Abstract
Peptidomimetics with a substituted imidazo[1,2-a]pyridine fragment were synthesized by a tandem of Groebke–Blackburn–Bienaymé and Ugi reactions. The target products contain substituted imidazo[1,2-a]pyridine and peptidomimetic moieties as pharmacophores with four diversity points introduced from readily available starting materials, including scaffold diversity. A small focused compound library of 20 Ugi products was prepared and screened for antibacterial activity.
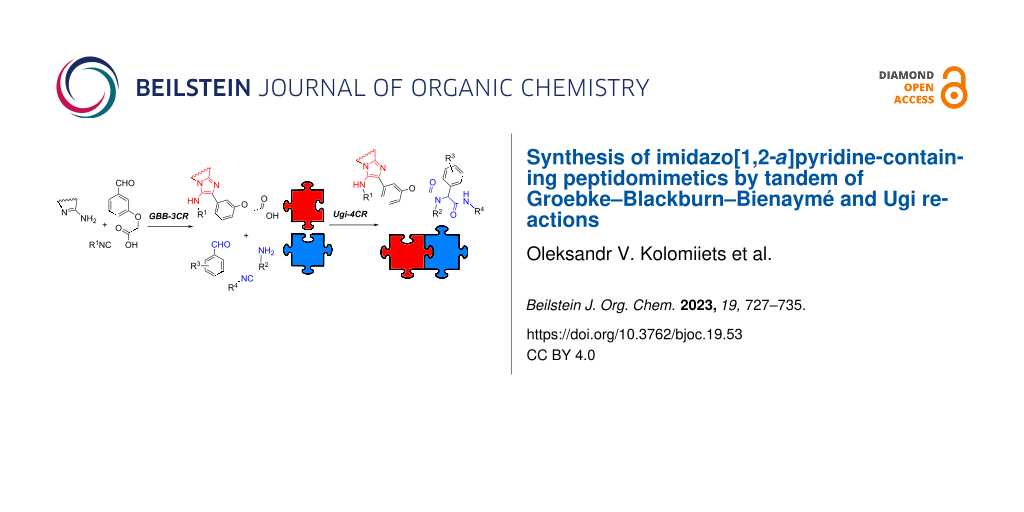
Graphical Abstract
Introduction
The use of isocyanide multicomponent reactions (IMCR) to prepare biologically active compounds is one of the most promising tools in modern organic and medicinal chemistry. Therefore, isocyanide multicomponent reactions are the way to prepare the necessary drugs "of tomorrow", and the possibility to combine such reactions gives a powerful method to rapidly and qualitatively expand the molecular diversity of biologically active compounds. The following possible combinations of IMCR have been described till today: Ugi and Ugi, azido-Ugi and Ugi, Ugi and Passerini, Groebke–Blackburn–Bienaymé (GBB-3CR) and Ugi, GBB-3CR and GBB-3CR, GBB-3CR and Passerini, Orru and Ugi, Orru and Passerini [1] (Scheme 1).
Scheme 1: Diversity of structures synthesized by combining IMCR’s.
Scheme 1: Diversity of structures synthesized by combining IMCR’s.
However, the issue of whether certain isocyanide multicomponent reactions can be combined is still unresolved, particularly due to the potential for varying the initial compounds involved.
Among all IMCRs, the Groebke–Blackburn–Bienaymé reaction is noteworthy [2-4]. The compounds obtained by this transformation demonstrate a wide range of biological activity, in particular structures containing a substituted imidazo[1,2-a]pyridine fragment, such as anticancer [5], antibacterial [6], antifungal [7], antiviral [8], anti-inflammatory [9], antimalarial [10], antiparkinsonian [11] and antituberculous activities [12]. In addition, some derivatives show enzyme inhibition [13] and can be used as potential drug candidate against Alzheimer's disease [14]. Compounds containing the imidazo[1,2-a]pyridine moiety are present in many natural products and marketed drugs, e.g., alpidem (an anxiolytic) [15], necopidem (anxiolytic) [16], zolpidem (hypnotic for the treatment of insomnia) [17], olprinon (cardiotonic agent for the treatment of acute heart failure) [18], GSK812397 (with anti-human immunodeficiency virus (HIV) properties) [19] (Figure 1).
Figure 1: Drugs possessing imidazo[1,2-a]pyridine unit.
Figure 1: Drugs possessing imidazo[1,2-a]pyridine unit.
In light of the numerous viral epidemics and even pandemics, antiviral drugs that can inhibit the activity of proteins and enzymes encoded by viruses, including influenza, SARS-CoV, and SARS-CoV-2, are becoming extremely important. For example, peptidomimetic structures such as lopinavir and ritonavir can inhibit 3CL protease, which is one of the major protein structures encoded by the SARS-CoV-2 genome [20]. Therefore, it is necessary to pay attention to another isocyanide multicomponent reaction – the Ugi reaction [21], which is one of the most important methods for the synthesis not only of peptidomimetics but also of other types of complex organic compounds. The presence of several diversity points and modification of starting components for a given reaction makes it possible to very quickly not only to insert important pharmacophoric fragments, but also to create a wide chemical space for the search for lead structures to solve problems of medicinal chemistry. Examples of drugs synthesized by MCR clearly demonstrate the immense advantages in this context, e.g., lidocaine [22], praziquantel [23,24], telaprevir [25], lacosamide [26], carfentanil [27], ivosidenib [28], and levetiracetam [29] (Figure 2). Epelsiban [30] and almorexant [31] are examples of agents currently or recently in clinical trials that have been synthesized using the MCR repertoire.
Therefore, an urgent problem is to synthesize structurally complex compounds, molecular hybrids containing pharmacophoric and peptidomimetic fragments, to explore their biological activity.
The possibility of inserting an unsubstituted imidazo[1,2-a]pyridine fragment into the peptidomimetic chain was already described in 2010 [32]. Thus, the corresponding acid components were synthesized with GBB-3CR and used in Ugi and Paserini reactions with various aldehydes, amines (for Ugi) and isocyanides. Moreover, in 2016 [33], an alternative route to use GBB-3CR products in Ugi reaction as carbonyl component was proposed, but these aldehydes did not have the structure of imidazo[1,2-a]pyridine. Interestingly, in 2019 [1], the synthesis of the amine component using GBB-3CR and the modification of the imidazo-pyrimidine scaffold by a peptidomimetic chain was carried out using the Ugi reaction (Scheme 2).
Scheme 2: Diversity of GBB reaction products as precursors for Ugi reaction.
Scheme 2: Diversity of GBB reaction products as precursors for Ugi reaction.
It is obvious that the exploration of the possibility to create hybrid molecules by combining GBB-3CR and Ugi is successful, so one of the promising areas is to increase the diversity of relevant compounds using substituted aminopyridines in GBB-3CR and the synthesis of new peptidomimetics.
Taking into account all these facts, we solved the task of developing a new approach for the synthesis of hybrid molecules containing substituted pharmacophoric heterocyclic and peptidomimetic fragments. An approach based on the preparation of an imidazo[1,2-a]pyridine-containing heterocyclic acid and its introduction as an acid component in the Ugi reaction was chosen. The route to the synthesis of the target molecules can be divided into two stages, with GBB-3CR in the first stage and the Ugi reaction in the second stage using the product of the first stage.
Results and Discussion
A model reaction between 2-amino-5-chloropyridine (1a), 2-(3-formylphenoxy)acetic acid (2) and tert-butyl isocyanide (3a) was chosen for the first step (Scheme 3). In one of the previous works [34], our group has shown that the optimal conditions for this type of reactions are stirring the starting materials for 24 hours at room temperature in DMF with catalytic amounts of HClO4. The yield of compound 4a in this case was 76%.
Scheme 3: Synthesis of new acids containing a substituted imidazo[1,2-a]pyridine fragment.
Scheme 3: Synthesis of new acids containing a substituted imidazo[1,2-a]pyridine fragment.
Then new heterocyclic acids were used as reagents for the Ugi four-component reaction. Due to the rather low solubility of the acids in methanol, it was necessary to increase the temperature and the reaction time. After a series of experiments, it was found that stirring the isocyanides 3a–d, heterocyclic acids 4a–c, aldehydes 5a–e, and primary amines 6a–d at 50 °C in methanol for 24–48 hours (depending on the nature of the starting materials) allowed obtaining the Ugi target products 7a–t in 28–72% yield (Scheme 4, Table 1).
Scheme 4: Synthesis of new peptidomimetics containing a substituted imidazo[1,2-a]pyridine fragment.
Scheme 4: Synthesis of new peptidomimetics containing a substituted imidazo[1,2-a]pyridine fragment.
Table 1: Library of peptidomimetics 7a–t containing a substituted imidazo[1,2-a]pyridine fragment.
| 7 | Amidine | R1 | R2 | R3 | R4 | Yield |
| a | 5-chloropyridine | t-Bu | 4-MeOPh | H | t-Bu | 63% |
| b | 5-chloropyridine | t-Bu | 4-MeOPh | 4-Cl | t-Bu | 71% |
| c | 5-chloropyridine | t-Bu | 4-MeOPh | 4-MeO | t-Bu | 61% |
| d | 5-chloropyridine | t-Bu | 4-MeOPh | methylenedioxy | t-Bu | 72% |
| e | 5-chloropyridine | t-Bu | 4-MeOPh | 4-CF3 | t-Bu | 45% |
| f | 5-chloropyridine | t-Bu | 4-MeOPh | 4-Cl | Cy | 51% |
| g | 5-chloropyridine | t-Bu | 4-MeOPh | 4-MeO | Cy | 56% |
| h | 5-chloropyridine | t-Bu | 4-MeOPh | methylenedioxy | Cy | 58% |
| i | 5-chloropyridine | t-Bu | 4-MeOPh | 4-CF3 | Cy | 62% |
| j | 5-chloropyridine | t-Bu | 4-MeOPh | 4-Cl | ethyloxycarbonyl | 61% |
| k | 5-chloropyridine | t-Bu | 4-MeOPh | 4-OMe | ethyloxycarbonyl | 40% |
| l | 5-chloropyridine | t-Bu | 4-MeOPh | methylenedioxy | ethyloxycarbonyl | 28% |
| m | 5-chloropyridine | t-Bu | 4-MeOPh | 4-Cl | o-NO2Bn | 53% |
| n | 5-chloropyridine | t-Bu | 4-MeOPh | 4-OMe | o-NO2Bn | 43% |
| o | 5-chloropyridine | t-Bu | Ph | 4-Cl | t-Bu | 63% |
| pa | 5-chloropyridine | t-Bu | 5-methylisoxazol-3-yl | 4-Cl | t-Bu | 30% |
| q | 5-chloropyridine | t-Bu | 5-methylisoxazol-3-yl | 4-OMe | t-Bu | 37% |
| r | 5-chloropyridine | Cy | 4-MeOPh | 4-Cl | t-Bu | 54% |
| s | 5-chloropyridine | Cy | 4-MeOPh | 4-MeO | t-Bu | 46% |
| t | 1,2,4-triazole | t-Bu | 4-MeOPh | 4-Cl | t-Bu | 30% |
aUgi 3-CR reaction – azomethine was inserted into the reaction.
Having obtained several new compounds containing substituted imidazo[1,2-a]pyridine and peptidomimetic fragments linked by a CH2O linker, we further explored the possibility of synthesizing similar hybrids without a CH2O linker between the two moieties. The heterocyclic acid 8a was obtained by a three-component reaction of 2-amino-5-chloropyridine (1a), 4-formylbenzoic acid (5f), and tert-butyl isocyanide (3a) according to the procedure described above (Scheme 3). In another step, we attempted to introduce the acid 8a into the Ugi four-component reaction with 4-chlorobenzaldehyde (5b), 4-methoxyaniline (6a) and tert-butyl isocyanide (3a). It should be especially noted that the solubility of compound 8a is very low (soluble in DMSO and N-methyl-2-pyrrolidone (NMP), slightly soluble in methanol, 1- and 2-propanol, acetone, DMF and insoluble in water, ethanol, acetonitrile, chloroform and some other typical solvents). Therefore, it was not surprising to us that the usual stirring of the starting materials in methanol at 50 °C was not successful for this multicomponent reaction. Increasing the temperature to 75 °C also did not give any positive results. Therefore, we tried to use DMSO, DMF and NMP as solvents for this Ugi treatment.
However, we found that the application of the solvent systems based on DMF and DMSO described in the literature [35,36] did not lead to the target compound 9a (Scheme 5, Table 2). Stirring at elevated temperatures or reaction in NMP also proved to be inefficient.
Scheme 5: Synthesis and reactivity of new acids containing a substituted imidazo[1,2-a]pyridine fragment without CH2O linker fragment.
Scheme 5: Synthesis and reactivity of new acids containing a substituted imidazo[1,2-a]pyridine fragment with...
In addition, we also tested the one-pot method for the synthesis of similar structures [32]. Aminopyridine, aldehyde and Sc(OTf)3 as catalyst were stirred in MeOH/DCM for 45 min at room temperature, then the isocyanide was added and the mixture was stirred for another 8 h. Without isolation, the corresponding aldehyde, amine and isocyanide were added to the resulting iminopyridine product and the mixture was then stirred at room temperature for 12 h. But again, the expected compounds could not be obtained.
Following this trend and based on published data, we synthesized heterocyclic acid 8b (Scheme 5) containing a carboxyl group at position 3 of the phenyl ring, which we introduced into the Ugi reaction with 4-methoxyaniline (6a), 4-chlorobenzaldehyde (5b), and tert-butyl isocyanide (3a) under various conditions (Table 2). In two cases (entries 1 and 6), the formation of the target reaction product was observed by 1H NMR spectroscopy of the crude reaction mixture. It was found that in the case of Table 2, entry 6, the ratio of product 9b to acid 8b was approximately 1:3.
Table 2: Screening conditions for synthesis of peptidomimetic 9а and 9b.
| Entry | Conditions | Ratio 8a/9aa | Ratio 8b/9ba |
| 1b | MeOH/DMF 1:2, 72 h, 75 °С | only 8a | 9:1 |
| 2b | MeOH, 72 h, 75 °С | only 8a | only 8b |
| 3 | DMF/DCM 1:2, 48 h, rt | only 8a | – |
| 4b | DMSO/DCM 1:2, 48 h, rt | only 8a | only 8b |
| 5 | NMP, 72 h, 75 °С | only 8a | – |
| 6b | DCM/MeOH 3:2, Sc(OTf)3 – 10 mol %, 48 h, rt (one pot) | only 8a | 3:1 |
aDetermined by 1H NMR; bconditions that were checked for the synthesis of both compounds.
The obtained results show that for the synthesis of new compounds containing precisely substituted imidazo[1,2-a]pyridine and peptidomimetic fragments it is better to use acids with a linker between the carboxyl group and aryl ring. This molecular fragment increases the solubility of the whole molecule, which leads to an improvement in the reactivity of the acids, whereas the reactivity of acids 8a and 8b is rather low in Ugi reactions.
Antibacterial activity
In the following experiment, we tested a specific group of compounds for their ability to act as antibacterial agents against Bacillus subtilis (strain 1211), Staphylococcus aureus (strain 2231) (Gram-positive), Escherichia coli (strain 1257), Pseudomonas aeruginosa (strain 1111) (Gram-negative). Compared to the reference substance, nitroxoline, the compounds generally showed lower levels of activity. However, some of the compounds demonstrated weak antimicrobial effects, as evidenced by their ability to inhibit the growth of the test microorganisms (see Table 3 for details).
Table 3: Antibacterial activity results.
| Entry | Compound | MICa/MBCb, mg/L | Strains of test cultures | |||
| Escherichia coli | Pseudomonas aeruginosa | Bacillus subtilis | Staphylococcus aureus | |||
| 1 | 7d | MIC | 500 | 500 | 500 | 500 |
| MBC | – | – | – | – | ||
| 2 | 7e | MIC | 500 | 500 | 500 | 500 |
| MBC | – | – | – | – | ||
| 3 | 7f | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 4 | 7g | MIC | – | – | 250 | – |
| MBC | – | – | – | – | ||
| 5 | 7i | MIC | 500 | –c | 500 | – |
| MBC | – | – | – | – | ||
| 6 | 7o | MIC | 500 | – | 125 | 250 |
| MBC | – | – | – | – | ||
| 7 | 7p | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 8 | 7q | MIC | 500 | 500 | 500 | 500 |
| MBC | – | – | – | – | ||
| 9 | 7t | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 10 | nitroxoline | MIC | 15.6 | 62.5 | 32.25 | 1.9 |
| MBC | 15.6 | 62.5 | 31.25 | 1.9 | ||
aMIC – minimum inhibitory concentration; bMBC – minimum bactericidal concentration; “–“ - the substance at concentration ≤ 500 mg/L does not inhibit culture growth; ccompounds improve the growth and development of bacteria compared to the control group.
Both Gram-positive and Gram-negative bacteria (specifically, strains of E. coli and B. subtilis) were equally inhibited in their growth. Compound 7o, in particular, was able to inhibit the growth of B. subtilis at a concentration of 125 mg/L. Bacteriostatic activity against E. coli was only observed at higher concentrations of 500 mg/L. This was also observed with the tested S. aureus strain. However, the Gram-negative bacterium P. aeruginosa showed resistance to a large number of synthesized compounds, with some compounds even promoting its growth and development at the given concentration range. Despite the low level of antibacterial activity observed with the synthesized heterocycles, this could be beneficial for screening these compounds for other types of activity, such as anticancer or antidiabetic effects, since it would minimize any negative impact on the organism's microflora.
Conclusion
To summarize, the study involved the combination of two multicomponent reactions – Groebke–Blackburn–Bienaymé and Ugi-type – to produce substituted 6-chloroimidazo[1,2-a]pyridin-2-ylphenoxy acetamides and 1H-imidazo[1,2-b][1,2,4]triazol-5-yl-phenoxy acetamide in a flexible manner. A method for the synthesis of the desired compounds was developed, and a small library of 20 Ugi products was prepared. The study also demonstrated that the use of a CH2O linker between the carboxyl group and the aryl ring in the Ugi reaction enhanced the reactivity of the acid component. The target compounds, which contained two pharmacophores – [1,2-a]pyridine (1H-imidazo[1,2-b][1,2,4]triazole) and peptidomimetic moieties – were evaluated for their antibacterial activity and exhibited a weak effect.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 6.8 MB | Download |
References
-
Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e
Return to citation in text: [1] [2] -
Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661–663. doi:10.1055/s-1998-1721
Return to citation in text: [1] -
Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. 1998, 37, 2234–2237. doi:10.1002/(sici)1521-3773(19980904)37:16<2234::aid-anie2234>3.0.co;2-r
Return to citation in text: [1] -
Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Tetrahedron Lett. 1998, 39, 3635–3638. doi:10.1016/s0040-4039(98)00653-4
Return to citation in text: [1] -
Perin, N.; Nhili, R.; Ester, K.; Laine, W.; Karminski-Zamola, G.; Kralj, M.; David-Cordonnier, M.-H.; Hranjec, M. Eur. J. Med. Chem. 2014, 80, 218–227. doi:10.1016/j.ejmech.2014.04.049
Return to citation in text: [1] -
Ramachandran, S.; Panda, M.; Mukherjee, K.; Choudhury, N. R.; Tantry, S. J.; Kedari, C. K.; Ramachandran, V.; Sharma, S.; Ramya, V. K.; Guptha, S.; Sambandamurthy, V. K. Bioorg. Med. Chem. Lett. 2013, 23, 4996–5001. doi:10.1016/j.bmcl.2013.06.043
Return to citation in text: [1] -
Takeshita, H.; Watanabe, J.; Kimura, Y.; Kawakami, K.; Takahashi, H.; Takemura, M.; Kitamura, A.; Someya, K.; Nakajima, R. Bioorg. Med. Chem. Lett. 2010, 20, 3893–3896. doi:10.1016/j.bmcl.2010.05.024
Return to citation in text: [1] -
Bode, M. L.; Gravestock, D.; Moleele, S. S.; van der Westhuyzen, C. W.; Pelly, S. C.; Steenkamp, P. A.; Hoppe, H. C.; Khan, T.; Nkabinde, L. A. Bioorg. Med. Chem. 2011, 19, 4227–4237. doi:10.1016/j.bmc.2011.05.062
Return to citation in text: [1] -
Lacerda, R. B.; de Lima, C. K. F.; da Silva, L. L.; Romeiro, N. C.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2009, 17, 74–84. doi:10.1016/j.bmc.2008.11.018
Return to citation in text: [1] -
Ndakala, A. J.; Gessner, R. K.; Gitari, P. W.; October, N.; White, K. L.; Hudson, A.; Fakorede, F.; Shackleford, D. M.; Kaiser, M.; Yeates, C.; Charman, S. A.; Chibale, K. J. Med. Chem. 2011, 54, 4581–4589. doi:10.1021/jm200227r
Return to citation in text: [1] -
McGuinness, B. F.; Cole, A. G.; Dong, G.; Brescia, M.-R.; Shao, Y.; Henderson, I.; Rokosz, L. L.; Stauffer, T. M.; Mannava, N.; Kimble, E. F.; Hicks, C.; White, N.; Wines, P. G.; Quadros, E. Bioorg. Med. Chem. Lett. 2010, 20, 6845–6849. doi:10.1016/j.bmcl.2010.08.064
Return to citation in text: [1] -
Sangani, C. B.; Jardosh, H. H.; Patel, M. P.; Patel, R. G. Med. Chem. Res. 2013, 22, 3035–3047. doi:10.1007/s00044-012-0322-5
Return to citation in text: [1] -
Hieke, M.; Rödl, C. B.; Wisniewska, J. M.; la Buscató, E.; Stark, H.; Schubert-Zsilavecz, M.; Steinhilber, D.; Hofmann, B.; Proschak, E. Bioorg. Med. Chem. Lett. 2012, 22, 1969–1975. doi:10.1016/j.bmcl.2012.01.038
Return to citation in text: [1] -
Zeng, F.; Southerland, J. A.; Voll, R. J.; Votaw, J. R.; Williams, L.; Ciliax, B. J.; Levey, A. I.; Goodman, M. M. Bioorg. Med. Chem. Lett. 2006, 16, 3015–3018. doi:10.1016/j.bmcl.2006.02.055
Return to citation in text: [1] -
Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Bioorg. Med. Chem. 2004, 12, 423–438. doi:10.1016/j.bmc.2003.10.050
Return to citation in text: [1] -
Jain, A. N. J. Med. Chem. 2004, 47, 947–961. doi:10.1021/jm030520f
Return to citation in text: [1] -
Hanson, S. M.; Morlock, E. V.; Satyshur, K. A.; Czajkowski, C. J. Med. Chem. 2008, 51, 7243–7252. doi:10.1021/jm800889m
Return to citation in text: [1] -
Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645
Return to citation in text: [1] -
Boggs, S.; Elitzin, V. I.; Gudmundsson, K.; Martin, M. T.; Sharp, M. J. Org. Process Res. Dev. 2009, 13, 781–785. doi:10.1021/op9000675
Return to citation in text: [1] -
Dömling, A.; Gao, L. Chem 2020, 6, 1283–1295. doi:10.1016/j.chempr.2020.04.023
Return to citation in text: [1] -
Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Angew. Chem. 1959, 71, 386. doi:10.1002/ange.19590711110
Return to citation in text: [1] -
Ugi, I.; Steinbrückner, C. Angew. Chem. 1960, 72, 267–268. doi:10.1002/ange.19600720709
Return to citation in text: [1] -
Dömling, A.; Khoury, K. ChemMedChem 2010, 5, 1420–1434. doi:10.1002/cmdc.201000202
Return to citation in text: [1] -
Cao, H.; Liu, H.; Dömling, A. Chem. – Eur. J. 2010, 16, 12296–12298. doi:10.1002/chem.201002046
Return to citation in text: [1] -
Znabet, A.; Polak, M. M.; Janssen, E.; de Kanter, F. J. J.; Turner, N. J.; Orru, R. V. A.; Ruijter, E. Chem. Commun. 2010, 46, 7918–7920. doi:10.1039/c0cc02823a
Return to citation in text: [1] -
Wehlan, H.; Oehme, J.; Schäfer, A.; Rossen, K. Org. Process Res. Dev. 2015, 19, 1980–1986. doi:10.1021/acs.oprd.5b00228
Return to citation in text: [1] -
Váradi, A.; Palmer, T. C.; Haselton, N.; Afonin, D.; Subrath, J. J.; Le Rouzic, V.; Hunkele, A.; Pasternak, G. W.; Marrone, G. F.; Borics, A.; Majumdar, S. ACS Chem. Neurosci. 2015, 6, 1570–1577. doi:10.1021/acschemneuro.5b00137
Return to citation in text: [1] -
Popovici-Muller, J.; Lemieux, R. M.; Artin, E.; Saunders, J. O.; Salituro, F. G.; Travins, J.; Cianchetta, G.; Cai, Z.; Zhou, D.; Cui, D.; Chen, P.; Straley, K.; Tobin, E.; Wang, F.; David, M. D.; Penard-Lacronique, V.; Quivoron, C.; Saada, V.; de Botton, S.; Gross, S.; Dang, L.; Yang, H.; Utley, L.; Chen, Y.; Kim, H.; Jin, S.; Gu, Z.; Yao, G.; Luo, Z.; Lv, X.; Fang, C.; Yan, L.; Olaharski, A.; Silverman, L.; Biller, S.; Su, S.-S. M.; Yen, K. ACS Med. Chem. Lett. 2018, 9, 300–305. doi:10.1021/acsmedchemlett.7b00421
Return to citation in text: [1] -
Cioc, R. C.; Schaepkens van Riempst, L.; Schuckman, P.; Ruijter, E.; Orru, R. V. A. Synthesis 2017, 49, 1664–1674. doi:10.1055/s-0036-1588672
Return to citation in text: [1] -
Borthwick, A. D.; Liddle, J. Retosiban and Epelsiban: Potent and Selective Orally Available Oxytocin Antagonists. Protein–Protein Interactions in Drug Discovery; Wiley-VCH: Weinheim, Germany, 2013; pp 225–256. doi:10.1002/9783527648207.ch10
Return to citation in text: [1] -
Aissaoui, H.; Cappi, M.; Clozel, M.; Fischli, W.; Koberstein, R. 1,2,3,4-Tetrahydroisoquinoline Derivatives. WO Patent WO2001068609A1, Sept 20, 2001.
Return to citation in text: [1] -
Al-Tel, T. H.; Al-Qawasmeh, R. A.; Voelter, W. Eur. J. Org. Chem. 2010, 5586–5593. doi:10.1002/ejoc.201000808
Return to citation in text: [1] [2] -
Kaur, T.; Gautam, R. N.; Sharma, A. Chem. – Asian J. 2016, 11, 2938–2945. doi:10.1002/asia.201601009
Return to citation in text: [1] -
Murlykina, M. V.; Kornet, M. N.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Brazhko, A. A.; Musatov, V. I.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2017, 13, 1050–1063. doi:10.3762/bjoc.13.104
Return to citation in text: [1] -
Söyler, Z.; Onwukamike, K. N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M. A. R. Green Chem. 2018, 20, 214–224. doi:10.1039/c7gc02577g
Return to citation in text: [1] -
Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126
Return to citation in text: [1]
| 31. | Aissaoui, H.; Cappi, M.; Clozel, M.; Fischli, W.; Koberstein, R. 1,2,3,4-Tetrahydroisoquinoline Derivatives. WO Patent WO2001068609A1, Sept 20, 2001. |
| 32. | Al-Tel, T. H.; Al-Qawasmeh, R. A.; Voelter, W. Eur. J. Org. Chem. 2010, 5586–5593. doi:10.1002/ejoc.201000808 |
| 33. | Kaur, T.; Gautam, R. N.; Sharma, A. Chem. – Asian J. 2016, 11, 2938–2945. doi:10.1002/asia.201601009 |
| 1. | Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e |
| 7. | Takeshita, H.; Watanabe, J.; Kimura, Y.; Kawakami, K.; Takahashi, H.; Takemura, M.; Kitamura, A.; Someya, K.; Nakajima, R. Bioorg. Med. Chem. Lett. 2010, 20, 3893–3896. doi:10.1016/j.bmcl.2010.05.024 |
| 17. | Hanson, S. M.; Morlock, E. V.; Satyshur, K. A.; Czajkowski, C. J. Med. Chem. 2008, 51, 7243–7252. doi:10.1021/jm800889m |
| 6. | Ramachandran, S.; Panda, M.; Mukherjee, K.; Choudhury, N. R.; Tantry, S. J.; Kedari, C. K.; Ramachandran, V.; Sharma, S.; Ramya, V. K.; Guptha, S.; Sambandamurthy, V. K. Bioorg. Med. Chem. Lett. 2013, 23, 4996–5001. doi:10.1016/j.bmcl.2013.06.043 |
| 18. | Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645 |
| 5. | Perin, N.; Nhili, R.; Ester, K.; Laine, W.; Karminski-Zamola, G.; Kralj, M.; David-Cordonnier, M.-H.; Hranjec, M. Eur. J. Med. Chem. 2014, 80, 218–227. doi:10.1016/j.ejmech.2014.04.049 |
| 15. | Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Bioorg. Med. Chem. 2004, 12, 423–438. doi:10.1016/j.bmc.2003.10.050 |
| 2. | Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661–663. doi:10.1055/s-1998-1721 |
| 3. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. 1998, 37, 2234–2237. doi:10.1002/(sici)1521-3773(19980904)37:16<2234::aid-anie2234>3.0.co;2-r |
| 4. | Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Tetrahedron Lett. 1998, 39, 3635–3638. doi:10.1016/s0040-4039(98)00653-4 |
| 11. | McGuinness, B. F.; Cole, A. G.; Dong, G.; Brescia, M.-R.; Shao, Y.; Henderson, I.; Rokosz, L. L.; Stauffer, T. M.; Mannava, N.; Kimble, E. F.; Hicks, C.; White, N.; Wines, P. G.; Quadros, E. Bioorg. Med. Chem. Lett. 2010, 20, 6845–6849. doi:10.1016/j.bmcl.2010.08.064 |
| 13. | Hieke, M.; Rödl, C. B.; Wisniewska, J. M.; la Buscató, E.; Stark, H.; Schubert-Zsilavecz, M.; Steinhilber, D.; Hofmann, B.; Proschak, E. Bioorg. Med. Chem. Lett. 2012, 22, 1969–1975. doi:10.1016/j.bmcl.2012.01.038 |
| 35. | Söyler, Z.; Onwukamike, K. N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M. A. R. Green Chem. 2018, 20, 214–224. doi:10.1039/c7gc02577g |
| 36. | Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126 |
| 10. | Ndakala, A. J.; Gessner, R. K.; Gitari, P. W.; October, N.; White, K. L.; Hudson, A.; Fakorede, F.; Shackleford, D. M.; Kaiser, M.; Yeates, C.; Charman, S. A.; Chibale, K. J. Med. Chem. 2011, 54, 4581–4589. doi:10.1021/jm200227r |
| 14. | Zeng, F.; Southerland, J. A.; Voll, R. J.; Votaw, J. R.; Williams, L.; Ciliax, B. J.; Levey, A. I.; Goodman, M. M. Bioorg. Med. Chem. Lett. 2006, 16, 3015–3018. doi:10.1016/j.bmcl.2006.02.055 |
| 32. | Al-Tel, T. H.; Al-Qawasmeh, R. A.; Voelter, W. Eur. J. Org. Chem. 2010, 5586–5593. doi:10.1002/ejoc.201000808 |
| 9. | Lacerda, R. B.; de Lima, C. K. F.; da Silva, L. L.; Romeiro, N. C.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2009, 17, 74–84. doi:10.1016/j.bmc.2008.11.018 |
| 1. | Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e |
| 8. | Bode, M. L.; Gravestock, D.; Moleele, S. S.; van der Westhuyzen, C. W.; Pelly, S. C.; Steenkamp, P. A.; Hoppe, H. C.; Khan, T.; Nkabinde, L. A. Bioorg. Med. Chem. 2011, 19, 4227–4237. doi:10.1016/j.bmc.2011.05.062 |
| 12. | Sangani, C. B.; Jardosh, H. H.; Patel, M. P.; Patel, R. G. Med. Chem. Res. 2013, 22, 3035–3047. doi:10.1007/s00044-012-0322-5 |
| 34. | Murlykina, M. V.; Kornet, M. N.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Brazhko, A. A.; Musatov, V. I.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2017, 13, 1050–1063. doi:10.3762/bjoc.13.104 |
| 21. | Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Angew. Chem. 1959, 71, 386. doi:10.1002/ange.19590711110 |
| 19. | Boggs, S.; Elitzin, V. I.; Gudmundsson, K.; Martin, M. T.; Sharp, M. J. Org. Process Res. Dev. 2009, 13, 781–785. doi:10.1021/op9000675 |
| 20. | Dömling, A.; Gao, L. Chem 2020, 6, 1283–1295. doi:10.1016/j.chempr.2020.04.023 |
| 29. | Cioc, R. C.; Schaepkens van Riempst, L.; Schuckman, P.; Ruijter, E.; Orru, R. V. A. Synthesis 2017, 49, 1664–1674. doi:10.1055/s-0036-1588672 |
| 30. | Borthwick, A. D.; Liddle, J. Retosiban and Epelsiban: Potent and Selective Orally Available Oxytocin Antagonists. Protein–Protein Interactions in Drug Discovery; Wiley-VCH: Weinheim, Germany, 2013; pp 225–256. doi:10.1002/9783527648207.ch10 |
| 27. | Váradi, A.; Palmer, T. C.; Haselton, N.; Afonin, D.; Subrath, J. J.; Le Rouzic, V.; Hunkele, A.; Pasternak, G. W.; Marrone, G. F.; Borics, A.; Majumdar, S. ACS Chem. Neurosci. 2015, 6, 1570–1577. doi:10.1021/acschemneuro.5b00137 |
| 28. | Popovici-Muller, J.; Lemieux, R. M.; Artin, E.; Saunders, J. O.; Salituro, F. G.; Travins, J.; Cianchetta, G.; Cai, Z.; Zhou, D.; Cui, D.; Chen, P.; Straley, K.; Tobin, E.; Wang, F.; David, M. D.; Penard-Lacronique, V.; Quivoron, C.; Saada, V.; de Botton, S.; Gross, S.; Dang, L.; Yang, H.; Utley, L.; Chen, Y.; Kim, H.; Jin, S.; Gu, Z.; Yao, G.; Luo, Z.; Lv, X.; Fang, C.; Yan, L.; Olaharski, A.; Silverman, L.; Biller, S.; Su, S.-S. M.; Yen, K. ACS Med. Chem. Lett. 2018, 9, 300–305. doi:10.1021/acsmedchemlett.7b00421 |
| 25. | Znabet, A.; Polak, M. M.; Janssen, E.; de Kanter, F. J. J.; Turner, N. J.; Orru, R. V. A.; Ruijter, E. Chem. Commun. 2010, 46, 7918–7920. doi:10.1039/c0cc02823a |
| 26. | Wehlan, H.; Oehme, J.; Schäfer, A.; Rossen, K. Org. Process Res. Dev. 2015, 19, 1980–1986. doi:10.1021/acs.oprd.5b00228 |
| 22. | Ugi, I.; Steinbrückner, C. Angew. Chem. 1960, 72, 267–268. doi:10.1002/ange.19600720709 |
| 23. | Dömling, A.; Khoury, K. ChemMedChem 2010, 5, 1420–1434. doi:10.1002/cmdc.201000202 |
| 24. | Cao, H.; Liu, H.; Dömling, A. Chem. – Eur. J. 2010, 16, 12296–12298. doi:10.1002/chem.201002046 |
© 2023 Kolomiiets et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.