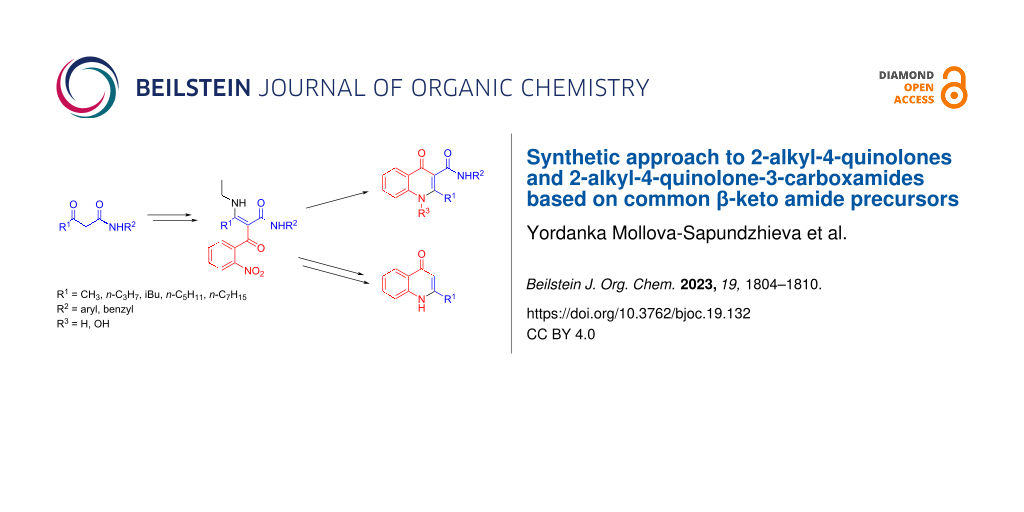
Abstract
β-Keto amides were used as convenient precursors to both 2-alkyl-4-quinolones and 2-alkyl-4-quinolone-3-carboxamides. The utility of this approach is demonstrated with the synthesis of fourteen novel and four known quinolone derivatives, including natural products of microbial origin such as HHQ and its C5-congener. Two compounds with high activity against S. aureus have been identified among the newly obtained quinolones, with MICs ≤ 3.12 and ≤ 6.25 µg/mL, respectively.
Graphical Abstract
Introduction
Among the vast number of biologically active quinoline derivatives [1,2], the subclass of 4-quinolones (also referred to as 4-oxo-1,4-dihydroquinolines, quinolin-4(1H)-ones, or 4-hydroxyquinolines) is of great importance with its rich variety of bioactive compounds. Perhaps the most prominent examples in this regard are the fluoroquinolone antimicrobials [3] – a remarkably successful drug class, used to treat bacterial infections caused by both Gram-positive and Gram-negative bacteria [4]. Other notable 4-quinolones of synthetic origin are ivacaftor [5] and elvitegravir [6], drugs used to treat cystic fibrosis and HIV infection, respectively.
A plethora of 4-quinolones with various substitution patterns and biological activities have been isolated from natural sources. This includes plant-derived alkaloids such as graveoline [7], evocarpine [8], leiokinine [9], evollionine C [10], leptomerine [11], and punarivine [12]. The fruit of Evodia rutaecarpa is a particularly rich source of 4-quinolones with long-chain substituents at position 2. Various alkaloids isolated from this source have been shown to possess anti-Helicobacter pylori activity [13], inhibitory effects on monoamine oxidase [14], cytotoxicity against cancer cell lines [15], activity against nuclear factor of activated T cells [16], and anti-inflammatory activity [17]. Alkaloids with similar structure and anti-inflammatory activity have been isolated from another member of the Rutaceae family – Zanthoxylum avicennae [18]. Inhibition of hepatitis C virus replication by 2-nonyl-4-quinolone, isolated from Ruta angustifolia leaves, has also been reported [19].
Another significant group of natural 4-quinolones are those of microbial origin. The function of these compounds in the microbial world is a matter of great research interest, with many reviews published in the recent years [20-22]. Some of the compounds are known to act as antibiotics [23-26], while others function as quorum-sensing signal molecules which regulate the production and release of virulence factors in bacteria, thus helping them to colonize niches and gain advantage over competitors [27,28]. Interference with this complicated communication mechanism is considered a viable strategy of combating bacterial infections and consequently a lot of research efforts have been devoted to it [29-33]. Many of the 4-quinolones produced by the Gram-negative opportunistic pathogen Pseudomonas aeruginosa and related species feature a saturated long-chain substituent at position 2 and are sometimes referred to as pseudanes [34,35]. Pseudomonas aeruginosa alone produces over 50 different quinolones, among which the most extensively studied is 2-heptyl-4-quinolone (HHQ) and its oxygenated derivatives 2-heptyl-3-hydroxy-4-quionolone (PQS) and 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) [27,36-38].
Considering the importance of 4-quinolones as potential drugs and biological probes, it is not surprising that the development of methods for their synthesis is a very active area of research. Recent contributions to the synthesis of 4-quinolones made use of phosphine-mediated redox cyclization of 1-(2-nitroaryl)prop-2-ynones [39], palladium-catalyzed carbonylative cyclization of 2-bromonitrobenzenes and alkynes [40], TsCl-mediated domino reaction of chromone-3-carboxaldehydes and amines [41], Pd-catalyzed redox-neutral C–N coupling reaction of iminoquinones with electron-deficient alkenes [42], NH3 insertion into o‑haloarylynones [43], gold(III)-catalyzed azide-yne cyclization [44], Michael/Truce-Smiles rearrangement cascade [45], and base-promoted annulations with isatoic anhydrides [46]. Many other contributions in this field up to 2019 have been extensively reviewed [47-49], with special attention to the total synthesis and functional analysis of 2-alkyl-4-quinolones as microbial signaling molecules [50,51].
Despite the variety of synthetic approaches to the construction and functionalization of the 4-quinolone ring system, most of the recent studies related to microbial 2-alkyl-4-quinolones relied on variations of the age-old Conrad–Limpach and Camps methods for the construction of the heterocyclic quinolone core [26,36,52-55]. These methods usually give poor overall yields of the target quinolone products and require rather harsh conditions during the ring-forming step, such as prolonged heating in Ph2O (270 °C) or in dioxane/NaOH (110 °C), respectively. This, along with the importance of the C-3 substitution in analogues of microbial behavioral modulators [54,56], prompted us to investigate a new synthetic approach that could provide a straightforward access to both 2-alkyl-4-quinolones and 2-alkyl-4-quinolone-3-carboxamides. Our approach falls within the broader methodological group of reductive cyclizations of o-nitrobenzoyl ketones [57,58], enamines [59,60], or isoxazoles [61]. The scope of these reductive cyclizations is limited by the availability of the necessary intermediates and has remained largely underexplored, especially with regard to 4-quinolones with long-chain substituents at the C-2 position. As a way of expanding the scope of this methodology, we resorted to the α-C-acylation of β-enamino amides, a reliable reaction, the utility of which we have already demonstrated in other contexts [62,63].
Results and Discussion
As the starting point of our synthetic experiments we used a set of β-keto amides 1. One of these compounds (1g) was acquired from a commercial supplier, others (1h and 1i) were prepared by acetoacetylation of the corresponding amine [64], and the remaining ones (1a–f) were prepared according to our previously published method [65,66]. The intermediate β-enamino amides 2 are easily available by condensation of the corresponding β-keto amide 1 and an amine (Scheme 1, conditions i). As the amine here plays only an auxiliary role, for the purpose of this research we opted for inexpensive ethylamine. Compounds 2 were obtained by simply stirring a dichloromethane solution of the corresponding keto amide 1 with a slight excess of 70% aqueous ethylamine over Na2SO4 and were used directly in the next step, without purification. These compounds are highly reactive at their α-position towards acylating reagents and this provides an opportunity to prepare the key o-nitrobenzoyl intermediates 3 in a reaction with o-nitrobenzoyl chloride (Scheme 1, conditions ii). The acylation of 2 to 3 proceeded with variable yields, depending on the substituents R1 and R2. Derivatives 2 with a primary carboxamide group (R2 = H) gave generally lower and poorly reproducible yields of the desired products 3. On the other hand, when R2 was aryl or benzyl the yields of 3 over two steps were very good, in the range of 75–92% (Table 1). The R1 substituent influenced the yield of 3 to a lower extent, but with an unfavorable effect of sterically bulkier substituents. Any α-substitution in R1 drove the yields of 3 below 50% and for this reason isolation and further elaboration of such products were considered impractical.
Scheme 1: Preparation of α-(o-nitrobenzoyl)-β-enamino amides 3. Reagents and conditions: i) EtNH2 (70% aq, 1.05–1.15 equiv), CH2Cl2, Na2SO4, 24 h, rt; ii) NMM (1 equiv), DMAP (0.2 equiv), o-nitrobenzoyl chloride (1 equiv), CH2Cl2, 2 h, rt.
Scheme 1: Preparation of α-(o-nitrobenzoyl)-β-enamino amides 3. Reagents and conditions: i) EtNH2 (70% aq, 1....
Table 1: Yields of α-(o-nitrobenzoyl)-β-enamino amides 3 prepared according to Scheme 1.
| 3 | R1 | R2 | Yield 3 [%]a |
| a | n-C3H7 | C6H5 | 90 |
| b | iBu | C6H5 | 75 |
| c | n-C5H11 | C6H5 | 89 |
| d | n-C7H15 | C6H5 | 88 |
| e | n-C7H15 | p-C6H4OCH3 | 86 |
| f | n-C7H15 | p-C6H4Cl | 91 |
| g | CH3 | p-C6H4Cl | 90 |
| h | CH3 | p-C6H4OCH3 | 92 |
| i | CH3 | CH2C6H5 | 90 |
aOver two steps, without purification of intermediate 2.
Once prepared, the key intermediates 3 could be transformed either directly to 2-alkyl-4-quinolone-3-carboxamides 5 or to 2-alkyl-4-quinolones 8, after an additional decarbamoylative step (Scheme 2). The decarbamoylation of compounds 3a–d was carried out by heating at 60 °C in neat H3PO4 for 90 minutes [62] and gave the corresponding β-enaminoketones 6a–d in good yields (Table 2). The NMR spectra of compounds 6 in DMSO-d6 in all cases indicated a mixture of Z/E isomers in approximately 85:15 ratio. The same spectra in CDCl3 showed broad coalescent signals for the characteristic vinyl CH protons, which is indicative of a dynamic equilibrium between the isomers.
Scheme 2: Alternative manipulations of intermediates 3, leading to either 2-alkyl-4-quinolones 8 (via enaminoketones 6) or 2-alkyl-4-quinolone-3-carboxamides 5 (by direct reduction/cyclocondensation). Reagents and conditions: iii) H3PO4, 60 °C, 90 min; iv) Zn/AcOH/CH2Cl2, rt, overnight; v) HCOONH4, Pd/C, CH3OH, rt. See main text for details.
Scheme 2: Alternative manipulations of intermediates 3, leading to either 2-alkyl-4-quinolones 8 (via enamino...
Table 2: Yields of β-enaminoketones 6 prepared by decarbamoylation of intermediates 3, according to Scheme 2:
| 6 | R1 | Yield 6 [%] |
| a | n-C3H7 | 90 |
| b | iBu | 91 |
| c | n-C5H11 | 91 |
| d | n-C7H15 | 93 |
For both types of nitro intermediates 3 and 6 the final ring-forming step required reduction of the nitro group with subsequent cyclization of the reduced intermediate (Scheme 2, conditions iv). We tried to carry out these reactions either with Zn in acetic acid/dichloromethane or by transfer hydrogenation with ammonium formate in the presence of Pd on charcoal. Both types of reductive conditions presented a challenge with regard to the chemoselectivity of the desired transformation, as they initially gave mixtures of 4-quinolones 5 or 8, respectively, and their corresponding N-hydroxy derivatives 4 or 7, respectively. Such a result is not surprising, considering that the reduction of the aromatic nitro derivatives 3 and 6 proceeds through the corresponding hydroxylamines, capable of intramolecular cyclization to products 4 or 7. Fortunately, under Zn/AcOH reductive conditions this was resolved by extending the duration of the reaction to 18–24 h, providing enough time for compounds 4/7 to get reduced to quinolones 5/8, which were isolated in good yields (Table 3 and Table 4).
Table 3: Yields of 2-alkyl-4-quinolone-3-carboxamides 5, prepared according to Scheme 2.
| 5 | R1 | R2 | Yield 5 [%] |
| a | n-C3H7 | C6H5 | 90 |
| b | iBu | C6H5 | 56 |
| c | n-C5H11 | C6H5 | 63 |
| d | n-C7H15 | C6H5 | 90 |
| e | n-C7H15 | p-C6H4OCH3 | 72 |
| f | n-C7H15 | p-C6H4Cl | 83 |
| g | CH3 | p-C6H4Cl | 92 |
| h | CH3 | p-C6H4OCH3 | 92 |
| i | CH3 | CH2C6H5 | 79 |
Table 4: Yields of 2-alkyl-4-quinolones 8, prepared according to Scheme 2:
| 8 | R1 | Yield 8 [%] |
| a | n-C3H7 | 72 |
| b | iBu | 74 |
| c | n-C5H11 | 90 |
| d | n-C7H15 | 90 |
In the case of the Pd-catalyzed transfer hydrogenation of intermediates 3 the yields of products 5 in most cases were lower than those obtained with Zn/AcOH, regardless of the reaction duration. On the other hand, limiting the reaction time to 60–90 min under these conditions allowed some of the N-hydroxy derivatives 4 to be isolated in good yield (Table 5), even though it did not entirely prevent the formation of products 5. Palladium catalysis was not appropriate for the hydrogenation of compounds 3f and 3g, because of concomitant reduction at the C–Cl bond.
Table 5: Yields of 1-hydroxy-2-alkyl-4-quinolone-3-carboxamides 4, prepared according to Scheme 2.
| 4 | R1 | R2 | Yield 4 [%] |
| a | n-C3H7 | C6H5 | 57 |
| b | iBu | C6H5 | 75 |
| c | n-C5H11 | C6H5 | 60 |
| d | n-C7H15 | C6H5 | 70 |
| e | n-C7H15 | p-C6H4OCH3 | 64 |
Intermediates 6, similarly to compounds 3, gave mixtures of products 7/8 under palladium-catalyzed transfer hydrogenation conditions. In contrast to 3, however, limiting the reaction time here did not help to develop a preparatively useful procedure for a preferential isolation of N-hydroxy derivatives 7. Further experiments for palladium-catalyzed hydrogenation with H2 at atmospheric pressure did not show any advantage over the transfer hydrogenation conditions.
Overall, the described synthetic approach (Scheme 1 and Scheme 2) allowed us to prepare in an operationally simple manner 2-alkyl-4-quinolones 8a–d, all of which are known from the literature [25,36,61,67,68] and two of them are natural products of microbial origin (8c [69] and 8d [70]). More importantly, the utility of the approach was demonstrated with the synthesis of the novel 2-alkyl-4-quinolone-3-carboxamides 5a–i and some of their N-hydroxy derivatives 4a–e. Compounds of this type with C-2 substitution other than methyl [71] have not been previously described.
All of the obtained products were screened for antimicrobial activity at a concentration of 1 mg/mL against S. aureus and E. coli, using the hole-plate method in Mueller–Hinton agar, with 100 µg loading of each compound in 100 µL DMSO (Table 6). Interestingly, at this concentration most of the compounds showed weak to moderate activity against E. coli, while S. aureus was inhibited only by C5 and C7-substituted analogs. Among the novel compounds, only compounds 4d and 4e gave inhibition zones of more than 20 mm and were further analyzed to determine their minimum inhibitory concentrations (MIC) by serial broth dilutions [72]. The MICs measured for 4d and 4e were ≤6.25 µg/mL and ≤3.12 µg/mL, respectively, with a MIC ≤ 0.78 µg/mL for levofloxacin as the positive control.
Table 6: Antibacterial activity of the synthesized quinolone derivatives 4, 5, and 8.
| Compounda | Sterile zone diameter (mm)b | |
|
S. aureus
ATCC 25923 |
E. coli
ATCC 25922 |
|
| 4b | – | 17 |
| 4d | 27 | 16 |
| 4e | 22 | – |
| 5a | – | 16 |
| 5b | – | 15 |
| 5c | – | 14 |
| 5d | 19 | 14 |
| 5e | 15 | 16 |
| 5f | 15 | 15 |
| 8a | – | 16 |
| 8b | – | 15 |
| 8c | 18 | 15 |
| 8d | 21 | 13 |
aCompounds giving sterile zones of less than 10 mm are not listed. bIncluding well diameter of 8 mm.
Conclusion
In conclusion, we have demonstrated that β-keto amides and 2-nitrobenzoyl chloride can be used as convenient precursors to a variety of 4-quinolone derivatives. The described approach is realized in a small number of steps, under mild conditions, and allows easy installation of long-chain substituents at the C-2 position of the quinolone core. These characteristics of the synthetic method could be particularly attractive in the search of novel mimics of the Pseudomonas quorum-sensing signal molecules. The high activity of compounds 4d and 4e against S. aureus provides a good lead for further structural optimizations.
Acknowledgements
We are grateful to Prof. Tsanko Gechev and the Center of Plant Systems Biology and Biotechnology, Plovdiv, for providing access to their Waters Acquity - Synapt XS UPLC - mass spectrometry system.
Funding
This research was funded by the Bulgarian National Science Fund, grant number KP-06-N59/14 and the Royal Society of Chemistry (UK), grant number R21-2062713611. Pavel Yanev acknowledges a postdoctoral grant from the National Program of Ministry of Education and Science “Young Scientists and Postdoctoral Students – 2 - 2022”.
References
-
Shang, X.-F.; Morris‐Natschke, S. L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Med. Res. Rev. 2018, 38, 775–828. doi:10.1002/med.21466
Return to citation in text: [1] -
Shang, X.-F.; Morris‐Natschke, S. L.; Yang, G.-Z.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Zhang, J.-Y.; Lee, K.-H. Med. Res. Rev. 2018, 38, 1614–1660. doi:10.1002/med.21492
Return to citation in text: [1] -
Bush, N. G.; Diez-Santos, I.; Abbott, L. R.; Maxwell, A. Molecules 2020, 25, 5662. doi:10.3390/molecules25235662
Return to citation in text: [1] -
Oliphant, C. M.; Green, G. M. Am. Fam. Physician 2002, 65, 455–464.
Return to citation in text: [1] -
Van Goor, F.; Hadida, S.; Grootenhuis, P. D. J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; Zhou, J.; McCartney, J.; Arumugam, V.; Decker, C.; Yang, J.; Young, C.; Olson, E. R.; Wine, J. J.; Frizzell, R. A.; Ashlock, M.; Negulescu, P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18825–18830. doi:10.1073/pnas.0904709106
Return to citation in text: [1] -
Sato, M.; Motomura, T.; Aramaki, H.; Matsuda, T.; Yamashita, M.; Ito, Y.; Kawakami, H.; Matsuzaki, Y.; Watanabe, W.; Yamataka, K.; Ikeda, S.; Kodama, E.; Matsuoka, M.; Shinkai, H. J. Med. Chem. 2006, 49, 1506–1508. doi:10.1021/jm0600139
Return to citation in text: [1] -
El Sayed, K.; Al-Said, M. S.; El-Feraly, F. S.; Ross, S. A. J. Nat. Prod. 2000, 63, 995–997. doi:10.1021/np000012y
Return to citation in text: [1] -
Kim, Y.-C.; Kim, N.-Y.; Jeong, S.-J.; Sohn, D.-H.; Miyamoto, T.; Higuchi, R. Planta Med. 1998, 64, 490. doi:10.1055/s-2006-957499
Return to citation in text: [1] -
Nakatsu, T.; Johns, T.; Kubo, I.; Milton, K.; Sakai, M.; Chatani, K.; Saito, K.; Yamagiwa, Y.; Kamikawa, T. J. Nat. Prod. 1990, 53, 1508–1513. doi:10.1021/np50072a017
Return to citation in text: [1] -
Li, Y.-H.; He, J.; Li, Y.; Wu, X.-D.; Peng, L.-Y.; Du, R.-N.; Cheng, X.; Zhao, Q.-S.; Li, R.-T. Helv. Chim. Acta 2014, 97, 1481–1486. doi:10.1002/hlca.201300449
Return to citation in text: [1] -
Akhmedzhanova, V. I.; Bessonova, I. A.; Yunusov, S. Y. Chem. Nat. Compd. 1986, 22, 78–79. doi:10.1007/bf00574586
Return to citation in text: [1] -
Dhingra, D.; Valecha, R. Indian J. Exp. Biol. 2014, 52, 799–807.
Return to citation in text: [1] -
Rho, T. C.; Bae, E.-A.; Kim, D.-H.; Oh, W. K.; Kim, B. Y.; Ahn, J. S.; Lee, H. S. Biol. Pharm. Bull. 1999, 22, 1141–1143. doi:10.1248/bpb.22.1141
Return to citation in text: [1] -
Han, X. H.; Hong, S. S.; Lee, D.; Lee, J. J.; Lee, M. S.; Moon, D.-C.; Han, K.; Oh, K.-W.; Lee, M. K.; Ro, J. S.; Hwang, B. Y. Arch. Pharmacal Res. 2007, 30, 397–401. doi:10.1007/bf02980210
Return to citation in text: [1] -
Zhao, N.; Li, Z.-L.; Li, D.-H.; Sun, Y.-T.; Shan, D.-T.; Bai, J.; Pei, Y.-H.; Jing, Y.-K.; Hua, H.-M. Phytochemistry 2015, 109, 133–139. doi:10.1016/j.phytochem.2014.10.020
Return to citation in text: [1] -
Jin, H. Z.; Lee, J. H.; Lee, D.; Lee, H. S.; Hong, Y. S.; Kim, Y. H.; Lee, J. J. Biol. Pharm. Bull. 2004, 27, 926–928. doi:10.1248/bpb.27.926
Return to citation in text: [1] -
Wang, T.-Y.; Wu, J.-B.; Hwang, T.-L.; Kuo, Y.-H.; Chen, J.-J. Chem. Biodiversity 2010, 7, 1828–1834. doi:10.1002/cbdv.200900289
Return to citation in text: [1] -
Ji, K.-L.; Liu, W.; Yin, W.-H.; Li, J.-Y.; Yue, J.-M. Org. Biomol. Chem. 2022, 20, 4176–4182. doi:10.1039/d2ob00711h
Return to citation in text: [1] -
Wahyuni, T. S.; Widyawaruyanti, A.; Lusida, M. I.; Fuad, A.; Soetjipto; Fuchino, H.; Kawahara, N.; Hayashi, Y.; Aoki, C.; Hotta, H. Fitoterapia 2014, 99, 276–283. doi:10.1016/j.fitote.2014.10.011
Return to citation in text: [1] -
Heeb, S.; Fletcher, M. P.; Chhabra, S. R.; Diggle, S. P.; Williams, P.; Cámara, M. FEMS Microbiol. Rev. 2011, 35, 247–274. doi:10.1111/j.1574-6976.2010.00247.x
Return to citation in text: [1] -
Reen, F. J.; McGlacken, G. P.; O’Gara, F. FEMS Microbiol. Lett. 2018, 365, fny076. doi:10.1093/femsle/fny076
Return to citation in text: [1] -
Lin, J.; Cheng, J.; Wang, Y.; Shen, X. Front. Cell. Infect. Microbiol. 2018, 8, 230. doi:10.3389/fcimb.2018.00230
Return to citation in text: [1] -
Wu, Y.; Seyedsayamdost, M. R. Cell Chem. Biol. 2017, 24, 1437–1444.e3. doi:10.1016/j.chembiol.2017.08.024
Return to citation in text: [1] -
Piochon, M.; Coulon, P. M. L.; Caulet, A.; Groleau, M.-C.; Déziel, E.; Gauthier, C. J. Nat. Prod. 2020, 83, 2145–2154. doi:10.1021/acs.jnatprod.0c00171
Return to citation in text: [1] -
Long, R. A.; Qureshi, A.; Faulkner, D. J.; Azam, F. Appl. Environ. Microbiol. 2003, 69, 568–576. doi:10.1128/aem.69.1.568-576.2003
Return to citation in text: [1] [2] -
Szamosvári, D.; Prothiwa, M.; Dieterich, C. L.; Böttcher, T. Chem. Commun. 2020, 56, 6328–6331. doi:10.1039/d0cc02498h
Return to citation in text: [1] [2] -
Huse, H.; Whiteley, M. Chem. Rev. 2011, 111, 152–159. doi:10.1021/cr100063u
Return to citation in text: [1] [2] -
Diggle, S. P.; Matthijs, S.; Wright, V. J.; Fletcher, M. P.; Chhabra, S. R.; Lamont, I. L.; Kong, X.; Hider, R. C.; Cornelis, P.; Cámara, M.; Williams, P. Chem. Biol. 2007, 14, 87–96. doi:10.1016/j.chembiol.2006.11.014
Return to citation in text: [1] -
Geske, G. D.; O’Neill, J. C.; Blackwell, H. E. Chem. Soc. Rev. 2008, 37, 1432–1447. doi:10.1039/b703021p
Return to citation in text: [1] -
Kamal, A. A. M.; Maurer, C. K.; Allegretta, G.; Haupenthal, J.; Empting, M.; Hartmann, R. W. Top. Med. Chem. 2017, 26, 185–210. doi:10.1007/7355_2017_17
Return to citation in text: [1] -
Schütz, C.; Empting, M. Beilstein J. Org. Chem. 2018, 14, 2627–2645. doi:10.3762/bjoc.14.241
Return to citation in text: [1] -
Ó Muimhneacháin, E.; Reen, F. J.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2018, 16, 169–179. doi:10.1039/c7ob02395b
Return to citation in text: [1] -
Abd El-Aleam, R. H.; George, R. F.; Georgey, H. H.; Abdel-Rahman, H. M. RSC Adv. 2021, 11, 36459–36482. doi:10.1039/d1ra06238g
Return to citation in text: [1] -
Kim, W. J.; Kim, Y. O.; Kim, J. H.; Nam, B.-H.; Kim, D.-G.; An, C. M.; Lee, J. S.; Kim, P. S.; Lee, H. M.; Oh, J.-S.; Lee, J. S. Mar. Drugs 2016, 14, 24. doi:10.3390/md14010024
Return to citation in text: [1] -
Bultel-Poncé, V.; Berge, J.-P.; Debitus, C.; Nicolas, J.-L.; Guyot, M. Mar. Biotechnol. 1999, 1, 384–390. doi:10.1007/pl00011792
Return to citation in text: [1] -
Reen, F. J.; Shanahan, R.; Cano, R.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2015, 13, 5537–5541. doi:10.1039/c5ob00315f
Return to citation in text: [1] [2] [3] -
Ramos, A. F.; Woods, D. F.; Shanahan, R.; Cano, R.; McGlacken, G. P.; Serra, C.; O'Gara, F.; Reen, F. J. Microbiology (London, U. K.) 2020, 166, 169–179. doi:10.1099/mic.0.000876
Return to citation in text: [1] -
Hodgkinson, J. T.; Galloway, W. R. J. D.; Welch, M.; Spring, D. R. Nat. Protoc. 2012, 7, 1184–1192. doi:10.1038/nprot.2012.054
Return to citation in text: [1] -
Dutta, L.; Ramasastry, S. S. V. Org. Lett. 2022, 24, 7665–7670. doi:10.1021/acs.orglett.2c03232
Return to citation in text: [1] -
Wang, J.-S.; Li, C.; Ying, J.; Xu, T.; Lu, W.; Li, C.-Y.; Wu, X.-F. J. Catal. 2022, 408, 81–87. doi:10.1016/j.jcat.2022.02.026
Return to citation in text: [1] -
Lei, J.; Ding, Y.; Zhou, H.-Y.; Gao, X.-Y.; Cao, Y.-H.; Tang, D.-Y.; Li, H.-y.; Xu, Z.-G.; Chen, Z.-Z. Green Chem. 2022, 24, 5755–5759. doi:10.1039/d2gc01689c
Return to citation in text: [1] -
Jillella, R.; Raju, S.; Hsiao, H.-C.; Hsu, D.-S.; Chuang, S.-C. Org. Lett. 2020, 22, 6252–6256. doi:10.1021/acs.orglett.0c01929
Return to citation in text: [1] -
Singh, S.; Nerella, S.; Pabbaraja, S.; Mehta, G. Org. Lett. 2020, 22, 1575–1579. doi:10.1021/acs.orglett.0c00172
Return to citation in text: [1] -
Huang, J.; Su, H.; Bao, M.; Qiu, L.; Zhang, Y.; Xu, X. Org. Biomol. Chem. 2020, 18, 3888–3892. doi:10.1039/d0ob00745e
Return to citation in text: [1] -
Xie, C.; Yang, D.; Wang, X.; Ma, C. J. Org. Chem. 2020, 85, 14937–14944. doi:10.1021/acs.joc.0c01662
Return to citation in text: [1] -
Khalifa, M. M.; Philkhana, S. C.; Golden, J. E. J. Org. Chem. 2020, 85, 464–481. doi:10.1021/acs.joc.9b02541
Return to citation in text: [1] -
Sajadikhah, S. S.; Lotfifar, N. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2018, 54, 587–589. doi:10.1007/s10593-018-2311-1
Return to citation in text: [1] -
Shen, C.; Wang, A.; Xu, J.; An, Z.; Loh, K. Y.; Zhang, P.; Liu, X. Chem 2019, 5, 1059–1107. doi:10.1016/j.chempr.2019.01.006
Return to citation in text: [1] -
Ghosh, P.; Das, S. Eur. J. Org. Chem. 2019, 4466–4516. doi:10.1002/ejoc.201900452
Return to citation in text: [1] -
Leichnitz, D.; Raguž, L.; Beemelmanns, C. Chem. Soc. Rev. 2017, 46, 6330–6344. doi:10.1039/c6cs00665e
Return to citation in text: [1] -
Horák, R.; Kvapil, L.; Motyka, K.; Slaninová, L.; Grepl, M.; Kořistek, K.; Urbášek, M.; Hradil, P.; Soural, M. Tetrahedron 2018, 74, 366–374. doi:10.1016/j.tet.2017.12.010
Return to citation in text: [1] -
Szamosvári, D.; Böttcher, T. Angew. Chem., Int. Ed. 2017, 56, 7271–7275. doi:10.1002/anie.201702944
Return to citation in text: [1] -
Li, J.; Clark, B. R. J. Nat. Prod. 2020, 83, 3181–3190. doi:10.1021/acs.jnatprod.0c00865
Return to citation in text: [1] -
Reen, F. J.; Clarke, S. L.; Legendre, C.; McSweeney, C. M.; Eccles, K. S.; Lawrence, S. E.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2012, 10, 8903–8910. doi:10.1039/c2ob26823j
Return to citation in text: [1] [2] -
Szamosvári, D.; Sylvester, K.; Schmid, P.; Lu, K.-Y.; Derbyshire, E. R.; Böttcher, T. Chem. Commun. 2019, 55, 7009–7012. doi:10.1039/c9cc01689a
Return to citation in text: [1] -
Lu, C.; Maurer, C. K.; Kirsch, B.; Steinbach, A.; Hartmann, R. W. Angew. Chem., Int. Ed. 2014, 53, 1109–1112. doi:10.1002/anie.201307547
Return to citation in text: [1] -
Walker, H. G., Jr.; Hauser, C. R. J. Am. Chem. Soc. 1946, 68, 2742–2743. doi:10.1021/ja01216a525
Return to citation in text: [1] -
Jung, J.-C.; Jung, Y.-J.; Park, O.-S. J. Heterocycl. Chem. 2001, 38, 61–67. doi:10.1002/jhet.5570380109
Return to citation in text: [1] -
Tois, J.; Vahermo, M.; Koskinen, A. Tetrahedron Lett. 2005, 46, 735–737. doi:10.1016/j.tetlet.2004.12.046
Return to citation in text: [1] -
Bunce, R. A.; Nammalwar, B. Org. Prep. Proced. Int. 2010, 42, 557–563. doi:10.1080/00304948.2010.526512
Return to citation in text: [1] -
Lohrer, B.; Bracher, F. Tetrahedron Lett. 2019, 60, 151327. doi:10.1016/j.tetlet.2019.151327
Return to citation in text: [1] [2] -
Venkov, A. P.; Angelov, P. A. Synthesis 2003, 2221–2225. doi:10.1055/s-2003-41067
Return to citation in text: [1] [2] -
Angelov, P.; Ivanova, S.; Yanev, P. Tetrahedron Lett. 2017, 58, 4776–4778. doi:10.1016/j.tetlet.2017.11.023
Return to citation in text: [1] -
Gama, F. H. S.; de Souza, R. O. M. A.; Garden, S. J. RSC Adv. 2015, 5, 70915–70928. doi:10.1039/c5ra14355a
Return to citation in text: [1] -
Angelov, P. Synlett 2010, 1273–1275. doi:10.1055/s-0029-1219836
Return to citation in text: [1] -
Yanev, P.; Angelov, P. Beilstein J. Org. Chem. 2018, 14, 2602–2606. doi:10.3762/bjoc.14.238
Return to citation in text: [1] -
Eidamshaus, C.; Triemer, T.; Reissig, H.-U. Synthesis 2011, 3261–3266. doi:10.1055/s-0030-1260198
Return to citation in text: [1] -
Thierbach, S.; Wienhold, M.; Fetzner, S.; Hennecke, U. Beilstein J. Org. Chem. 2019, 15, 187–193. doi:10.3762/bjoc.15.18
Return to citation in text: [1] -
Wratten, S. J.; Wolfe, M. S.; Andersen, R. J.; Faulkner, D. J. Antimicrob. Agents Chemother. 1977, 11, 411–414. doi:10.1128/aac.11.3.411
Return to citation in text: [1] -
Wells, I. C.; Elliott, W. H.; Thayer, S. A.; Doisy, E. A. J. Biol. Chem. 1952, 196, 321–330. doi:10.1016/s0021-9258(18)55736-7
Return to citation in text: [1] -
Yamato, M.; Horiuchi, J.; Takeuchi, Y. Chem. Pharm. Bull. 1981, 29, 3124–3129. doi:10.1248/cpb.29.3124
Return to citation in text: [1] -
Andrews, J. M. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. doi:10.1093/jac/48.suppl_1.5
Return to citation in text: [1]
| 42. | Jillella, R.; Raju, S.; Hsiao, H.-C.; Hsu, D.-S.; Chuang, S.-C. Org. Lett. 2020, 22, 6252–6256. doi:10.1021/acs.orglett.0c01929 |
| 43. | Singh, S.; Nerella, S.; Pabbaraja, S.; Mehta, G. Org. Lett. 2020, 22, 1575–1579. doi:10.1021/acs.orglett.0c00172 |
| 44. | Huang, J.; Su, H.; Bao, M.; Qiu, L.; Zhang, Y.; Xu, X. Org. Biomol. Chem. 2020, 18, 3888–3892. doi:10.1039/d0ob00745e |
| 1. | Shang, X.-F.; Morris‐Natschke, S. L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Med. Res. Rev. 2018, 38, 775–828. doi:10.1002/med.21466 |
| 2. | Shang, X.-F.; Morris‐Natschke, S. L.; Yang, G.-Z.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Zhang, J.-Y.; Lee, K.-H. Med. Res. Rev. 2018, 38, 1614–1660. doi:10.1002/med.21492 |
| 6. | Sato, M.; Motomura, T.; Aramaki, H.; Matsuda, T.; Yamashita, M.; Ito, Y.; Kawakami, H.; Matsuzaki, Y.; Watanabe, W.; Yamataka, K.; Ikeda, S.; Kodama, E.; Matsuoka, M.; Shinkai, H. J. Med. Chem. 2006, 49, 1506–1508. doi:10.1021/jm0600139 |
| 16. | Jin, H. Z.; Lee, J. H.; Lee, D.; Lee, H. S.; Hong, Y. S.; Kim, Y. H.; Lee, J. J. Biol. Pharm. Bull. 2004, 27, 926–928. doi:10.1248/bpb.27.926 |
| 57. | Walker, H. G., Jr.; Hauser, C. R. J. Am. Chem. Soc. 1946, 68, 2742–2743. doi:10.1021/ja01216a525 |
| 58. | Jung, J.-C.; Jung, Y.-J.; Park, O.-S. J. Heterocycl. Chem. 2001, 38, 61–67. doi:10.1002/jhet.5570380109 |
| 5. | Van Goor, F.; Hadida, S.; Grootenhuis, P. D. J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; Zhou, J.; McCartney, J.; Arumugam, V.; Decker, C.; Yang, J.; Young, C.; Olson, E. R.; Wine, J. J.; Frizzell, R. A.; Ashlock, M.; Negulescu, P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18825–18830. doi:10.1073/pnas.0904709106 |
| 17. | Wang, T.-Y.; Wu, J.-B.; Hwang, T.-L.; Kuo, Y.-H.; Chen, J.-J. Chem. Biodiversity 2010, 7, 1828–1834. doi:10.1002/cbdv.200900289 |
| 59. | Tois, J.; Vahermo, M.; Koskinen, A. Tetrahedron Lett. 2005, 46, 735–737. doi:10.1016/j.tetlet.2004.12.046 |
| 60. | Bunce, R. A.; Nammalwar, B. Org. Prep. Proced. Int. 2010, 42, 557–563. doi:10.1080/00304948.2010.526512 |
| 14. | Han, X. H.; Hong, S. S.; Lee, D.; Lee, J. J.; Lee, M. S.; Moon, D.-C.; Han, K.; Oh, K.-W.; Lee, M. K.; Ro, J. S.; Hwang, B. Y. Arch. Pharmacal Res. 2007, 30, 397–401. doi:10.1007/bf02980210 |
| 26. | Szamosvári, D.; Prothiwa, M.; Dieterich, C. L.; Böttcher, T. Chem. Commun. 2020, 56, 6328–6331. doi:10.1039/d0cc02498h |
| 36. | Reen, F. J.; Shanahan, R.; Cano, R.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2015, 13, 5537–5541. doi:10.1039/c5ob00315f |
| 52. | Szamosvári, D.; Böttcher, T. Angew. Chem., Int. Ed. 2017, 56, 7271–7275. doi:10.1002/anie.201702944 |
| 53. | Li, J.; Clark, B. R. J. Nat. Prod. 2020, 83, 3181–3190. doi:10.1021/acs.jnatprod.0c00865 |
| 54. | Reen, F. J.; Clarke, S. L.; Legendre, C.; McSweeney, C. M.; Eccles, K. S.; Lawrence, S. E.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2012, 10, 8903–8910. doi:10.1039/c2ob26823j |
| 55. | Szamosvári, D.; Sylvester, K.; Schmid, P.; Lu, K.-Y.; Derbyshire, E. R.; Böttcher, T. Chem. Commun. 2019, 55, 7009–7012. doi:10.1039/c9cc01689a |
| 3. | Bush, N. G.; Diez-Santos, I.; Abbott, L. R.; Maxwell, A. Molecules 2020, 25, 5662. doi:10.3390/molecules25235662 |
| 15. | Zhao, N.; Li, Z.-L.; Li, D.-H.; Sun, Y.-T.; Shan, D.-T.; Bai, J.; Pei, Y.-H.; Jing, Y.-K.; Hua, H.-M. Phytochemistry 2015, 109, 133–139. doi:10.1016/j.phytochem.2014.10.020 |
| 54. | Reen, F. J.; Clarke, S. L.; Legendre, C.; McSweeney, C. M.; Eccles, K. S.; Lawrence, S. E.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2012, 10, 8903–8910. doi:10.1039/c2ob26823j |
| 56. | Lu, C.; Maurer, C. K.; Kirsch, B.; Steinbach, A.; Hartmann, R. W. Angew. Chem., Int. Ed. 2014, 53, 1109–1112. doi:10.1002/anie.201307547 |
| 10. | Li, Y.-H.; He, J.; Li, Y.; Wu, X.-D.; Peng, L.-Y.; Du, R.-N.; Cheng, X.; Zhao, Q.-S.; Li, R.-T. Helv. Chim. Acta 2014, 97, 1481–1486. doi:10.1002/hlca.201300449 |
| 47. | Sajadikhah, S. S.; Lotfifar, N. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2018, 54, 587–589. doi:10.1007/s10593-018-2311-1 |
| 48. | Shen, C.; Wang, A.; Xu, J.; An, Z.; Loh, K. Y.; Zhang, P.; Liu, X. Chem 2019, 5, 1059–1107. doi:10.1016/j.chempr.2019.01.006 |
| 49. | Ghosh, P.; Das, S. Eur. J. Org. Chem. 2019, 4466–4516. doi:10.1002/ejoc.201900452 |
| 9. | Nakatsu, T.; Johns, T.; Kubo, I.; Milton, K.; Sakai, M.; Chatani, K.; Saito, K.; Yamagiwa, Y.; Kamikawa, T. J. Nat. Prod. 1990, 53, 1508–1513. doi:10.1021/np50072a017 |
| 13. | Rho, T. C.; Bae, E.-A.; Kim, D.-H.; Oh, W. K.; Kim, B. Y.; Ahn, J. S.; Lee, H. S. Biol. Pharm. Bull. 1999, 22, 1141–1143. doi:10.1248/bpb.22.1141 |
| 50. | Leichnitz, D.; Raguž, L.; Beemelmanns, C. Chem. Soc. Rev. 2017, 46, 6330–6344. doi:10.1039/c6cs00665e |
| 51. | Horák, R.; Kvapil, L.; Motyka, K.; Slaninová, L.; Grepl, M.; Kořistek, K.; Urbášek, M.; Hradil, P.; Soural, M. Tetrahedron 2018, 74, 366–374. doi:10.1016/j.tet.2017.12.010 |
| 8. | Kim, Y.-C.; Kim, N.-Y.; Jeong, S.-J.; Sohn, D.-H.; Miyamoto, T.; Higuchi, R. Planta Med. 1998, 64, 490. doi:10.1055/s-2006-957499 |
| 45. | Xie, C.; Yang, D.; Wang, X.; Ma, C. J. Org. Chem. 2020, 85, 14937–14944. doi:10.1021/acs.joc.0c01662 |
| 7. | El Sayed, K.; Al-Said, M. S.; El-Feraly, F. S.; Ross, S. A. J. Nat. Prod. 2000, 63, 995–997. doi:10.1021/np000012y |
| 11. | Akhmedzhanova, V. I.; Bessonova, I. A.; Yunusov, S. Y. Chem. Nat. Compd. 1986, 22, 78–79. doi:10.1007/bf00574586 |
| 46. | Khalifa, M. M.; Philkhana, S. C.; Golden, J. E. J. Org. Chem. 2020, 85, 464–481. doi:10.1021/acs.joc.9b02541 |
| 20. | Heeb, S.; Fletcher, M. P.; Chhabra, S. R.; Diggle, S. P.; Williams, P.; Cámara, M. FEMS Microbiol. Rev. 2011, 35, 247–274. doi:10.1111/j.1574-6976.2010.00247.x |
| 21. | Reen, F. J.; McGlacken, G. P.; O’Gara, F. FEMS Microbiol. Lett. 2018, 365, fny076. doi:10.1093/femsle/fny076 |
| 22. | Lin, J.; Cheng, J.; Wang, Y.; Shen, X. Front. Cell. Infect. Microbiol. 2018, 8, 230. doi:10.3389/fcimb.2018.00230 |
| 18. | Ji, K.-L.; Liu, W.; Yin, W.-H.; Li, J.-Y.; Yue, J.-M. Org. Biomol. Chem. 2022, 20, 4176–4182. doi:10.1039/d2ob00711h |
| 61. | Lohrer, B.; Bracher, F. Tetrahedron Lett. 2019, 60, 151327. doi:10.1016/j.tetlet.2019.151327 |
| 19. | Wahyuni, T. S.; Widyawaruyanti, A.; Lusida, M. I.; Fuad, A.; Soetjipto; Fuchino, H.; Kawahara, N.; Hayashi, Y.; Aoki, C.; Hotta, H. Fitoterapia 2014, 99, 276–283. doi:10.1016/j.fitote.2014.10.011 |
| 62. | Venkov, A. P.; Angelov, P. A. Synthesis 2003, 2221–2225. doi:10.1055/s-2003-41067 |
| 63. | Angelov, P.; Ivanova, S.; Yanev, P. Tetrahedron Lett. 2017, 58, 4776–4778. doi:10.1016/j.tetlet.2017.11.023 |
| 64. | Gama, F. H. S.; de Souza, R. O. M. A.; Garden, S. J. RSC Adv. 2015, 5, 70915–70928. doi:10.1039/c5ra14355a |
| 40. | Wang, J.-S.; Li, C.; Ying, J.; Xu, T.; Lu, W.; Li, C.-Y.; Wu, X.-F. J. Catal. 2022, 408, 81–87. doi:10.1016/j.jcat.2022.02.026 |
| 72. | Andrews, J. M. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. doi:10.1093/jac/48.suppl_1.5 |
| 41. | Lei, J.; Ding, Y.; Zhou, H.-Y.; Gao, X.-Y.; Cao, Y.-H.; Tang, D.-Y.; Li, H.-y.; Xu, Z.-G.; Chen, Z.-Z. Green Chem. 2022, 24, 5755–5759. doi:10.1039/d2gc01689c |
| 27. | Huse, H.; Whiteley, M. Chem. Rev. 2011, 111, 152–159. doi:10.1021/cr100063u |
| 36. | Reen, F. J.; Shanahan, R.; Cano, R.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2015, 13, 5537–5541. doi:10.1039/c5ob00315f |
| 37. | Ramos, A. F.; Woods, D. F.; Shanahan, R.; Cano, R.; McGlacken, G. P.; Serra, C.; O'Gara, F.; Reen, F. J. Microbiology (London, U. K.) 2020, 166, 169–179. doi:10.1099/mic.0.000876 |
| 38. | Hodgkinson, J. T.; Galloway, W. R. J. D.; Welch, M.; Spring, D. R. Nat. Protoc. 2012, 7, 1184–1192. doi:10.1038/nprot.2012.054 |
| 70. | Wells, I. C.; Elliott, W. H.; Thayer, S. A.; Doisy, E. A. J. Biol. Chem. 1952, 196, 321–330. doi:10.1016/s0021-9258(18)55736-7 |
| 39. | Dutta, L.; Ramasastry, S. S. V. Org. Lett. 2022, 24, 7665–7670. doi:10.1021/acs.orglett.2c03232 |
| 71. | Yamato, M.; Horiuchi, J.; Takeuchi, Y. Chem. Pharm. Bull. 1981, 29, 3124–3129. doi:10.1248/cpb.29.3124 |
| 29. | Geske, G. D.; O’Neill, J. C.; Blackwell, H. E. Chem. Soc. Rev. 2008, 37, 1432–1447. doi:10.1039/b703021p |
| 30. | Kamal, A. A. M.; Maurer, C. K.; Allegretta, G.; Haupenthal, J.; Empting, M.; Hartmann, R. W. Top. Med. Chem. 2017, 26, 185–210. doi:10.1007/7355_2017_17 |
| 31. | Schütz, C.; Empting, M. Beilstein J. Org. Chem. 2018, 14, 2627–2645. doi:10.3762/bjoc.14.241 |
| 32. | Ó Muimhneacháin, E.; Reen, F. J.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2018, 16, 169–179. doi:10.1039/c7ob02395b |
| 33. | Abd El-Aleam, R. H.; George, R. F.; Georgey, H. H.; Abdel-Rahman, H. M. RSC Adv. 2021, 11, 36459–36482. doi:10.1039/d1ra06238g |
| 25. | Long, R. A.; Qureshi, A.; Faulkner, D. J.; Azam, F. Appl. Environ. Microbiol. 2003, 69, 568–576. doi:10.1128/aem.69.1.568-576.2003 |
| 36. | Reen, F. J.; Shanahan, R.; Cano, R.; O'Gara, F.; McGlacken, G. P. Org. Biomol. Chem. 2015, 13, 5537–5541. doi:10.1039/c5ob00315f |
| 61. | Lohrer, B.; Bracher, F. Tetrahedron Lett. 2019, 60, 151327. doi:10.1016/j.tetlet.2019.151327 |
| 67. | Eidamshaus, C.; Triemer, T.; Reissig, H.-U. Synthesis 2011, 3261–3266. doi:10.1055/s-0030-1260198 |
| 68. | Thierbach, S.; Wienhold, M.; Fetzner, S.; Hennecke, U. Beilstein J. Org. Chem. 2019, 15, 187–193. doi:10.3762/bjoc.15.18 |
| 34. | Kim, W. J.; Kim, Y. O.; Kim, J. H.; Nam, B.-H.; Kim, D.-G.; An, C. M.; Lee, J. S.; Kim, P. S.; Lee, H. M.; Oh, J.-S.; Lee, J. S. Mar. Drugs 2016, 14, 24. doi:10.3390/md14010024 |
| 35. | Bultel-Poncé, V.; Berge, J.-P.; Debitus, C.; Nicolas, J.-L.; Guyot, M. Mar. Biotechnol. 1999, 1, 384–390. doi:10.1007/pl00011792 |
| 69. | Wratten, S. J.; Wolfe, M. S.; Andersen, R. J.; Faulkner, D. J. Antimicrob. Agents Chemother. 1977, 11, 411–414. doi:10.1128/aac.11.3.411 |
| 23. | Wu, Y.; Seyedsayamdost, M. R. Cell Chem. Biol. 2017, 24, 1437–1444.e3. doi:10.1016/j.chembiol.2017.08.024 |
| 24. | Piochon, M.; Coulon, P. M. L.; Caulet, A.; Groleau, M.-C.; Déziel, E.; Gauthier, C. J. Nat. Prod. 2020, 83, 2145–2154. doi:10.1021/acs.jnatprod.0c00171 |
| 25. | Long, R. A.; Qureshi, A.; Faulkner, D. J.; Azam, F. Appl. Environ. Microbiol. 2003, 69, 568–576. doi:10.1128/aem.69.1.568-576.2003 |
| 26. | Szamosvári, D.; Prothiwa, M.; Dieterich, C. L.; Böttcher, T. Chem. Commun. 2020, 56, 6328–6331. doi:10.1039/d0cc02498h |
| 65. | Angelov, P. Synlett 2010, 1273–1275. doi:10.1055/s-0029-1219836 |
| 66. | Yanev, P.; Angelov, P. Beilstein J. Org. Chem. 2018, 14, 2602–2606. doi:10.3762/bjoc.14.238 |
| 27. | Huse, H.; Whiteley, M. Chem. Rev. 2011, 111, 152–159. doi:10.1021/cr100063u |
| 28. | Diggle, S. P.; Matthijs, S.; Wright, V. J.; Fletcher, M. P.; Chhabra, S. R.; Lamont, I. L.; Kong, X.; Hider, R. C.; Cornelis, P.; Cámara, M.; Williams, P. Chem. Biol. 2007, 14, 87–96. doi:10.1016/j.chembiol.2006.11.014 |
| 62. | Venkov, A. P.; Angelov, P. A. Synthesis 2003, 2221–2225. doi:10.1055/s-2003-41067 |
© 2023 Mollova-Sapundzhieva et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.