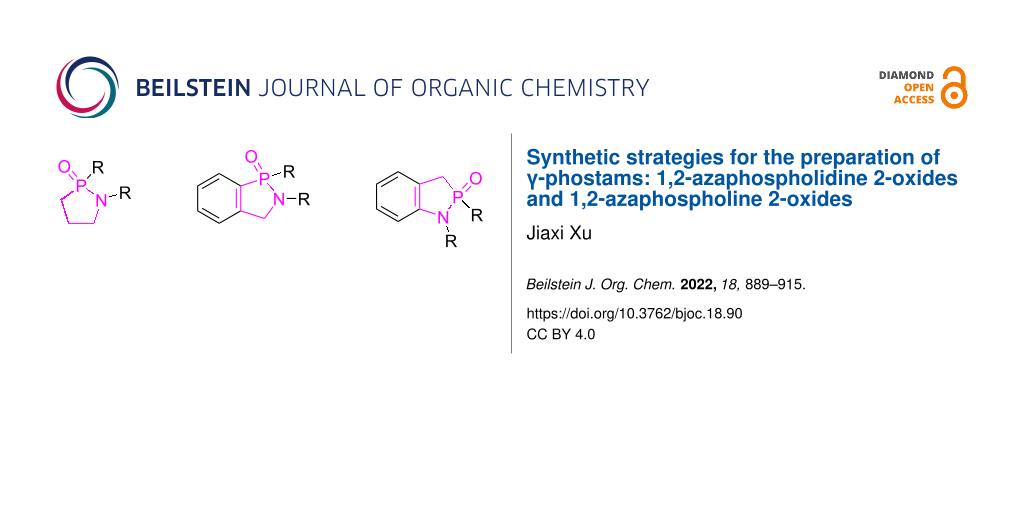
Abstract
γ-Phostams include γ-phosphonolactams and γ-phosphinolactams and their fused derivatives, phosphorus analogues of γ-lactams. They are 1,2-azaphospholidine 2-oxides and 1,2-azaphospholine 2-oxides and important biological five-membered azaphosphaheterocycles. They have been prepared through two major strategies of cyclizations and annulations. Cyclizations achieve ring construction through the formation of any bond in the ring, while annulations build the ring via [4 + 1] and [3 + 2] fashions with the simultaneous formation of two bonds. The review includes the synthesis of 1,2-azaphospholidine and 1,2-azaphospholine 2-oxides/sulfides and their fused derivatives.
Graphical Abstract
Introduction
Phosphaheterocycles are a class of important organic compounds [1-8] and have been widely applied in agrochemicals, medicinal agents, and materials science [9,10]. They are also organic synthetic intermediates and building blocks [7,11,13]. 1,2-Azaphosphaheterocycle oxide derivatives, phosphonolactams and phosphinolactams, are phosphorus analogues of the corresponding lactams [11-14]. 1,2-Azaphospholidine 2-oxides and 1,2-azaphospholine 2-oxides, also called γ-phosphonolactams and γ-phosphinolactams, and their fused derivatives are important five-membered 1,2-azaphosphaheterocyclic derivatives. They are γ-phostams, phosphorus analogues of γ-lactams, showing various biological activities, such as anti-inflammatory [15,16], antioxidant [17,18], and antitumor [18-20] (Figure 1).
Figure 1: Biologically active 1,2-azaphospholine 2-oxide derivatives.
Figure 1: Biologically active 1,2-azaphospholine 2-oxide derivatives.
Various synthetic methods of 1,2-azaphospholidine 2-oxide and 1,2-azaphospholine 2-oxide derivatives have been developed to date. Two major synthetic strategies are cyclization reactions and annulation reactions. The cyclization reactions have been applied in the construction of any ring bonds of 1,2-azaphospholidine and 1,2-azaphospholine rings, while [4 + 1] and [3 + 2] annulations are alternative routes for the formation of 1,2-azaphospholidine and 1,2-azaphospholine rings (Figure 2). This review includes the synthesis of 1,2-azaphospholidine and 1,2-azaphospholine 2-oxides/sulfides and their fused derivatives.
Figure 2: Diverse synthetic strategies for the preparation of 1,2-azaphospholidine and 1,2-azaphospholine 2-oxide derivatives.
Figure 2: Diverse synthetic strategies for the preparation of 1,2-azaphospholidine and 1,2-azaphospholine 2-o...
Review
Synthesis of 1,2-azaphospholidine 2-oxide derivatives via cyclization
Various cyclization strategies have been developed for the synthesis of 1,2-azaphospholidine 2-oxides/sulfides and their fused derivatives. The 1,2-azaphospholidine 2-oxide/sulfide derivatives have been prepared by construction of any of their ring bonds.
Synthesis via C–N bond formation
In 1962, Helferich and Curtius reported the first synthesis of a 1,2-azaphospholidine 2-oxide (γ-phosphonolactam) from N,N’-diphenyl 3-chloropropylphosphondiamide (1), which was cyclized to 1-phenyl-2-phenylamino-γ-phosphonolactam (2) in the presence of NaOH in methanol via the C–N bond formation (Scheme 1) [21].
Scheme 1: Synthesis of 1-phenyl-2-phenylamino-γ-phosphonolactam (2) from N,N’-diphenyl 3-chloropropylphosphondiamide (1).
Scheme 1: Synthesis of 1-phenyl-2-phenylamino-γ-phosphonolactam (2) from N,N’-diphenyl 3-chloropropylphosphon...
In 1974, the strategy was applied for the synthesis of 2-ethoxy-1-methyl-γ-phosphonolactam (6) for potential insecticides. Diethyl 3-bromopropylphosphonate (3) was first monochlorinated with phosphorus pentachloride in carbon tetrachloride followed by aminolysis with methylamine, affording ethyl N-methyl-(3-bromopropyl)phosphonamidate (5). Compound 5 was then treated with NaH in refluxing xylene to give the desired 2-ethoxy-1-methyl-γ-phosphonolactam (6) in approximate 40% yield (Scheme 2) [22].
Scheme 2: Synthesis of 2-ethoxy-1-methyl-γ-phosphonolactam (6) from ethyl N-methyl-(3-bromopropyl)phosphonamidate (5).
Scheme 2: Synthesis of 2-ethoxy-1-methyl-γ-phosphonolactam (6) from ethyl N-methyl-(3-bromopropyl)phosphonami...
In 1981, Miles and co-workers synthesized 2-aryl-1-methyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 13 from 2-iodotoluene (7). The reaction of 2-iodotoluene (7) and diethyl methylphosphonite gave ethyl 2-methylphenyl(methyl)phosphinate (8) in an excellent yield under the catalysis of anhydrous NiCl2 (Scheme 3) [23]. Ethyl 2-methylphenyl(methyl)phosphinate (8) was converted into 2-methylphenyl(methyl)phosphinic chloride (9) in 94% yield by treatment with excess PCl5. However, the radical chlorination of 2-methylphenyl(methyl)phosphinic chloride (9) gave the desired 2-chloromethylphenyl(methyl)phosphinic chloride (10) in 65% yield with unreacted starting 9 in 25–30%, and the dichlorinated product 11 in 5–10%. The reaction of 2-chloromethylphenyl(methyl)phosphinic chloride (10) with amines generated N-aryl-2-chloromethylphenyl(methyl)phosphinamides 12 in 52–99% yields, which were further treated with DBU in refluxing THF, affording 2-aryl-1-methyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 13 in 40–100% yield (Scheme 3) [23]. This is a general method to synthesize 1,2-azaphospholidine 2-oxide derivatives 13.
Scheme 3: Synthesis of 2-aryl-1-methyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 13 from N-aryl-2-chloromethylphenyl(methyl)phosphinamides 12.
Scheme 3: Synthesis of 2-aryl-1-methyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 13 from N-aryl-2-chlorom...
Arylphosphinyl azides generate arylphosphinyl nitrenes under photoirradiation. The phosphinyl nitrenes underwent an intramolecular insertion into the ortho C–H bond of the aryl group accompanied with the Curtius-like rearrangement as well [24]. Both monomesityl and dimesitylphosphinyl azides 14 generated 2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 15 in 31% and 20%, respectively, via an intramolecular nitrene C–H insertion, for dimesitylphosphinyl azide (14b), with 51% yield of phosphonamidate 16 as byproduct. Bis(2,4,6-triisopropylphenyl)phosphinyl azide (17) gave the corresponding 2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxide 18 in 51% and phosphonamidate 19 in 28% yield (Scheme 4). The synthetic method was applied occasionally [24].
Scheme 4: Synthesis of 2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides from alkylarylphosphinyl or diarylphosphinyl azides via an intramolecular nitrene C–H insertion.
Scheme 4: Synthesis of 2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides from alkylarylphosphinyl or diarylphosph...
P-Stereogenic 3-arylmethylidene-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides 21 and 23 were prepared in good to excellent yields with moderate to good E/Z selectivities via the TBAF-mediated cyclization of enantiopure N-alkyl-P-(2-ethynylaryl)-P-phenylphosphinamides 20 and 22. The synthetic strategy provided optically active 2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxide derivatives 21 and 23 (Scheme 5) [25].
Scheme 5: Synthesis of 3-arylmethylidene-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides via the TBAF-mediated cyclization of N-alkyl-P-(2-ethynylaryl)-P-phenylphosphinamides.
Scheme 5: Synthesis of 3-arylmethylidene-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxides via the TBAF-mediated ...
The metal-free intramolecular oxidative C–H bond amidation of methyl and ethyl 2,6-dimethylphenylphosphonamidates 24, 26, and 28 is an interesting strategy for the synthesis of 1-methoxy/ethoxy-7-methyl-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxide derivatives 25, 27, and 29 in satisfactory to good yields (Scheme 6) [26]. The 2,6-dimethylphenyl (26) and 4-aryl-2,4-dimethylphenyl (28) groups can be substituted by various functional groups. The same reaction using ethyl 3-substituted 2,4,6-trimethylphenylphosphonamidates 30 generated two regioisomeric 5,7-dimethyl-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides 31 and 32. Also, the ethyl 6-bromo-2,4-dimethylphenylphosphonamidate (33a) and the electron-rich 6-methoxy substrate 33b gave the corresponding 7-bromo and 4-iodo products 34a and 34b. However, ethyl N-substituted 2,6-dimethylphenylphosphonamidates 35, ethyl 2-methylphenylphosphonamidate (36), and ethyl P-phenyl 2,6-dimethylphenylphosphinamide (37) did not undergo the reaction, showing a limited application of the synthetic strategy (Scheme 6) [26]. The products are carboxylic phosphonic imides.
Scheme 6: Synthesis of 2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides via the metal-free intramolecular oxidative C–H bond formation of alkyl 6-substituted 2-methylphenylphosphonamidates.
Scheme 6: Synthesis of 2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides via the metal-free intramolecular oxida...
In 2021, Montchamp and co-workers synthesized 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 42 and 44 in moderate 54–63% yields via the intramolecular copper-catalyzed cross-coupling of ethyl/benzyl 2-bromobenzylphosphonamidates 41 or P-(2-bromobenzyl)-P-(methyl)phosphinamide (43) as a key step. They were prepared from 2-bromobenzyl bromide (38) via three and four steps, respectively (Scheme 7) [27].
Scheme 7: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 42 and 44 from ethyl/benzyl 2-bromobenzylphosphonamidates 41 and P-(2-bromobenzyl)-P-(methyl)phosphinamide (43).
Scheme 7: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 42 and 44 from ethyl/benzyl 2-bromobenzy...
Synthesis via P–N bond formation
Since 1980, new strategies for the synthesis of γ-phosphonolactams and γ-phosphinolactams have been explored via cyclization by P–N bond formation [28]. Kleiner prepared 1-aryl-2-methyl-1,2-azaphospholidine 2-oxides 46 in 40–78% yields by heating 3-arylaminopropyl(methyl)phosphinic acids 45 under reduced pressure. The reactions of 1,2-oxaphospholane 2-oxides 47/2-sulfides 50 and anilines 48 generated 1-aryl-2-methyl-1,2-azaphospholidine 2-oxides 49 and 46a as well, while the reaction of 1,2-thiaphospholane 2-sulfide (51) with aniline (48a) gave azaphospholidine 2-sulfide 52 in only 21% yield by heating at 200 °C for 60 h (Scheme 8) [28].
Scheme 8: Synthesis of azaphospholidine 2-oxides/sulfide from 1,2-oxaphospholane 2-oxides/sulfides and 1,2-thiaphospholane 2-sulfide with anilines.
Scheme 8: Synthesis of azaphospholidine 2-oxides/sulfide from 1,2-oxaphospholane 2-oxides/sulfides and 1,2-th...
In 1982, Collins and co-workers prepared both 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56 and 2-sulfide 60 through heating zwitterionic 2-aminobenzyl(phenyl)phosphinic acid 54 and 2-aminobenzyl(phenyl)dithiophosphinic acid 58, respectively. On the other hand, methyl 2-aminobenzyl(phenyl)phosphinate (53) was hydrolyzed under acidic conditions and neutralized with ammonia to give zwitterionic 2-aminobenzyl(phenyl)phosphinic acid 54. It was heated at 230 °C under reduced pressure to generate 2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (55), which was further alkylated with alkyl halides in the presence of NaH, affording 1-alkyl-2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56. Alternatively, under heating, methyl 2-aminobenzyl(phenyl)phosphinate (53) underwent a transmethylation from the O to N atom to generate the zwitterionic 2-((methylamino)benzyl)(phenyl)phosphinic acid 53’, which was further converted into 1-methyl-2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (56a, R = Me) in excellent yield under heating or treatment with DCC. On the other way, methyl (2-aminobenzyl)(phenyl)phosphinate (53) was reduced with lithium aluminum hydride to 2-aminobenzyl(phenyl)phosphine (57). It was oxidized with sulfur to give zwitterionic 2-aminobenzyl(phenyl)dithiophosphinic acid (58), which underwent thermal elimination of hydrogen sulfide to yield 2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfide (59). Sulfide 59 was similarly transformed to 1-alkyl-2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfides 60 by treatment with alkyl halides in the presence of NaH. All 1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfides 60 were converted into the corresponding 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56 by oxidation with mCPBA, while treatment of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56 with phosphorus pentasulfide transformed them back to 1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfides 60. Heating dimethyl 2-aminobenzylphosphonate (61) generated 1-methyl-2-methoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (62) in 34% yield via transmethylation and intramolecular cyclocondensation (Scheme 9) [29].
Scheme 9: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides/sulfides from 2-aminobenzyl(phenyl)phosphinic acid.
Scheme 9: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides/sulfides from 2-aminobenzyl(phenyl)phosp...
In 1983, Collins and co-workers prepared 2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfide (59) following the similar procedure. They first oxidized 2-aminobenzyl(phenyl)phosphine (57) with two atom-equivalents of sulfur in refluxing benzene, affording the zwitterionic 2-aminobenzyl(phenyl)dithiophosphinic acid 58 in 80% yield. It was converted to 2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfide (59) in 91% yield by heating at 100–120 °C under vacuum (Scheme 10) [30].
Scheme 10: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfide (59) from zwitterionic 2-aminobenzyl(phenyl)dithiophosphinic acid (58).
Scheme 10: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-sulfide (59) from zwitterionic 2-aminobenzyl(ph...
In the same year, they also realized the synthesis of 2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (55) in 75% yield by heating at 190–200 °C and in 47% yield by DCC condensation of compound 54 – the oxygen analogue of 2-aminobenzyl(phenyl)dithiophosphinic acid 58. The cyclodehydration method was more efficient than the DCC coupling one. The similar treatment of alkyl 2-aminobenzyl(phenyl)phosphinates 53 and 63 gave 1-alkyl-2-phenyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56 in 51–65% yield through the alkyl transfer from the O to the N atom followed by dehydration under heating. The direct heating of 2-aminobenzyl(methyl)phosphinic acid hydrochloric acid salt (65) generated 2-methyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (44) in 66% yield. However, when compound 65 was carefully neutralized with ammonia to give the zwitterionic 2-aminobenzylmethylphosphinic acid (66), the conversion into 2-methyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (44) proceeded in a quantitative yield in the presence of DCC in refluxing chloroform. Also, dimethyl 2-aminobenzylphosphonate (61) was transformed to 1-methyl-2-methoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide (62) via a similar alkyl transfer from the O to N atom followed by dehydration under heating (Scheme 11) [31].
Scheme 11: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides from 2-aminobenzyl(methyl/phenyl)phosphinic acids, alkyl 2-aminobenzyl(phenyl)phosphinates, and dimethyl 2-aminobenzylphosphonate (61).
Scheme 11: Synthesis of 1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides from 2-aminobenzyl(methyl/phenyl)phosphi...
Natchev prepared racemic and optically active ethyl 2-methyl-1,2-azaphospholidine-5-carboxylate 2-oxide (69) as cyclic analogue of the herbicide phosphinothricin (glufosinate, ᴅʟ-68) from ᴅʟ-, ᴅ-, and ʟ-2-amino-4-(hydroxy(methyl)phosphoryl)butanoic acid (68) by treatment with phosphorus pentachloride or thionyl chloride, respectively, in the presence of triethylamine when he synthesized phosphonopeptides. Similar yields were obtained for these two different chlorinating reagents. Ethyl 2-methyl-1,2-azaphospholidine-5-carboxylate 2-oxide (69) was sensible to glutaminase, an enzyme that could ring-open 1,2-azaphospholidine-5-carboxylate 2-oxides (Scheme 12) [32].
Scheme 12: Synthesis of ethyl 2-methyl-1,2-azaphospholidine-5-carboxylate 2-oxide 69 from 2-amino-4-(hydroxy(methyl)phosphoryl)butanoic acids 68.
Scheme 12: Synthesis of ethyl 2-methyl-1,2-azaphospholidine-5-carboxylate 2-oxide 69 from 2-amino-4-(hydroxy(m...
Griffiths and co-workers mentioned the synthesis of dimethyl (2-methoxy-1,3-dimethyl-2-oxido-1,3-dihydrobenzo[d][1,2]azaphosphol-3-yl)phosphonate (71) from the reaction of dimethyl 2-(methylamino)benzoylphosphonate (70) and trimethyl phosphite at 105 °C through an ylide intermediate D. The ylide D was generated via deoxygenation of benzoylphosphonate 70 with trimethyl phosphite to form a carbene intermediate B, and trimethyl phosphite nucleophilic attacking to the carbene B followed by an intramolecular displacement. The yield was not reported (Scheme 13) [33].
Scheme 13: Synthesis of 2-methoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide 71 from dimethyl 2-(methylamino)benzoylphosphonate (70) and trimethyl phosphite.
Scheme 13: Synthesis of 2-methoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide 71 from dimethyl 2-(methylamino...
Synthesis via P–C bond formation
In 1979, Coppola at Sandoz, Inc. reported an alternative strategy for the synthesis of tricyclic γ-phosphonolactams 74, 78, and 81 from N-(3-chloropropyl)-2-methylaminobenzamides 72, 76 and N-methyl-2-(3-bromopropylamino)benzamides 79 via the P–C bond formation as the crucial step. N-(3-Chloropropyl)-2-methylaminobenzamides 72 and 76 and N-methyl-2-(3-bromopropylamino)benzamides 79 were first treated with phosphorus trichloride followed by intramolecular cyclization in the presence of sodium hydride in dioxane, affording tricyclic γ-phosphonolactams 74, 78, and 81 in low to moderate yields (Scheme 14) [34].
Scheme 14: Synthesis of tricyclic γ-phosphonolactams via formation of the P–C bond.
Scheme 14: Synthesis of tricyclic γ-phosphonolactams via formation of the P–C bond.
In 2005, Aladzheva and co-workers prepared γ-phosphonolactams 85 from the substitution of ethyl 2-(3-chloropropyl)aminoalkanoates 82 derived from glycine and ᴅʟ-alanine ethyl esters and diethyl chlorophosphite (83a, R = EtO) or ethyl N,N-diethylchlorophosphamidite (83b, R = NEt2) followed by an intramolecular cyclization via an intramolecular Arbuzov reaction under heating [35]. The obtained γ-phosphonolactams 85 were further hydrolyzed into 2-(3-phosphonopropyl)aminoalkanoic acids 86 (Scheme 15) [35,36]. This is a convenient way to synthesize γ-phosphonolactams 85.
Scheme 15: Synthesis of γ-phosphonolactams 85 from ethyl 2-(3-chloropropyl)aminoalkanoates with diethyl chlorophosphite and ethyl N,N-diethylchlorophosphamidite.
Scheme 15: Synthesis of γ-phosphonolactams 85 from ethyl 2-(3-chloropropyl)aminoalkanoates with diethyl chloro...
They further extended their method to synthesize cyclic O,O- and O,S-bidentate ligands with a P–N–P backbone. The substitution reaction of 3-bromopropylamine hydrogen bromide (87) and chloroethoxyphosphine derivatives 83 followed by an intramolecular cyclization via the intramolecular Arbuzov reaction under heating generated 1,2-azaphospholidine 2-oxides 89. Compounds 89 were further transformed into N-phosphoryl- and N-thiophosphoryl-1,2-azaphospholidine 2-oxides 90/2-sulfides 91 via oxidation with hydrogen peroxide and sulfurization with sulfur, respectively (Scheme 16) [37,38].
Scheme 16: Synthesis of N-phosphoryl- and N-thiophosphoryl-1,2-azaphospholidine 2-oxides 90/2-sulfides 91 from 3-bromopropylamine hydrogen bromide and chloroethoxyphosphine derivatives.
Scheme 16: Synthesis of N-phosphoryl- and N-thiophosphoryl-1,2-azaphospholidine 2-oxides 90/2-sulfides 91 from...
Synthesis via formation of the C–C bond neighboring at the ring phosphorus atom
In 1984, Collins and co-workers attempted the synthesis of benzo-γ-phosphonolactams 56a and 93 from (chloromethyl)(phenyl)-N-methyl-N-phenylphosphinamide (92a) and chloromethyl N,N’-dimethyl-N,N’-diphenylphosphondiamide (92b) via an intramolecular Friedel–Crafts alkylation. Although they tried several different amide derivatives, only phosphinamide 92a and phosphonic diamide 92b gave the corresponding 1-methyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56a in 23% and 93 in 63% yields, respectively, showing the limited scope of the synthetic method (Scheme 17) [39].
Scheme 17: Synthesis of 1-methyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56a and 93 from P-(chloromethyl)amide precursors 92a and 92b through intramolecular Friedel–Crafts alkylation.
Scheme 17: Synthesis of 1-methyl-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 56a and 93 from P-(chloromethyl...
Ring-closing metathesis (RCM) is an efficient strategy for the construction of cyclic compounds via the formation of a C=C bond [40,41], which can be reduced to the C–C bond.
2-Allylamino-1,5-dihydro-1,2-azaphosphole 2-oxide derivatives 95 were prepared in 13–76% yield from N,N’-diallyl-vinylphosphonodiamides 94 via the Grubbs ruthenium-catalyzed RCM. N,N’-Dicinnamyl-N,N’-dimethyl-vinylphosphonodiamide (94a) (R = R’ = H) generated the 2-(N-methyl-N-cinnamylamino)-1,5-dihydro-1,2-azaphosphole 2-oxide (95a) in the lowest yield. The (E)-prop-1-enylphosphonodiamide 96 also underwent the RCM well. However, N,N’-dicinnamyl-vinylphosphonodiamides 94b,c (R’ = H, Me, R = Ph) always generated the corresponding N,N’-dicinnamyl-styrylphosphonodiamides 98 in 12–17% yields as byproducts via the RCM with the generated styrene in the reaction mixture (Scheme 18) [42].
Scheme 18: Synthesis of 2-allylamino-1,5-dihydro-1,2-azaphosphole 2-oxides from N,N’-diallyl-vinylphosphonodiamides.
Scheme 18: Synthesis of 2-allylamino-1,5-dihydro-1,2-azaphosphole 2-oxides from N,N’-diallyl-vinylphosphonodia...
One year later, the same group investigated the influence of the double bond geometry and the substitution pattern on the alkene. The results indicated that the double bond geometry plays a moderate role, while the bulkiness of the R group and the nature of its terminal substituents (aromatic phenyl and aliphatic isopropyl) are the crucial factors impacting the diastereoselectivity. Generally, the substrates with bulky R and aromatic phenyl terminal substituent in their allyl groups gave rise to the desired 1,5-dihydro-1,2-azaphosphole 2-oxides 100, 102, and 104 in higher diastereoselectivity (Scheme 19) [43]. The RCM reaction is a powerful strategy for the synthesis of P-stereogenic 1,5-dihydro-1,2-azaphosphole 2-oxide derivatives, which can be further reduced to 1,2-azaphospholidine 2-oxide derivatives. Thus, the strategy is an efficient method for the synthesis of 1,2-azaphospholidine 2-oxides via the C–C bond formation.
Scheme 19: Diastereoselective synthesis of 2-allylamino-1,5-dihydro-1,2-azaphosphole 2-oxides from N,N’-diallyl-vinylphosphonodiamides.
Scheme 19: Diastereoselective synthesis of 2-allylamino-1,5-dihydro-1,2-azaphosphole 2-oxides from N,N’-dially...
Our research group achieved the synthesis of 1-alkyl-3-benzoyl-2-ethoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxide derivatives 106 from ethyl N-alkyl-N-aryl-1-diazo-2-oxo-2-phenylethylphosphonamidates 105 via the copper-catalyzed intramolecular carbene aromatic C–H bond insertion (Scheme 20) [44]. This is an efficient synthetic strategy for 3-benzoyl-2-ethoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 106 through the formation of the C–C bond neighboring at the ring phosphorus atom.
Scheme 20: Synthesis of 1-alkyl-3-benzoyl-2-ethoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 106 from ethyl N-alkyl-N-aryl-1-diazo-2-oxo-2-phenylethylphosphonamidates 105.
Scheme 20: Synthesis of 1-alkyl-3-benzoyl-2-ethoxy-1,3-dihydrobenzo[d][1,2]azaphosphole 2-oxides 106 from ethy...
Synthesis via formation of the C–C bond neighboring at the ring nitrogen atom
In 2001, the Ortiz group developed a strategy for the synthesis of γ-phosphinolactam derivatives via formation of the C–C bond neighboring the nitrogen atom. Like the dearomatizing anionic cyclization of N-alkyl-N-benzylbenzamides [45], the strategy is the dearomatizing anionic cyclization of diaryl-N-alkyl-N-benzylphosphinamides by treatment with sec-butyllithium followed by reactions with different electrophiles, such as water, alcohols, alkyl halides, aldehydes, etc. They first realized the dearomatizing anionic cyclization of diphenyl-N-benzyl-N-methylphosphinamide (107) in the presence of sec-butyllithium followed by treatment with methanol, deuterium oxide, methyl iodide, and benzaldehyde, affording a series of cyclohexadiene-fused γ-phosphinolactams 108–112 in low regio- and stereoselectivies (Scheme 21) [46].
Scheme 21: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-benzyl-N-methylphosphinamide (107).
Scheme 21: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-benzyl-N-methylphosphinamide (...
They further performed a detailed investigation on the dearomatizing anionic cyclization of diphenyl-N-alkyl-N-benzylphosphinamides 107, 116, and 119 by treatment with sec-butyllithium and protonation with various alcohols (MeOH, iPrOH, t-BuOH), phenols (PhOH, 2-tert-butyl-4-methylphenol, and 2,6-di(tert-butyl)-4-methylphenol (DTBMP)), trifluoroacetic acid (TFA), and 4-methylbenzenesulfonic acid (TsOH) as the proton donors. The results indicated that the less bulky methanol, more bulky DTBMP, more sterically hindered and acidic TsOH show certain regio- and stereoselectivities, which also depended upon the steric hindrance of the N-alkyl group, although several regio- and diastereoisomers were generated in the reactions (Scheme 22) [19].
Scheme 22: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-alkyl-N-benzylphosphinamides.
Scheme 22: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-alkyl-N-benzylphosphinamides.
They further investigated the influences of the time of metalation, the concentration of base, cosolvents (additives), and the time of contact with the electrophiles on the regio- and stereoselectivities in the tandem intramolecular nucleophilic dearomatization of diphenyl-N-alkyl-N-benzylphosphinamides and reactions with different electrophiles under various reaction conditions [47].
They used both racemic and enantiopure diphenyl-N-methyl-N-(1-phenylethyl)phosphinamides 124 as starting materials, realizing an unprecedented asymmetric induction in the synthesis of cyclohexadiene-fused γ-phosphinolactams 126–131, through the formation of configurationally stable lithium salts. When aldehydes 125 were applied as electrophiles, three new stereocenters were generated in the reaction and the corresponding hydroxymethyl cyclohexadiene-fused γ-phosphinolactams 126–129 were obtained in good to excellent yields with moderate to good diastereoselectivities and excellent enantioselectivities (for both enantiopure starting materials). When DTBMP and benzyl bromide were utilized as electrophiles, only two and one new stereocenter(s) were generated, respectively, and the corresponding protonated and benzylated cyclohexadiene-fused γ-phosphinolactams 130–133 were obtained in good yields, with excellent enantioselectivities for both enantiopure starting materials. In each of the cases, a minor diastereoisomeric epimer in the phosphorus atom was also isolated in less than 8% yield (Scheme 23) [48].
Scheme 23: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-methyl-N-(1-phenylethyl)phosphinamides 124.
Scheme 23: Synthesis of cyclohexadiene-fused γ-phosphinolactams from diphenyl-N-methyl-N-(1-phenylethyl)phosph...
The authors further performed detailed mechanistic investigations experimentally and theoretically [49,50].
Dinaphth-1-yl-N-alkyl-N-benzylphosphinamides 134 were also applied to the tandem intramolecular nucleophilic dearomatization and protonation or electrophilic alkylation reactions, affording the corresponding dihydronaphthylene-fused γ-phosphinolactams 135–142. Methanol was used as the electrophile for protonation, while methyl iodide and allyl bromide were used as electrophiles for alkylation. A remarkable difference compared with the diphenylphosphinamides is the fact that the current reactions proceeded with excellent regio- and stereoselectivities and yields in THF without the use of the carcinogenic cosolvent HMPA (Scheme 24) [51].
Scheme 24: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-alkyl-N-benzylphosphinamides 134.
Scheme 24: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-alkyl-N-benzylphosph...
With benzaldehyde as an electrophile, both alkylated and protonated benzocyclohexadiene-fused γ-phosphinolactams 143 and 136a were generated in 25% and 15% yield, respectively. However, two pairs of alkylated and protonated benzocyclohexadiene-fused γ-phosphinolactams 144 and 145, 136a and 146 were obtained with 4-chlorobenzaldehyde as electrophile. When acetic anhydride was employed as electrophile, besides the protonated product 136a, two different alkylated products 147 and 148 were formed depending on the reaction time. The 1-acetoxylvinylated and 3-hydroxybut-2-enoylated products 147 and 148 were obtained, respectively, through O-acetylation and C-acetylation followed by enolization of the first generated acetylated benzocyclohexadiene-fused γ-phosphinolactams 149. The results indicated that short reaction times favored O-acetylation, while long reaction times preferred C-acetylation (Scheme 25) [52].
Scheme 25: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-benzyl-N-methylphosphinamides.
Scheme 25: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-benzyl-N-methylphosp...
They further investigated various α,β-unsaturated carbonyl compounds as electrophiles. Interestingly, for propenal (150), besides two pairs of alkylated products 151 and 152, the second naphthyl group was alkylated by sec-butyllithium, leading to the corresponding product 153 in 5% yield. For but-3-en-2-one (154), methyl propenoate, cyclopent-2-enone (160a), and cyclohex-2-enone (160b), all gave the corresponding diastereomeric alkylated products 155–159 and 161–163 (Scheme 26) [52].
Scheme 26: Synthesis of carbonyl-containing benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-benzyl-N-methylphosphinamide.
Scheme 26: Synthesis of carbonyl-containing benzocyclohexadiene-fused γ-phosphinolactams from dinaphth-1-yl-N-...
By contrast, dinaphth-2-yl-N-benzyl-N-methylphosphinamide (164) generated only the sec-butylated product 165 by the treatment with sec-butyllithium and methanol. However, it gave the corresponding protonated product 166 when LDA was used as base instead of sec-butyllithium. Similar as dinaphth-1-yl-N-benzyl-N-methylphosphinamide (134a), the reactions of dinaphth-1-yl-N-alkyl-N-benzylphosphinamides 134 with but-3-en-2-one generated the corresponding products 167–171. With benzyl bromide as electrophile, dinaphth-1-yl-N-benzyl-N-methylphosphinamide (134a) gave the corresponding diastereomeric products 171–173. The second naphthyl group was alkylated by sec-butyllithium, affording product 174 in 4% yield as well. In the presence of LDA as base instead of sec-butyllithium and with MeI as electrophile, dinaphth-2-yl-N-benzyl-N-methylphosphinamide (164) gave three pairs of methylated diastereomeric products 175–177 (Scheme 27) [53].
Scheme 27: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphthyl-N-benzyl-N-methylphosphinamides.
Scheme 27: Synthesis of benzocyclohexadiene-fused γ-phosphinolactams from dinaphthyl-N-benzyl-N-methylphosphin...
They also explored the application of their strategy in the dearomatizing cyclization of aryl-N,N’-dibenzyl-N,N’-dimethylphosphonodiamides 178, 181, and 183. The corresponding cyclohexadiene-fused γ-phosphinolactam derivatives 179, 180, 182, and 184–188 were obtained after treatment with sec-butyllithium in a mixture of THF and DMPU (1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone) followed by addition of various electrophiles, such as methanol, phenolic DTBMP, benzyl bromide, aldehydes, and benzophenone. For both benzyl bromide and benzophenone, their alkylations occurred only at the γ-position of the phenyl group in phenylphosphonodiamides due to less bulkyness (Scheme 28) [54].
Scheme 28: Synthesis of cyclohexadiene-fused 1-(N-benzyl-N-methyl)amino-γ-phosphinolactams from aryl-N,N’-dibenzyl-N,N’-dimethylphosphonodiamides.
Scheme 28: Synthesis of cyclohexadiene-fused 1-(N-benzyl-N-methyl)amino-γ-phosphinolactams from aryl-N,N’-dibe...
They finally extended their strategy to alkyl-linked bis(diphenyl-N-benzylphosphinamide)s 189. Both pairs of meso-bis(cyclohexadiene-fused γ-phosphinolactam)s 190 and 192, 190 as major products, and two pairs of racemic diastereomeric bis(cyclohexadiene-fused γ-phosphinolactam)s 191 and 193 were obtained when the reactions were quenched with less steric methanol as the electrophile because the protonation occurred at both the α- and γ-positions of the phenyl group in their diphenylphosphinamide moieties. However, only one pair of meso-bis(cyclohexadiene-fused γ-phosphinolactam)s 192 as major products and one pair of racemic diastereomeric bis(cyclohexadiene-fused γ-phosphinolactam)s 193 were obtained when the reactions were quenched with more bulky DTBMP as the electrophile due to protonation at only the less bulky γ-position of the phenyl group in their diphenylphosphinamide moieties (Scheme 29) [55].
Scheme 29: Synthesis of bis(cyclohexadiene-fused γ-phosphinolactam)s from bis(diphenyl-N-benzylphosphinamide)s.
Scheme 29: Synthesis of bis(cyclohexadiene-fused γ-phosphinolactam)s from bis(diphenyl-N-benzylphosphinamide)s....
When tetramethylene-linked bis(diphenyl-N-benzylphosphinamide) (189c, n = 3) was treated with sec-butyllithium followed by addition of benzaldehyde and methanol sequentially, the reaction afforded four diastereomeric tetramethylene-linked bis(hydroxymethyl-derived cyclohexadiene-fused γ-phosphinolactam)s 194–197 generated by alkylation at the γ-position of the phenyl group in their diphenylphosphinamide moieties due to favorable steric hindrance. One pair (194) of meso-diastereomers is the major product (Scheme 30) [55].
Scheme 30: Synthesis of bis(hydroxymethyl-derived cyclohexadiene-fused γ-phosphinolactam)s from tetramethylene-linked bis(diphenyl-N-benzylphosphinamide).
Scheme 30: Synthesis of bis(hydroxymethyl-derived cyclohexadiene-fused γ-phosphinolactam)s from tetramethylene...
Synthesis of 1,2-azaphospholidine 2-oxide derivatives via annulations
Annulations are alternative strategies for the construction of 1,2-azaphospholidine 2-oxides and their fused derivative, [4 + 1] annulations and more occasionally a [3 + 2] annulation.
[4 + 1] Annulation via formations of both C–N and N–P bonds
Miles and Street prepared 2-aryl/dimethylamino-1-ethoxy-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides 201 from 2-(diethoxyphosphoryl)benzoic acid (198) and amine derivatives. They first converted 2-(diethoxyphosphoryl)benzoic acid (198) into its anhydride 1-ethoxy-3H-benzo[c][1,2]oxaphosphol-3-one 1-oxide (199) by treatment with thionyl chloride under nitrogen. The mixed anhydride 199 was further treated with phosphorus pentachloride to generate ethyl (2-(chlorocarbonyl)phenyl)phosphonochloridate (200), which reacted with amine and hydrazine derivatives in the presence of triethylamine, affording the desired 1-ethoxy-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides 201 but the yields were not reported (Scheme 31) [56].
Scheme 31: Synthesis of 2-aryl/dimethylamino-1-ethoxy-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides from ethyl (2-(chlorocarbonyl)phenyl)phosphonochloridate 200 with amines and N,N-dimethylhydrazine.
Scheme 31: Synthesis of 2-aryl/dimethylamino-1-ethoxy-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxides from ethy...
Two ethyl 1-substituted 2-ethoxy-1,2-azaphospholidine-4-carboxylate 2-oxides 203 were synthesized in 70% and 39% yields, respectively, from ethyl 2-((chloro(ethoxy)phosphoryl)methyl)acrylate (202) and benzyl- and adamantylmethylamines via aza-Michael addition and intramolecular nucleophilic substitution. The synthetic method showed very limited substrate scope. Only less bulky primary amines underwent the first aza-Michael addition and then intramolecular nucleophilic substitution. However, aromatic amines, aniline, 2,3-dihydro-1H-inden-4-amine, and the bulky aliphatic primary amine adamantylamine did not proceed due to their reduced nucleophilicities, generating the corresponding phosphonamidates only through the reaction with the more electrophilic phosphonochloridate (Scheme 32) [57].
Scheme 32: Synthesis of ethyl 2-ethoxy-1,2-azaphospholidine-4-carboxylate 2-oxides from ethyl 2-((chloro(ethoxy)phosphoryl)methyl)acrylate (202) and primary amines.
Scheme 32: Synthesis of ethyl 2-ethoxy-1,2-azaphospholidine-4-carboxylate 2-oxides from ethyl 2-((chloro(ethox...
[4 + 1] Annulation via formations of both C–P and P–N bonds
(1S,3R)-2-(tert-Butyldiphenylsilyl)-3-methyl-1-phenyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxide (210) was synthesized in low yields of 20–43% with diastereomeric ratios of 10:1 to >20:1 from (R)-1-tert-butyl-1,1-diphenyl-N-(1-phenylethyl)silanamine (204) via the treatment with butyllithium followed by double displacement with four different phosphorus electrophiles 206–209, which include three 1,3,2-oxazaphospholidine 2-oxide derivatives 206–208 and phenylphosphonic dichloride (209). The product 210 was further transformed into (1S,3R)-3-methyl-1-phenyl-2,3-dihydro-1H-benzo[c][1,2]azaphosphole 212, which can be applied as a precatalyst. However, its borane complex showed 23% enantiomeric excess in the asymmetric borane reduction of acetophenone in THF at room temperature (Scheme 33) [58].
Scheme 33: Synthesis of (1S,3R)-2-(tert-butyldiphenylsilyl)-3-methyl-1-phenyl-2,3-dihydrobenzo[c][1,2]azaphosphole 1-oxide via double displacement of phosphorus electrophiles with (R)-1-tert-butyl-1,1-diphenyl-N-(1-phenylethyl)silanamine (204).
Scheme 33: Synthesis of (1S,3R)-2-(tert-butyldiphenylsilyl)-3-methyl-1-phenyl-2,3-dihydrobenzo[c][1,2]azaphosp...
The reaction of 3-(phenylaminomethylene)-2-phenylamino-6-methyl-2,3-dihydro-4H-chromen-4-one (213) and diethyl phosphite at 90–100 °C generated 2-ethoxy-6-methyl-2-oxo-1-phenyl-3-phenylamino-2,3,3a,9a-tetrahydro-4H-1,2-azaphospholo[5,4-b]chromen-4-one (215) in 44% yield through the Michael addition and subsequent intramolecular aminolysis (Scheme 34) [15]. The products are potential anti-inflammatory agents.
Scheme 34: Synthesis of 2,3,3a,9a-tetrahydro-4H-1,2-azaphospholo[5,4-b]chromen-4-one (215) from 3-(phenylaminomethylene)-2-phenylamino-6-methyl-2,3-dihydro-4H-chromen-4-one (213) and diethyl phosphite.
Scheme 34: Synthesis of 2,3,3a,9a-tetrahydro-4H-1,2-azaphospholo[5,4-b]chromen-4-one (215) from 3-(phenylamino...
In 2013, to develop potent anti-inflammatory agents, Abdou and co-workers prepared quinoline-fused 1,2-azaphospholine 2-oxides 217 in approximate 80% yield from 2-azidoquinoline-3-carbaldehydes 216 and tris(dimethylamino)phosphine in THF with water as solvent. It was mentioned that 2-azidoquinoline-3-carbaldehyde 216 and tris(dimethylamino)phosphine first generated phosphinimines 218 followed by quenching with water and sequential loss of two molecules of dimethylamine. However, the mechanism of this transformation was not clearly investigated (Scheme 35) [16].
Scheme 35: Synthesis of quinoline-fused 1,2-azaphospholine 2-oxides from 2-azidoquinoline-3-carbaldehydes and tris(dimethylamino)phosphine.
Scheme 35: Synthesis of quinoline-fused 1,2-azaphospholine 2-oxides from 2-azidoquinoline-3-carbaldehydes and ...
To develop new antitumor agents composed of chromene and 5-oxo-1,2-azaphospolidine 2-oxide motifs, Ali’s group performed the reaction of (E)-2-cyano-N'-((4-oxo-4H-chromen-3-yl)methylene)acetohydrazide (220) and phosphonic acid in the presence of 4-toluenesulfonic acid in dioxane, affording (E)-2,3-dihydroxy-1-(((4-oxo-4H-chromen-3-yl)methylene)amino)-1-hydro-1,2-azaphosphol-5-one 2-oxide (223) in 38% yield via the acid-catalyzed addition to the cyano group and subsequent cyclocondensation, hydrolysis, and tautomerization. Alternatively, the reaction of (E)-2-cyano-N'-((4-oxo-4H-chromen-3-yl)methylene)acetohydrazide (220) and phosphorus tribromide in the presence of triethylamine in dioxane followed by two step hydrolysis gave the same product 223 in 28% yield. Phosphorus tribromide first reacted with more nucleophilic amide nitrogen atom to form dibromophosphanamine derivative 224 by loss of HBr. The more nucleophilic dibromophosphanamine derivative 224 further underwent a nucleophilic addition to the cyano group followed by hydrolysis to give the final product 223 (Scheme 36). The product 223 showed antitumor activity in a biological assay [20].
Scheme 36: Synthesis of 1-hydro-1,2-azaphosphol-5-one 2-oxide from cyanoacetohydrazide with phosphonic acid and phosphorus tribromide.
Scheme 36: Synthesis of 1-hydro-1,2-azaphosphol-5-one 2-oxide from cyanoacetohydrazide with phosphonic acid an...
To develop antioxidants and antitumor agents, the same group synthesized chromene-fused 5-oxo-1,2-azaphospholidine 2-oxide derivatives. The reaction of 2-imino-2H-chromene-3-carboxamide (228) and diethyl phosphite at 80–90 °C under the catalysis of boron trifluoride, afforded 4-amino-1-ethoxy-9b-hydrochromeno[4,3-c][1,2]azaphosphol-3(2H)-one 1-oxide (229) in 40% yield through the Michael addition and subsequent tautomerization and intramolecular aminolysis. Under similar conditions, the reaction of 2-imino-2H-chromene-3-carboxamide (228) and tris(2-chloroethyl) phosphite (232) generated 4-amino-1-(2-chloroethoxy)-9b-hydrochromeno[4,3-c][1,2]azaphosphol-3(2H)-one 1-oxide (233) in 30% yield through the Michael addition and subsequent tautomerization and intramolecular aminolysis. Non-aminolysis byproduct 234 was obtained in 25% (Scheme 37). Both of the chromene-fused 5-oxo-1,2-azaphospholidine 2-oxide derivatives 233 and 234 showed good antioxidant and antitumor activities [17].
Scheme 37: Synthesis of chromene-fused 5-oxo-1,2-azaphospolidine 2-oxides.
Scheme 37: Synthesis of chromene-fused 5-oxo-1,2-azaphospolidine 2-oxides.
[4 + 1] Annulation via formations of both C–C and C–N bonds
When the Ortiz group investigated the directed ortho-lithiation of aminophosphazenes, they realized one example synthesis of (R)-1-phenyl-2-((R)-1-phenylethyl)-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxide (239) from methyl (R)-(diphenyl((1-phenylethyl)amino)-λ5-phosphanylidene)carbamate (237) via the ortho-directed lithiation with tert-butyllithium with carbamate as the directing group followed by electrophilic quench with methyl chloroformate and intramolecular aminolysis. Finally, hydrolysis removed the directing group, affording the final product 239 in 50% yield (Scheme 38) [59].
Scheme 38: Synthesis of (R)-1-phenyl-2-((R)-1-phenylethyl)-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxide (239) from methyl (R)-(diphenyl((1-phenylethyl)amino)-λ5-phosphanylidene)carbamate (237) via ortho-directed lithiation followed by electrophilic quench and intramolecular aminolysis.
Scheme 38: Synthesis of (R)-1-phenyl-2-((R)-1-phenylethyl)-2-hydrobenzo[c][1,2]azaphosphol-3-one 1-oxide (239)...
Structurally diverse methyl 2-(1-ethoxy-1-oxido-2-phenyl-2,3-dihydrobenzo[c][1,2]azaphosphol-3-yl)acetates 242a (R’ = OEt) and methyl 2-(1-aryl-1-oxido-2-phenyl-2,3-dihydrobenzo[c][1,2]azaphosphol-3-yl)acetates 242b–d (R’ = Ar) were prepared in 50–98% yield with diastereomeric ratio of 1:1.6–1:5.2 from ethyl aryl-N-phenylphosphonamidates 240a (R’ = OEt) and diaryl-N-phenylphosphinamides 240b–d (R’ = Ar), respectively, with methyl acrylate (241) via the rhodium-catalyzed oxidative coupling and subsequent intramolecular aza-Michael addition. Methyl acrylate (241) could be replaced by various electron-deficient olefins 244, including ethyl and butyl acrylates, but-3-en-2-one, N,N-dimethylacrylamide, acrylonitrile, and phenyl vinyl sulfone. In addition, ethyl 1-arylvinyl-N-phenylphosphonamidates 246 were able to react with methyl acrylate (241), affording methyl 2-(3-aryl-2-ethoxy-2-oxo-1-phenyl-1,5-dihydro-1,2-azaphosphol-5-yl)acetates 247 in moderate yields of 53–71% with diastereomeric ratios of 1:1.7 to 1:3. The synthetic strategy is more versatile (Scheme 39) [60].
Scheme 39: Synthesis of dihydro[1,2]azaphosphole 1-oxides from aryl/vinyl-N-phenylphosphonamidates and aryl-N-phenylphosphinamides with electron-withdrawing ethenes via the rhodium-catalyzed oxidative coupling and subsequent intramolecular aza-Michael addition.
Scheme 39: Synthesis of dihydro[1,2]azaphosphole 1-oxides from aryl/vinyl-N-phenylphosphonamidates and aryl-N-...
[3 + 2] Annulation via formations of both C–C and P–N bonds
After oxidation with K3Fe(CN)6, diphenyl 3,5-di(tert-butyl)-4-hydroxybenzylphosphonate (248) was oxidized into diphenyl (3,5-di-tert-butyl-4-oxocyclohexa-2,5-dienylidene)methylphosphonate (249), which was reacted with 2,6-diaminopyridine (250) in acetonitrile at room temperature to give diphenyl 3,5-di(tert-butyl)-4-hydroxyphenyl-(2,6-diaminopyridin-3-yl)methylphosphonate (251) with 6-amino-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-phenoxy-1,3-dihydro-[1,2]azaphospholo[5,4-b]pyridine 2-oxide (252) as byproduct. The yield of 6-amino-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-phenoxy-1,3-dihydro-[1,2]azaphospholo[5,4-b]pyridine 2-oxide (252) was improved to 35% when the reaction was conducted in refluxing dioxane for 2 h. It was generated from the intramolecular aminolysis of diphenyl 3,5-di(tert-butyl)-4-hydroxyphenyl-(2,6-diaminopyridin-3-yl)methylphosphonate (251) and showed excellent antioxidant activity (Scheme 40) [18].
Scheme 40: Synthesis of 1,3-dihydro-[1,2]azaphospholo[5,4-b]pyridine 2-oxides.
Scheme 40: Synthesis of 1,3-dihydro-[1,2]azaphospholo[5,4-b]pyridine 2-oxides.
Conclusion
1,2-Azaphospholidine 2-oxides and 1,2-azaphospholine 2-oxides are important five-membered azaphosphaheterocycles, They are known as γ-phostams, including γ-phosphonolactams and γ-phosphinolactams. Benzo[1,2]azaphospholine 2-oxides are phosphorus analogues of indolinone derivatives and show important biological activities. Both γ-phosphonolactams and γ-phosphinolactams and their fused derivatives have been synthesized through various synthetic strategies. The synthetic strategies can be categorized into cyclization and annulation strategies. The cyclizations have been widely applied for the formation of any C–N, C–P, P–N, and C–C bonds in the 1,2-azaphospholidine ring. [4 + 1] Annulations have been mainly utilized in the construction of the 1,2-azaphospholidine ring, while the [3 + 2] annulation has been seldomly used in the synthesis of pyridine-fused 1,2-azaphospholidine 2-oxide only. Few asymmetric synthetic methods have been developed to date. Thus, highly stereoselective asymmetric synthetic methods to access 1,2-azaphospholidine 2-oxides and 1,2-azaphospholine 2-oxides, and their fused and spiro derivatives are in high demand and should be developed in the near future.
References
-
Neidlein, R. Stud. Nat. Prod. Chem. 1991, 9, 509–528.
Return to citation in text: [1] -
Kamalov, R. M.; Schmidpeter, A.; Polborn, K. Phosphorus, Sulfur Silicon Relat. Elem. 1993, 83, 111–118. doi:10.1080/10426509308034353
Return to citation in text: [1] -
Jenner, G. Pol. J. Chem. 1992, 66, 1535–1549.
Return to citation in text: [1] -
Bogachenkov, A. S.; Dogadina, A. V.; Boyarskaya, I. A.; Boyarskiy, V. P.; Vasilyev, A. V. Org. Biomol. Chem. 2016, 14, 1370–1381. doi:10.1039/c5ob02143j
Return to citation in text: [1] -
Son, J.-Y.; Kim, H.; Jeon, W. H.; Baek, Y.; Seo, B.; Um, K.; Lee, K.; Lee, P. H. Adv. Synth. Catal. 2017, 359, 3194–3206. doi:10.1002/adsc.201700742
Return to citation in text: [1] -
Fu, Z.; Sun, S.; Yang, A.; Sun, F.; Xu, J. Chem. Commun. 2019, 55, 13124–13127. doi:10.1039/c9cc06352h
Return to citation in text: [1] -
Xu, J. Chem. Heterocycl. Compd. 2020, 56, 979–981. doi:10.1007/s10593-020-02763-9
Return to citation in text: [1] [2] -
Luo, Y.; Fu, Z.; Fu, X.; Du, C.; Xu, J. Org. Biomol. Chem. 2020, 18, 9526–9537. doi:10.1039/d0ob02011g
Return to citation in text: [1] -
Yang, K.-W.; Feng, L.; Yang, S.-K.; Aitha, M.; LaCuran, A. E.; Oelschlaeger, P.; Crowder, M. W. Bioorg. Med. Chem. Lett. 2013, 23, 5855–5859. doi:10.1016/j.bmcl.2013.08.098
Return to citation in text: [1] -
Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 84, 8131–8139. doi:10.1021/acs.joc.9b00994
Return to citation in text: [1] -
Xu, J. Chem. Heterocycl. Compd. 2020, 56, 308–310. doi:10.1007/s10593-020-02660-1
Return to citation in text: [1] [2] -
Fu, X.; Li, X.; Xu, J. Org. Lett. 2021, 23, 8733–8737. doi:10.1021/acs.orglett.1c03182
Return to citation in text: [1] -
Xu, J. X. Asian J. Org. Chem. 2022, 11, e202200176.
Return to citation in text: [1] [2] -
Luo, Y.; Xu, J. Org. Lett. 2020, 22, 7780–7785. doi:10.1021/acs.orglett.0c02346
Return to citation in text: [1] -
Ali, T. E. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 185, 88–96. doi:10.1080/10426500802713309
Return to citation in text: [1] [2] -
Abdou, W. M.; Khidre, R. E.; Shaddy, A. A. J. Heterocycl. Chem. 2013, 50, 33–41. doi:10.1002/jhet.968
Return to citation in text: [1] [2] -
Ali, T. E.; Assiri, M. A.; El-Shaaer, H. M.; Hassan, M. M.; Fouda, A. M.; Hassanin, N. M. Synth. Commun. 2019, 49, 2983–2994. doi:10.1080/00397911.2019.1652323
Return to citation in text: [1] [2] -
Gibadullina, E.; Nguyen, T. T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. Eur. J. Med. Chem. 2019, 184, 111735. doi:10.1016/j.ejmech.2019.111735
Return to citation in text: [1] [2] [3] -
Fernández, I.; Ortiz, F. L.; Velázquez, A. M.; Granda, S. G. J. Org. Chem. 2002, 67, 3852–3860. doi:10.1021/jo025587+
Return to citation in text: [1] [2] -
Ali, T. E.; Ali, M. M.; Abdel-Kariem, S. M.; Ahmed, M. M. Synth. Commun. 2017, 47, 1458–1470. doi:10.1080/00397911.2017.1332224
Return to citation in text: [1] [2] -
Helferich, B.; Curtius, U. Justus Liebigs Ann. Chem. 1962, 655, 59–69. doi:10.1002/jlac.19626550109
Return to citation in text: [1] -
Collins, D. J.; Hetherington, J. W.; Swan, J. M. Aust. J. Chem. 1974, 27, 1759–1765. doi:10.1071/ch9741759
Return to citation in text: [1] -
Miles, J. A.; Grabiak, R. C.; Beeny, M. T. J. Org. Chem. 1981, 46, 3486–3492. doi:10.1021/jo00330a021
Return to citation in text: [1] [2] -
Harger, M. J. P.; Shimmin, P. A. J. Chem. Soc., Perkin Trans. 1 1993, 227–232. doi:10.1039/p19930000227
Return to citation in text: [1] [2] -
Soengas, R.; Belmonte Sánchez, E.; Iglesias, M. J.; López Ortiz, F. ACS Omega 2018, 3, 5116–5124. doi:10.1021/acsomega.8b00491
Return to citation in text: [1] -
Kim, Y. R.; Cho, S.; Lee, P. H. Org. Lett. 2014, 16, 3098–3101. doi:10.1021/ol501207w
Return to citation in text: [1] [2] -
Sabourin, A.; Dufour, J.; Vors, J.-P.; Bernier, D.; Montchamp, J.-L. J. Org. Chem. 2021, 86, 14684–14694. doi:10.1021/acs.joc.1c01501
Return to citation in text: [1] -
Kleiner, H.-J. Liebigs Ann. Chem. 1980, 324–332. doi:10.1002/jlac.198019800217
Return to citation in text: [1] [2] -
Collins, D. J.; Drygala, P. F.; Swan, J. M. Tetrahedron Lett. 1982, 23, 1117–1120. doi:10.1016/s0040-4039(00)87037-9
Return to citation in text: [1] -
Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1983, 36, 2095–2110. doi:10.1071/ch9832095
Return to citation in text: [1] -
Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1983, 36, 2517–2536. doi:10.1071/ch9832517
Return to citation in text: [1] -
Natchev, I. A. Bull. Chem. Soc. Jpn. 1988, 61, 3699–3704. doi:10.1246/bcsj.61.3699
Return to citation in text: [1] -
Griffiths, D. V.; Harris, J. E.; Whitehead, B. J. J. Chem. Soc., Perkin Trans. 1 1997, 2545–2548. doi:10.1039/a701147d
Return to citation in text: [1] -
Coppola, G. M. J. Heterocycl. Chem. 1979, 16, 897–901. doi:10.1002/jhet.5570160513
Return to citation in text: [1] -
Aladzheva, I. M.; Bykhovskaya, O. V.; Petrovskii, P. V.; Lyssenko, K. A.; Antipin, M. Y.; Mastryukova, T. A. Russ. Chem. Bull. 2005, 54, 2635–2641. doi:10.1007/s11172-006-0168-4
Return to citation in text: [1] [2] -
Bykhovskaya, O. V.; Aladzheva, I. M.; Lobanov, D. I.; Petrovskii, P. V.; Lyssenko, K. A.; Fedyanin, I. V.; Mastryukova, T. A. Russ. Chem. Bull. 2005, 54, 2642–2647. doi:10.1007/s11172-006-0169-3
Return to citation in text: [1] -
Aladzheva, I. M.; Bykhovskaya, O. V.; Nelyubina, Y. V.; Korlyukov, A. A.; Petrovskii, P. V.; Odinets, I. L. Synthesis 2010, 613–618. doi:10.1055/s-0029-1218600
Return to citation in text: [1] -
Aladzheva, I. M.; Bykhovskaya, O. V.; Nelyubina, Y. V.; Petrovskii, P. V.; Odinets, I. L. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 769–771. doi:10.1080/10426507.2010.499549
Return to citation in text: [1] -
Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1984, 37, 1009–1021. doi:10.1071/ch9841009
Return to citation in text: [1] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1] -
McReynolds, M. D.; Dougherty, J. M.; Hanson, P. R. Chem. Rev. 2004, 104, 2239–2258. doi:10.1021/cr020109k
Return to citation in text: [1] -
Hanson, P. R.; Stoianova, D. S. Tetrahedron Lett. 1999, 40, 3297–3300. doi:10.1016/s0040-4039(99)00479-7
Return to citation in text: [1] -
Stoianova, D. S.; Hanson, P. R. Org. Lett. 2000, 2, 1769–1772. doi:10.1021/ol005952o
Return to citation in text: [1] -
Huang, P.; Xu, J. RSC Adv. 2016, 6, 63736–63748. doi:10.1039/c6ra10555f
Return to citation in text: [1] -
Ahmed, A.; Clayden, J.; Yasin, S. A. Chem. Commun. 1999, 231–232. doi:10.1039/a808218i
Return to citation in text: [1] -
Fernández, I.; Ortiz, F. L.; Tejerina, B.; Granda, S. G. Org. Lett. 2001, 3, 1339–1342. doi:10.1021/ol015716t
Return to citation in text: [1] -
Fernández, I.; Forcén-Acebal, A.; García-Granda, S.; López-Ortiz, F. J. Org. Chem. 2003, 68, 4472–4485. doi:10.1021/jo0342370
Return to citation in text: [1] -
Fernández, I.; Gómez, G. R.; Alfonso, I.; Iglesias, M. J.; Ortiz, F. L. Chem. Commun. 2005, 5408–5410. doi:10.1039/b510685k
Return to citation in text: [1] -
Fernández, I.; González, J.; López-Ortiz, F. J. Am. Chem. Soc. 2004, 126, 12551–12564. doi:10.1021/ja039863t
Return to citation in text: [1] -
Ramallal, A. M.; Fernández, I.; López Ortiz, F.; González, J. Chem. – Eur. J. 2005, 11, 3022–3031. doi:10.1002/chem.200400987
Return to citation in text: [1] -
Gómez, G. R.; Ortiz, F. L. Synlett 2002, 781–783. doi:10.1055/s-2002-25365
Return to citation in text: [1] -
Ruiz-Gómez, G.; Francesch, A.; Cuevas, C.; Serrano-Ruiz, M.; Iglesias, M. J.; López-Ortiz, F. Tetrahedron 2012, 68, 995–1004. doi:10.1016/j.tet.2011.11.094
Return to citation in text: [1] [2] -
Ruiz-Gómez, G.; Iglesias, M. J.; Serrano-Ruiz, M.; López-Ortiz, F. J. Org. Chem. 2007, 72, 9704–9712. doi:10.1021/jo701607s
Return to citation in text: [1] -
Ruiz-Gómez, G.; Francesch, A.; José Iglesias, M.; López-Ortiz, F.; Cuevas, C.; Serrano-Ruiz, M. Org. Lett. 2008, 10, 3981–3984. doi:10.1021/ol801463g
Return to citation in text: [1] -
Ruiz-Gómez, G.; Iglesias, M. J.; Serrano-Ruiz, M.; García-Granda, S.; Francesch, A.; López-Ortiz, F.; Cuevas, C. J. Org. Chem. 2007, 72, 3790–3799. doi:10.1021/jo070276q
Return to citation in text: [1] [2] -
Miles, J. A.; Street, R. W. J. Org. Chem. 1978, 43, 4668–4670. doi:10.1021/jo00418a034
Return to citation in text: [1] -
Boufroura, H.; Abdelli, A.; Bourdreux, F.; Gaucher, A.; Clavier, G.; Efrit, M. L.; M'rabet, H.; Prim, D. RSC Adv. 2017, 7, 18211–18216. doi:10.1039/c6ra28420e
Return to citation in text: [1] -
Burns, B.; Merifield, E.; Mahon, M. F.; Molloy, K. C.; Wills, M. J. Chem. Soc., Perkin Trans. 1 1993, 2243–2246. doi:10.1039/p19930002243
Return to citation in text: [1] -
Casimiro, M.; Roces, L.; García-Granda, S.; Iglesias, M. J.; López Ortiz, F. Org. Lett. 2013, 15, 2378–2381. doi:10.1021/ol400971q
Return to citation in text: [1] -
Park, S.; Seo, B.; Shin, S.; Son, J.-Y.; Lee, P. H. Chem. Commun. 2013, 49, 8671–8673. doi:10.1039/c3cc44995e
Return to citation in text: [1]
| 37. | Aladzheva, I. M.; Bykhovskaya, O. V.; Nelyubina, Y. V.; Korlyukov, A. A.; Petrovskii, P. V.; Odinets, I. L. Synthesis 2010, 613–618. doi:10.1055/s-0029-1218600 |
| 38. | Aladzheva, I. M.; Bykhovskaya, O. V.; Nelyubina, Y. V.; Petrovskii, P. V.; Odinets, I. L. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 769–771. doi:10.1080/10426507.2010.499549 |
| 39. | Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1984, 37, 1009–1021. doi:10.1071/ch9841009 |
| 40. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 41. | McReynolds, M. D.; Dougherty, J. M.; Hanson, P. R. Chem. Rev. 2004, 104, 2239–2258. doi:10.1021/cr020109k |
| 47. | Fernández, I.; Forcén-Acebal, A.; García-Granda, S.; López-Ortiz, F. J. Org. Chem. 2003, 68, 4472–4485. doi:10.1021/jo0342370 |
| 48. | Fernández, I.; Gómez, G. R.; Alfonso, I.; Iglesias, M. J.; Ortiz, F. L. Chem. Commun. 2005, 5408–5410. doi:10.1039/b510685k |
| 46. | Fernández, I.; Ortiz, F. L.; Tejerina, B.; Granda, S. G. Org. Lett. 2001, 3, 1339–1342. doi:10.1021/ol015716t |
| 19. | Fernández, I.; Ortiz, F. L.; Velázquez, A. M.; Granda, S. G. J. Org. Chem. 2002, 67, 3852–3860. doi:10.1021/jo025587+ |
| 45. | Ahmed, A.; Clayden, J.; Yasin, S. A. Chem. Commun. 1999, 231–232. doi:10.1039/a808218i |
| 42. | Hanson, P. R.; Stoianova, D. S. Tetrahedron Lett. 1999, 40, 3297–3300. doi:10.1016/s0040-4039(99)00479-7 |
| 43. | Stoianova, D. S.; Hanson, P. R. Org. Lett. 2000, 2, 1769–1772. doi:10.1021/ol005952o |
| 49. | Fernández, I.; González, J.; López-Ortiz, F. J. Am. Chem. Soc. 2004, 126, 12551–12564. doi:10.1021/ja039863t |
| 50. | Ramallal, A. M.; Fernández, I.; López Ortiz, F.; González, J. Chem. – Eur. J. 2005, 11, 3022–3031. doi:10.1002/chem.200400987 |
| 52. | Ruiz-Gómez, G.; Francesch, A.; Cuevas, C.; Serrano-Ruiz, M.; Iglesias, M. J.; López-Ortiz, F. Tetrahedron 2012, 68, 995–1004. doi:10.1016/j.tet.2011.11.094 |
| 57. | Boufroura, H.; Abdelli, A.; Bourdreux, F.; Gaucher, A.; Clavier, G.; Efrit, M. L.; M'rabet, H.; Prim, D. RSC Adv. 2017, 7, 18211–18216. doi:10.1039/c6ra28420e |
| 58. | Burns, B.; Merifield, E.; Mahon, M. F.; Molloy, K. C.; Wills, M. J. Chem. Soc., Perkin Trans. 1 1993, 2243–2246. doi:10.1039/p19930002243 |
| 55. | Ruiz-Gómez, G.; Iglesias, M. J.; Serrano-Ruiz, M.; García-Granda, S.; Francesch, A.; López-Ortiz, F.; Cuevas, C. J. Org. Chem. 2007, 72, 3790–3799. doi:10.1021/jo070276q |
| 56. | Miles, J. A.; Street, R. W. J. Org. Chem. 1978, 43, 4668–4670. doi:10.1021/jo00418a034 |
| 54. | Ruiz-Gómez, G.; Francesch, A.; José Iglesias, M.; López-Ortiz, F.; Cuevas, C.; Serrano-Ruiz, M. Org. Lett. 2008, 10, 3981–3984. doi:10.1021/ol801463g |
| 55. | Ruiz-Gómez, G.; Iglesias, M. J.; Serrano-Ruiz, M.; García-Granda, S.; Francesch, A.; López-Ortiz, F.; Cuevas, C. J. Org. Chem. 2007, 72, 3790–3799. doi:10.1021/jo070276q |
| 52. | Ruiz-Gómez, G.; Francesch, A.; Cuevas, C.; Serrano-Ruiz, M.; Iglesias, M. J.; López-Ortiz, F. Tetrahedron 2012, 68, 995–1004. doi:10.1016/j.tet.2011.11.094 |
| 53. | Ruiz-Gómez, G.; Iglesias, M. J.; Serrano-Ruiz, M.; López-Ortiz, F. J. Org. Chem. 2007, 72, 9704–9712. doi:10.1021/jo701607s |
| 16. | Abdou, W. M.; Khidre, R. E.; Shaddy, A. A. J. Heterocycl. Chem. 2013, 50, 33–41. doi:10.1002/jhet.968 |
| 20. | Ali, T. E.; Ali, M. M.; Abdel-Kariem, S. M.; Ahmed, M. M. Synth. Commun. 2017, 47, 1458–1470. doi:10.1080/00397911.2017.1332224 |
| 15. | Ali, T. E. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 185, 88–96. doi:10.1080/10426500802713309 |
| 1. | Neidlein, R. Stud. Nat. Prod. Chem. 1991, 9, 509–528. |
| 2. | Kamalov, R. M.; Schmidpeter, A.; Polborn, K. Phosphorus, Sulfur Silicon Relat. Elem. 1993, 83, 111–118. doi:10.1080/10426509308034353 |
| 3. | Jenner, G. Pol. J. Chem. 1992, 66, 1535–1549. |
| 4. | Bogachenkov, A. S.; Dogadina, A. V.; Boyarskaya, I. A.; Boyarskiy, V. P.; Vasilyev, A. V. Org. Biomol. Chem. 2016, 14, 1370–1381. doi:10.1039/c5ob02143j |
| 5. | Son, J.-Y.; Kim, H.; Jeon, W. H.; Baek, Y.; Seo, B.; Um, K.; Lee, K.; Lee, P. H. Adv. Synth. Catal. 2017, 359, 3194–3206. doi:10.1002/adsc.201700742 |
| 6. | Fu, Z.; Sun, S.; Yang, A.; Sun, F.; Xu, J. Chem. Commun. 2019, 55, 13124–13127. doi:10.1039/c9cc06352h |
| 7. | Xu, J. Chem. Heterocycl. Compd. 2020, 56, 979–981. doi:10.1007/s10593-020-02763-9 |
| 8. | Luo, Y.; Fu, Z.; Fu, X.; Du, C.; Xu, J. Org. Biomol. Chem. 2020, 18, 9526–9537. doi:10.1039/d0ob02011g |
| 15. | Ali, T. E. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 185, 88–96. doi:10.1080/10426500802713309 |
| 16. | Abdou, W. M.; Khidre, R. E.; Shaddy, A. A. J. Heterocycl. Chem. 2013, 50, 33–41. doi:10.1002/jhet.968 |
| 26. | Kim, Y. R.; Cho, S.; Lee, P. H. Org. Lett. 2014, 16, 3098–3101. doi:10.1021/ol501207w |
| 11. | Xu, J. Chem. Heterocycl. Compd. 2020, 56, 308–310. doi:10.1007/s10593-020-02660-1 |
| 12. | Fu, X.; Li, X.; Xu, J. Org. Lett. 2021, 23, 8733–8737. doi:10.1021/acs.orglett.1c03182 |
| 13. | Xu, J. X. Asian J. Org. Chem. 2022, 11, e202200176. |
| 14. | Luo, Y.; Xu, J. Org. Lett. 2020, 22, 7780–7785. doi:10.1021/acs.orglett.0c02346 |
| 26. | Kim, Y. R.; Cho, S.; Lee, P. H. Org. Lett. 2014, 16, 3098–3101. doi:10.1021/ol501207w |
| 7. | Xu, J. Chem. Heterocycl. Compd. 2020, 56, 979–981. doi:10.1007/s10593-020-02763-9 |
| 11. | Xu, J. Chem. Heterocycl. Compd. 2020, 56, 308–310. doi:10.1007/s10593-020-02660-1 |
| 13. | Xu, J. X. Asian J. Org. Chem. 2022, 11, e202200176. |
| 24. | Harger, M. J. P.; Shimmin, P. A. J. Chem. Soc., Perkin Trans. 1 1993, 227–232. doi:10.1039/p19930000227 |
| 9. | Yang, K.-W.; Feng, L.; Yang, S.-K.; Aitha, M.; LaCuran, A. E.; Oelschlaeger, P.; Crowder, M. W. Bioorg. Med. Chem. Lett. 2013, 23, 5855–5859. doi:10.1016/j.bmcl.2013.08.098 |
| 10. | Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 84, 8131–8139. doi:10.1021/acs.joc.9b00994 |
| 25. | Soengas, R.; Belmonte Sánchez, E.; Iglesias, M. J.; López Ortiz, F. ACS Omega 2018, 3, 5116–5124. doi:10.1021/acsomega.8b00491 |
| 22. | Collins, D. J.; Hetherington, J. W.; Swan, J. M. Aust. J. Chem. 1974, 27, 1759–1765. doi:10.1071/ch9741759 |
| 23. | Miles, J. A.; Grabiak, R. C.; Beeny, M. T. J. Org. Chem. 1981, 46, 3486–3492. doi:10.1021/jo00330a021 |
| 60. | Park, S.; Seo, B.; Shin, S.; Son, J.-Y.; Lee, P. H. Chem. Commun. 2013, 49, 8671–8673. doi:10.1039/c3cc44995e |
| 21. | Helferich, B.; Curtius, U. Justus Liebigs Ann. Chem. 1962, 655, 59–69. doi:10.1002/jlac.19626550109 |
| 24. | Harger, M. J. P.; Shimmin, P. A. J. Chem. Soc., Perkin Trans. 1 1993, 227–232. doi:10.1039/p19930000227 |
| 18. | Gibadullina, E.; Nguyen, T. T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. Eur. J. Med. Chem. 2019, 184, 111735. doi:10.1016/j.ejmech.2019.111735 |
| 18. | Gibadullina, E.; Nguyen, T. T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. Eur. J. Med. Chem. 2019, 184, 111735. doi:10.1016/j.ejmech.2019.111735 |
| 19. | Fernández, I.; Ortiz, F. L.; Velázquez, A. M.; Granda, S. G. J. Org. Chem. 2002, 67, 3852–3860. doi:10.1021/jo025587+ |
| 20. | Ali, T. E.; Ali, M. M.; Abdel-Kariem, S. M.; Ahmed, M. M. Synth. Commun. 2017, 47, 1458–1470. doi:10.1080/00397911.2017.1332224 |
| 17. | Ali, T. E.; Assiri, M. A.; El-Shaaer, H. M.; Hassan, M. M.; Fouda, A. M.; Hassanin, N. M. Synth. Commun. 2019, 49, 2983–2994. doi:10.1080/00397911.2019.1652323 |
| 17. | Ali, T. E.; Assiri, M. A.; El-Shaaer, H. M.; Hassan, M. M.; Fouda, A. M.; Hassanin, N. M. Synth. Commun. 2019, 49, 2983–2994. doi:10.1080/00397911.2019.1652323 |
| 18. | Gibadullina, E.; Nguyen, T. T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. Eur. J. Med. Chem. 2019, 184, 111735. doi:10.1016/j.ejmech.2019.111735 |
| 23. | Miles, J. A.; Grabiak, R. C.; Beeny, M. T. J. Org. Chem. 1981, 46, 3486–3492. doi:10.1021/jo00330a021 |
| 59. | Casimiro, M.; Roces, L.; García-Granda, S.; Iglesias, M. J.; López Ortiz, F. Org. Lett. 2013, 15, 2378–2381. doi:10.1021/ol400971q |
| 28. | Kleiner, H.-J. Liebigs Ann. Chem. 1980, 324–332. doi:10.1002/jlac.198019800217 |
| 27. | Sabourin, A.; Dufour, J.; Vors, J.-P.; Bernier, D.; Montchamp, J.-L. J. Org. Chem. 2021, 86, 14684–14694. doi:10.1021/acs.joc.1c01501 |
| 28. | Kleiner, H.-J. Liebigs Ann. Chem. 1980, 324–332. doi:10.1002/jlac.198019800217 |
| 35. | Aladzheva, I. M.; Bykhovskaya, O. V.; Petrovskii, P. V.; Lyssenko, K. A.; Antipin, M. Y.; Mastryukova, T. A. Russ. Chem. Bull. 2005, 54, 2635–2641. doi:10.1007/s11172-006-0168-4 |
| 35. | Aladzheva, I. M.; Bykhovskaya, O. V.; Petrovskii, P. V.; Lyssenko, K. A.; Antipin, M. Y.; Mastryukova, T. A. Russ. Chem. Bull. 2005, 54, 2635–2641. doi:10.1007/s11172-006-0168-4 |
| 36. | Bykhovskaya, O. V.; Aladzheva, I. M.; Lobanov, D. I.; Petrovskii, P. V.; Lyssenko, K. A.; Fedyanin, I. V.; Mastryukova, T. A. Russ. Chem. Bull. 2005, 54, 2642–2647. doi:10.1007/s11172-006-0169-3 |
| 33. | Griffiths, D. V.; Harris, J. E.; Whitehead, B. J. J. Chem. Soc., Perkin Trans. 1 1997, 2545–2548. doi:10.1039/a701147d |
| 34. | Coppola, G. M. J. Heterocycl. Chem. 1979, 16, 897–901. doi:10.1002/jhet.5570160513 |
| 31. | Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1983, 36, 2517–2536. doi:10.1071/ch9832517 |
| 32. | Natchev, I. A. Bull. Chem. Soc. Jpn. 1988, 61, 3699–3704. doi:10.1246/bcsj.61.3699 |
| 29. | Collins, D. J.; Drygala, P. F.; Swan, J. M. Tetrahedron Lett. 1982, 23, 1117–1120. doi:10.1016/s0040-4039(00)87037-9 |
| 30. | Collins, D. J.; Drygala, P. F.; Swan, J. M. Aust. J. Chem. 1983, 36, 2095–2110. doi:10.1071/ch9832095 |
© 2022 Xu; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.