495. doi:10.1021/ja00305a027 |
| 30. | Ma, X.; Zhang, X.; Xie, G.; Awad, J. M.; Zhang, W. Tetrahedron Lett. 2019, 60, 151127. doi:10.1016/j.tetlet.2019.151127 |
| 31. | Zhang, W.; Lu, Y.; Hiu-Tung Chen, C.; Zeng, L.; Kassel, D. B. J. Comb. Chem. 2006, 8, 687–695. doi:10.1021/cc060061e |
| 32. | Lu, Q.; Huang, X.; Song, G.; Sun, C.-M.; Jasinski, J. P.; Keeley, A. C.; Zhang, W. ACS Comb. Sci. 2013, 15, 350–355. doi:10.1021/co400026s |
| 33. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Zhang, W. Green Chem. 2019, 21, 4489–4494. doi:10.1039/c9gc01642b |
| 34. | Muthengi, A.; Zhang, X.; Dhawan, G.; Zhang, W.; Corsini, F.; Zhang, W. Green Chem. 2018, 20, 3134–3139. doi:10.1039/c8gc01099d |
| 35. | Zhang, X.; Qiu, W.; Ma, X.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. J. Org. Chem. 2018, 83, 13536–13542. doi:10.1021/acs.joc.8b02046 |
| 36. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392 |
| 37. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 38. | Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487 |
| 39. | Muthengi, A.; Erickson, J.; Muriph, R. E.; Zhang, W. J. Org. Chem. 2019, 84, 5927–5935. doi:10.1021/acs.joc.9b00448 |
| 5. | Parsons, A. F.; Palframan, M. J. Erythrina and related alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G. A., Ed.; Academic Press: London, UK, 2010; Vol. 68, pp 39–81. doi:10.1016/s1099-4831(10)06802-1 |
| 15. | Dondas, H. A.; Fishwick, C. W. G.; Gai, X.; Grigg, R.; Kilner, C.; Dumrongchai, N.; Kongkathip, B.; Kongkathip, N.; Polysuk, C.; Sridharan, V. Angew. Chem., Int. Ed. 2005, 44, 7570–7574. doi:10.1002/anie.200502066 |
| 40. | Grigg, R.; Coulter, T. Tetrahedron Lett. 1991, 32, 1359–1362. doi:10.1016/s0040-4039(00)79667-5 |
| 4. | Zhang, F.; Simpkins, N. S.; Blake, A. J. Org. Biomol. Chem. 2009, 7, 1963–1979. doi:10.1039/b900189a |
| 23. | Amornraksa, K.; Grigg, R.; Gunaratne, H. Q. N.; Kemp, J.; Sridharan, V. J. Chem. Soc., Perkin Trans. 1 1987, 2285–2296. doi:10.1039/p19870002285 |
| 24. | Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c |
| 25. | Harju, K.; Yli-Kauhaluoma, J. Mol. Diversity 2005, 9, 187–207. doi:10.1007/s11030-005-1339-1 |
| 26. | Zhang, W.; Zhang, X.; Ma, X.; Zhang, W. Tetrahedron Lett. 2018, 59, 3845–3847. doi:10.1016/j.tetlet.2018.09.023 |
| 3. | Zhang, Q.; Tu, G.; Zhao, Y.; Cheng, T. Tetrahedron 2002, 58, 6795–6798. doi:10.1016/s0040-4020(02)00792-5 |
| 27. | Tsuge, O.; Kanemasa, S.; Ohe, M.; Takenaka, S. Bull. Chem. Soc. Jpn. 1987, 60, 4079–4089. doi:10.1246/bcsj.60.4079 |
| 28. | Grigg, R.; Idle, J.; McMeekin, P.; Vipond, D. J. Chem. Soc., Chem. Commun. 1987, 49–51. doi:10.1039/c39870000049 |
| 29. | Grigg, R.; Surendrakumar, S.; Thianpatanagul, S.; Vipond, D. J. Chem. Soc., Perkin Trans. 1 1988, 2693–2701. doi:10.1039/p19880002693 |
| 11. | Lin, W.; Ma, S. Org. Chem. Front. 2017, 4, 958–966. doi:10.1039/c7qo00062f |
| 12. | Umihara, H.; Yoshino, T.; Shimokawa, J.; Kitamura, M.; Fukuyama, T. Angew. Chem., Int. Ed. 2016, 55, 6915–6918. doi:10.1002/anie.201602650 |
| 13. | Komatsubara, M.; Umeki, T.; Fukuda, T.; Iwao, M. J. Org. Chem. 2014, 79, 529–537. doi:10.1021/jo402181w |
| 14. | Kapat, A.; Kumar, P. S.; Baskaran, S. Beilstein J. Org. Chem. 2007, 3, No. 49. doi:10.1186/1860-5397-3-49 |
| 15. | Dondas, H. A.; Fishwick, C. W. G.; Gai, X.; Grigg, R.; Kilner, C.; Dumrongchai, N.; Kongkathip, B.; Kongkathip, N.; Polysuk, C.; Sridharan, V. Angew. Chem., Int. Ed. 2005, 44, 7570–7574. doi:10.1002/anie.200502066 |
| 18. | Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e |
| 19. | Haji, M. Beilstein J. Org. Chem. 2016, 12, 1269–1301. doi:10.3762/bjoc.12.121 |
| 20. | Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r |
| 52. | Wang, G.; Liu, C.; Li, B.; Wang, Y.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Tetrahedron 2017, 73, 6372–6380. doi:10.1016/j.tet.2017.09.034 |
| 10. | Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phytother. Res. 2009, 23, 1032–1035. doi:10.1002/ptr.2742 |
| 21. | Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484–4517. doi:10.1021/cr050011g |
| 22. | Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182 |
| 50. | Link, J. T. The intramolecular heck reaction. In Organic Reactions; Overman, L. E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; Vol. 60, pp 157–213. doi:10.1002/0471264180.or060.02 |
| 51. | Bharath Kumar Reddy, P.; Ravi, K.; Mahesh, K.; Leelavathi, P. Tetrahedron Lett. 2018, 59, 4039–4043. doi:10.1016/j.tetlet.2018.09.068 |
| 9. | Wang, R.-F.; Yang, X.-W.; Ma, C. M.; Cai, S.-Q.; Li, J.-N.; Shoyama, Y. Heterocycles 2004, 63, 1443–1448. doi:10.3987/com-04-10062 |
| 30. | Ma, X.; Zhang, X.; Xie, G.; Awad, J. M.; Zhang, W. Tetrahedron Lett. 2019, 60, 151127. doi:10.1016/j.tetlet.2019.151127 |
| 37. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 7. | Reddy, M. V. R.; Rao, M. R.; Rhodes, D.; Hansen, M. S. T.; Rubins, K.; Bushman, F. D.; Venkateswarlu, Y.; Faulkner, D. J. J. Med. Chem. 1999, 42, 1901–1907. doi:10.1021/jm9806650 |
| 8. | Malla Reddy, S.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A. K.; Venkateswarlu, Y. Tetrahedron 2005, 61, 9242–9247. doi:10.1016/j.tet.2005.07.067 |
| 16. | Sun, H.; Tawa, G.; Wallqvist, A. Drug Discovery Today 2012, 17, 310–324. doi:10.1016/j.drudis.2011.10.024 |
| 17. | Hu, Y.; Stumpfe, D.; Bajorath, J. J. Med. Chem. 2017, 60, 1238–1246. doi:10.1021/acs.jmedchem.6b01437 |
| 50. | Link, J. T. The intramolecular heck reaction. In Organic Reactions; Overman, L. E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; Vol. 60, pp 157–213. doi:10.1002/0471264180.or060.02 |
| 51. | Bharath Kumar Reddy, P.; Ravi, K.; Mahesh, K.; Leelavathi, P. Tetrahedron Lett. 2018, 59, 4039–4043. doi:10.1016/j.tetlet.2018.09.068 |
© 2020 Ma et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)