Abstract
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) is a commonly known oxidant. Herein, we report that DDQ can be used to synthesize 1,2-disubstituted benzimidazoles and quinazolin-4(3H)-ones via the intra- and intermolecular C–N coupling reaction under solvent-free mechanochemical (ball milling) conditions. In the presence of DDQ, the intramolecular C(sp2)–H amidation of N-(2-(arylideneamino)phenyl)-p-toluenesulfonamides leads to 1,2-disubstituted benzimidazoles and the one-pot coupling of 2-aminobenzamides with aryl/alkyl aldehydes resulted in substituted quinazolin-4(3H)-one derivatives in high yields.
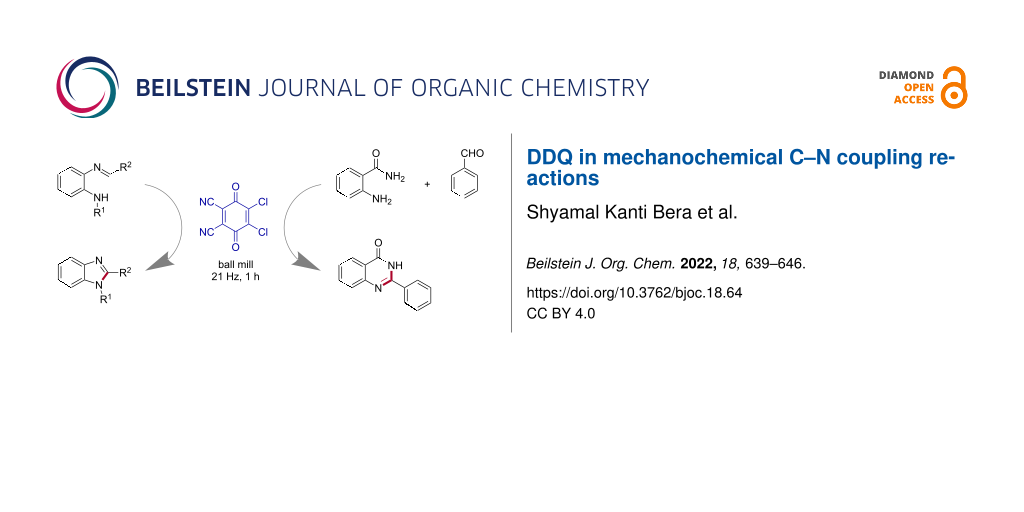
Graphical Abstract
Introduction
The reawakening approaches to use solvent-free and environmentally benign conditions in organic synthesis have facilitated new opportunities [1-4]. The research area of mechanochemistry [5,6] mainly focuses on conducting synthetic transformations in solid-state or solvent-free conditions. Mechanochemistry is one of the emerging avenues in chemistry that can make the world more sustainable by following the “Twelve Principles of Green Chemistry” [2]. Mechanochemistry is one of the ten innovative technologies that IUPAC recognized [7]. To perform organic transformations in a greener way, the mechanochemical methods can also be considered as one of the alternative approaches [8-10]. The one-pot multicomponent synthesis of important heterocycles can be the state of art practice by applying the strategies like domino, cascade, or tandem [11-13]. These environmentally friendly approaches set forth the journey of facilitating sustainable systems by using mechanochemical methods to access small organic compounds [3].
Due to the high reduction potential of the quinone moiety in 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), it was well established as a hydride transfer reagent in various organic reactions [14,15]. Generally, DDQ assists in dehydrogenation reactions in organic synthesis [16]. In this context, various carbon–heteroatom bond formation reactions such as C–P [17], C–O [18-20], and C–S [21] were achieved using DDQ as an oxidant [22,23]. In addition, the utilization of DDQ as a photoredox catalyst [24] and co-catalyst [25,26] have also been documented in organic synthesis [27]. DDQ-mediated oxidative C–N cross-coupling reactions are well known, but limited reports are available for reactions carried out under solvent-free conditions [28,29].
However, improving environmentally benign methods [30,31] of C–N bond synthesis is of enormous significance [32-34]. In comparison to the metal-mediated C–N coupling reactions [35], the direct C–H amination is vital to provide many amine derivatives by sustainable methods [36,37]. The dehydrogenative C–N cross-coupling reactions from unreactive N–H and C–H bonds can lead to various nitrogen-containing heterocycles [32,38]. Herein, we disclose the DDQ-mediated oxidative C–N coupling toward the synthesis of 1,2-disubstituted benzimidazoles [39] under mechanochemical (ball milling) conditions (Figure 1a). In addition, the one-pot coupling of 2-aminobenzamides with aryl/alkyl aldehydes in the presence of DDQ resulted in substituted quinazolin-4(3H)-one [40] derivatives (Figure 1b).
Figure 1: Our work on mechanochemical C–N coupling reactions using DDQ. The newly formed C–N bonds are shown as red lines for clarity. a) The intramolecular C(sp2)-H amidation leading to 1,2-disubstituted benzimidazoles. b) One-pot coupling to synthesize substituted quinazolin-4(3H)-ones.
Figure 1: Our work on mechanochemical C–N coupling reactions using DDQ. The newly formed C–N bonds are shown ...
Results and Discussion
Towards the optimization study, (E)-N-(2-((2-bromobenzylidene)amino)phenyl)-4-methylbenzenesulfonamide (1a) was considered as a model substrate for the synthesis of 2-(2-bromophenyl)-1-tosyl-1H-benzo[d]imidazole (2a, Table 1). Initially, with 1.0 equiv of DDQ, product 2a was obtained in 88% yield (Table 1, entry 1). However, in the presence of 1.2 equiv, the yield of 2a increased to 97% (Table 1, entry 2). Further, with the increase of the amount of DDQ to 1.5 equiv, no further improvement was obtained (Table 1, entry 3). In addition, we have screened several iodine oxidation reagents, but none of them gave better yields (Table 1, entries 4–7). On the other hand, oxone as an oxidant yielded product 2a with up to 43% yield (Table 1, entry 8). Similarly, we have optimized the reaction conditions for the synthesis of 2-phenylquinazolin-4(3H)-one (5a) from anthranilamide and benzaldehyde under the solvent-free conditions (Table S1, Supporting Information File 1). Notably, the use of 1.0 equiv of DDQ as oxidant, afforded the product 2-phenylquinazolin-4(3H)-one (5a) in 98% yield within 1 h. However, when reducing the amount of DDQ to 0.5 equiv the of product 5a decreased to 48%. On the other hand, increasing the amount of DDQ to 1.2 equiv resulted in 98% yield of the product (Supporting Information File 1, Table S1, entries 1 and 3). Further studies revealed that other commonly used oxidants such as PIDA and oxone gave 30% and 61% the desired product, respectively (Supporting Information File 1, Table S1, entries 4 and 5). When molecular iodine or NIS were used as oxidants, product 5a was obtained in 83% and 80% yield, respectively (Supporting Information File 1, Table S1, entries 6 and 7). However, the yield of the desired product 5a slightly decreased to 92% with lowering of the operating milling frequency from 21 to 16 Hz (Supporting Information File 1, Table S1, entry 9). On the other hand, the yield of product 5a was unaffected by increasing the operating frequency from 21 Hz to 25 Hz (Supporting Information File 1, Table S1, entry 8).
Table 1: Optimization of the reaction conditions.a
|
|
||
| Entry | Reagent (equiv) | Yield (%)b |
| 1 | DDQ (1.0) | 88 |
| 2 | DDQ (1.2) | 97 |
| 3 | DDQ (1.5) | 96 |
| 4 | NIS (1.2) | 65 |
| 5 | I2 (1.2) | 32 |
| 6 | I2 (1.2)/K2CO3 (1.2) | 23 |
| 7 | PIDA (1.2) | 60 |
| 8 | oxone (1.2) | 43 |
aReaction conditions: 0.14 mmol of 1a and 0.167 mmol of DDQ (1.2 equiv) under solvent-free conditions were milled at 21 Hz in a 10 mL milling jar containing one stainless-steel grinding ball (15 mm in diameter) for 1 h. bYield of the isolated product after purification through silica-gel column chromatography.
1,2-Disubstituted benzimidazoles are heterocyclic scaffolds holding a broad range of biological activities [41-43]. For example, telmisartan, a 1,2-disubstituted benzimidazole derivative, is extensively used as an antihypertensive agent [44]. The substrate scope for the synthesis of variously substituted benzimidazoles is shown in Figure 2. Benzimidazoles with a variety of aryl substituents (such as bromo, nitro, chloro, and 2,4,6-trimethyl) in the 2-position of the benzimidazole (2a–d,g) were obtained in good yields. Furthermore, the synthesis of the corresponding benzimidazoles with fused aromatic systems in the 2-position such as anthracenyl (2e) and naphthyl (2f) proved to be efficient. Similarly, the 5,6-dimethyl- or 5,6-dichloro-1,2-disubstituted benzimidazoles 2i, 2j, and 2k were synthesized with 82, 85, and 79% yield, respectively. In addition, the structure of the synthesized 2-(4-(phenylethynyl)phenyl)-substituted product 2h was established from the X-ray crystallography data.
Figure 2: Scope of the mechanochemical synthesis of substituted benzimidazoles.
Figure 2: Scope of the mechanochemical synthesis of substituted benzimidazoles.
Various methodologies are available in the literature toward constructing quinazolin-4(3H)-one derivatives [40,45,46]. Quinazolin-4(3H)-ones and its derivatives possess several biological activities such as antibacterial [47], antiviral [48], antitumor [49,50], antimalarial [51], anti-inflammatory [52], etc. We therefore investigated the scope of the one-pot coupling of 2-aminobenzamides with aryl/alkyl aldehydes in the presence of DDQ under the optimized mechanochemical conditions and the results are shown in Figure 3.
Figure 3: Synthesis of quinazolin-4(3H)-one derivatives.
Figure 3: Synthesis of quinazolin-4(3H)-one derivatives.
Unsubstituted anthranilamides reacted smoothly with various substituted aldehydes (containing a bromo, fluoro, hydroxy, chloro, ethyl, or methyl group) affording the corresponding products 5b–g with good to excellent yields. Also, 3,4,5-trimethoxy-, 2,4,6-trimethyl-, anthracene-9-yl-, and naphthalene-1-yl-substituted benzaldehydes were well tolerated and gave the desired products 5h–k with high yields. In this context, biphenyl aldehyde with a chloro group was efficiently converted to 5l with 93% yield. Furthermore, aromatic aldehydes having a strong electron-withdrawing group (such as NO2) were smoothly converted to the corresponding products 5m with 92% yield, respectively. In addition to this, aliphatic aldehydes (cyclohexanecarbaldehyde, butyraldehyde) were well tolerated under the standard reaction conditions to produce the product 5n and 5o with good yield.
The substrate scope of this methodology was extended to chloro and fluoro-substituted anthranilamides and aldehydes (Figure 4). Initially, 2-amino-5-fluorobenzamide was reacted with various benzaldehydes having bromo, ethyl, methyl, styryl, and cyclohexyl groups to produce the respective cyclized products 6b–f with good to excellent yields. Similarly, biphenyl aldehydes with -OMe and -COMe groups were well tolerated under the standard reaction conditions and delivered the corresponding products 6g and 6h with 83 and 85% yields, respectively. In addition, we have also explored the substrate scope with 2-amino-5-chlorobenzamide and various aldehydes. Benzaldehyde containing fluoro, bromo, ethyl, and anthryl groups led to the corresponding products 6j, 6k, 6l, and 6p in good to excellent yield. Aliphatic aldehydes such as butyraldehyde gave the cyclized product 6m with an 86% yield. In this regard, an -OMe and -COMe group-containing biphenyl aldehyde resulted in the corresponding products 6n and 6o with 79 and 82% yields, respectively. Nitro and fluoro-substituted aromatic aldehydes efficiently reacted with the chloro and fluoro-substituted anthranilamides and delivered the corresponding products 6q and 6r with good yields.
Figure 4: The substrate scope for the synthesis of quinazolin-4(3H)-one derivatives.
Figure 4: The substrate scope for the synthesis of quinazolin-4(3H)-one derivatives.
To understand the reaction mechanism, we have performed radical trapping experiments using TEMPO and BHT in the reaction of substrate 1c (Figure 5a). Under the standard reaction conditions in the presence of TEMPO or BHT, the expected product 2c was formed in 66 and 72% yields. These results indicate that a radical pathway may not be involved in the reaction. So, based on literature reports [53-55], we have proposed a reaction mechanism in Figure 5b. Initially, DDQ abstracts a hydride ion from substrate 1a to generate the intermediate A. Then intermediate A undergoes an electrophilic intramolecular cyclization to form the cationic intermediate B, followed by hydride abstraction to generate the desired product 2a. On the other hand, the formation of quinazolin-4(3H)-ones starts with the formation of an imine intermediate and then it will follow the similar mechanistic pathway.
Figure 5: a) Control experiment and b) Plausible mechanism.
Figure 5: a) Control experiment and b) Plausible mechanism.
To explore the synthetic utility of the oxidative C–N cross-coupling reaction, we have performed the large-scale synthesis under the solvent-free (ball milling) conditions as shown in Figure 6. In this context, milling of the substrate (E)-N-(2-((4-bromobenzylidene)amino)phenyl)-4-methylbenzenesulfonamide (1c, 2.795 mmol) in the presence of 1.2 equiv of DDQ delivered 1.098 g (92%) of the cyclized product 2-(4-bromophenyl)-1-tosyl-1H-benzo[d]imidazole (2c). Similarly, we also carried out the large-scale synthesis with 4.04 mmol each of anthranilamide and 4-bromobenzaldehyde (4), which produced 1.16 g (95%) of the desired product 2-(4-bromophenyl)quinazolin-4(3H)-one (5m).
Figure 6: Large-scale synthesis. a) 1,2-Disubstituted benzimidazoles. b) Substituted quinazolin-4(3H)-ones. Reaction conditions: reactants were milled at 21 Hz in a 25 mL milling jar containing one stainless-steel grinding ball (15 mm in diameter).
Figure 6: Large-scale synthesis. a) 1,2-Disubstituted benzimidazoles. b) Substituted quinazolin-4(3H)-ones. R...
Conclusion
In summary, we have successfully developed efficient methodologies for synthesizing 1,2-disubstituted benzimidazoles and quinazolin-4(3H)-one derivatives under mechanochemical (ball milling at 21 Hz) conditions in the presence of DDQ. The developed methodology can be considered as a green and eco-friendly methodology due to its solvent-free and metal-free nature. So, it can also be regarded as an alternative pathway to the traditional solution-based protocols. We anticipate that our developed strategy will have a substantial impact on the field of organic synthesis.
Supporting Information
| Supporting Information File 1: Experimental details, characterization data, copies of NMR spectra and X-ray crystallography details. | ||
| Format: PDF | Size: 7.0 MB | Download |
References
-
Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887
Return to citation in text: [1] -
Ardila‐Fierro, K. J.; Hernández, J. G. ChemSusChem 2021, 14, 2145–2162. doi:10.1002/cssc.202100478
Return to citation in text: [1] [2] -
Egorov, I. N.; Santra, S.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Majee, A.; Ranu, B. C.; Rusinov, V. L.; Chupakhin, O. N. Green Chem. 2020, 22, 302–315. doi:10.1039/c9gc03414e
Return to citation in text: [1] [2] -
Tan, D.; Friščić, T. Eur. J. Org. Chem. 2018, 18–33. doi:10.1002/ejoc.201700961
Return to citation in text: [1] -
Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h
Return to citation in text: [1] -
Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A. M.; Yang, W.; Chen, Y. I. Chem. Commun. 2021, 57, 1080–1092. doi:10.1039/d0cc06581a
Return to citation in text: [1] -
Gomollón-Bel, F. Chem. Int. 2019, 41 (2), 12–17. doi:10.1515/ci-2019-0203
Return to citation in text: [1] -
Bose, A.; Mal, P. Beilstein J. Org. Chem. 2019, 15, 881–900. doi:10.3762/bjoc.15.86
Return to citation in text: [1] -
Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186
Return to citation in text: [1] -
O’Neill, R. T.; Boulatov, R. Nat. Rev. Chem. 2021, 5, 148–167. doi:10.1038/s41570-020-00249-y
Return to citation in text: [1] -
Lou, S.-J.; Mao, Y.-J.; Xu, D.-Q.; He, J.-Q.; Chen, Q.; Xu, Z.-Y. ACS Catal. 2016, 6, 3890–3894. doi:10.1021/acscatal.6b00861
Return to citation in text: [1] -
Das, D.; Bhosle, A. A.; Panjikar, P. C.; Chatterjee, A.; Banerjee, M. ACS Sustainable Chem. Eng. 2020, 8, 19105–19116. doi:10.1021/acssuschemeng.0c07465
Return to citation in text: [1] -
Bera, S. K.; Mal, P. J. Org. Chem. 2021, 86, 14144–14159. doi:10.1021/acs.joc.1c01742
Return to citation in text: [1] -
Alsharif, M. A.; Raja, Q. A.; Majeed, N. A.; Jassas, R. S.; Alsimaree, A. A.; Sadiq, A.; Naeem, N.; Mughal, E. U.; Alsantali, R. I.; Moussa, Z.; Ahmed, S. A. RSC Adv. 2021, 11, 29826–29858. doi:10.1039/d1ra04575j
Return to citation in text: [1] -
Wendlandt, A. E.; Stahl, S. S. Angew. Chem., Int. Ed. 2015, 54, 14638–14658. doi:10.1002/anie.201505017
Return to citation in text: [1] -
Cheng, D.; Wu, L.; Deng, Z.; Xu, X.; Yan, J. Adv. Synth. Catal. 2017, 359, 4317–4321. doi:10.1002/adsc.201700853
Return to citation in text: [1] -
Chen, Q.; Wen, C.; Wang, X.; Yu, G.; Ou, Y.; Huo, Y.; Zhang, K. Adv. Synth. Catal. 2018, 360, 3590–3594. doi:10.1002/adsc.201800804
Return to citation in text: [1] -
Li, J.-S.; Xue, Y.; Fu, D.-M.; Li, D.-L.; Li, Z.-W.; Liu, W.-D.; Pang, H.-L.; Zhang, Y.-F.; Cao, Z.; Zhang, L. RSC Adv. 2014, 4, 54039–54042. doi:10.1039/c4ra08627a
Return to citation in text: [1] -
Yi, H.; Liu, Q.; Liu, J.; Zeng, Z.; Yang, Y.; Lei, A. ChemSusChem 2012, 5, 2143–2146. doi:10.1002/cssc.201200458
Return to citation in text: [1] -
Pan, D.; Pan, Z.; Hu, Z.; Li, M.; Hu, X.; Jin, L.; Sun, N.; Hu, B.; Shen, Z. Eur. J. Org. Chem. 2019, 5650–5655. doi:10.1002/ejoc.201900773
Return to citation in text: [1] -
Li, C.; Li, J.; Tan, C.; Wu, W.; Jiang, H. Org. Chem. Front. 2018, 5, 3158–3162. doi:10.1039/c8qo00799c
Return to citation in text: [1] -
Yan, B.; Fu, Y.; Zhu, H.; Chen, Z. J. Org. Chem. 2019, 84, 4246–4262. doi:10.1021/acs.joc.9b00231
Return to citation in text: [1] -
Zhai, L.; Shukla, R.; Rathore, R. Org. Lett. 2009, 11, 3474–3477. doi:10.1021/ol901331p
Return to citation in text: [1] -
Natarajan, P.; König, B. Eur. J. Org. Chem. 2021, 2145–2161. doi:10.1002/ejoc.202100011
Return to citation in text: [1] -
Song, C.; Dong, X.; Yi, H.; Chiang, C.-W.; Lei, A. ACS Catal. 2018, 8, 2195–2199. doi:10.1021/acscatal.7b04434
Return to citation in text: [1] -
Shen, Z.; Dai, J.; Xiong, J.; He, X.; Mo, W.; Hu, B.; Sun, N.; Hu, X. Adv. Synth. Catal. 2011, 353, 3031–3038. doi:10.1002/adsc.201100429
Return to citation in text: [1] -
Mandal, T.; Azim, A.; Das, S.; De Sarkar, S. Asian J. Org. Chem. 2022, 11, e202100601. doi:10.1002/ajoc.202100601
Return to citation in text: [1] -
Sun, C.; Zheng, L.; Xu, W.; Dushkin, A. V.; Su, W. Green Chem. 2020, 22, 3489–3494. doi:10.1039/d0gc00372g
Return to citation in text: [1] -
Hernández, J. G.; Turberg, M.; Schiffers, I.; Bolm, C. Chem. – Eur. J. 2016, 22, 14513–14517. doi:10.1002/chem.201603057
Return to citation in text: [1] -
Li, C.-J. Chem 2016, 1, 423–437. doi:10.1016/j.chempr.2016.08.007
Return to citation in text: [1] -
Hermann, G. N.; Bolm, C. ACS Catal. 2017, 7, 4592–4596. doi:10.1021/acscatal.7b00582
Return to citation in text: [1] -
Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283–9303. doi:10.1039/c3cs60228a
Return to citation in text: [1] [2] -
Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534–1544. doi:10.1021/ar800098p
Return to citation in text: [1] -
Majumdar, B.; Sarma, D.; Bhattacharya, T.; Sarma, T. K. ACS Sustainable Chem. Eng. 2017, 5, 9286–9294. doi:10.1021/acssuschemeng.7b02267
Return to citation in text: [1] -
Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512
Return to citation in text: [1] -
Sun, C.-L.; Shi, Z.-J. Chem. Rev. 2014, 114, 9219–9280. doi:10.1021/cr400274j
Return to citation in text: [1] -
Louillat, M.-L.; Patureau, F. W. Chem. Soc. Rev. 2014, 43, 901–910. doi:10.1039/c3cs60318k
Return to citation in text: [1] -
Carvalho, L. C. R.; Fernandes, E.; Marques, M. M. B. Chem. – Eur. J. 2011, 17, 12544–12555. doi:10.1002/chem.201101508
Return to citation in text: [1] -
Maiti, S.; Mal, P. Adv. Synth. Catal. 2015, 357, 1416–1424. doi:10.1002/adsc.201401110
Return to citation in text: [1] -
Alam, M. T.; Maiti, S.; Mal, P. Beilstein J. Org. Chem. 2018, 14, 2396–2403. doi:10.3762/bjoc.14.216
Return to citation in text: [1] [2] -
Li, Y.-F.; Wang, G.-F.; He, P.-L.; Huang, W.-G.; Zhu, F.-H.; Gao, H.-Y.; Tang, W.; Luo, Y.; Feng, C.-L.; Shi, L.-P.; Ren, Y.-D.; Lu, W.; Zuo, J.-P. J. Med. Chem. 2006, 49, 4790–4794. doi:10.1021/jm060330f
Return to citation in text: [1] -
Valdez, J.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Navarrete-Vázquez, G.; Tapia, A.; Cortés, R.; Hernández, M.; Castillo, R. Bioorg. Med. Chem. Lett. 2002, 12, 2221–2224. doi:10.1016/s0960-894x(02)00346-3
Return to citation in text: [1] -
Sondhi, S. M.; Rajvanshi, S.; Johar, M.; Bharti, N.; Azam, A.; Singh, A. K. Eur. J. Med. Chem. 2002, 37, 835–843. doi:10.1016/s0223-5234(02)01403-4
Return to citation in text: [1] -
Sharpe, M.; Jarvis, B.; Goa, K. L. Drugs 2001, 61, 1501–1529. doi:10.2165/00003495-200161100-00009
Return to citation in text: [1] -
Parua, S.; Das, S.; Sikari, R.; Sinha, S.; Paul, N. D. J. Org. Chem. 2017, 82, 7165–7175. doi:10.1021/acs.joc.7b00643
Return to citation in text: [1] -
Sahoo, S.; Pal, S. J. Org. Chem. 2021, 86, 18067–18080. doi:10.1021/acs.joc.1c02343
Return to citation in text: [1] -
Kung, P.-P.; Casper, M. D.; Cook, K. L.; Wilson-Lingardo, L.; Risen, L. M.; Vickers, T. A.; Ranken, R.; Blyn, L. B.; Wyatt, J. R.; Cook, P. D.; Ecker, D. J. J. Med. Chem. 1999, 42, 4705–4713. doi:10.1021/jm9903500
Return to citation in text: [1] -
Chen, M.; Li, P.; Hu, D.; Zeng, S.; Li, T.; Jin, L.; Xue, W.; Song, B. Bioorg. Med. Chem. Lett. 2016, 26, 168–173. doi:10.1016/j.bmcl.2015.11.006
Return to citation in text: [1] -
Chandrika, P. M.; Yakaiah, T.; Rao, A. R. R.; Narsaiah, B.; Reddy, N. C.; Sridhar, V.; Rao, J. V. Eur. J. Med. Chem. 2008, 43, 846–852. doi:10.1016/j.ejmech.2007.06.010
Return to citation in text: [1] -
Cao, S.-L.; Feng, Y.-P.; Jiang, Y.-Y.; Liu, S.-Y.; Ding, G.-Y.; Li, R.-T. Bioorg. Med. Chem. Lett. 2005, 15, 1915–1917. doi:10.1016/j.bmcl.2005.01.083
Return to citation in text: [1] -
Kikuchi, H.; Yamamoto, K.; Horoiwa, S.; Hirai, S.; Kasahara, R.; Hariguchi, N.; Matsumoto, M.; Oshima, Y. J. Med. Chem. 2006, 49, 4698–4706. doi:10.1021/jm0601809
Return to citation in text: [1] -
Ozaki, K.-i.; Yamada, Y.; Oine, T.; Ishizuka, T.; Iwasawa, Y. J. Med. Chem. 1985, 28, 568–576. doi:10.1021/jm50001a006
Return to citation in text: [1] -
Batra, A.; Singh, K. N. Eur. J. Org. Chem. 2020, 6676–6703. doi:10.1002/ejoc.202000785
Return to citation in text: [1] -
Cheng, D.; Bao, W. J. Org. Chem. 2008, 73, 6881–6883. doi:10.1021/jo8010039
Return to citation in text: [1] -
Guo, X.; Zipse, H.; Mayr, H. J. Am. Chem. Soc. 2014, 136, 13863–13873. doi:10.1021/ja507598y
Return to citation in text: [1]
| 47. | Kung, P.-P.; Casper, M. D.; Cook, K. L.; Wilson-Lingardo, L.; Risen, L. M.; Vickers, T. A.; Ranken, R.; Blyn, L. B.; Wyatt, J. R.; Cook, P. D.; Ecker, D. J. J. Med. Chem. 1999, 42, 4705–4713. doi:10.1021/jm9903500 |
| 48. | Chen, M.; Li, P.; Hu, D.; Zeng, S.; Li, T.; Jin, L.; Xue, W.; Song, B. Bioorg. Med. Chem. Lett. 2016, 26, 168–173. doi:10.1016/j.bmcl.2015.11.006 |
| 49. | Chandrika, P. M.; Yakaiah, T.; Rao, A. R. R.; Narsaiah, B.; Reddy, N. C.; Sridhar, V.; Rao, J. V. Eur. J. Med. Chem. 2008, 43, 846–852. doi:10.1016/j.ejmech.2007.06.010 |
| 50. | Cao, S.-L.; Feng, Y.-P.; Jiang, Y.-Y.; Liu, S.-Y.; Ding, G.-Y.; Li, R.-T. Bioorg. Med. Chem. Lett. 2005, 15, 1915–1917. doi:10.1016/j.bmcl.2005.01.083 |
| 1. | Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887 |
| 2. | Ardila‐Fierro, K. J.; Hernández, J. G. ChemSusChem 2021, 14, 2145–2162. doi:10.1002/cssc.202100478 |
| 3. | Egorov, I. N.; Santra, S.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Majee, A.; Ranu, B. C.; Rusinov, V. L.; Chupakhin, O. N. Green Chem. 2020, 22, 302–315. doi:10.1039/c9gc03414e |
| 4. | Tan, D.; Friščić, T. Eur. J. Org. Chem. 2018, 18–33. doi:10.1002/ejoc.201700961 |
| 8. | Bose, A.; Mal, P. Beilstein J. Org. Chem. 2019, 15, 881–900. doi:10.3762/bjoc.15.86 |
| 9. | Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186 |
| 10. | O’Neill, R. T.; Boulatov, R. Nat. Rev. Chem. 2021, 5, 148–167. doi:10.1038/s41570-020-00249-y |
| 25. | Song, C.; Dong, X.; Yi, H.; Chiang, C.-W.; Lei, A. ACS Catal. 2018, 8, 2195–2199. doi:10.1021/acscatal.7b04434 |
| 26. | Shen, Z.; Dai, J.; Xiong, J.; He, X.; Mo, W.; Hu, B.; Sun, N.; Hu, X. Adv. Synth. Catal. 2011, 353, 3031–3038. doi:10.1002/adsc.201100429 |
| 27. | Mandal, T.; Azim, A.; Das, S.; De Sarkar, S. Asian J. Org. Chem. 2022, 11, e202100601. doi:10.1002/ajoc.202100601 |
| 2. | Ardila‐Fierro, K. J.; Hernández, J. G. ChemSusChem 2021, 14, 2145–2162. doi:10.1002/cssc.202100478 |
| 22. | Yan, B.; Fu, Y.; Zhu, H.; Chen, Z. J. Org. Chem. 2019, 84, 4246–4262. doi:10.1021/acs.joc.9b00231 |
| 23. | Zhai, L.; Shukla, R.; Rathore, R. Org. Lett. 2009, 11, 3474–3477. doi:10.1021/ol901331p |
| 5. | Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h |
| 6. | Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A. M.; Yang, W.; Chen, Y. I. Chem. Commun. 2021, 57, 1080–1092. doi:10.1039/d0cc06581a |
| 24. | Natarajan, P.; König, B. Eur. J. Org. Chem. 2021, 2145–2161. doi:10.1002/ejoc.202100011 |
| 16. | Cheng, D.; Wu, L.; Deng, Z.; Xu, X.; Yan, J. Adv. Synth. Catal. 2017, 359, 4317–4321. doi:10.1002/adsc.201700853 |
| 18. | Li, J.-S.; Xue, Y.; Fu, D.-M.; Li, D.-L.; Li, Z.-W.; Liu, W.-D.; Pang, H.-L.; Zhang, Y.-F.; Cao, Z.; Zhang, L. RSC Adv. 2014, 4, 54039–54042. doi:10.1039/c4ra08627a |
| 19. | Yi, H.; Liu, Q.; Liu, J.; Zeng, Z.; Yang, Y.; Lei, A. ChemSusChem 2012, 5, 2143–2146. doi:10.1002/cssc.201200458 |
| 20. | Pan, D.; Pan, Z.; Hu, Z.; Li, M.; Hu, X.; Jin, L.; Sun, N.; Hu, B.; Shen, Z. Eur. J. Org. Chem. 2019, 5650–5655. doi:10.1002/ejoc.201900773 |
| 53. | Batra, A.; Singh, K. N. Eur. J. Org. Chem. 2020, 6676–6703. doi:10.1002/ejoc.202000785 |
| 54. | Cheng, D.; Bao, W. J. Org. Chem. 2008, 73, 6881–6883. doi:10.1021/jo8010039 |
| 55. | Guo, X.; Zipse, H.; Mayr, H. J. Am. Chem. Soc. 2014, 136, 13863–13873. doi:10.1021/ja507598y |
| 14. | Alsharif, M. A.; Raja, Q. A.; Majeed, N. A.; Jassas, R. S.; Alsimaree, A. A.; Sadiq, A.; Naeem, N.; Mughal, E. U.; Alsantali, R. I.; Moussa, Z.; Ahmed, S. A. RSC Adv. 2021, 11, 29826–29858. doi:10.1039/d1ra04575j |
| 15. | Wendlandt, A. E.; Stahl, S. S. Angew. Chem., Int. Ed. 2015, 54, 14638–14658. doi:10.1002/anie.201505017 |
| 21. | Li, C.; Li, J.; Tan, C.; Wu, W.; Jiang, H. Org. Chem. Front. 2018, 5, 3158–3162. doi:10.1039/c8qo00799c |
| 3. | Egorov, I. N.; Santra, S.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Majee, A.; Ranu, B. C.; Rusinov, V. L.; Chupakhin, O. N. Green Chem. 2020, 22, 302–315. doi:10.1039/c9gc03414e |
| 51. | Kikuchi, H.; Yamamoto, K.; Horoiwa, S.; Hirai, S.; Kasahara, R.; Hariguchi, N.; Matsumoto, M.; Oshima, Y. J. Med. Chem. 2006, 49, 4698–4706. doi:10.1021/jm0601809 |
| 11. | Lou, S.-J.; Mao, Y.-J.; Xu, D.-Q.; He, J.-Q.; Chen, Q.; Xu, Z.-Y. ACS Catal. 2016, 6, 3890–3894. doi:10.1021/acscatal.6b00861 |
| 12. | Das, D.; Bhosle, A. A.; Panjikar, P. C.; Chatterjee, A.; Banerjee, M. ACS Sustainable Chem. Eng. 2020, 8, 19105–19116. doi:10.1021/acssuschemeng.0c07465 |
| 13. | Bera, S. K.; Mal, P. J. Org. Chem. 2021, 86, 14144–14159. doi:10.1021/acs.joc.1c01742 |
| 17. | Chen, Q.; Wen, C.; Wang, X.; Yu, G.; Ou, Y.; Huo, Y.; Zhang, K. Adv. Synth. Catal. 2018, 360, 3590–3594. doi:10.1002/adsc.201800804 |
| 52. | Ozaki, K.-i.; Yamada, Y.; Oine, T.; Ishizuka, T.; Iwasawa, Y. J. Med. Chem. 1985, 28, 568–576. doi:10.1021/jm50001a006 |
| 32. | Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283–9303. doi:10.1039/c3cs60228a |
| 33. | Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534–1544. doi:10.1021/ar800098p |
| 34. | Majumdar, B.; Sarma, D.; Bhattacharya, T.; Sarma, T. K. ACS Sustainable Chem. Eng. 2017, 5, 9286–9294. doi:10.1021/acssuschemeng.7b02267 |
| 28. | Sun, C.; Zheng, L.; Xu, W.; Dushkin, A. V.; Su, W. Green Chem. 2020, 22, 3489–3494. doi:10.1039/d0gc00372g |
| 29. | Hernández, J. G.; Turberg, M.; Schiffers, I.; Bolm, C. Chem. – Eur. J. 2016, 22, 14513–14517. doi:10.1002/chem.201603057 |
| 30. | Li, C.-J. Chem 2016, 1, 423–437. doi:10.1016/j.chempr.2016.08.007 |
| 31. | Hermann, G. N.; Bolm, C. ACS Catal. 2017, 7, 4592–4596. doi:10.1021/acscatal.7b00582 |
| 44. | Sharpe, M.; Jarvis, B.; Goa, K. L. Drugs 2001, 61, 1501–1529. doi:10.2165/00003495-200161100-00009 |
| 40. | Alam, M. T.; Maiti, S.; Mal, P. Beilstein J. Org. Chem. 2018, 14, 2396–2403. doi:10.3762/bjoc.14.216 |
| 45. | Parua, S.; Das, S.; Sikari, R.; Sinha, S.; Paul, N. D. J. Org. Chem. 2017, 82, 7165–7175. doi:10.1021/acs.joc.7b00643 |
| 46. | Sahoo, S.; Pal, S. J. Org. Chem. 2021, 86, 18067–18080. doi:10.1021/acs.joc.1c02343 |
| 40. | Alam, M. T.; Maiti, S.; Mal, P. Beilstein J. Org. Chem. 2018, 14, 2396–2403. doi:10.3762/bjoc.14.216 |
| 41. | Li, Y.-F.; Wang, G.-F.; He, P.-L.; Huang, W.-G.; Zhu, F.-H.; Gao, H.-Y.; Tang, W.; Luo, Y.; Feng, C.-L.; Shi, L.-P.; Ren, Y.-D.; Lu, W.; Zuo, J.-P. J. Med. Chem. 2006, 49, 4790–4794. doi:10.1021/jm060330f |
| 42. | Valdez, J.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Navarrete-Vázquez, G.; Tapia, A.; Cortés, R.; Hernández, M.; Castillo, R. Bioorg. Med. Chem. Lett. 2002, 12, 2221–2224. doi:10.1016/s0960-894x(02)00346-3 |
| 43. | Sondhi, S. M.; Rajvanshi, S.; Johar, M.; Bharti, N.; Azam, A.; Singh, A. K. Eur. J. Med. Chem. 2002, 37, 835–843. doi:10.1016/s0223-5234(02)01403-4 |
| 32. | Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283–9303. doi:10.1039/c3cs60228a |
| 38. | Carvalho, L. C. R.; Fernandes, E.; Marques, M. M. B. Chem. – Eur. J. 2011, 17, 12544–12555. doi:10.1002/chem.201101508 |
| 39. | Maiti, S.; Mal, P. Adv. Synth. Catal. 2015, 357, 1416–1424. doi:10.1002/adsc.201401110 |
| 35. | Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512 |
| 36. | Sun, C.-L.; Shi, Z.-J. Chem. Rev. 2014, 114, 9219–9280. doi:10.1021/cr400274j |
| 37. | Louillat, M.-L.; Patureau, F. W. Chem. Soc. Rev. 2014, 43, 901–910. doi:10.1039/c3cs60318k |
© 2022 Bera et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.