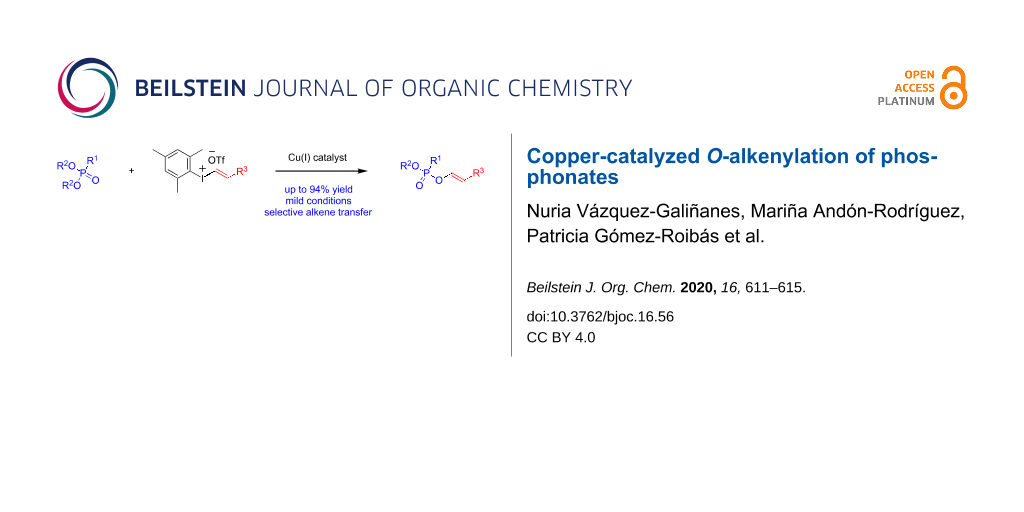
Abstract
Copper catalysis allows the direct oxygen alkenylation of dialkyl phosphonates with alkenyl(aryl)iodonium salts with selective transfer of the alkenyl group. This novel methodology proceeds with a wide range of phosphonates under mild conditions and gives straightforward access to valuable enol phosphonates in very good yields.
Graphical Abstract
Introduction
Organophosphorus compounds represent an important class of products with a wide range of applications in biology, agriculture and synthetic organic chemistry [1-3]. In particular, O-alkenyl phosphonate esters (i.e., enol phosphonates) have been described as potent insecticides and show antifungal activity [4]. While several methods are available for the preparation of cyclic enol phosphonates [5-10], the synthesis of the acyclic counterparts has received less attention. Current methodologies for the synthesis of acyclic mixed enol phosphonates include the Perkow-type reaction between phosphonites and α-halocarbonyl compounds [11], the mercury-catalyzed addition of phosphonic acid monoesters to terminal alkynes [12,13] and multistep procedures involving a Mitsunobu reaction between 2-hydroxyalkyl phenyl selenides and phosphonic acid monoesters followed by an oxidation/elimination step [14] or reaction of an enolate with a phosphonic dichloride and subsequent treatment with an alcohol [15] (Scheme 1a). However, these procedures are subject to selectivity problems, involve toxic and hazardous materials or are limited to the restricted availability of the corresponding phosphorus reagents. Therefore, the development of alternative methods for the synthesis of acyclic enol phosphonates is highly desirable.
Scheme 1: Synthesis of mixed alkyl alkenyl phosphonates.
Scheme 1: Synthesis of mixed alkyl alkenyl phosphonates.
Diaryliodonium and aryl(alkenyl)iodonium salts, which are air- and moisture-stable, nontoxic and easy to prepare compounds, have become efficient reagents for mild and selective arylation and alkenylation reactions in organic synthesis [16-18]. In particular, the use of these hypervalent iodine reagents in copper catalysis has allowed to perform a wide range of previously unknown synthetic transformations [19-29]. In these reactions, aryl(vinyl)Cu(III) species [30,31] have been proposed as key intermediates to undergo reactions with a variety of nucleophiles. Fañanás-Mastral and Feringa recently reported a catalytic method for the synthesis of mixed alkyl aryl phosphonates based on a copper-catalyzed arylation of phosphonates with diaryliodonium salts [32]. Encouraged by this work, in the context of an electrophilic alkenylation of phosphonates, we reasoned that the action of a copper catalyst on an alkenyl(aryl)iodonium salt [33,34] would generate an alkenyl–copper(III) species which might undergo nucleophilic attack of the Lewis-basic oxygen of a dialkyl phosphonate. The resulting phosphonium-like intermediate would evolve by Arbuzov-type substitution of one of the alkyl groups, and final reductive elimination would form the new C(sp2)–O bond, providing an acyclic enol phosphonate with concomitant regeneration of the Cu(I) catalyst (Scheme 1b). Herein we report the successful realization of such a copper-catalyzed oxygen-alkenylation strategy and show that a range of readily available, dialkyl phosphonates and alkenyl(aryl)iodonium salts can be combined to form enol phosphonates in high yield and excellent selectivity.
Results and Discussion
We started our studies by investigating the reaction between diethyl phosphonate 1a and styryl(mesityl)iodonium triflate (2a, Table 1). We first run the reaction under the conditions reported for the copper-catalyzed O-arylation of phosphonates (CuCl as catalyst, 2,6-di-tert-butylpyridine (dtbpy) as additive in dichloromethane at 40 °C) [32]. Under those conditions, enol phosphonate 3a was the only product of the reaction, although low conversion and yield were observed (Table 1, entry 1). A screening of copper complexes at a higher temperature (50 °C) revealed that CuTC (TC = thiophene-2-carboxylate) is the most efficient catalyst for this transformation (Table 1, entries 2–6). Finally, by using 2 equiv of 2a full conversion was achieved and enol phosphonate 3a was isolated in 78% yield with full selectivity towards the monoalkenylation product (Table 1, entry 7). Importantly, no reaction was observed in the absence of copper catalyst (Table 1, entry 8), while the absence of dtbpy led to a minimal conversion (Table 1, entry 9). The structure of the alkenyliodonium salt also plays an important role in the outcome of the reaction since the use of a phenyl group instead of the mesityl ligand caused a dramatic decrease in conversion and reaction yield likely due to a faster decomposition of the salt (Table 1, entry 10).
Table 1: Optimization studiesa.
|
|
|||||
| entry | 2a (equiv) | [Cu] | T (°C) | conv (%)b | 3a (%)b |
| 1 | 1.5 | CuCl | 40 | 42 | 34 |
| 2 | 1.5 | CuCl | 50 | 63 | 53 |
| 3 | 1.5 | CuOTf·PhCH3 | 50 | 32 | 25 |
| 4 | 1.5 | Cu(OTf)2 | 50 | 65 | 60 |
| 5 | 1.5 | CuI | 50 | 50 | 50 |
| 6 | 1.5 | CuTC | 50 | 75 | 69 |
| 7 | 2 | CuTC | 50 | full | 82 (78)c |
| 8 | 2 | – | 50 | – | – |
| 9d | 2 | CuTC | 50 | 10 | 5 |
| 10e | 2e | CuTC | 50 | 30 | 15 |
aReactions run on a 0.2 mmol scale; bDetermined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard. cYield of isolated product shown in brackets. dReaction run in the absence of dtbpy. eStyryl(phenyl)iodonium triflate used instead of 2a.
Having established optimized conditions for the copper-catalyzed O-alkenylation of phosphonates, we set out to investigate the scope of the reaction (Scheme 2). This catalytic transformation proved to be very efficient for several diethyl phosphonates bearing alkyl, benzyl and aryl groups providing in all cases the corresponding enol phosphonates 3a–d in good yields. Importantly, no double alkenylation product was observed in any case. Benzyl and alkyl diethyl phosphonates bearing halide groups also worked well and led to enol phosphonates 3e and 3f in good yields without any traces of side products. An acetal-protected aldehyde could also be used providing enol phosphonate 3g in 52% yield. In this case, prolonged reaction times led to partial evolution of 3g into enol ether 4. This transformation may be explained by an acid-mediated elimination of ethanol likely caused by trace formation of triflic acid via decomposition of ethyl triflate. As a limitation, substrates bearing a vinyl substituent or an enolizable ester group did not give any conversion. This methodology is also applicable to other dialkyl phosphonates as illustrated by the synthesis of enol phosphonates 3j, 3k and 3l. Interestingly, the copper-catalyzed alkenylation of phosphonates followed the same reactivity trend as the one described for the arylation reaction [32] with the diisopropyl phosphonates being more efficient than the dimethyl phosphonate esters. It is also important to remark that, in sharp contrast to the copper-catalyzed reaction between H-phosphonates and vinyliodonium salts described by Eustache and co-workers [35], no formation of the P-alkenylation product was observed in any case.
Scheme 2: Scope of the copper-catalyzed alkenylation of dialkyl phosphonates. Reactions run on a 0.2 mmol scale. Yields refer to isolated pure products. aReaction time = 10 h. bWhen reaction was stirred over 18 h a 3g:4 mixture was obtained in a 1:1 ratio.
Scheme 2: Scope of the copper-catalyzed alkenylation of dialkyl phosphonates. Reactions run on a 0.2 mmol sca...
Different alkenyliodonium salts were also used for this transformation. Styryl(mesityl)iodonium salts bearing both electron-donating and electron-withdrawing substituents worked well and allowed access to the corresponding enol phosphonates 3m–p in very good yields. Importantly, the bulky mesityl ligand allowed the selective transfer of the alkenyl group in all cases. In sharp contrast, no alkenylation product was observed when alkenyliodonium salts bearing aliphatic substituents were used likely due to a faster decomposition of the salt [36,37].
Conclusion
In summary, we have developed an efficient copper-catalyzed oxygen alkenylation of dialkyl phosphonates with alkenyl(aryl)iodonium salts. The reaction proceeds under mild conditions, with excellent levels of selectivity and affords acyclic enol phosphonates in high yields. We believe that the reaction occurs through the formation of a high valent alkenyl–copper(III) species which gets attacked by the phosphoryl oxygen of the phosphonate.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of enol phosphonates 3. | ||
| Format: PDF | Size: 3.5 MB | Download |
Funding
Financial support from the AEI (CTQ2017-88451-R), Xunta de Galicia (ED431F 2016/006; ED431C 2018/04; Centro singular de investigación de Galicia accreditation 2016-2019, ED431G/09) and the European Union (ERDF) is gratefully acknowledged. N. V.-G. thanks AEI for a predoctoral FPI fellowship.
References
-
McGrath, J. W.; Chin, J. P.; Quinn, J. P. Nat. Rev. Microbiol. 2013, 11, 412–419. doi:10.1038/nrmicro3011
Return to citation in text: [1] -
Duke, S. O.; Powles, S. B. Pest Manage. Sci. 2008, 64, 319–325. doi:10.1002/ps.1518
Return to citation in text: [1] -
Quin, L. D., Ed. A Guide to Organophosphorus Chemistry; Wiley-Interscience: New York, NY, USA, 2000.
Return to citation in text: [1] -
Engel, R., Ed. Handbook of Organophosphorus Chemistry; Marcel Dekker: New York, NY, USA, 1992. doi:10.1201/9781482277241
Return to citation in text: [1] -
Peng, A.-Y.; Ding, Y.-X. J. Am. Chem. Soc. 2003, 125, 15006–15007. doi:10.1021/ja038627f
Return to citation in text: [1] -
Peng, A.-Y.; Ding, Y.-X. Org. Lett. 2004, 6, 1119–1121. doi:10.1021/ol0499506
Return to citation in text: [1] -
Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258–3261. doi:10.1021/ol4012794
Return to citation in text: [1] -
Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358–3361. doi:10.1021/ol401407v
Return to citation in text: [1] -
Park, Y.; Seo, J.; Park, S.; Yoo, E. J.; Lee, P. H. Chem. – Eur. J. 2013, 19, 16461–16468. doi:10.1002/chem.201302652
Return to citation in text: [1] -
Pérez-Saavedra, B.; Vázquez-Galiñanes, N.; Saá, C.; Fañanás-Mastral, M. ACS Catal. 2017, 7, 6104–6109. doi:10.1021/acscatal.7b02434
Return to citation in text: [1] -
Despax, C.; Navech, J. Tetrahedron Lett. 1990, 31, 4471–4472. doi:10.1016/s0040-4039(00)97651-2
Return to citation in text: [1] -
Peng, A.; Ding, Y. Synthesis 2003, 205–208. doi:10.1055/s-2003-36818
Return to citation in text: [1] -
Wasserman, H. H.; Cohen, D. J. Am. Chem. Soc. 1960, 82, 4435–4436. doi:10.1021/ja01501a084
Return to citation in text: [1] -
Sheng, S.-R.; Sun, W.-K.; Hu, M.-G.; Liu, X.-L.; Wang, Q.-Y. J. Chem. Res. 2007, 97–99. doi:10.3184/030823407x198221
Return to citation in text: [1] -
Campbell, I. B.; Guo, J.; Jones, E.; Steel, P. G. Org. Biomol. Chem. 2004, 2, 2725–2727. doi:10.1039/b411111g
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] -
Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689
Return to citation in text: [1] -
Aradi, K.; Tóth, B. L.; Tolnai, G. L.; Novák, Z. Synlett 2016, 27, 1456–1485. doi:10.1055/s-0035-1561369
Return to citation in text: [1] -
Fañanás-Mastral, M. Synthesis 2017, 49, 1905–1930. doi:10.1055/s-0036-1589483
Return to citation in text: [1] -
Phipps, R. J.; Grimster, N. P.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 8172–8174. doi:10.1021/ja801767s
Return to citation in text: [1] -
Phipps, R. J.; Gaunt, M. J. Science 2009, 323, 1593–1597. doi:10.1126/science.1169975
Return to citation in text: [1] -
Zhu, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2012, 134, 10815–10818. doi:10.1021/ja305100g
Return to citation in text: [1] -
Suero, M. G.; Bayle, E. D.; Collins, B. S. L.; Gaunt, M. J. J. Am. Chem. Soc. 2013, 135, 5332–5335. doi:10.1021/ja401840j
Return to citation in text: [1] -
Collins, B. S. L.; Suero, M. G.; Gaunt, M. J. Angew. Chem., Int. Ed. 2013, 52, 5799–5802. doi:10.1002/anie.201301529
Return to citation in text: [1] -
Xu, Z.-F.; Cai, C.-X.; Liu, J.-T. Org. Lett. 2013, 15, 2096–2099. doi:10.1021/ol4003543
Return to citation in text: [1] -
Wang, Y.; Chen, C.; Peng, J.; Li, M. Angew. Chem., Int. Ed. 2013, 52, 5323–5357. doi:10.1002/anie.201300586
Return to citation in text: [1] -
Cahard, E.; Male, H. P. J.; Tissot, M.; Gaunt, M. J. J. Am. Chem. Soc. 2015, 137, 7986–7989. doi:10.1021/jacs.5b03937
Return to citation in text: [1] -
Beaud, R.; Phipps, R. J.; Gaunt, M. J. J. Am. Chem. Soc. 2016, 138, 13183–13186. doi:10.1021/jacs.6b09334
Return to citation in text: [1] -
Teskey, C. J.; Sohel, S. M. A.; Bunting, D. L.; Modha, S. G.; Greaney, M. F. Angew. Chem., Int. Ed. 2017, 56, 5263–5266. doi:10.1002/anie.201701523
Return to citation in text: [1] -
Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008
Return to citation in text: [1] -
Casitas, A.; Ribas, X. Chem. Sci. 2013, 4, 2301–2318. doi:10.1039/c3sc21818j
Return to citation in text: [1] -
Fañanás-Mastral, M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 9894–9897. doi:10.1021/ja505281v
Return to citation in text: [1] [2] [3] -
Ochiai, M.; Sumi, K.; Nagao, Y.; Fujita, E. Tetrahedron Lett. 1985, 26, 2351–2354. doi:10.1016/s0040-4039(00)95096-2
Return to citation in text: [1] -
Okuyama, T.; Takino, T.; Sato, K.; Ochiai, M. J. Am. Chem. Soc. 1998, 120, 2275–2282. doi:10.1021/ja972267c
Return to citation in text: [1] -
Thielges, S.; Bisseret, P.; Eustache, J. Org. Lett. 2005, 7, 681–684. doi:10.1021/ol047516y
Return to citation in text: [1] -
Beringer, F. M.; Bodlaender, P. J. Org. Chem. 1969, 34, 1981–1985. doi:10.1021/jo01258a107
Return to citation in text: [1] -
Lockhart, T. P. J. Am. Chem. Soc. 1983, 105, 1940–1946. doi:10.1021/ja00345a045
Return to citation in text: [1]
| 1. | McGrath, J. W.; Chin, J. P.; Quinn, J. P. Nat. Rev. Microbiol. 2013, 11, 412–419. doi:10.1038/nrmicro3011 |
| 2. | Duke, S. O.; Powles, S. B. Pest Manage. Sci. 2008, 64, 319–325. doi:10.1002/ps.1518 |
| 3. | Quin, L. D., Ed. A Guide to Organophosphorus Chemistry; Wiley-Interscience: New York, NY, USA, 2000. |
| 12. | Peng, A.; Ding, Y. Synthesis 2003, 205–208. doi:10.1055/s-2003-36818 |
| 13. | Wasserman, H. H.; Cohen, D. J. Am. Chem. Soc. 1960, 82, 4435–4436. doi:10.1021/ja01501a084 |
| 35. | Thielges, S.; Bisseret, P.; Eustache, J. Org. Lett. 2005, 7, 681–684. doi:10.1021/ol047516y |
| 11. | Despax, C.; Navech, J. Tetrahedron Lett. 1990, 31, 4471–4472. doi:10.1016/s0040-4039(00)97651-2 |
| 36. | Beringer, F. M.; Bodlaender, P. J. Org. Chem. 1969, 34, 1981–1985. doi:10.1021/jo01258a107 |
| 37. | Lockhart, T. P. J. Am. Chem. Soc. 1983, 105, 1940–1946. doi:10.1021/ja00345a045 |
| 5. | Peng, A.-Y.; Ding, Y.-X. J. Am. Chem. Soc. 2003, 125, 15006–15007. doi:10.1021/ja038627f |
| 6. | Peng, A.-Y.; Ding, Y.-X. Org. Lett. 2004, 6, 1119–1121. doi:10.1021/ol0499506 |
| 7. | Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2013, 15, 3258–3261. doi:10.1021/ol4012794 |
| 8. | Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Org. Lett. 2013, 15, 3358–3361. doi:10.1021/ol401407v |
| 9. | Park, Y.; Seo, J.; Park, S.; Yoo, E. J.; Lee, P. H. Chem. – Eur. J. 2013, 19, 16461–16468. doi:10.1002/chem.201302652 |
| 10. | Pérez-Saavedra, B.; Vázquez-Galiñanes, N.; Saá, C.; Fañanás-Mastral, M. ACS Catal. 2017, 7, 6104–6109. doi:10.1021/acscatal.7b02434 |
| 32. | Fañanás-Mastral, M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 9894–9897. doi:10.1021/ja505281v |
| 4. | Engel, R., Ed. Handbook of Organophosphorus Chemistry; Marcel Dekker: New York, NY, USA, 1992. doi:10.1201/9781482277241 |
| 32. | Fañanás-Mastral, M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 9894–9897. doi:10.1021/ja505281v |
| 19. | Fañanás-Mastral, M. Synthesis 2017, 49, 1905–1930. doi:10.1055/s-0036-1589483 |
| 20. | Phipps, R. J.; Grimster, N. P.; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 8172–8174. doi:10.1021/ja801767s |
| 21. | Phipps, R. J.; Gaunt, M. J. Science 2009, 323, 1593–1597. doi:10.1126/science.1169975 |
| 22. | Zhu, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2012, 134, 10815–10818. doi:10.1021/ja305100g |
| 23. | Suero, M. G.; Bayle, E. D.; Collins, B. S. L.; Gaunt, M. J. J. Am. Chem. Soc. 2013, 135, 5332–5335. doi:10.1021/ja401840j |
| 24. | Collins, B. S. L.; Suero, M. G.; Gaunt, M. J. Angew. Chem., Int. Ed. 2013, 52, 5799–5802. doi:10.1002/anie.201301529 |
| 25. | Xu, Z.-F.; Cai, C.-X.; Liu, J.-T. Org. Lett. 2013, 15, 2096–2099. doi:10.1021/ol4003543 |
| 26. | Wang, Y.; Chen, C.; Peng, J.; Li, M. Angew. Chem., Int. Ed. 2013, 52, 5323–5357. doi:10.1002/anie.201300586 |
| 27. | Cahard, E.; Male, H. P. J.; Tissot, M.; Gaunt, M. J. J. Am. Chem. Soc. 2015, 137, 7986–7989. doi:10.1021/jacs.5b03937 |
| 28. | Beaud, R.; Phipps, R. J.; Gaunt, M. J. J. Am. Chem. Soc. 2016, 138, 13183–13186. doi:10.1021/jacs.6b09334 |
| 29. | Teskey, C. J.; Sohel, S. M. A.; Bunting, D. L.; Modha, S. G.; Greaney, M. F. Angew. Chem., Int. Ed. 2017, 56, 5263–5266. doi:10.1002/anie.201701523 |
| 32. | Fañanás-Mastral, M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 9894–9897. doi:10.1021/ja505281v |
| 16. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 17. | Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689 |
| 18. | Aradi, K.; Tóth, B. L.; Tolnai, G. L.; Novák, Z. Synlett 2016, 27, 1456–1485. doi:10.1055/s-0035-1561369 |
| 33. | Ochiai, M.; Sumi, K.; Nagao, Y.; Fujita, E. Tetrahedron Lett. 1985, 26, 2351–2354. doi:10.1016/s0040-4039(00)95096-2 |
| 34. | Okuyama, T.; Takino, T.; Sato, K.; Ochiai, M. J. Am. Chem. Soc. 1998, 120, 2275–2282. doi:10.1021/ja972267c |
| 15. | Campbell, I. B.; Guo, J.; Jones, E.; Steel, P. G. Org. Biomol. Chem. 2004, 2, 2725–2727. doi:10.1039/b411111g |
| 14. | Sheng, S.-R.; Sun, W.-K.; Hu, M.-G.; Liu, X.-L.; Wang, Q.-Y. J. Chem. Res. 2007, 97–99. doi:10.3184/030823407x198221 |
| 30. | Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008 |
| 31. | Casitas, A.; Ribas, X. Chem. Sci. 2013, 4, 2301–2318. doi:10.1039/c3sc21818j |
© 2020 Vázquez-Galiñanes et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)