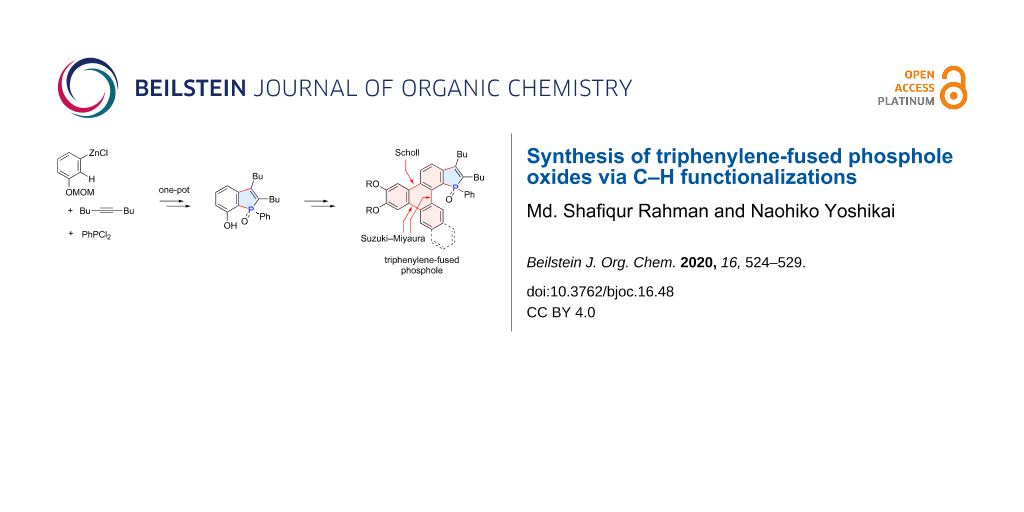
Abstract
The synthesis of triphenylene-fused phosphole oxides has been achieved through two distinct C–H functionalization reactions as key steps. The phosphole ring was constructed by a three-component coupling of 3-(methoxymethoxy)phenylzinc chloride, an alkyne, and dichlorophenylphosphine, involving the regioselective C–H activation of the C2 position of the arylzinc intermediate via 1,4-cobalt migration. The resulting 7-hydroxybenzo[b]phosphole derivative was used for further π-extension through Suzuki–Miyaura couplings and a Scholl reaction, the latter closing the triphenylene ring. The absorption and emission spectra of the thus-synthesized compounds illustrated their nature as hybrids of triphenylene and benzo[b]phosphole.
Graphical Abstract
Introduction
The phosphorus-containing five-membered ring, phosphole, has attracted significant attention as a structural motif in π-conjugated functional molecules [1-9]. Its inherently unique electronic structure, along with opportunities to modify the phosphorus center and the periphery by substitution or ring fusion, have stimulated chemists to explore a structurally diverse range of phosphole derivatives with extended π-system. These included, in particular, those fused with polycyclic aromatic hydrocarbons (PAHs) for possible applications in organic electronics, bioimaging and sensing, and asymmetric catalysis (Figure 1). To name a few examples, Yamaguchi et al. described synthetic routes to novel phosphorus-containing ladder molecules and their application as fluorescence probes for biological imaging [10,11]. Marinetti extensively studied the synthesis of phosphahelicenes with linear fusion of the phosphole and the carbohelicene units [12], and their applications in asymmetric catalysis [13] and organic light-emitting diodes [14]. Recently, Saito et al. reported the synthesis of phosphorus-bridged triphenylenes, that is, triphosphasumanene trisulfides, and demonstrated their capability as a junction for single-molecule conductors [15].
Figure 1: Examples of functional molecules based on π-extended phospholes.
Figure 1: Examples of functional molecules based on π-extended phospholes.
The synthesis of PAH-fused phospholes typically requires efficient methods for the construction of the phosphole ring as well as for the π-extension/fusion of the PAH moiety, which should be smoothly implemented into the overall synthetic planning. In this context, we have recently reported the synthesis of novel phosphahelicenes that featured angular fusion of the phosphole and the carbohelicene moieties (Scheme 1) [16]. The approach focused on the regioselective one-pot synthesis of a 7-hydroxybenzo[b]phosphole derivative from an 3-alkoxyphenylzinc reagent, an alkyne, and dichlorophenylphosphine [17]. The hydroxy group of this key intermediate served as a handle for the π-extension through a Suzuki–Miyaura coupling, Sonogashira coupling, and electrophilic alkyne carbocyclization [18].
Scheme 1: Syntheses of PAH-fused phospholes featuring a 7-hydroxybenzo[b]phosphole as a key intermediate.
Scheme 1: Syntheses of PAH-fused phospholes featuring a 7-hydroxybenzo[b]phosphole as a key intermediate.
Given the successful synthesis of the angularly fused phosphahelicenes, we became interested in the further exploitation of 7-hydroxybenzo[b]phosphole as an intermediate for the synthesis of π-extended phospholes. In this respect, our attention focused on the fusion of phosphole with triphenylene, which represents one of the most common disc-like PAH motifs in organic materials chemistry [19-25]. Herein, we report on the synthesis of triphenylene-fused phosphole oxides, which are distinct from Saito’s compounds [15] as well as from other reported examples [26-28] in terms of the mode of fusion of the phosphole and triphenylene units. The present phosphole/triphenylene hybrid molecules displayed absorption and emission profiles that reflected the characteristics of both triphenylene and benzo[b]phosphole.
Results and Discussion
The present synthetic study commenced with the recently reported preparation of 7-hydroxybenzo[b]phosphole derivative 3 from 3-(methoxymethoxy)phenylzinc (1), 5-decyne (2), and PhPCl2 in the presence of a cobalt–diphosphine catalyst (Scheme 2). This one-pot construction of the benzo[b]phosphole core ensured the preferential phosphole ring closure in proximity of the alkoxy group of the arylzinc reagent 1 (regioselectivity of ≈3:1), presumably due to a secondary interaction between the MOM group and the cobalt catalyst during the key C–H activation step, i.e., 1,4-cobalt migration in the alkenylcobalt intermediate [29]. The oxidation of the benzo[b]phosphole phosphorous atom and cleavage of the MOM group took place simultaneously, and thus afforded compound 3 in 33% yield on a 5 mmol scale [16]. Compound 3 was then converted to the triflate, and subjected to Suzuki–Miyaura couplings with 2-bromophenylboronic acid (5a) or 3-bromonaphth-2-ylboronic acid (5b) to afford the phosphole-fused biaryls 6a and 6b, respectively, in decent yields. Subsequent Suzuki–Miyaura couplings of 6a or 6b with 3,4-dialkoxyarylboronic acids furnished the phosphole-fused ortho-teraryl products 7a–c in moderate to high yields.
Scheme 2: Synthesis of phosphole-fused ortho-teraryl compounds 7.
Scheme 2: Synthesis of phosphole-fused ortho-teraryl compounds 7.
With the phosphole-fused ortho-teraryl compounds 7 in hand, we next examined their cyclization into triphenylene derivatives by the Scholl reaction (Scheme 3) [30]. The reaction of 7a (0.1 mmol) in the presence of [bis(trifluoroacetoxy)iodo]benzene] (PIFA) and BF3·OEt2 in dichloromethane at −78 °C afforded, after 12 h, the desired cyclized product 8a in 59% yield. The reaction could be performed on a 0.5 mmol scale in a similar yield of 58%. Note that other typical reagents used for the Scholl reaction, such as DDQ/CF3CO2H, FeCl3, Cu(OTf)2, and AlCl3 failed to promote the cyclization of 7a to 8a. The PIFA/BF3·OEt2 system also promoted the Scholl reaction of terphenyl 7b bearing a methylenedioxy moiety with a comparable efficiency to afford 8b in 56% yield. Compound 8c, a naphthylene-linked analogue of 8a, also underwent cyclization under the same conditions to give the corresponding product 8c albeit in a somewhat lower yield of 40%.
Scheme 3: Oxidative cyclization of phosphole-fused ortho-teraryl compounds 7 into triphenylene-fused phosphole oxides 8. The yields in parentheses were obtained in a 0.5 mmol-scale reaction.
Scheme 3: Oxidative cyclization of phosphole-fused ortho-teraryl compounds 7 into triphenylene-fused phosphol...
The triphenylene-fused phosphole oxide 8a was recrystallized from CH2Cl2, and the molecular structure was unambiguously confirmed by single crystal X-ray analysis (Figure 2) [31]. As can be seen from the side view, the triphenylene moiety slightly deviated from planarity, because the fusion with the phosphole ring caused a subtle steric repulsion between the phosphorus substituents and the triphenylene edge within the phospha[4]helicene moiety. Unlike many triphenylene derivatives, the crystal packing of 8a did not involve columnar π–π stacking of the PAH moiety (Figure S1, Supporting Information File 1). This is likely due to the fact that such π-stacking is inhibited by the steric bulk of the phosphole substituents (i.e., the butyl groups, the phenyl group, and the oxygen atom).
![[1860-5397-16-48-2]](/bjoc/content/figures/1860-5397-16-48-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP drawings of compound 8a (thermal ellipsoids set at 50% probability). a) top view; b) side view).
Figure 2: ORTEP drawings of compound 8a (thermal ellipsoids set at 50% probability). a) top view; b) side vie...
Upon the successful synthesis of the triphenylene-fused phosphole oxides 8, we studied the absorption and emission properties of these compounds in CH2Cl2 solution. Figure 3 shows the absorption and emission spectra of compounds 8a–c, and Table 1 provides a summary of these spectra and reported spectral data of structurally related benzo[b]phosphole and triphenylene derivatives. The optical data illustrate the nature of compounds 8a–c as hybrids of triphenylene and benzo[b]phosphole oxide. Regarding the absorption, 8a and 8b displayed multiple absorption bands from 250–400 nm (Table 1, entries 1 and 2), which reflected the characteristics of PAHs including triphenylene derivatives (Table 1, entries 5 and 6) [32-34], rather than the 2,3-dialkylbenzo[b]phosphole core (Table 1, entry 4). As expected, the absorption of 8c showed a bathochromic shift compared to 8a and 8b as a result of π-extension (Table 1, entry 3). Like many 2,3-dialkylbenzo[b]phospholes [17,35], 8a–c showed strong fluorescence in solution (ΦF = 0.67 and 0.34 for 8a and 8b, respectively). In contrast to the highly resolved bands in the absorption spectra, the fluorescence spectra were rather simple, with distinct emission peaks at 452 nm (8a), 450 nm (8b), and 477 nm (8c). Such a behavior was distinct from the fluorescence of the parent triphenylene and 2,3-dialkoxytriphenylene, which have been reported to show multiple emission peaks (Table 1, entries 5 and 6).
![[1860-5397-16-48-3]](/bjoc/content/figures/1860-5397-16-48-3.png?scale=2.4&max-width=1024&background=FFFFFF)
Figure 3: UV–vis absorption (solid lines) and fluorescence (dashed lines) spectra of compounds 8a–c.
Figure 3: UV–vis absorption (solid lines) and fluorescence (dashed lines) spectra of compounds 8a–c.
Table 1: Summary of the absorption and emission spectra.a
| entry | compound | λabs (nm)b | log εmaxc | λem (nm) | ΦFd |
| 1 | 8a | 289, 330, 393 (sh) | 4.16 | 452 | 0.67 |
| 2 | 8b | 284, 324, 387 (sh) | 4.27 | 450 | 0.34 |
| 3 | 8c | 301, 361, 420 (sh) | 4.41 | 477 | – |
| 4e | BP | 320 | 3.27 | 387 | 0.11 |
| 5f | TP | 260, 285, 320 (sh) | 4.23 | 355, 364, 371 | 0.02 |
| 6g | TP(OC12H25)2 | 277, 298, 356 | – | 364, 382 | – |
aMeasured in CH2Cl2 at 5 × 10−6 M. bRepresentative absorption maxima (sh stands for a shoulder peak). cMolar absorption coefficient for the longest-wavelength absorption maximum (except the shoulder). dDetermined using quinine sulfate as the standard (54% in 0.1 M H2SO4). eBP = 1-phenyl-2,3-dibutylbenzo[b]phosphole oxide. Data taken from [17]. fTP = triphenylene. Data taken from [32] (λabs and log ε) and [33] (λem and ΦF). gTP(OC12H25)2 = 2,3-di(n-dodecyloxy)triphenylene. Data taken from [34].
Conclusion
In summary, we synthesized novel triphenylene-fused phosphole oxides through C–H functionalization and cross-coupling reactions. The phosphole ring was constructed in the early stage of the synthesis by a three-component assembly method featuring a 1,4-cobalt migration as the key step. Unlike other C–H activation/alkyne annulation approaches to benzo[b]phospholes [36-40], this three-component method guarantees a good regioselectivity for the formation of the desired 7-hydroxybenzo[b]phosphole derivatives. The triphenylene moiety was completed in the last step through a Scholl reaction. The synthesized triphenylene-fused phosphole oxides showed strong blue fluorescence in solution. The absorption and emission profiles of the π-extended phosphole oxide revealed their characteristics as hybrids of 2,3-dialkoxytriphenylene and 1-phenyl-2,3-dialkylbenzo[b]phosphole. We anticipate that the key intermediate of the present synthesis, 3, and related benzo[b]phospholes accessible by the three-component assembly hold promise for further explorations inot novel π-extended phosphole derivatives.
References
-
Baumgartner, T.; Réau, R. Chem. Rev. 2006, 106, 4681–4727. doi:10.1021/cr040179m
Return to citation in text: [1] -
Crassous, J.; Réau, R. Dalton Trans. 2008, 6865–6876. doi:10.1039/b810976a
Return to citation in text: [1] -
Matano, Y.; Imahori, H. Org. Biomol. Chem. 2009, 7, 1258–1271. doi:10.1039/b819255n
Return to citation in text: [1] -
Ren, Y.; Baumgartner, T. Dalton Trans. 2012, 41, 7792–7800. doi:10.1039/c2dt00024e
Return to citation in text: [1] -
Baumgartner, T. Acc. Chem. Res. 2014, 47, 1613–1622. doi:10.1021/ar500084b
Return to citation in text: [1] -
Stolar, M.; Baumgartner, T. Chem. – Asian J. 2014, 9, 1212–1225. doi:10.1002/asia.201301670
Return to citation in text: [1] -
Duffy, M. P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. Chem. Soc. Rev. 2016, 45, 5296–5310. doi:10.1039/c6cs00257a
Return to citation in text: [1] -
Shameem, M. A.; Orthaber, A. Chem. – Eur. J. 2016, 22, 10718–10735. doi:10.1002/chem.201600005
Return to citation in text: [1] -
Hibner-Kulicka, P.; Joule, J. A.; Skalik, J.; Bałczewski, P. RSC Adv. 2017, 7, 9194–9236. doi:10.1039/c6ra26333j
Return to citation in text: [1] -
Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Higashiyama, T.; Yamaguchi, S. Angew. Chem., Int. Ed. 2015, 54, 15213–15217. doi:10.1002/anie.201507939
Return to citation in text: [1] -
Wang, C.; Taki, M.; Sato, Y.; Fukazawa, A.; Higashiyama, T.; Yamaguchi, S. J. Am. Chem. Soc. 2017, 139, 10374–10381. doi:10.1021/jacs.7b04418
Return to citation in text: [1] -
Yavari, K.; Moussa, S.; Ben Hassine, B.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem., Int. Ed. 2012, 51, 6748–6752. doi:10.1002/anie.201202024
Return to citation in text: [1] -
Yavari, K.; Aillard, P.; Zhang, Y.; Nuter, F.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem., Int. Ed. 2014, 53, 861–865. doi:10.1002/anie.201308377
Return to citation in text: [1] -
Yavari, K.; Delaunay, W.; De Rycke, N.; Reynaldo, T.; Aillard, P.; Srebro-Hooper, M.; Chang, V. Y.; Muller, G.; Tondelier, D.; Geffroy, B.; Voituriez, A.; Marinetti, A.; Hissler, M.; Crassous, J. Chem. – Eur. J. 2019, 25, 5303–5310. doi:10.1002/chem.201806140
Return to citation in text: [1] -
Furukawa, S.; Suda, Y.; Kobayashi, J.; Kawashima, T.; Tada, T.; Fujii, S.; Kiguchi, M.; Saito, M. J. Am. Chem. Soc. 2017, 139, 5787–5792. doi:10.1021/jacs.6b12119
Return to citation in text: [1] [2] -
Rahman, M. S.; Yoshikai, N. Org. Lett. 2019, 21, 3232–3236. doi:10.1021/acs.orglett.9b00955
Return to citation in text: [1] [2] -
Wu, B.; Santra, M.; Yoshikai, N. Angew. Chem., Int. Ed. 2014, 53, 7543–7546. doi:10.1002/anie.201404019
Return to citation in text: [1] [2] [3] -
Wakatsuki, A.; Yukimoto, M.; Minoura, M.; Fujii, K.; Kimura, Y.; Matano, Y. Dalton Trans. 2018, 47, 7123–7127. doi:10.1039/c8dt01503a
Return to citation in text: [1] -
Watson, M. D.; Fechtenkötter, A.; Müllen, K. Chem. Rev. 2001, 101, 1267–1300. doi:10.1021/cr990322p
Return to citation in text: [1] -
Pérez, D.; Guitián, E. Chem. Soc. Rev. 2004, 33, 274–283. doi:10.1039/b305549n
Return to citation in text: [1] -
Kumar, S. Chem. Soc. Rev. 2006, 35, 83–109. doi:10.1039/b506619k
Return to citation in text: [1] -
Sergeyev, S.; Pisula, W.; Geerts, Y. H. Chem. Soc. Rev. 2007, 36, 1902–1929. doi:10.1039/b417320c
Return to citation in text: [1] -
Alam, M. A.; Motoyanagi, J.; Yamamoto, Y.; Fukushima, T.; Kim, J.; Kato, K.; Takata, M.; Saeki, A.; Seki, S.; Tagawa, S.; Aida, T. J. Am. Chem. Soc. 2009, 131, 17722–17723. doi:10.1021/ja905373d
Return to citation in text: [1] -
Percec, V.; Imam, M. R.; Peterca, M.; Wilson, D. A.; Graf, R.; Spiess, H. W.; Balagurusamy, V. S. K.; Heiney, P. A. J. Am. Chem. Soc. 2009, 131, 7662–7677. doi:10.1021/ja8094944
Return to citation in text: [1] -
Badjic, J. D.; Ronconi, C. M.; Stoddart, J. F.; Balzani, V.; Silvi, S.; Credi, A. J. Am. Chem. Soc. 2006, 128, 1489–1499. doi:10.1021/ja0543954
Return to citation in text: [1] -
Wang, S.; Yan, C.; Shang, J.; Wang, W.; Yuan, C.; Zhang, H.-L.; Shao, X. Angew. Chem., Int. Ed. 2019, 58, 3819–3823. doi:10.1002/anie.201813070
Return to citation in text: [1] -
Wu, B.; Chopra, R.; Yoshikai, N. Org. Lett. 2015, 17, 5666–5669. doi:10.1021/acs.orglett.5b02950
Return to citation in text: [1] -
Aillard, P.; Gicquel, M.; Yavari, K.; Retailleau, P.; Voituriez, A.; Marinetti, A. Eur. J. Org. Chem. 2018, 5853–5860. doi:10.1002/ejoc.201800438
Return to citation in text: [1] -
Tan, B.-H.; Dong, J.; Yoshikai, N. Angew. Chem., Int. Ed. 2012, 51, 9610–9614. doi:10.1002/anie.201204388
Return to citation in text: [1] -
Grzybowski, M.; Skonieczny, K.; Butenschön, H.; Gryko, D. T. Angew. Chem., Int. Ed. 2013, 52, 9900–9930. doi:10.1002/anie.201210238
Return to citation in text: [1] -
CCDC 1984209 (8a) contains crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Return to citation in text: [1] -
Rieger, R.; Müllen, K. J. Phys. Org. Chem. 2010, 23, 315–325. doi:10.1002/poc.1644
Return to citation in text: [1] [2] -
Verbitskiy, E. V.; Eltsov, O. S.; Zhilina, E. F.; Pakhomov, I. M.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Tetrahedron 2019, 75, 2687–2696. doi:10.1016/j.tet.2019.03.044
Return to citation in text: [1] [2] -
Schwab, M. G.; Qin, T.; Pisula, W.; Mavrinskiy, A.; Feng, X.; Baumgarten, M.; Kim, H.; Laquai, F.; Schuh, S.; Trattnig, R.; List, E. J. W.; Müllen, K. Chem. – Asian J. 2011, 6, 3001–3010. doi:10.1002/asia.201100258
Return to citation in text: [1] [2] -
Yoshikai, N.; Santra, M.; Wu, B. Organometallics 2017, 36, 2637–2645. doi:10.1021/acs.organomet.7b00244
Return to citation in text: [1] -
Wu, B.; Yoshikai, N. Org. Biomol. Chem. 2016, 14, 5402–5416. doi:10.1039/c6ob00219f
Return to citation in text: [1] -
Chen, Y.-R.; Duan, W.-L. J. Am. Chem. Soc. 2013, 135, 16754–16757. doi:10.1021/ja407373g
Return to citation in text: [1] -
Unoh, Y.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 12975–12979. doi:10.1002/anie.201307211
Return to citation in text: [1] -
Quint, V.; Morlet-Savary, F.; Lohier, J.-F.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S. J. Am. Chem. Soc. 2016, 138, 7436–7441. doi:10.1021/jacs.6b04069
Return to citation in text: [1] -
Liu, W.-Q.; Lei, T.; Zhou, S.; Yang, X.-L.; Li, J.; Chen, B.; Sivaguru, J.; Tung, C.-H.; Wu, L.-Z. J. Am. Chem. Soc. 2019, 141, 13941–13947. doi:10.1021/jacs.9b06920
Return to citation in text: [1]
| 17. | Wu, B.; Santra, M.; Yoshikai, N. Angew. Chem., Int. Ed. 2014, 53, 7543–7546. doi:10.1002/anie.201404019 |
| 32. | Rieger, R.; Müllen, K. J. Phys. Org. Chem. 2010, 23, 315–325. doi:10.1002/poc.1644 |
| 33. | Verbitskiy, E. V.; Eltsov, O. S.; Zhilina, E. F.; Pakhomov, I. M.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Tetrahedron 2019, 75, 2687–2696. doi:10.1016/j.tet.2019.03.044 |
| 34. | Schwab, M. G.; Qin, T.; Pisula, W.; Mavrinskiy, A.; Feng, X.; Baumgarten, M.; Kim, H.; Laquai, F.; Schuh, S.; Trattnig, R.; List, E. J. W.; Müllen, K. Chem. – Asian J. 2011, 6, 3001–3010. doi:10.1002/asia.201100258 |
| 17. | Wu, B.; Santra, M.; Yoshikai, N. Angew. Chem., Int. Ed. 2014, 53, 7543–7546. doi:10.1002/anie.201404019 |
| 35. | Yoshikai, N.; Santra, M.; Wu, B. Organometallics 2017, 36, 2637–2645. doi:10.1021/acs.organomet.7b00244 |
| 1. | Baumgartner, T.; Réau, R. Chem. Rev. 2006, 106, 4681–4727. doi:10.1021/cr040179m |
| 2. | Crassous, J.; Réau, R. Dalton Trans. 2008, 6865–6876. doi:10.1039/b810976a |
| 3. | Matano, Y.; Imahori, H. Org. Biomol. Chem. 2009, 7, 1258–1271. doi:10.1039/b819255n |
| 4. | Ren, Y.; Baumgartner, T. Dalton Trans. 2012, 41, 7792–7800. doi:10.1039/c2dt00024e |
| 5. | Baumgartner, T. Acc. Chem. Res. 2014, 47, 1613–1622. doi:10.1021/ar500084b |
| 6. | Stolar, M.; Baumgartner, T. Chem. – Asian J. 2014, 9, 1212–1225. doi:10.1002/asia.201301670 |
| 7. | Duffy, M. P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. Chem. Soc. Rev. 2016, 45, 5296–5310. doi:10.1039/c6cs00257a |
| 8. | Shameem, M. A.; Orthaber, A. Chem. – Eur. J. 2016, 22, 10718–10735. doi:10.1002/chem.201600005 |
| 9. | Hibner-Kulicka, P.; Joule, J. A.; Skalik, J.; Bałczewski, P. RSC Adv. 2017, 7, 9194–9236. doi:10.1039/c6ra26333j |
| 14. | Yavari, K.; Delaunay, W.; De Rycke, N.; Reynaldo, T.; Aillard, P.; Srebro-Hooper, M.; Chang, V. Y.; Muller, G.; Tondelier, D.; Geffroy, B.; Voituriez, A.; Marinetti, A.; Hissler, M.; Crassous, J. Chem. – Eur. J. 2019, 25, 5303–5310. doi:10.1002/chem.201806140 |
| 30. | Grzybowski, M.; Skonieczny, K.; Butenschön, H.; Gryko, D. T. Angew. Chem., Int. Ed. 2013, 52, 9900–9930. doi:10.1002/anie.201210238 |
| 13. | Yavari, K.; Aillard, P.; Zhang, Y.; Nuter, F.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem., Int. Ed. 2014, 53, 861–865. doi:10.1002/anie.201308377 |
| 31. | CCDC 1984209 (8a) contains crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. |
| 12. | Yavari, K.; Moussa, S.; Ben Hassine, B.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem., Int. Ed. 2012, 51, 6748–6752. doi:10.1002/anie.201202024 |
| 29. | Tan, B.-H.; Dong, J.; Yoshikai, N. Angew. Chem., Int. Ed. 2012, 51, 9610–9614. doi:10.1002/anie.201204388 |
| 10. | Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Higashiyama, T.; Yamaguchi, S. Angew. Chem., Int. Ed. 2015, 54, 15213–15217. doi:10.1002/anie.201507939 |
| 11. | Wang, C.; Taki, M.; Sato, Y.; Fukazawa, A.; Higashiyama, T.; Yamaguchi, S. J. Am. Chem. Soc. 2017, 139, 10374–10381. doi:10.1021/jacs.7b04418 |
| 16. | Rahman, M. S.; Yoshikai, N. Org. Lett. 2019, 21, 3232–3236. doi:10.1021/acs.orglett.9b00955 |
| 18. | Wakatsuki, A.; Yukimoto, M.; Minoura, M.; Fujii, K.; Kimura, Y.; Matano, Y. Dalton Trans. 2018, 47, 7123–7127. doi:10.1039/c8dt01503a |
| 15. | Furukawa, S.; Suda, Y.; Kobayashi, J.; Kawashima, T.; Tada, T.; Fujii, S.; Kiguchi, M.; Saito, M. J. Am. Chem. Soc. 2017, 139, 5787–5792. doi:10.1021/jacs.6b12119 |
| 34. | Schwab, M. G.; Qin, T.; Pisula, W.; Mavrinskiy, A.; Feng, X.; Baumgarten, M.; Kim, H.; Laquai, F.; Schuh, S.; Trattnig, R.; List, E. J. W.; Müllen, K. Chem. – Asian J. 2011, 6, 3001–3010. doi:10.1002/asia.201100258 |
| 17. | Wu, B.; Santra, M.; Yoshikai, N. Angew. Chem., Int. Ed. 2014, 53, 7543–7546. doi:10.1002/anie.201404019 |
| 26. | Wang, S.; Yan, C.; Shang, J.; Wang, W.; Yuan, C.; Zhang, H.-L.; Shao, X. Angew. Chem., Int. Ed. 2019, 58, 3819–3823. doi:10.1002/anie.201813070 |
| 27. | Wu, B.; Chopra, R.; Yoshikai, N. Org. Lett. 2015, 17, 5666–5669. doi:10.1021/acs.orglett.5b02950 |
| 28. | Aillard, P.; Gicquel, M.; Yavari, K.; Retailleau, P.; Voituriez, A.; Marinetti, A. Eur. J. Org. Chem. 2018, 5853–5860. doi:10.1002/ejoc.201800438 |
| 36. | Wu, B.; Yoshikai, N. Org. Biomol. Chem. 2016, 14, 5402–5416. doi:10.1039/c6ob00219f |
| 37. | Chen, Y.-R.; Duan, W.-L. J. Am. Chem. Soc. 2013, 135, 16754–16757. doi:10.1021/ja407373g |
| 38. | Unoh, Y.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 12975–12979. doi:10.1002/anie.201307211 |
| 39. | Quint, V.; Morlet-Savary, F.; Lohier, J.-F.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S. J. Am. Chem. Soc. 2016, 138, 7436–7441. doi:10.1021/jacs.6b04069 |
| 40. | Liu, W.-Q.; Lei, T.; Zhou, S.; Yang, X.-L.; Li, J.; Chen, B.; Sivaguru, J.; Tung, C.-H.; Wu, L.-Z. J. Am. Chem. Soc. 2019, 141, 13941–13947. doi:10.1021/jacs.9b06920 |
| 16. | Rahman, M. S.; Yoshikai, N. Org. Lett. 2019, 21, 3232–3236. doi:10.1021/acs.orglett.9b00955 |
| 32. | Rieger, R.; Müllen, K. J. Phys. Org. Chem. 2010, 23, 315–325. doi:10.1002/poc.1644 |
| 15. | Furukawa, S.; Suda, Y.; Kobayashi, J.; Kawashima, T.; Tada, T.; Fujii, S.; Kiguchi, M.; Saito, M. J. Am. Chem. Soc. 2017, 139, 5787–5792. doi:10.1021/jacs.6b12119 |
| 19. | Watson, M. D.; Fechtenkötter, A.; Müllen, K. Chem. Rev. 2001, 101, 1267–1300. doi:10.1021/cr990322p |
| 20. | Pérez, D.; Guitián, E. Chem. Soc. Rev. 2004, 33, 274–283. doi:10.1039/b305549n |
| 21. | Kumar, S. Chem. Soc. Rev. 2006, 35, 83–109. doi:10.1039/b506619k |
| 22. | Sergeyev, S.; Pisula, W.; Geerts, Y. H. Chem. Soc. Rev. 2007, 36, 1902–1929. doi:10.1039/b417320c |
| 23. | Alam, M. A.; Motoyanagi, J.; Yamamoto, Y.; Fukushima, T.; Kim, J.; Kato, K.; Takata, M.; Saeki, A.; Seki, S.; Tagawa, S.; Aida, T. J. Am. Chem. Soc. 2009, 131, 17722–17723. doi:10.1021/ja905373d |
| 24. | Percec, V.; Imam, M. R.; Peterca, M.; Wilson, D. A.; Graf, R.; Spiess, H. W.; Balagurusamy, V. S. K.; Heiney, P. A. J. Am. Chem. Soc. 2009, 131, 7662–7677. doi:10.1021/ja8094944 |
| 25. | Badjic, J. D.; Ronconi, C. M.; Stoddart, J. F.; Balzani, V.; Silvi, S.; Credi, A. J. Am. Chem. Soc. 2006, 128, 1489–1499. doi:10.1021/ja0543954 |
| 33. | Verbitskiy, E. V.; Eltsov, O. S.; Zhilina, E. F.; Pakhomov, I. M.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Tetrahedron 2019, 75, 2687–2696. doi:10.1016/j.tet.2019.03.044 |
© 2020 Rahman and Yoshikai; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)