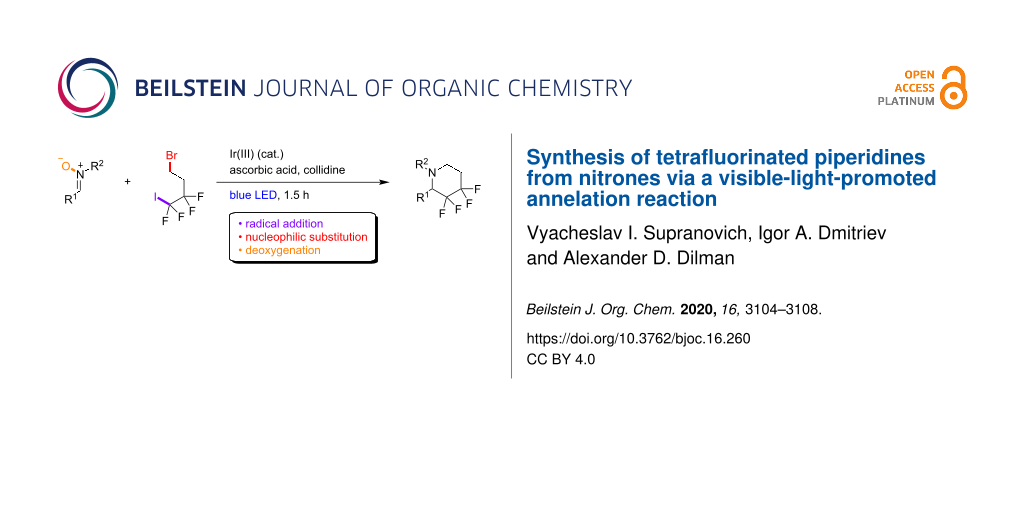
Abstract
A method for the one-step construction of 3,3,4,4-tetrafluorinated piperidines from nitrones and readily accessible tetrafluorinated iodobromobutane is described. The reaction requires an excess amount of ascorbic acid as the terminal reductant and is performed in the presence of an iridium photocatalyst activated by blue light. The annelation is a result of a radical addition at the nitrone, intramolecular nucleophilic substitution, and reduction of the N–O bond.
Graphical Abstract
Introduction
Nitrogen-containing heterocyclic compounds play an important role in pharmaceutical industry and related areas [1,2]. Among the variety of aromatic and saturated structures, the piperidine ring has a special role, as it is the most widely occurring form of nitrogen in FDA-approved drugs [3]. The ability of fluorine atoms to modify basicity, lipophilicity, as well as the hydrogen-bonding properties of amines [4,5] makes fluorinated piperidines [6,7] attractive targets in medicinal chemistry [8,9]. Previous efforts were mainly focused on the synthesis of mono- and difluorinated compounds. A single fluorine atom is typically introduced into a saturated cycle by nucleophilic fluorination reactions [10-17]. For compounds bearing gem-difluorinated fragments, an alternative pathway based on the construction of the cycle from building blocks may be considered [18]. However, the latter frequently implies multistep protocols, since two distinct bond-forming reactions are necessary for the cycle formation. While mono- and difluorinated piperidines are well known, tetrafluorinated piperidines are rare [19]. At the same time, the chemistry of compounds containing the tetrafluoroethylene fragment (CF2CF2) has advanced considerably over the last decade [20], and the use of preformed tetrafluorinated building blocks [21] provides the most efficient way of making these molecules.
Recently, we disclosed a photoredox method for the reductive radical fluoroalkylation of nitrones [22-24]. We have also evaluated the construction of fluorinated tetrahydroisoquinoline structures starting from nitrones, but this required four consecutive reactions with different conditions and chromatographic separation at each step [25] (Scheme 1). Herein we report a single-step protocol for the construction of fluorinated piperidines based on an accidentally discovered annelation reaction proceeding under reductive conditions.
Scheme 1: The construction of tetrafluorinated piperidines from nitrones.
Scheme 1: The construction of tetrafluorinated piperidines from nitrones.
Results and Discussion
Nitrone 1a was selected as a model substrate and it was combined with commercially available dibromide 2a under blue light irradiation in the presence of an iridium photocatalyst and stoichiometric quantities (1.2 equiv) of a reducing system (ascorbic acid/collidine). In this reaction no products were formed with reactant 2a remaining unconsumed (Table 1). To obtain a more reactive fluorinated halide, the bromine atom residing at the fluorinated moiety was exchanged for iodine by treatment with zinc, followed by the reaction with iodine monochloride. The iodide 2b reacted quite rapidly with the nitrone but the expected fluoroalkylation product was not observed. Instead, the tetrafluorinated piperidine 3a was obtained in a moderate yield (Table 1, entry 2). Apparently, after the fluoroalkylation event, the reduction of the N–O bond had occurred. The corresponding addition of an additional amount of the reductant and performing the reaction in DMF led to product 3a in 84% isolated yield (Table 1, entry 4).
Table 1: Optimization studies for the synthesis of 3a.
|
|
|||||
| entry | 2a | ascorbic acida | collidinea | solvent | yield, % |
| 1 | 2a, 1.2 | 1.2 | 1.2 | DMSO | – |
| 2 | 2b, 1.2 | 1.2 | 1.2 | DMSO | 56b |
| 3 | 2b, 1.5 | 2.5 | 3.5 | DMSO | 70b |
| 4 | 2b, 1.5 | 2.5 | 3.5 | DMF | 84c |
aEquivalents are shown; bdetermined by 19F NMR; cisolated yield.
Under the optimized conditions, a series of nitrones were involved in the reductive annelation reaction (Scheme 2). The process worked with nitrones containing alkyl, halogen, and electron-donating groups in the aromatic ring. With ester and cyano groups, the piperidines 3h and 3j were obtained in decreased yields, which may be tentatively attributed to a greater propensity of the corresponding nitrones towards nucleophilic addition of ascorbate. The nitrones derived from α-unbranched aliphatic aldehydes also provided the expected piperidines (products 3m–q) in reasonable yields. In case of 3n there was some unidentified side product formed under the standard conditions. In the previous research on the radical addition to nitrones we sometimes encountered a fluoroalkylation of the aromatic rings, if an excess of the fluorinated alkyl iodide was used [22-24]. This prompted us to attempt the reaction with an equimolar amount of the iodide 2b, which resulted in a cleaner process. However, the reaction of a nitrone obtained from cyclohexyl carboxaldehyde gave a complex mixture containing unidentified products. The structures of compounds 3f and 3h were established by X-ray analysis (CCDC, 3f: 2032382; 3h: 2032383).
Scheme 2: The scope of the annelation reaction for the synthesis of piperidines. Isolated yields are shown. a1.0 equiv of 2b was used.
Scheme 2: The scope of the annelation reaction for the synthesis of piperidines. Isolated yields are shown. a...
The proposed annelation mechanism is shown in Scheme 3. The iridium(III) photocatalyst under the action of light and ascorbic acid generates the iridium(II) species. The latter serves as a key reducing agent, and importantly, its formation is maintained throughout the process while an excess amount of ascorbate is present. The annelation likely starts from the addition of the fluorinated radical to the C=N bond followed by a conversion of the nitroxyl radical via hydrogen atom transfer [26,27] providing hydroxylamine 4. At the next stage, the intramolecular N-alkylation occurs leading to an N-oxide. This step of nucleophilic substitution could be catalyzed by iodide anions accumulating in the reaction mixture. Finally, the deoxygenation of the N-oxide fragment may proceed via consecutive protonation and electron-transfer steps [28].
Scheme 3: The proposed mechanism of the photoredox annelation reaction (asc = ascorbic acid).
Scheme 3: The proposed mechanism of the photoredox annelation reaction (asc = ascorbic acid).
Conclusion
In summary, a one-step method for the synthesis of tetrafluorinated piperidines starting from nitrones and a fluorinated building block is described. The annelation is based on a sequence of visible-light-promoted redox processes and a substitution reaction, and involves the cleavage of the N–O bond.
Supporting Information
| Supporting Information File 1: Full experimental details, compound characterization, X-ray data, and copies of NMR spectra. | ||
| Format: PDF | Size: 4.9 MB | Download |
| Supporting Information File 2: Crystallographic information file for compound 3f. | ||
| Format: CIF | Size: 1.8 MB | Download |
| Supporting Information File 3: Crystallographic information file for compound 3h. | ||
| Format: CIF | Size: 1.6 MB | Download |
References
-
Taylor, A. P.; Robinson, R. P.; Fobian, Y. M.; Blakemore, D. C.; Jones, L. H.; Fadeyi, O. Org. Biomol. Chem. 2016, 14, 6611–6637. doi:10.1039/c6ob00936k
Return to citation in text: [1] -
Wu, Y. J.; Gribble, G. W.; Joule, J. Heterocycles and Medicine: A Survey of the Heterocyclic Drugs Approved by the U.S. FDA from 2000 to Present. Progress in Heterocyclic Chemistry; Elsevier: Oxford, U.K., 2012; Vol. 24, pp 1–53. doi:10.1016/b978-0-08-096807-0.00001-4
Return to citation in text: [1] -
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b
Return to citation in text: [1] -
Morgenthaler, M.; Schweizer, E.; Hoffmann-Röder, A.; Benini, F.; Martin, R. E.; Jaeschke, G.; Wagner, B.; Fischer, H.; Bendels, S.; Zimmerli, D.; Schneider, J.; Diederich, F.; Kansy, M.; Müller, K. ChemMedChem 2007, 2, 1100–1115. doi:10.1002/cmdc.200700059
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Bhuma, N.; Burade, S. S.; Louat, T.; Herman, J.; Kawade, S.; Doshi, P. J.; Dhavale, D. D. Tetrahedron 2018, 74, 852–858. doi:10.1016/j.tet.2017.12.059
Return to citation in text: [1] -
Giacoboni, J.; Clausen, R. P.; Marigo, M. Synlett 2016, 27, 2803–2806. doi:10.1055/s-0036-1588313
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Zou, L.; Ruan, Y.; Jiang, W.; Yan, N.; Liu, D.-Y.; Yu, C.-Y.; Hu, X.-G. ChemistrySelect 2019, 4, 12683–12688. doi:10.1002/slct.201903403
Return to citation in text: [1] -
Yan, N.; Fang, Z.; Liu, Q.-Q.; Guo, X.-H.; Hu, X.-G. Org. Biomol. Chem. 2016, 14, 3469–3475. doi:10.1039/c6ob00063k
Return to citation in text: [1] -
Okoromoba, O. E.; Hammond, G. B.; Xu, B. Org. Lett. 2015, 17, 3975–3977. doi:10.1021/acs.orglett.5b01919
Return to citation in text: [1] -
Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75.
Return to citation in text: [1] -
Nielsen, M. K.; Ahneman, D. T.; Riera, O.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 5004–5008. doi:10.1021/jacs.8b01523
Return to citation in text: [1] -
Surmont, R.; Verniest, G.; De Weweire, A.; Thuring, J. W.; Macdonald, G.; Deroose, F.; De Kimpe, N. Synlett 2009, 1933–1936. doi:10.1055/s-0029-1217533
Return to citation in text: [1] -
González-Esguevillas, M.; Miró, J.; Jeffrey, J. L.; MacMillan, D. W. C. Tetrahedron 2019, 75, 4222–4227. doi:10.1016/j.tet.2019.05.043
Return to citation in text: [1] -
Nairoukh, Z.; Wollenburg, M.; Schlepphorst, C.; Bergander, K.; Glorius, F. Nat. Chem. 2019, 11, 264–270. doi:10.1038/s41557-018-0197-2
Return to citation in text: [1] -
Qing, F.-L.; Zheng, F. Synlett 2011, 1052–1072. doi:10.1055/s-0030-1259947
Return to citation in text: [1] -
Konno, T.; Hoshino, T.; Kida, T.; Takano, S.; Ishihara, T. J. Fluorine Chem. 2013, 152, 106–113. doi:10.1016/j.jfluchem.2013.02.013
Return to citation in text: [1] -
Václavík, J.; Klimánková, I.; Budinská, A.; Beier, P. Eur. J. Org. Chem. 2018, 3554–3593. doi:10.1002/ejoc.201701590
Return to citation in text: [1] -
Dmowski, W. J. Fluorine Chem. 2012, 142, 6–13. doi:10.1016/j.jfluchem.2012.06.018
Return to citation in text: [1] -
Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2018, 20, 840–843. doi:10.1021/acs.orglett.7b03987
Return to citation in text: [1] [2] -
Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Adv. Synth. Catal. 2018, 360, 3788–3792. doi:10.1002/adsc.201800802
Return to citation in text: [1] [2] -
Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Dilman, A. D. Eur. J. Org. Chem. 2019, 4119–4122. doi:10.1002/ejoc.201900274
Return to citation in text: [1] [2] -
Supranovich, V. I.; Dilman, A. D. Mendeleev Commun. 2019, 29, 515–516. doi:10.1016/j.mencom.2019.09.012
Return to citation in text: [1] -
Warren, J. J.; Mayer, J. M. J. Am. Chem. Soc. 2008, 130, 7546–7547. doi:10.1021/ja802055t
Return to citation in text: [1] -
Warren, J. J.; Mayer, J. M. J. Am. Chem. Soc. 2010, 132, 7784–7793. doi:10.1021/ja102337n
Return to citation in text: [1] -
Jeong, J.; Suzuki, K.; Hibino, M.; Yamaguchi, K.; Mizuno, N. ChemistrySelect 2016, 1, 5042–5048. doi:10.1002/slct.201601146
Return to citation in text: [1]
| 1. | Taylor, A. P.; Robinson, R. P.; Fobian, Y. M.; Blakemore, D. C.; Jones, L. H.; Fadeyi, O. Org. Biomol. Chem. 2016, 14, 6611–6637. doi:10.1039/c6ob00936k |
| 2. | Wu, Y. J.; Gribble, G. W.; Joule, J. Heterocycles and Medicine: A Survey of the Heterocyclic Drugs Approved by the U.S. FDA from 2000 to Present. Progress in Heterocyclic Chemistry; Elsevier: Oxford, U.K., 2012; Vol. 24, pp 1–53. doi:10.1016/b978-0-08-096807-0.00001-4 |
| 8. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 9. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 28. | Jeong, J.; Suzuki, K.; Hibino, M.; Yamaguchi, K.; Mizuno, N. ChemistrySelect 2016, 1, 5042–5048. doi:10.1002/slct.201601146 |
| 6. | Bhuma, N.; Burade, S. S.; Louat, T.; Herman, J.; Kawade, S.; Doshi, P. J.; Dhavale, D. D. Tetrahedron 2018, 74, 852–858. doi:10.1016/j.tet.2017.12.059 |
| 7. | Giacoboni, J.; Clausen, R. P.; Marigo, M. Synlett 2016, 27, 2803–2806. doi:10.1055/s-0036-1588313 |
| 4. | Morgenthaler, M.; Schweizer, E.; Hoffmann-Röder, A.; Benini, F.; Martin, R. E.; Jaeschke, G.; Wagner, B.; Fischer, H.; Bendels, S.; Zimmerli, D.; Schneider, J.; Diederich, F.; Kansy, M.; Müller, K. ChemMedChem 2007, 2, 1100–1115. doi:10.1002/cmdc.200700059 |
| 5. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 22. | Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2018, 20, 840–843. doi:10.1021/acs.orglett.7b03987 |
| 23. | Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Adv. Synth. Catal. 2018, 360, 3788–3792. doi:10.1002/adsc.201800802 |
| 24. | Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Dilman, A. D. Eur. J. Org. Chem. 2019, 4119–4122. doi:10.1002/ejoc.201900274 |
| 3. | Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b |
| 26. | Warren, J. J.; Mayer, J. M. J. Am. Chem. Soc. 2008, 130, 7546–7547. doi:10.1021/ja802055t |
| 27. | Warren, J. J.; Mayer, J. M. J. Am. Chem. Soc. 2010, 132, 7784–7793. doi:10.1021/ja102337n |
| 20. | Václavík, J.; Klimánková, I.; Budinská, A.; Beier, P. Eur. J. Org. Chem. 2018, 3554–3593. doi:10.1002/ejoc.201701590 |
| 22. | Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2018, 20, 840–843. doi:10.1021/acs.orglett.7b03987 |
| 23. | Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Adv. Synth. Catal. 2018, 360, 3788–3792. doi:10.1002/adsc.201800802 |
| 24. | Dmitriev, I. A.; Supranovich, V. I.; Levin, V. V.; Dilman, A. D. Eur. J. Org. Chem. 2019, 4119–4122. doi:10.1002/ejoc.201900274 |
| 19. | Konno, T.; Hoshino, T.; Kida, T.; Takano, S.; Ishihara, T. J. Fluorine Chem. 2013, 152, 106–113. doi:10.1016/j.jfluchem.2013.02.013 |
| 25. | Supranovich, V. I.; Dilman, A. D. Mendeleev Commun. 2019, 29, 515–516. doi:10.1016/j.mencom.2019.09.012 |
| 10. | Zou, L.; Ruan, Y.; Jiang, W.; Yan, N.; Liu, D.-Y.; Yu, C.-Y.; Hu, X.-G. ChemistrySelect 2019, 4, 12683–12688. doi:10.1002/slct.201903403 |
| 11. | Yan, N.; Fang, Z.; Liu, Q.-Q.; Guo, X.-H.; Hu, X.-G. Org. Biomol. Chem. 2016, 14, 3469–3475. doi:10.1039/c6ob00063k |
| 12. | Okoromoba, O. E.; Hammond, G. B.; Xu, B. Org. Lett. 2015, 17, 3975–3977. doi:10.1021/acs.orglett.5b01919 |
| 13. | Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75. |
| 14. | Nielsen, M. K.; Ahneman, D. T.; Riera, O.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 5004–5008. doi:10.1021/jacs.8b01523 |
| 15. | Surmont, R.; Verniest, G.; De Weweire, A.; Thuring, J. W.; Macdonald, G.; Deroose, F.; De Kimpe, N. Synlett 2009, 1933–1936. doi:10.1055/s-0029-1217533 |
| 16. | González-Esguevillas, M.; Miró, J.; Jeffrey, J. L.; MacMillan, D. W. C. Tetrahedron 2019, 75, 4222–4227. doi:10.1016/j.tet.2019.05.043 |
| 17. | Nairoukh, Z.; Wollenburg, M.; Schlepphorst, C.; Bergander, K.; Glorius, F. Nat. Chem. 2019, 11, 264–270. doi:10.1038/s41557-018-0197-2 |
| 21. | Dmowski, W. J. Fluorine Chem. 2012, 142, 6–13. doi:10.1016/j.jfluchem.2012.06.018 |
© 2020 Supranovich et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)