Abstract
Thiazoles and their hydrogenated analogues are not only key structural units in a wide variety of natural products but they also constitute important building blocks in medicinal chemistry. Therefore, the synthesis of these compounds using new protocols is always interesting. It is well known that N-propargylamines can undergo a number of cyclization reactions to produce various nitrogen-containing heterocycles. In this review, we highlight the most important developments on the synthesis of thiazole and its derivatives starting from N-propargylamines. This review will be helpful in the development of improved methods for the synthesis of natural and biologically important compounds.
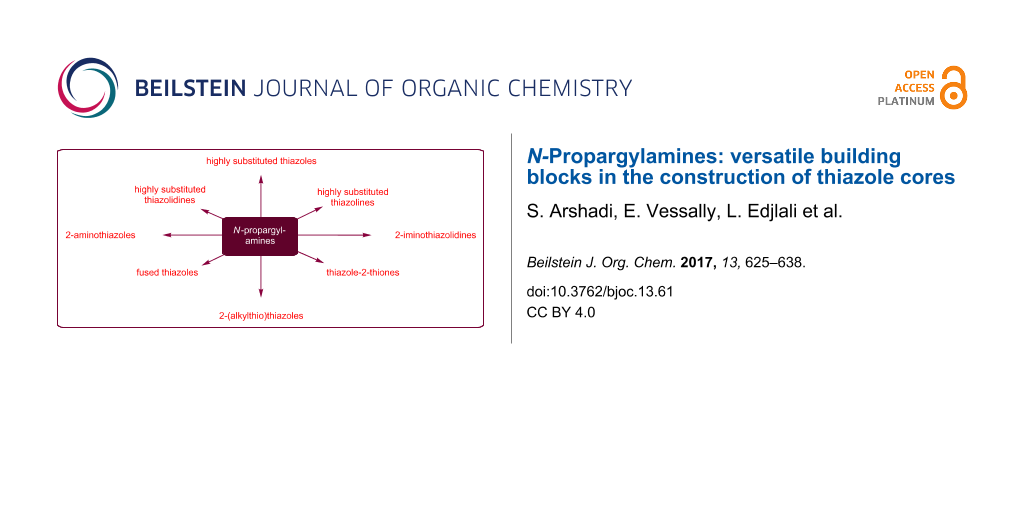
Graphical Abstract
Introduction
Thiazoles are an important class of azole compounds that have attracted considerable attention due to the fact that they exhibit a wide variety of pharmacological activities. For example, abafungin (Figure 1) is an antifungal drug marketed worldwide for the treatment of dermatomycoses. It works by inhibiting the enzyme sterol 24-C-methyltransferase [1-4]. Febuxostat, also known by its brand name adenuric is a xanthine oxidase inhibitor that helps to prevent gout flare-ups [5-7]. Ritonavir (norvir), is an HIV protease inhibitor. It works by blocking the growth of HIV [8,9]. Tiazofurin is a C-nucleoside analogue with antineoplastic activity and acts by inhibition of the guanosine triphosphate (GTP) biosynthesis through a reduction of PI and PIP kinase activity [10-14] (Figure 1). This compound class is also a crucial part of many natural products such as vitamin B1 (thiamine), epothilone, dolastatin, and many more (Figure 2) [15-24]. Moreover, thiazoles are widely applied as pesticides and dyes [25]. As a consequence, many routes for the synthesis of thiazole derivatives are reported in the literature [26-33]. Among them, the Hantzsch thiazole synthesis (condensation of α-haloketones with thioamides) is the most efficient and straight forward procedure [34-43]. However, the general applicability of this method is limited by the narrow substitution patterns [31], by the harsh reaction conditions [26,30] or both. Therefore, methods that overcome these drawbacks are required.
Figure 1: Selected examples of bioactive thiazole derivatives.
Figure 1: Selected examples of bioactive thiazole derivatives.
Figure 2: Some natural sources of thiazoles.
Figure 2: Some natural sources of thiazoles.
The hydrogenated thiazoles (thiazoline and thiazolidine derivatives) are also important structural motifs that are widely found in biologically active natural or synthetic products [44-50]. Compounds containing these rings have widespread biological applications as anticancer [51-53], anti-HIV [54,55], anti-inflammatory [56], antimicrobial [57-59], and specially antibiotic [60-64] agents. Despite their great relevance in drug design, only very few synthetic methods towards these compounds have been reported to date [44].
N-Propargylamines are one of the most specific class of alkynes having diverse reaction patterns. It is well known that they can undergo a number of cyclization reactions to produce various N-heterocycles and complex natural products. In this context we recently reviewed their role in the syntheses of pyrrole [65], pyridine [66], quinoline [67], pyrazine [68], 1,4-oxazepane, and 1,4-diazepane [69] derivatives. The synthesis of thiazoles and their hydrogenated analogues from N-propargylamines offers several advantages, such as high functional group tolerance and high atom and step economy. In continuation of our works [69-74], in this review, we will highlight the most important developments on the synthesis of thiazole and its derivatives from N-propargylamines (Figure 3) which will be helpful in the development of improved methods for the synthesis of natural and biologically important compounds. The review is organized by the type of starting materials.
Figure 3: Some important thiazole-based compounds derived from N-propargylamines.
Figure 3: Some important thiazole-based compounds derived from N-propargylamines.
Review
1 From N-propargylamines and carbon disulfide
The first example of a cyclization of N-propargylamines 1 with carbon disulfide to lead to 5-methylenethiazolidine-2-thiones 2 was reported in 1949 by Batty and Weedon. The reaction took place in refluxing ethanol and generally afforded the corresponding products in good yields. It was also observed that products 2 rapidly formed by reaction of 1 with carbon disulfide in the presence of sodium hydroxide as the base in water at 20 °C. Further, the authors showed that treatment of methylene compound 2 with cold concentrated sulfuric acid gave the corresponding isomeric thiazole-2-thiones 3 in high yields (Scheme 1) [75]. Thirty-six years later, Hanefeld and Bercin synthesized a series of 2-(alkylthio)thiazoles by employing the aforementioned method as the key step [76]. In 2001, Shi and Shen found that using a Pd(PPh3)4/toluene system clearly accelerated this cyclocondensation and the desired products were obtained in excellent yields [77]. Other systems such as Pd(OAc)2/THF [78], D301R (a tertiary amine-functionalized ion-exchange resin)/biphenyl [79], and diethylamine/NaOH/H2O [80] were also successfully employed in this transformation. Despite all these successes, the number of reported examples in this interesting field is limited. There is still further need to study the scope and limitations of this approach for the preparation of thiazolidine-2-thione derivatives.
Scheme 1: The synthesis of thiazole-2-thiones 3 through the thermal cyclocondensation of N-propargylamines 1 with carbon disulfide as developed by Batty and Weedon [75].
Scheme 1: The synthesis of thiazole-2-thiones 3 through the thermal cyclocondensation of N-propargylamines 1 ...
A straightforward way towards 2-benzylthiazolo[3,2-a]benzimidazole derivatives 6 has been proposed by Balova et al. In their approach, a sequential cyclocondensation/5-exo-dig cyclization process between 2-amino-N-propargylanilines 4 and CS2 afforded heterocyclic systems of type 5. Isomerization of the latter compounds upon heating in the presence of KOH in ethanol gave the corresponding 2-benzylthiazolo[3,2-a]benzimidazoles 6 in good yields (Scheme 2a) [81]. In a closely related investigation, Shafiee and co-workers also found that the cyclocondensation of 2-amino-N-propargylbenzamides 7 with CS2 in a KOH/EtOH system gave the corresponding 2-methylenethiazolo[2,3-b]quinazolinones 8 in good to high yields (Scheme 2b) [82].
Scheme 2: (a) One-pot synthesis of 2-benzylthiazolo[3,2-a]benzimidazoles 6 through a base-catalyzed cascade reaction of internal N-propargylamines 4 and CS2. (b) Synthesis of 2-methylene-thiazolo[2,3-b]quinazolinones 8 using 2-amino-propargylbenzamides 7 as substrates.
Scheme 2: (a) One-pot synthesis of 2-benzylthiazolo[3,2-a]benzimidazoles 6 through a base-catalyzed cascade r...
2 From N-propargylamines and isothiocyanates
The first example of a synthesis of thiazole derivatives from N-propargylamines and isothiocyanates was reported in 1964 by Easton et al. The authors obtained 2-iminothiazolidines 11 in good yields by the treatment of secondary α,α-disubstituted N-propargylamines 9 with isothiocyanates 10 through a catalyst-free thiourea formation/intramolecular thia-Michael cyclization in ether (Scheme 3a). They also showed that the treatment of primary α,α-disubstituted N-propargylamines with isothiocyanates led to the corresponding N-propargylthioureas that converted to the cyclic forms upon standing for several days at room temperature [83]. Thirteen years later, Arya and co-workers applied this method for the synthesis of 3-thia-1-azaspiro[4,5]decane ring systems [84]. In 1993, U. Urleb extended the scope of the reaction from isothiocyanates to heterocyclic isothiocyanates and some reported examples are shown in Scheme 3b [85].
Scheme 3: (a) Synthesis of 2-iminothiazolidines 11 from N-propargylamines 9 and isothiocyanates 10. (b) Synthesis of 4,4-disubstituted-5-methylenethiazoles 14 using heterocyclic isothiocyanates 12 and α,α-disubstituted N-propargylamines 13 as substrates.
Scheme 3: (a) Synthesis of 2-iminothiazolidines 11 from N-propargylamines 9 and isothiocyanates 10. (b) Synth...
This strategy was elegantly used by Sasmal and co-workers in the preparation of 2-aminothiazoles 17 from ethyl 4-aminobut-2-ynoate salts 15 and isothiocyanates 16. Several bases and solvents were screened and the combination of Et3N and THF at room temperature was found to be superior. Under the optimized conditions, the reaction tolerates both aryl and alkyl isothiocyanates 16 and gave the corresponding 2-aminothiazoles 17 in good to high yields (Scheme 4a). The authors further expanded the scope of N-propargylamines to diethyl 3-aminoprop-1-ynylphosphonate salts 18 leading to 5-diethyl methylphosphonate-substituted 2-aminothiazoles 19 in good yields (Scheme 4b) [86].
Scheme 4: (a) Synthesis of 2-aminothiazoles 17 through the reaction of ethyl 4-aminobut-2-ynoate salts 15 with isothiocyanates 16. (b) Synthesis of 5-diethyl methylphosphonate-substituted 2-aminothiazoles 19 through reaction of diethyl 3-aminoprop-1-ynylphosphonate salts 18 with 16.
Scheme 4: (a) Synthesis of 2-aminothiazoles 17 through the reaction of ethyl 4-aminobut-2-ynoate salts 15 wit...
An interesting approach towards the synthesis of 2-aminothiazole derivatives by treatment of N-propargylamines with isothiocyanates in the presence of p-toluenesulfonic acid (PTSA) as catalyst under microwave irradiation was developed by Castagnolo et al. Following this route, several 4-substituted 5-methylthiazol-2-amines 22 were synthesized from terminal N-propargylamines 20 and isothiocyanates 21 in DMF at 160 °C. Interestingly, when internal N-propargylamines were treated with 21, exclusively imidazolthiones 24 in yields ranging from 15 to 33% instead of the expected 2-aminothiazoles were obtained. The authors also found that with decreasing reaction temperature the yield of 22 decreased in favor of the thiazolines 23. Some reported examples are collected in Table 1 [87].
Table 1: Microwave-assisted domino reactions of N-propargylamines 20 with isothiocyanates 21 developed by Castagnolo.
|
|
||||||||
| Entry | R1 | R2 | R3 | Solvent | Temp (°C) | Product | Ratio 22:23:24 | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | H | Ph | All | DMF | 160 | 3a | 100:0:0 | 47 |
| 2 | H | Ph | Bn | DMF | 160 | 3b | 100:0:0 | 56 |
| 3 | H | 4-Cl-Ph | All | DMF | 160 | 3c | 100:0:0 | 55 |
| 4 | H | 2,4-Cl2-Ph | Bn | DMF | 160 | 3d | 100:0:0 | 62 |
| 5 | H | 2,4-Cl2-Ph | All | DMF | 160 | 3e | 100:0:0 | 62 |
| 6 | Ph | H | Bn | DMF | 160 | 5a | 0:0:100 | 15 |
| 7 | Ph | H | All | DMF | 160 | 5b | 0:0:100 | 33 |
| 8 | Ph | H | Ph | DMF | 160 | 5c | 0:0:100 | 21 |
| 9 | H | H | Bn | MeCN | 100 | 3f/4a | 25:75:0 | 18:60 |
| 10 | H | H | Ph | MeCN | 100 | 3g/4b | 20:80:0 | 12:57 |
| 12 | H | 2,4-Cl2-Ph | All | DCE | 130 | 4c | 0:100:0 | 60 |
Recently, to develop an efficient protocol for the synthesis of 5-(iodomethylene)-3-methylthiazolidines 27 from N-propargylamines, X. Zhou and co-workers have investigated the three-component halocyclization of N-propargylamines 25, aryl isothiocyanates 26, and iodine in ethyl acetate. Excellent yields of desired products were observed (Scheme 5). The mechanism shown in Scheme 6 was proposed for this transformation and comprises the following key steps: (i) the reaction of N-propargylamine 25 and isothiocyanate 26 forms the thiourea intermediate A, (ii) electrophilic addition of I2 to the alkyne moiety of this intermediate produces the iodonium intermediate B, (iii) isomerization of iodonium B gives intermediate C and (iv) a sequential intramolecular cyclization and HI elimination of C finally affords thiazolidines 27 [88].
Scheme 5: Synthesis of 5-(iodomethylene)-3-methylthiazolidines 27 described by Zhou.
Scheme 5: Synthesis of 5-(iodomethylene)-3-methylthiazolidines 27 described by Zhou.
Scheme 6: Mechanism that accounts for the formation of 27.
Scheme 6: Mechanism that accounts for the formation of 27.
Seeking for a greener approach towards thiazolidines of type 30, the group of Clausen has proposed a base-catalyzed protocol using t-BuOH in water at 20 °C for a quite efficient cyclization between secondary N-propargylamines 28 and fluorescein isothiocyanate 29 (Scheme 7) [89].
Scheme 7: Clausen’s synthesis of fluorescein thiazolidines 30.
Scheme 7: Clausen’s synthesis of fluorescein thiazolidines 30.
More recently, Beauchemin and co-workers reported the syntheses of a series of multiply substituted thiazolidines 33 via the cyclization reaction of secondary N-propargylamines 32 with blocked N-isothiocyanate precursors 31. The desired N-isocyanates A are produced in situ upon heating or treatment with a base, in acetonitrile under microwave irradiation conditions (Scheme 8). The reaction tolerated a variety of functional groups such as fluoro, cyano, hydroxy, and methoxy, allowing a further derivatization of the products [90].
Scheme 8: Synthesis of multiply substituted thiazolidines 33 from N-propargylamines 32 and blocked N-isothiocyanate precursors 31.
Scheme 8: Synthesis of multiply substituted thiazolidines 33 from N-propargylamines 32 and blocked N-isothioc...
3 From N-propargyl thioamides
The first example of a thiazole synthesis from N-propargyl thioamides has been reported by Short and Ziegler in 1993. N-Propargyl thiocarbamate 34 cyclized to disubstituted thiazole 35 through an addition–cycloelimination strategy by the treatment with sodium benzenesulfinate and I2 in ethyl acetate and water at 80 °C (Scheme 9a) [91]. Later, the P. Wipf research team found that N-propargylamines 36 were converted to the corresponding vinylthiazolines 38 through the treatment with dithioic acids 37 in the presence of EDCI in dichloromethane. This transformation is believed to occur through a tandem coupling–cyclization reaction. The authors showed that the treatment of 38 with DBU at 0 °C provided thiazoles 39 in good yields (Scheme 9b) [92].
Scheme 9: (a) Microwave-assisted cyclization of N-propargyl thiocarbamate 34. (b) Synthesis of thiazoles 39 through a tandem coupling–cyclization–isomerization sequential process.
Scheme 9: (a) Microwave-assisted cyclization of N-propargyl thiocarbamate 34. (b) Synthesis of thiazoles 39 t...
Along this line, Junjappa and co-workers reported an efficient route for the synthesis of 2-substituted 5-methylenethiazolidines 42 through the reaction of β-oxodithioesters 40 with N-propargylamine (41). The mechanism proposed by the authors to explain this reaction is based on the formation of β-oxo-N-propargyl thioamides A as intermediates, followed by their spontaneous ring closure. This reaction was run in refluxing ethanol and provided in all cases the desired thiazolidines 42 in high to excellent yields (Scheme 10) [93].
Scheme 10: Synthesis of thiazolidines 42 (42’) from the reaction of β-oxodithioesters 40 (40’) with N-propargylamine (41) through an N-propargylthioamide intermediate A.
Scheme 10: Synthesis of thiazolidines 42 (42’) from the reaction of β-oxodithioesters 40 (40’) with N-propargy...
In 2009, Yarovenko and co-workers developed the synthesis of 5-(dibromomethyl)thiazole derivatives 44 by treatment of N-propargyl thioamides 43 with bromine in an ionic liquid (1-butyl-3-methylimidazolium hexafluorophosphate). Mechanistically, the reaction involves: i) bromination of triple bond of thioamide 43 which resulted in a bridged bromonium ion intermediate A; ii) regioselective 5-exo-dig cyclization of intermediate A to give dihydrothiazole B; and iii) addition of a second bromine to the alkene moiety in intermediate B to provide the corresponding thiazole 44 (Scheme 11) [94].
Scheme 11: Synthesis of 5-(dibromomethyl)thiazoles 44 via halocyclization of N-propargylamines 43 described by Yarovenko.
Scheme 11: Synthesis of 5-(dibromomethyl)thiazoles 44 via halocyclization of N-propargylamines 43 described by...
Recently, Alhalib and Moran reported two examples for the preparation of fully substituted dihydrothiazoles 46 through the treatment of N-propargylamides 45 with Lawesson’s reagent in toluene. It is suggested that the N-(propargyl)thioamide intermediate A is initially formed, followed by a facile 5-exo-dig cyclization process to give the final products 46 in moderate yields (Scheme 12) [95].
Scheme 12: Synthesis of dihydrothiazoles 46 through the treatment of N-propargylamides 45 with Lawesson’s reagent.
Scheme 12: Synthesis of dihydrothiazoles 46 through the treatment of N-propargylamides 45 with Lawesson’s reag...
An important study on 2,5-disubstituted thiazoles 49 was carried out by Sasmal, Sridhar, and Iqbal. The authors converted silyl-protected N-propargylamines 47 into thiazoles 49 by their treatment with benzotriazolylthiones 48 in a THF/MeOH/Et3N system (Scheme 13). The proposed mechanism for the reaction starts with the generation of the N-(propargyl)thioamide intermediates A through a thioacylation of N-propargylamine 47 with benzotriazolylthione 48. Then N-desilylation of A furnishes intermediate B which undergoes a base-promoted cyclization to give the intermediate C. Finally, the isomerization of C affords the observed products 49 (Scheme 14) [96].
Scheme 13: Synthesis of thiazoles 49 by treatment of silyl-protected N-propargylamines 47 with benzotriazolylthiones 48.
Scheme 13: Synthesis of thiazoles 49 by treatment of silyl-protected N-propargylamines 47 with benzotriazolylt...
Scheme 14: Mechanism proposed to explain the synthesis of 2,5-disubstituted thiazoles 49 developed by Sasmal.
Scheme 14: Mechanism proposed to explain the synthesis of 2,5-disubstituted thiazoles 49 developed by Sasmal.
In 2011, X. Meng and S. Kim reported an example of thiazolidine preparation through a Mo-catalyzed 5-exo-dig cyclization of the N-propargylthiocarbamate 50 in toluene under irradiation at 350 nm. As shown in Scheme 15 the target 2-phenoxy-substituted thiazolidine 51 was obtained in a yield of 54% along with the product originating from a 6-endo-dig cyclization [97].
Scheme 15: Mo-catalyzed cyclization of N-propargylthiocarbamate 50.
Scheme 15: Mo-catalyzed cyclization of N-propargylthiocarbamate 50.
Recently, Foroumadi and co-workers studied the possibility of synthesizing thiazole derivatives from N-propargylthioureas through a regioselective 5-exo-dig cyclization–proton transfer–isomerization sequential process. They found that the easily available N-(propargylcarbamothioyl)amides 53 in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO) as the base in refluxing ethanol, rapidly cyclized and produced the corresponding dihydrothiazol-2-ylamides 54 in good yields (Scheme 16a). The mechanism for this cyclization as proposed by the authors is depicted in Scheme 16b [98].
Scheme 16: (a) DABCO-mediated intramolecular cyclization of N-(propargylcarbamothioyl)amides 53 to the corresponding dihydrothiazol-2-ylamides 54. (b) Possible reaction pathway for the generation of product 54.
Scheme 16: (a) DABCO-mediated intramolecular cyclization of N-(propargylcarbamothioyl)amides 53 to the corresp...
Following this work, the Čikotienė group studied the metal-free halogen, chalcogen, or oxocarbenium ion-mediated cyclization of a series of N-propargylthioureas 55 (Table 2). Some important information of the reactions are listed below: (1) iodine-mediated cyclizations of terminal N-propargylthioureas 55 gave exclusively 4,5-dihydrothiazoles 57 through a 5-exo-dig cyclization, whereas internal N-propargylthioureas 55 under the same reaction conditions gave a mixture of 4H-1,3-thiazines 56 and 4,5-dihydrothiazoles 57. The mechanistic course of this reaction sequence is shown in Scheme 17 and involves the initial formation of the charge-transfer complex A between the iodonium ion and the triple bond. The 5-exo-dig cyclization of this intermediate gives rise to 4,5-dihydrothiazoles and the competing 6-endo-dig ring-closing process affords 4H-1,3-thiazines after conversion of the charge-transfer complex into the ring-opened iodovinyl B or bridged iodirenium C ions; (2) bromine-mediated cyclizations of both electron-poor and electron-rich N-propargylthioureas 55 gave exclusively 4,5-dihydrothiazoles 57 in moderate to good yields; (3) phenyl hypochloroselenoite-mediated cyclizations of terminal N-propargylthioureas 55 underwent a regioselective 5-exo-dig cyclization giving the corresponding 4,5-dihydrothiazoles 57 in moderate yields. On the other hand internal N-propargylamines 55 under the same reaction conditions gave a mixture of 57 and 56; (4) arylideneoxonium ion-mediated cyclization of internal N-propargylamines 55 afforded exclusively the corresponding 4H-1,3-thiazines 56 in good yields. However, terminal N-propargylamines failed to participate in this reaction [99].
Table 2: Electrophile-mediated cyclization of N-propargylthioureas 55.
|
|
|||||
| Entry | R | Reaction conditions | E+ |
56
Yield (%) |
57
Yield (%) |
|---|---|---|---|---|---|
| 1 | H | I2 (2 equiv), DCM, 0 °C | I+ | – | 20 |
| 2 | Ph | I2 (2 equiv), DCM, 0 °C | I+ | 18 | 25 |
| 3 | 4-OMe-Ph | I2 (2 equiv), DCM, 0 °C | I+ | 59 | traces |
| 4 | Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 67 |
| 5 | 4-OMe-Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 68 |
| 6 | 4-Cl-Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 44 |
| 7 | H | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | – | 45 |
| 8 | Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 56 | traces |
| 9 | 4-OMe-Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 73 | – |
| 10 | 4-Cl-Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 51 | – |
| 11 | H | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
– | – |
| 12 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
81 | – |
| 13 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
60 | – |
| 14 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
57 | – |
| 15 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
76 | – |
| 16 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
59 | – |
| 17 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
58 | – |
| 18 | 4-Cl-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
57 | – |
| 19 | 4-Cl-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C |
|
65 | – |
Scheme 17: Proposed mechanism for the generation of the iodine-substituted 4H-1,3-thiazines 56 and 4,5-dihydrothiazoles 57.
Scheme 17: Proposed mechanism for the generation of the iodine-substituted 4H-1,3-thiazines 56 and 4,5-dihydro...
4 Miscellaneous
Recently, Stevens and co-workers reported a robust protocol towards dihydrothiazoles through an Au(III)-catalyzed intramolecular cyclization of the corresponding dithiocarboimidates. Thus, the corresponding 5-alkylidene-dihydrothiazoles 58 were synthesized in good to excellent yields from N-(propargyldithiocarbo)imidates 57 through a 5-exo-dig cyclization followed by a thio-Claisen-type rearrangement with AuCl3 as the catalyst in dichloromethane (Scheme 18). It is worth mentioning that the required N-(propargyldithiocarbo)imidates were easily prepared in high yields through a condensation of commercially available and cheap N-propargylamine, allyl bromide, and carbon disulfide [100].
Scheme 18: Au(III)-catalyzed synthesis of 5-alkylidenedihydrothiazoles 58 developed by Stevens.
Scheme 18: Au(III)-catalyzed synthesis of 5-alkylidenedihydrothiazoles 58 developed by Stevens.
Conclusion
Much work has been carried out during the past decade and has demonstrated that N-propargylamines are one of the most useful and versatile precursors in the synthesis of various nitrogen heterocycles and complex natural products. In this regard, recently an impressive increase in the number of publications on the preparation of thiazoles and their hydrogenated analogues through inter- and intramolecular cyclization of N-propargylamine derivatives appeared in the literature. In this review we discussed the most representative and interesting reports on this emerging field. As illustrated, the processes provided the title compounds in good yields with fewer steps and higher atom economy than previously reported examples. We hope that this review will encourage synthetic organic chemists to employ these valuable methodologies to the synthesis of important new thiazole derivatives.
References
-
Villars, V.; Jones, T. C. Clin. Exp. Dermatol. 1989, 14, 124–127. doi:10.1111/j.1365-2230.1989.tb00908.x.
Return to citation in text: [1] -
Kagawa, S. Clin. Exp. Dermatol. 1989, 14, 114–115. doi:10.1111/j.1365-2230.1989.tb00905.x
Return to citation in text: [1] -
Tamura, T.; Asahara, M.; Yamamoto, M.; Yamaura, M.; Matsumura, M.; Goto, K.; Rezaei-Matehkolaei, A.; Mirhendi, H.; Makimura, M.; Makimura, K. Microbiol. Immunol. 2014, 58, 1–8. doi:10.1111/1348-0421.12109
Return to citation in text: [1] -
Borelli, C.; Schaller, M.; Niewerth, M.; Nocker, K.; Baasner, B.; Berg, D.; Tiemann, R.; Tietjen, K.; Fugmann, B.; Lang-Fugmann, S.; Korting, H. C. Chemotherapy 2008, 54, 245–259. doi:10.1159/000142334
Return to citation in text: [1] -
Takano, Y.; Hase-Aoki, K.; Horiuchi, H.; Zhao, L.; Kasahara, Y.; Kondo, S.; Becker, M. A. Life Sci. 2005, 76, 1835–1847. doi:10.1016/j.lfs.2004.10.031
Return to citation in text: [1] -
Sánchez-Lozada, L. G.; Tapia, E.; Soto, V.; Ávila-Casado, C.; Franco, M.; Zhao, L.; Johnson, R. J. Nephrol., Dial., Transplant. 2008, 23, 1179–1185. doi:10.1093/ndt/gfm783
Return to citation in text: [1] -
Kataoka, H.; Yang, K.; Rock, K. L. Eur. J. Pharmacol. 2015, 746, 174–179. doi:10.1016/j.ejphar.2014.11.013
Return to citation in text: [1] -
Molla, A.; Korneyeva, M.; Gao, Q.; Vasavanonda, S.; Schipper, P. J.; Mo, H.-M.; Markowitz, M.; Chernyavskiy, T.; Niu, P.; Lyons, N.; Hsu, A.; Granneman, G. R.; Ho, D. D.; Boucher, C. A. B.; Leonard, J. M.; Norbeck, D. W.; Kempf, D. J. Nat. Med. 1996, 2, 760–766. doi:10.1038/nm0796-760
Return to citation in text: [1] -
Boffito, M.; Jackson, A.; Pozniak, A.; Giraudon, M.; Kulkarni, R.; Abelardo, M. C.; Patel, I. H.; Morcos, P. N. Drugs R&D 2015, 15, 141–153. doi:10.1007/s40268-015-0087-7
Return to citation in text: [1] -
Vitale, M.; Zamai, L.; Falcieri, E.; Zauli, G.; Gobbi, P.; Santi, S.; Cinti, C.; Weber, G. Cytometry 1997, 30, 61–66. doi:10.1002/(SICI)1097-0320(19970215)30:1<61::AID-CYTO9>3.0.CO;2-I
Return to citation in text: [1] -
Pankiewicz, K. W. Pharmacol. Ther. 1997, 76, 89–100. doi:10.1016/S0163-7258(97)00092-2
Return to citation in text: [1] -
Savić, D.; Stanković, T.; Lavrnja, I.; Podolski-Renić, A.; Banković, J.; Peković, S.; Stojiljković, M.; Rakić, L.; Ruždijić, S.; Pešić, M. Mol. Inhibit. Target. Ther. 2015, 1, 3–14. doi:10.1515/motth-2015-0002
Return to citation in text: [1] -
Weber, G.; Natsumeda, Y.; Lui, M. S.; Faderan, M. A.; Liepnieks, J. J.; Elliott, W. L. Adv. Enzyme Regul. 1984, 22, 69–93. doi:10.1016/0065-2571(84)90009-8
Return to citation in text: [1] -
https://pixabay.com/en/syringe-pill-bottle-morphine-small-1884784/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
Jin, Z. J. Nat. Prod. 2016, 33, 1268–1318. doi:10.1039/c6np00067c
Return to citation in text: [1] -
Ramu, E.; Rao, B. V. Tetrahedron: Asymmetry 2009, 20, 2201–2204. doi:10.1016/j.tetasy.2009.09.003
Return to citation in text: [1] -
Le Bozec, L.; Moody, C. J. Aust. J. Chem. 2009, 62, 639–647. doi:10.1071/CH09126
Return to citation in text: [1] -
Davyt, D.; Serra, G. Mar. Drugs 2010, 8, 2755–2780. doi:10.3390/md8112755
Return to citation in text: [1] -
https://pixabay.com/en/nudibranch-snorkeling-diving-scuba-274937/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
https://pixabay.com/en/pine-cones-pine-nuts-tap-forest-820759/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
https://pixabay.com/en/glow-worm-glowworm-bug-firefly-147679/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
https://pixabay.com/en/cranberries-berries-red-berry-112151/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
https://pixabay.com/en/sea-slugs-pests-snail-plague-nature-590491/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
https://pixabay.com/en/sponge-beach-sea-water-sun-summer-62721/. (accessed Feb 14, 2017).
Return to citation in text: [1] -
Harikrishna, N.; Isloor, A. M.; Ananda, K.; Obaid, A.; Fun, H.-K. RSC Adv. 2015, 5, 43648–43659. doi:10.1039/C5RA04995D
Return to citation in text: [1] -
Zagade, A. A.; Senthilkumar, G. P. Pharma Chem. 2011, 3, 523–537.
Return to citation in text: [1] [2] -
Smirnova, N. G.; Zavarzin, I. V.; Krayushkin, M. M. Chem. Heterocycl. Compd. 2006, 42, 144–165. doi:10.1007/s10593-006-0064-8
Return to citation in text: [1] -
Halimehjani, A. Z.; Hasani, L.; Alaei, M. A.; Saidi, M. R. Tetrahedron Lett. 2016, 57, 883–886. doi:10.1016/j.tetlet.2016.01.045
Return to citation in text: [1] -
Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Org. Lett. 2015, 17, 4698–4701. doi:10.1021/acs.orglett.5b02152
Return to citation in text: [1] -
Miura, T.; Funakoshi, Y.; Fujimoto, Y.; Nakahashi, J.; Murakami, M. Org. Lett. 2015, 17, 2454–2457. doi:10.1021/acs.orglett.5b00960
Return to citation in text: [1] [2] -
Kumar, S. V.; Parameshwarappa, G.; Ila, H. J. Org. Chem. 2013, 78, 7362–7369. doi:10.1021/jo401208u
Return to citation in text: [1] [2] -
Maltsev, O. V.; Walter, V.; Brandl, M. J.; Hintermann, L. Synthesis 2013, 45, 2763–2767. doi:10.1055/s-0033-1339492
Return to citation in text: [1] -
Chhabria, M. T.; Patel, S.; Modi, P.; Brahmkshatriya, P. S. Curr. Top. Med. Chem. 2016, 16, 2841–2862. doi:10.2174/1568026616666160506130731
Return to citation in text: [1] -
Hantzsch, A.; Weber, J. H. Ber. Dtsch. Chem. Ges. 1887, 20, 3118–3132. doi:10.1002/cber.188702002200
Return to citation in text: [1] -
Bramley, S. E.; Dupplin, V.; Goberdhan, D. G. C.; Meakins, G. D. J. Chem. Soc., Perkin Trans. 1 1987, 639–643. doi:10.1039/P19870000639
Return to citation in text: [1] -
Aguilar, E.; Meyers, A. I. Tetrahedron Lett. 1994, 35, 2473–2476. doi:10.1016/S0040-4039(00)77147-4
Return to citation in text: [1] -
Prakash, R.; Kumar, A.; Aggarwal, R.; Prakash, O.; Singh, S. P. Synth. Commun. 2007, 37, 2501–2505. doi:10.1080/00397910701462476
Return to citation in text: [1] -
Merritt, E. A.; Bagley, M. C. Synthesis 2007, 3535–3541. doi:10.1055/s-2007-990851
Return to citation in text: [1] -
Guernon, J. M.; Wu, Y.-J. Tetrahedron Lett. 2011, 52, 3633–3635. doi:10.1016/j.tetlet.2011.05.028
Return to citation in text: [1] -
Chidananda, N.; Poojary, B.; Sumangala, V.; Kumari, N. S. Med. Chem. Res. 2014, 23, 3979–3997. doi:10.1007/s00044-014-0975-3
Return to citation in text: [1] -
Kamila, S.; Mendoza, K.; Biehl, E. R. Tetrahedron Lett. 2012, 53, 4921–4924. doi:10.1016/j.tetlet.2012.06.116
Return to citation in text: [1] -
Gu, C.; Zhai, J.; Jiang, J.; Liu, H.; Wang, L.; Zhu, D.; Ji, Y. Chin. J. Chem. 2014, 32, 179–190. doi:10.1002/cjoc.201300878
Return to citation in text: [1] -
Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Chu, L.; Liu, H.; Shen, Q.; Zhou, J. J. Med. Chem. 2013, 56, 5048–5058. doi:10.1021/jm400367n
Return to citation in text: [1] -
Gaumont, A.-C.; Gulea, M.; Levillain, J. Chem. Rev. 2009, 109, 1371–1401. doi:10.1021/cr800189z
Return to citation in text: [1] [2] -
Tan, K. C.; Wakimoto, T.; Takada, K.; Ohtsuki, T.; Uchiyama, N.; Goda, Y.; Abe, I. J. Nat. Prod. 2013, 76, 1388–1391. doi:10.1021/np400404r
Return to citation in text: [1] -
Budovská, M.; Kutschy, P.; Kožár, T.; Gondová, T.; Petrovaj, J. Tetrahedron 2013, 69, 1092–1104. doi:10.1016/j.tet.2012.11.067
Return to citation in text: [1] -
Segade, Y.; Montaos, M. A.; Rodríguez, J.; Jiménez, C. Org. Lett. 2014, 16, 5820–5823. doi:10.1021/ol502958u
Return to citation in text: [1] -
Pedras, M. S. C.; Sarma-Mamillapalle, V. K. Bioorg. Med. Chem. Lett. 2012, 20, 3991–3996. doi:10.1016/j.bmc.2012.05.020
Return to citation in text: [1] -
Kreutzer, M. F.; Kage, H.; Herrmann, J.; Pauly, J.; Hermenau, R.; Müller, R.; Hoffmeister, D.; Nett, M. Org. Biomol. Chem. 2014, 12, 113–118. doi:10.1039/c3ob41839a
Return to citation in text: [1] -
Aeluri, M.; Dasari, B.; Arya, P. Org. Lett. 2015, 17, 472–475. doi:10.1021/ol503465p
Return to citation in text: [1] -
Han, F. S.; Osajima, H.; Cheung, M.; Tokuyama, H.; Fukuyama, T. Chem. – Eur. J. 2007, 13, 3026–3038. doi:10.1002/chem.200601446
Return to citation in text: [1] -
Sondhi, S. M.; Rani, R.; Gupta, P. P.; Agrawal, S. K.; Saxena, A. K. Mol. Diversity 2009, 13, 357–366. doi:10.1007/s11030-009-9125-0
Return to citation in text: [1] -
Havrylyuk, D.; Kovach, N.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Arch. Pharm. 2011, 344, 514–522. doi:10.1002/ardp.201100055
Return to citation in text: [1] -
Meleddu, R.; Distinto, S.; Corona, A.; Tramontano, E.; Bianco, G.; Melis, C.; Cottiglia, F.; Maccioni, E. J. Enzyme Inhib. Med. Chem. 2016, 32, 130–136. doi:10.1080/14756366.2016.1238366
Return to citation in text: [1] -
Lu, Z.; Harper, M. K.; Pond, C. D.; Barrows, L. R.; Ireland, C. M.; Van Wagoner, R. M. J. Nat. Prod. 2012, 75, 1436–1440. doi:10.1021/np300270p
Return to citation in text: [1] -
Kim, E.-A.; Choi, J.; Han, A. R.; Choi, S. Y.; Hahn, H.-G.; Cho, S.-W. NeuroToxicology 2013, 38, 106–114. doi:10.1016/j.neuro.2013.07.001
Return to citation in text: [1] -
Bonde, C. G.; Gaikwad, N. J. Bioorg. Med. Chem. 2004, 12, 2151–2161. doi:10.1016/j.bmc.2004.02.024
Return to citation in text: [1] -
Bondock, S.; Khalifa, W.; Fadda, A. A. Eur. J. Med. Chem. 2007, 42, 948–954. doi:10.1016/j.ejmech.2006.12.025
Return to citation in text: [1] -
Altıntop, M. D.; Kaplancıklı, Z. A.; Çiftçi, G. A.; Demirel, R. Eur. J. Med. Chem. 2014, 74, 264–277. doi:10.1016/j.ejmech.2013.12.060
Return to citation in text: [1] -
Zipperer, A.; Konnerth, M. C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N. A.; Slavetinsky, C.; Marschal, M.; Willmann, M.; Kalbacher, H.; Schittek, B.; Brötz-Oesterhelt, H.; Grond, S.; Peschel, A.; Krismer, B. Nature 2016, 535, 511–516. doi:10.1038/nature18634
Return to citation in text: [1] -
Faine, S.; Harper, M. Antimicrob. Agents Chemother. 1973, 3, 15–18. doi:10.1128/AAC.3.1.15
Return to citation in text: [1] -
Garrod, L. Br. Med. J. 1960, 1, 527–529. doi:10.1136/bmj.1.5172.527
Return to citation in text: [1] -
Bodin, N.-O.; Ekström, B.; Forsgren, U.; Jalar, L.-P.; Magni, L.; Ramsay, C.-H.; Sjöberg, B. Antimicrob. Agents Chemother. 1975, 8, 518–525. doi:10.1128/AAC.8.5.518
Return to citation in text: [1] -
Akova, M. Clin. Microbiol. Infect. 2008, 14, 185–188. doi:10.1111/j.1469-0691.2007.01847.x
Return to citation in text: [1] -
Vessally, E. RSC Adv. 2016, 6, 18619–18631. doi:10.1039/C5RA20706A
Return to citation in text: [1] -
Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 71662–71675. doi:10.1039/C6RA08720E
Return to citation in text: [1] -
Vessally, E.; Edjlali, L.; Hosseinian, A.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 49730–49746. doi:10.1039/C6RA05221E
Return to citation in text: [1] -
Vessally, E.; Hosseinian, A.; Bekhradnia, A.; Esrafili, M. D. Curr. Org. Synth. 2017, 14, 557–567. doi:10.2174/1570179413666160818144816
Return to citation in text: [1] -
Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 99781–99793. doi:10.1039/C6RA20718A
Return to citation in text: [1] [2] -
Vessally, E.; Soleimani-Amiri, S.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A. RSC Adv. 2017, 7, 7079–7091. doi:10.1039/c6ra25816f
Return to citation in text: [1] -
Arshadi, S.; Vessally, E.; Edjlali, L.; Ghorbani-Kalhor, E.; Hosseinzadeh-Khanmiri, R. RSC Adv. 2017, 7, 13198–13211. doi:10.1039/c7ra00746a
Return to citation in text: [1] -
Vessally, E.; Hosseinzadeh-Khanmiri, R.; Ghorbani-Kalhor, E.; Es’haghi, M.; Bekhradnia, A. RSC Adv. 2017, 7, 19061–19072. doi:10.1039/c7ra01371j
Return to citation in text: [1] -
Vessally, E.; Abdoli, M. J. Iran. Chem. Soc. 2016, 13, 1235–1256. doi:10.1007/s13738-016-0838-6
Return to citation in text: [1] -
Vessally, E.; Saeidian, H.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A. Curr. Org. Chem. 2017, 21, 249–271. doi:10.2174/1385272820666161018150925
Return to citation in text: [1] -
Batty, J. W.; Weedon, B. C. L. J. Chem. Soc. 1949, 786–789. doi:10.1039/JR9490000786
Return to citation in text: [1] [2] -
Hanefeld, W.; Bercin, E. Liebigs Ann. Chem. 1985, 58–64. doi:10.1002/jlac.198519850107
Return to citation in text: [1] -
Shi, M.; Shen, Y.-M. Heteroat. Chem. 2001, 12, 610–616. doi:10.1002/hc.1092
Return to citation in text: [1] -
Shi, M.; Shen, Y.-M. J. Org. Chem. 2002, 67, 16–21. doi:10.1021/jo0014966
Return to citation in text: [1] -
Liu, A.; He, L.; Peng, S.; Pan, Z.; Wang, J.; Gao, J. Sci. China: Chem. 2010, 53, 1578–1585. doi:10.1007/s11426-010-4028-6
Return to citation in text: [1] -
Maddani, M. R.; Prabhu, K. R. J. Org. Chem. 2010, 75, 2327–2332. doi:10.1021/jo1001593
Return to citation in text: [1] -
Novikov, R. V.; Danilkina, N. A.; Balova, I. A. Chem. Heterocycl. Compd. 2011, 47, 758–766. doi:10.1007/s10593-011-0831-z
Return to citation in text: [1] -
Mahdavi, M.; Bialam, M.; Saeedi, M.; Jafarpour, F.; Foroumadi, A.; Shafiee, A. Synlett 2015, 26, 173–176. doi:10.1055/s-0034-1379499
Return to citation in text: [1] -
Easton, N. R.; Cassady, D. R.; Dillard, R. D. J. Org. Chem. 1964, 29, 1851–1855. doi:10.1021/jo01030a044
Return to citation in text: [1] -
Arya, V.; Grewal, R.; Kaul, C.; David, J.; Honkan, V. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1977, 15, 133–140.
Return to citation in text: [1] -
Urleb, U.; Neidlein, R.; Kramer, W. Helv. Chim. Acta 1993, 76, 431–440. doi:10.1002/hlca.19930760127
Return to citation in text: [1] -
Sasmal, P. K.; Chandrasekhar, A.; Sridhar, S.; Iqbal, J. Tetrahedron 2008, 64, 11074–11080. doi:10.1016/j.tet.2008.09.074
Return to citation in text: [1] -
Scalacci, N.; Pelloja, C.; Radi, M.; Castagnolo, D. Synlett 2016, 27, 1883–1887. doi:10.1055/s-0035-1561985
Return to citation in text: [1] -
Huang, S.; Shao, Y.; Liu, R.; Zhou, X. Tetrahedron 2015, 71, 4219–4226. doi:10.1016/j.tet.2015.04.080
Return to citation in text: [1] -
Viart, H. M.-F.; Larsen, T. S.; Tassone, C.; Andresen, T. L.; Clausen, M. H. Chem. Commun. 2014, 50, 7800–7802. doi:10.1039/c4cc00863d
Return to citation in text: [1] -
Vincent-Rocan, J.-F.; Derasp, J. S.; Beauchemin, A. M. Chem. Commun. 2015, 51, 16405–16408. doi:10.1039/c5cc07212c
Return to citation in text: [1] -
Short, K. M.; Ziegler, C. B. Tetrahedron Lett. 1993, 34, 71–74. doi:10.1016/S0040-4039(00)60060-6
Return to citation in text: [1] -
Wipf, P.; Rahman, L. T.; Rector, S. R. J. Org. Chem. 1998, 63, 7132–7133. doi:10.1021/jo981542q
Return to citation in text: [1] -
Chandrasekharam, M.; Singh, O. M.; Ila, H.; Junjappa, H. Synth. Commun. 1998, 28, 3073–3079. doi:10.1080/00397919808004887
Return to citation in text: [1] -
Yarovenko, V. N.; Polushina, A. V.; Zavarzin, I. V.; Krayushkin, M. M.; Kotovskaya, S. K.; Charushin, V. N. J. Sulfur Chem. 2009, 30, 327–337. doi:10.1080/17415990902774194
Return to citation in text: [1] -
Alhalib, A.; Moran, W. J. Org. Biomol. Chem. 2014, 12, 795–800. doi:10.1039/c3ob42030b
Return to citation in text: [1] -
Sasmal, P. K.; Sridhar, S.; Iqbal, J. Tetrahedron Lett. 2006, 47, 8661–8665. doi:10.1016/j.tetlet.2006.09.157
Return to citation in text: [1] -
Meng, X.; Kim, S. Org. Biomol. Chem. 2011, 9, 4429–4431. doi:10.1039/c1ob05512g
Return to citation in text: [1] -
Saeedi, M.; Goli, F.; Mahdavi, M.; Sarihi, P.; Asadipour, A.; Shafiee, A.; Foroumadi, A. J. Chem. Res. 2014, 38, 131–133. doi:10.3184/174751914X13899480812215
Return to citation in text: [1] -
Urbanaitė, A.; Jonušis, M.; Bukšnaitienė, R.; Balkaitis, S.; Čikotienė, I. Eur. J. Org. Chem. 2015, 7091–7113. doi:10.1002/ejoc.201501063
Return to citation in text: [1] -
Heugebaert, T. S. A.; Vervaecke, L. P. D.; Stevens, C. V. Org. Biomol. Chem. 2011, 9, 4791–4794. doi:10.1039/c1ob05509g
Return to citation in text: [1]
| 79. | Liu, A.; He, L.; Peng, S.; Pan, Z.; Wang, J.; Gao, J. Sci. China: Chem. 2010, 53, 1578–1585. doi:10.1007/s11426-010-4028-6 |
| 80. | Maddani, M. R.; Prabhu, K. R. J. Org. Chem. 2010, 75, 2327–2332. doi:10.1021/jo1001593 |
| 75. | Batty, J. W.; Weedon, B. C. L. J. Chem. Soc. 1949, 786–789. doi:10.1039/JR9490000786 |
| 87. | Scalacci, N.; Pelloja, C.; Radi, M.; Castagnolo, D. Synlett 2016, 27, 1883–1887. doi:10.1055/s-0035-1561985 |
| 88. | Huang, S.; Shao, Y.; Liu, R.; Zhou, X. Tetrahedron 2015, 71, 4219–4226. doi:10.1016/j.tet.2015.04.080 |
| 85. | Urleb, U.; Neidlein, R.; Kramer, W. Helv. Chim. Acta 1993, 76, 431–440. doi:10.1002/hlca.19930760127 |
| 86. | Sasmal, P. K.; Chandrasekhar, A.; Sridhar, S.; Iqbal, J. Tetrahedron 2008, 64, 11074–11080. doi:10.1016/j.tet.2008.09.074 |
| 83. | Easton, N. R.; Cassady, D. R.; Dillard, R. D. J. Org. Chem. 1964, 29, 1851–1855. doi:10.1021/jo01030a044 |
| 84. | Arya, V.; Grewal, R.; Kaul, C.; David, J.; Honkan, V. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1977, 15, 133–140. |
| 81. | Novikov, R. V.; Danilkina, N. A.; Balova, I. A. Chem. Heterocycl. Compd. 2011, 47, 758–766. doi:10.1007/s10593-011-0831-z |
| 82. | Mahdavi, M.; Bialam, M.; Saeedi, M.; Jafarpour, F.; Foroumadi, A.; Shafiee, A. Synlett 2015, 26, 173–176. doi:10.1055/s-0034-1379499 |
| 89. | Viart, H. M.-F.; Larsen, T. S.; Tassone, C.; Andresen, T. L.; Clausen, M. H. Chem. Commun. 2014, 50, 7800–7802. doi:10.1039/c4cc00863d |
| 90. | Vincent-Rocan, J.-F.; Derasp, J. S.; Beauchemin, A. M. Chem. Commun. 2015, 51, 16405–16408. doi:10.1039/c5cc07212c |
| 91. | Short, K. M.; Ziegler, C. B. Tetrahedron Lett. 1993, 34, 71–74. doi:10.1016/S0040-4039(00)60060-6 |
| 98. | Saeedi, M.; Goli, F.; Mahdavi, M.; Sarihi, P.; Asadipour, A.; Shafiee, A.; Foroumadi, A. J. Chem. Res. 2014, 38, 131–133. doi:10.3184/174751914X13899480812215 |
| 99. | Urbanaitė, A.; Jonušis, M.; Bukšnaitienė, R.; Balkaitis, S.; Čikotienė, I. Eur. J. Org. Chem. 2015, 7091–7113. doi:10.1002/ejoc.201501063 |
| 96. | Sasmal, P. K.; Sridhar, S.; Iqbal, J. Tetrahedron Lett. 2006, 47, 8661–8665. doi:10.1016/j.tetlet.2006.09.157 |
| 97. | Meng, X.; Kim, S. Org. Biomol. Chem. 2011, 9, 4429–4431. doi:10.1039/c1ob05512g |
| 94. | Yarovenko, V. N.; Polushina, A. V.; Zavarzin, I. V.; Krayushkin, M. M.; Kotovskaya, S. K.; Charushin, V. N. J. Sulfur Chem. 2009, 30, 327–337. doi:10.1080/17415990902774194 |
| 95. | Alhalib, A.; Moran, W. J. Org. Biomol. Chem. 2014, 12, 795–800. doi:10.1039/c3ob42030b |
| 92. | Wipf, P.; Rahman, L. T.; Rector, S. R. J. Org. Chem. 1998, 63, 7132–7133. doi:10.1021/jo981542q |
| 93. | Chandrasekharam, M.; Singh, O. M.; Ila, H.; Junjappa, H. Synth. Commun. 1998, 28, 3073–3079. doi:10.1080/00397919808004887 |
| 100. | Heugebaert, T. S. A.; Vervaecke, L. P. D.; Stevens, C. V. Org. Biomol. Chem. 2011, 9, 4791–4794. doi:10.1039/c1ob05509g |
| 1. | Villars, V.; Jones, T. C. Clin. Exp. Dermatol. 1989, 14, 124–127. doi:10.1111/j.1365-2230.1989.tb00908.x. |
| 2. | Kagawa, S. Clin. Exp. Dermatol. 1989, 14, 114–115. doi:10.1111/j.1365-2230.1989.tb00905.x |
| 3. | Tamura, T.; Asahara, M.; Yamamoto, M.; Yamaura, M.; Matsumura, M.; Goto, K.; Rezaei-Matehkolaei, A.; Mirhendi, H.; Makimura, M.; Makimura, K. Microbiol. Immunol. 2014, 58, 1–8. doi:10.1111/1348-0421.12109 |
| 4. | Borelli, C.; Schaller, M.; Niewerth, M.; Nocker, K.; Baasner, B.; Berg, D.; Tiemann, R.; Tietjen, K.; Fugmann, B.; Lang-Fugmann, S.; Korting, H. C. Chemotherapy 2008, 54, 245–259. doi:10.1159/000142334 |
| 15. | Jin, Z. J. Nat. Prod. 2016, 33, 1268–1318. doi:10.1039/c6np00067c |
| 16. | Ramu, E.; Rao, B. V. Tetrahedron: Asymmetry 2009, 20, 2201–2204. doi:10.1016/j.tetasy.2009.09.003 |
| 17. | Le Bozec, L.; Moody, C. J. Aust. J. Chem. 2009, 62, 639–647. doi:10.1071/CH09126 |
| 18. | Davyt, D.; Serra, G. Mar. Drugs 2010, 8, 2755–2780. doi:10.3390/md8112755 |
| 19. | https://pixabay.com/en/nudibranch-snorkeling-diving-scuba-274937/. (accessed Feb 14, 2017). |
| 20. | https://pixabay.com/en/pine-cones-pine-nuts-tap-forest-820759/. (accessed Feb 14, 2017). |
| 21. | https://pixabay.com/en/glow-worm-glowworm-bug-firefly-147679/. (accessed Feb 14, 2017). |
| 22. | https://pixabay.com/en/cranberries-berries-red-berry-112151/. (accessed Feb 14, 2017). |
| 23. | https://pixabay.com/en/sea-slugs-pests-snail-plague-nature-590491/. (accessed Feb 14, 2017). |
| 24. | https://pixabay.com/en/sponge-beach-sea-water-sun-summer-62721/. (accessed Feb 14, 2017). |
| 57. | Bonde, C. G.; Gaikwad, N. J. Bioorg. Med. Chem. 2004, 12, 2151–2161. doi:10.1016/j.bmc.2004.02.024 |
| 58. | Bondock, S.; Khalifa, W.; Fadda, A. A. Eur. J. Med. Chem. 2007, 42, 948–954. doi:10.1016/j.ejmech.2006.12.025 |
| 59. | Altıntop, M. D.; Kaplancıklı, Z. A.; Çiftçi, G. A.; Demirel, R. Eur. J. Med. Chem. 2014, 74, 264–277. doi:10.1016/j.ejmech.2013.12.060 |
| 10. | Vitale, M.; Zamai, L.; Falcieri, E.; Zauli, G.; Gobbi, P.; Santi, S.; Cinti, C.; Weber, G. Cytometry 1997, 30, 61–66. doi:10.1002/(SICI)1097-0320(19970215)30:1<61::AID-CYTO9>3.0.CO;2-I |
| 11. | Pankiewicz, K. W. Pharmacol. Ther. 1997, 76, 89–100. doi:10.1016/S0163-7258(97)00092-2 |
| 12. | Savić, D.; Stanković, T.; Lavrnja, I.; Podolski-Renić, A.; Banković, J.; Peković, S.; Stojiljković, M.; Rakić, L.; Ruždijić, S.; Pešić, M. Mol. Inhibit. Target. Ther. 2015, 1, 3–14. doi:10.1515/motth-2015-0002 |
| 13. | Weber, G.; Natsumeda, Y.; Lui, M. S.; Faderan, M. A.; Liepnieks, J. J.; Elliott, W. L. Adv. Enzyme Regul. 1984, 22, 69–93. doi:10.1016/0065-2571(84)90009-8 |
| 14. | https://pixabay.com/en/syringe-pill-bottle-morphine-small-1884784/. (accessed Feb 14, 2017). |
| 60. | Zipperer, A.; Konnerth, M. C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N. A.; Slavetinsky, C.; Marschal, M.; Willmann, M.; Kalbacher, H.; Schittek, B.; Brötz-Oesterhelt, H.; Grond, S.; Peschel, A.; Krismer, B. Nature 2016, 535, 511–516. doi:10.1038/nature18634 |
| 61. | Faine, S.; Harper, M. Antimicrob. Agents Chemother. 1973, 3, 15–18. doi:10.1128/AAC.3.1.15 |
| 62. | Garrod, L. Br. Med. J. 1960, 1, 527–529. doi:10.1136/bmj.1.5172.527 |
| 63. | Bodin, N.-O.; Ekström, B.; Forsgren, U.; Jalar, L.-P.; Magni, L.; Ramsay, C.-H.; Sjöberg, B. Antimicrob. Agents Chemother. 1975, 8, 518–525. doi:10.1128/AAC.8.5.518 |
| 64. | Akova, M. Clin. Microbiol. Infect. 2008, 14, 185–188. doi:10.1111/j.1469-0691.2007.01847.x |
| 8. | Molla, A.; Korneyeva, M.; Gao, Q.; Vasavanonda, S.; Schipper, P. J.; Mo, H.-M.; Markowitz, M.; Chernyavskiy, T.; Niu, P.; Lyons, N.; Hsu, A.; Granneman, G. R.; Ho, D. D.; Boucher, C. A. B.; Leonard, J. M.; Norbeck, D. W.; Kempf, D. J. Nat. Med. 1996, 2, 760–766. doi:10.1038/nm0796-760 |
| 9. | Boffito, M.; Jackson, A.; Pozniak, A.; Giraudon, M.; Kulkarni, R.; Abelardo, M. C.; Patel, I. H.; Morcos, P. N. Drugs R&D 2015, 15, 141–153. doi:10.1007/s40268-015-0087-7 |
| 54. | Meleddu, R.; Distinto, S.; Corona, A.; Tramontano, E.; Bianco, G.; Melis, C.; Cottiglia, F.; Maccioni, E. J. Enzyme Inhib. Med. Chem. 2016, 32, 130–136. doi:10.1080/14756366.2016.1238366 |
| 55. | Lu, Z.; Harper, M. K.; Pond, C. D.; Barrows, L. R.; Ireland, C. M.; Van Wagoner, R. M. J. Nat. Prod. 2012, 75, 1436–1440. doi:10.1021/np300270p |
| 5. | Takano, Y.; Hase-Aoki, K.; Horiuchi, H.; Zhao, L.; Kasahara, Y.; Kondo, S.; Becker, M. A. Life Sci. 2005, 76, 1835–1847. doi:10.1016/j.lfs.2004.10.031 |
| 6. | Sánchez-Lozada, L. G.; Tapia, E.; Soto, V.; Ávila-Casado, C.; Franco, M.; Zhao, L.; Johnson, R. J. Nephrol., Dial., Transplant. 2008, 23, 1179–1185. doi:10.1093/ndt/gfm783 |
| 7. | Kataoka, H.; Yang, K.; Rock, K. L. Eur. J. Pharmacol. 2015, 746, 174–179. doi:10.1016/j.ejphar.2014.11.013 |
| 56. | Kim, E.-A.; Choi, J.; Han, A. R.; Choi, S. Y.; Hahn, H.-G.; Cho, S.-W. NeuroToxicology 2013, 38, 106–114. doi:10.1016/j.neuro.2013.07.001 |
| 31. | Kumar, S. V.; Parameshwarappa, G.; Ila, H. J. Org. Chem. 2013, 78, 7362–7369. doi:10.1021/jo401208u |
| 44. | Gaumont, A.-C.; Gulea, M.; Levillain, J. Chem. Rev. 2009, 109, 1371–1401. doi:10.1021/cr800189z |
| 45. | Tan, K. C.; Wakimoto, T.; Takada, K.; Ohtsuki, T.; Uchiyama, N.; Goda, Y.; Abe, I. J. Nat. Prod. 2013, 76, 1388–1391. doi:10.1021/np400404r |
| 46. | Budovská, M.; Kutschy, P.; Kožár, T.; Gondová, T.; Petrovaj, J. Tetrahedron 2013, 69, 1092–1104. doi:10.1016/j.tet.2012.11.067 |
| 47. | Segade, Y.; Montaos, M. A.; Rodríguez, J.; Jiménez, C. Org. Lett. 2014, 16, 5820–5823. doi:10.1021/ol502958u |
| 48. | Pedras, M. S. C.; Sarma-Mamillapalle, V. K. Bioorg. Med. Chem. Lett. 2012, 20, 3991–3996. doi:10.1016/j.bmc.2012.05.020 |
| 49. | Kreutzer, M. F.; Kage, H.; Herrmann, J.; Pauly, J.; Hermenau, R.; Müller, R.; Hoffmeister, D.; Nett, M. Org. Biomol. Chem. 2014, 12, 113–118. doi:10.1039/c3ob41839a |
| 50. | Aeluri, M.; Dasari, B.; Arya, P. Org. Lett. 2015, 17, 472–475. doi:10.1021/ol503465p |
| 34. | Hantzsch, A.; Weber, J. H. Ber. Dtsch. Chem. Ges. 1887, 20, 3118–3132. doi:10.1002/cber.188702002200 |
| 35. | Bramley, S. E.; Dupplin, V.; Goberdhan, D. G. C.; Meakins, G. D. J. Chem. Soc., Perkin Trans. 1 1987, 639–643. doi:10.1039/P19870000639 |
| 36. | Aguilar, E.; Meyers, A. I. Tetrahedron Lett. 1994, 35, 2473–2476. doi:10.1016/S0040-4039(00)77147-4 |
| 37. | Prakash, R.; Kumar, A.; Aggarwal, R.; Prakash, O.; Singh, S. P. Synth. Commun. 2007, 37, 2501–2505. doi:10.1080/00397910701462476 |
| 38. | Merritt, E. A.; Bagley, M. C. Synthesis 2007, 3535–3541. doi:10.1055/s-2007-990851 |
| 39. | Guernon, J. M.; Wu, Y.-J. Tetrahedron Lett. 2011, 52, 3633–3635. doi:10.1016/j.tetlet.2011.05.028 |
| 40. | Chidananda, N.; Poojary, B.; Sumangala, V.; Kumari, N. S. Med. Chem. Res. 2014, 23, 3979–3997. doi:10.1007/s00044-014-0975-3 |
| 41. | Kamila, S.; Mendoza, K.; Biehl, E. R. Tetrahedron Lett. 2012, 53, 4921–4924. doi:10.1016/j.tetlet.2012.06.116 |
| 42. | Gu, C.; Zhai, J.; Jiang, J.; Liu, H.; Wang, L.; Zhu, D.; Ji, Y. Chin. J. Chem. 2014, 32, 179–190. doi:10.1002/cjoc.201300878 |
| 43. | Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Chu, L.; Liu, H.; Shen, Q.; Zhou, J. J. Med. Chem. 2013, 56, 5048–5058. doi:10.1021/jm400367n |
| 51. | Han, F. S.; Osajima, H.; Cheung, M.; Tokuyama, H.; Fukuyama, T. Chem. – Eur. J. 2007, 13, 3026–3038. doi:10.1002/chem.200601446 |
| 52. | Sondhi, S. M.; Rani, R.; Gupta, P. P.; Agrawal, S. K.; Saxena, A. K. Mol. Diversity 2009, 13, 357–366. doi:10.1007/s11030-009-9125-0 |
| 53. | Havrylyuk, D.; Kovach, N.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Arch. Pharm. 2011, 344, 514–522. doi:10.1002/ardp.201100055 |
| 26. | Zagade, A. A.; Senthilkumar, G. P. Pharma Chem. 2011, 3, 523–537. |
| 27. | Smirnova, N. G.; Zavarzin, I. V.; Krayushkin, M. M. Chem. Heterocycl. Compd. 2006, 42, 144–165. doi:10.1007/s10593-006-0064-8 |
| 28. | Halimehjani, A. Z.; Hasani, L.; Alaei, M. A.; Saidi, M. R. Tetrahedron Lett. 2016, 57, 883–886. doi:10.1016/j.tetlet.2016.01.045 |
| 29. | Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Org. Lett. 2015, 17, 4698–4701. doi:10.1021/acs.orglett.5b02152 |
| 30. | Miura, T.; Funakoshi, Y.; Fujimoto, Y.; Nakahashi, J.; Murakami, M. Org. Lett. 2015, 17, 2454–2457. doi:10.1021/acs.orglett.5b00960 |
| 31. | Kumar, S. V.; Parameshwarappa, G.; Ila, H. J. Org. Chem. 2013, 78, 7362–7369. doi:10.1021/jo401208u |
| 32. | Maltsev, O. V.; Walter, V.; Brandl, M. J.; Hintermann, L. Synthesis 2013, 45, 2763–2767. doi:10.1055/s-0033-1339492 |
| 33. | Chhabria, M. T.; Patel, S.; Modi, P.; Brahmkshatriya, P. S. Curr. Top. Med. Chem. 2016, 16, 2841–2862. doi:10.2174/1568026616666160506130731 |
| 25. | Harikrishna, N.; Isloor, A. M.; Ananda, K.; Obaid, A.; Fun, H.-K. RSC Adv. 2015, 5, 43648–43659. doi:10.1039/C5RA04995D |
| 26. | Zagade, A. A.; Senthilkumar, G. P. Pharma Chem. 2011, 3, 523–537. |
| 30. | Miura, T.; Funakoshi, Y.; Fujimoto, Y.; Nakahashi, J.; Murakami, M. Org. Lett. 2015, 17, 2454–2457. doi:10.1021/acs.orglett.5b00960 |
| 66. | Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 71662–71675. doi:10.1039/C6RA08720E |
| 44. | Gaumont, A.-C.; Gulea, M.; Levillain, J. Chem. Rev. 2009, 109, 1371–1401. doi:10.1021/cr800189z |
| 75. | Batty, J. W.; Weedon, B. C. L. J. Chem. Soc. 1949, 786–789. doi:10.1039/JR9490000786 |
| 76. | Hanefeld, W.; Bercin, E. Liebigs Ann. Chem. 1985, 58–64. doi:10.1002/jlac.198519850107 |
| 69. | Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 99781–99793. doi:10.1039/C6RA20718A |
| 69. | Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 99781–99793. doi:10.1039/C6RA20718A |
| 70. | Vessally, E.; Soleimani-Amiri, S.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A. RSC Adv. 2017, 7, 7079–7091. doi:10.1039/c6ra25816f |
| 71. | Arshadi, S.; Vessally, E.; Edjlali, L.; Ghorbani-Kalhor, E.; Hosseinzadeh-Khanmiri, R. RSC Adv. 2017, 7, 13198–13211. doi:10.1039/c7ra00746a |
| 72. | Vessally, E.; Hosseinzadeh-Khanmiri, R.; Ghorbani-Kalhor, E.; Es’haghi, M.; Bekhradnia, A. RSC Adv. 2017, 7, 19061–19072. doi:10.1039/c7ra01371j |
| 73. | Vessally, E.; Abdoli, M. J. Iran. Chem. Soc. 2016, 13, 1235–1256. doi:10.1007/s13738-016-0838-6 |
| 74. | Vessally, E.; Saeidian, H.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A. Curr. Org. Chem. 2017, 21, 249–271. doi:10.2174/1385272820666161018150925 |
| 67. | Vessally, E.; Edjlali, L.; Hosseinian, A.; Bekhradnia, A.; Esrafili, M. D. RSC Adv. 2016, 6, 49730–49746. doi:10.1039/C6RA05221E |
| 68. | Vessally, E.; Hosseinian, A.; Bekhradnia, A.; Esrafili, M. D. Curr. Org. Synth. 2017, 14, 557–567. doi:10.2174/1570179413666160818144816 |
© 2017 Arshadi et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)