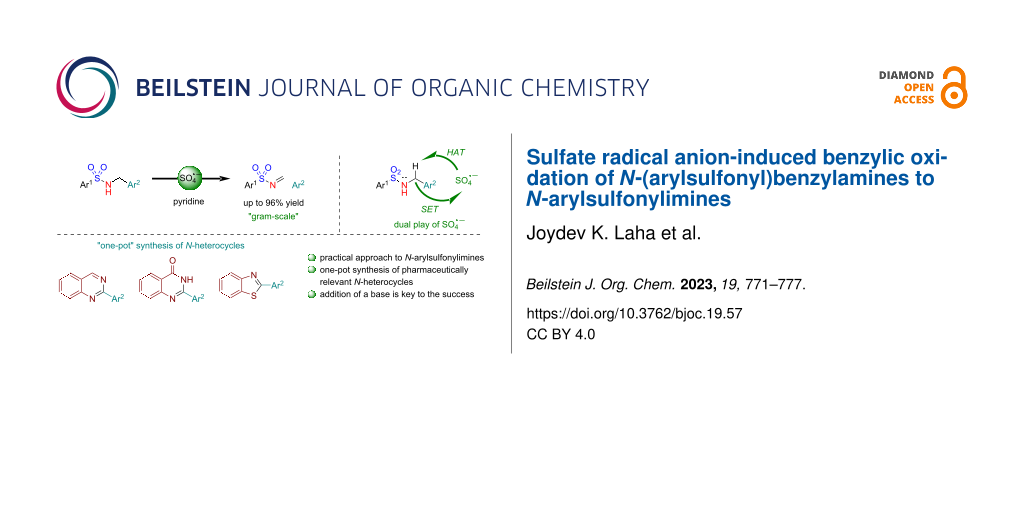
Abstract
A mild, operationally convenient, and practical method for the synthesis of synthetically useful N-arylsulfonylimines from N-(arylsulfonyl)benzylamines using K2S2O8 in the presence of pyridine as a base is reported herein. In addition, a “one-pot” tandem synthesis of pharmaceutically relevant N-heterocycles by the reaction of N-arylsulfonylimines, generated in situ with ortho-substituted anilines is also reported. The key features of the protocol include the use of a green oxidant, a short reaction time (30 min), chromatography-free isolation, scalability, and economical, delivering N-arylsulfonylimines in excellent yields of up to 96%. While the oxidation of N-aryl(benzyl)amines to N-arylimines using K2S2O8 is reported to be problematic, the oxidation of N-(arylsulfonyl)benzylamines to N-arylsulfonylimines using K2S2O8 has been achieved for the first time. The dual role of the sulfate radical anion (SO4·−), including hydrogen atom abstraction (HAT) and single electron transfer (SET), is proposed to be involved in the plausible reaction mechanism.
Graphical Abstract
Introduction
Among various imine compounds [1], N-arylsulfonylimines are perhaps the most prominent due to their unique stability, defined reactivity, and versatility in organic synthesis [2]. Leveraging their electron-deficient nature, N-arylsulfonylimines are widely used in organic transformations including nucleophilic addition, cycloaddition, imino-aldol reaction, ene reactions, aza-Friedel–Crafts reactions, and C–H functionalizations ([3] and references therein), leading to the synthesis of diverse nitrogen heterocycles of pharmaceutical relevance [4]. The traditional synthetic method for the preparation of N-arylsulfonylimines, similar to the preparation of N-arylimines, is based on the condensation of aromatic aldehydes and sulfonamides (Scheme 1a) [3,5-8]. Because of the poor nucleophilicity of sulfonamides, the condensation reactions generally require harsh reaction conditions involving the use of strong acids, elevated temperature, and metal catalysts. Other methods include a non-dehydrative reaction of aldehydes with isocyanate analogs ([3] and references therein) (Scheme 1b) and an oxidative reaction of primary benzylic alcohols with sulfonamides or chloramine-T ([3] and references therein), and although they are elegant, they use substrates that are not readily accessible or toxic in nature. To overcome these limitations, oxidation of N-(arylsulfonyl)benzylamines to N-arylsulfonylimines, as opposed to the traditional methods, under mild and neutral reaction conditions has been reported, although limited to a few methods. However, these methods of oxidation involving the use of CrO2 [9], PhI(OAc)2/I2 [10], TEMPO [11], NHPI [12], and metal catalysts [13], suffer from serious limitations including the use of metal catalysts, high temperature, risk of explosive hazards, production of large waste, and often low yield (Scheme 1c). Thus, an environmentally benign method that could deliver N-arylsulfonylimines under mild reaction conditions is highly desirable.
Scheme 1: Various synthetic approaches to N-arylsulfonylimines.
Scheme 1: Various synthetic approaches to N-arylsulfonylimines.
Previously, we reported a tandem oxidative intramolecular cyclization of N-aryl(benzyl)amines, having an internal nucleophile substituted at the ortho-position in the aniline ring, to nitrogen heterocycles using potassium persulfate (K2S2O8) as the exclusive reagent [14]. The mechanistic study revealed that an initial oxidation to an iminium ion could be the key intermediate in the intramolecular cyclization step. In sharp contrast, when N-aryl(benzyl)amines that do not have an ortho-substituted nucleophile in aniline ring were used as the substrates in this reaction, N-arylimines were not isolated. Rather, an amide, in some cases, was isolated via oxidation of the benzylic methylene to a carbonyl group [14]. In the quest of a new method for the synthesis of N-arylsulfonylimines, we questioned ourselves whether N-(arylsulfonyl)benzylamines would behave similarly as N-aryl(benzyl)amines under K2S2O8-mediated oxidative conditions and could provide a platform for the synthesis of N-arylsulfonylimines.
To this endeavor, we have developed a method for the synthesis of N-arylsulfonylimines from N-(arylsulfonyl)benzylamines using K2S2O8 in the presence of pyridine as a base. The key findings include a) requirement of a mild base for the formation N-arylsulfonylimines, and b) stability of N-arylsulfonylimines, unlike N-arylimines, under the oxidative conditions. Further, to demonstrate the scope and applicability of this approach, a gram-scale synthesis and a “one-pot” tandem synthesis of pharmaceutically relevant N-heterocycles by the reaction of in situ-generated N-arylsulfonylimines with various ortho-substituted anilines were also developed. The mechanism of the oxidation is believed to occur via hydrogen atom abstraction (HAT) followed by single electron transfer (SET) enabled by the sulfate radical anion (SO4·−).
Results and Discussion
Initially, we investigated the reaction of N-benzenesulfonyl(benzyl)amine (1a) as a model substrate with K2S2O8 in MeCN at 80 °C for 12 h, conditions that were used earlier in our previous study [14]. Unfortunately, no product formation was observed under these conditions, while substrate 1a remained unreacted (Table 1, entry 1). When the solvent was changed to H2O, a trace quantity of product formation was observed (Table 1, entry 2). To our surprise, when 2 equiv of pyridine were used as an additive along with the oxidant K2S2O8 in MeCN, the desired product N-benzenesulfonylimine 2a was obtained in 90% yield (Table 1, entry 3). Subsequently, we carried out further optimization studies by changing the additive, solvent, temperature, and reaction time to obtain the best possible yield of the product 2a (Table 1). Interestingly, when duration of the reaction was reduced to 1 h, product 2a was obtained in 96% yield with complete conversion of substrate 1a (Table 1, entry 4). Further shortening the reaction time to 30 min resulted in the formation of 2a also in 96% yield (Table 1, entry 5). Lowering the temperature to 60 °C had a deleterious effect (Table 1, entry 6). Likewise, reducing the stoichiometry of pyridine to 1 equiv proved detrimental (Table 1, entry 7). Replacing pyridine with other organic and inorganic bases such as Et3N, DBU, DABCO or K2CO3 also gave product 2a, however, in varying yields (Table 1, entries 8–11). While replacing the solvent MeCN with DCE delivered 2a in 89% yield, and a dramatic reduction in the yield of 2a was observed when H2O was used as the solvent (Table 1, entries 12 and 13). Therefore, the conditions listed in entry 5 of Table 1 were chosen as the best conditions for further evaluating the substrate scope. Unlike the oxidation of N-aryl(benzyl)amines to N-arylimines using K2S2O8 in the presence or absence of a base is unsuccessful, the oxidation of N-arylsulfonyl(benzyl)amines 1a to imines 2a was achieved under the optimized conditions. Distinctly, the use of a base is the key to success in this oxidation. Perhaps more importantly, the stability of N-benzenesulfonylimine 2a, unlike N-arylimines, under the oxidative conditions is noteworthy.
Table 1: Optimization of reaction conditions.a
|
|
|||||
| Entry | Additive (equiv) | Solvent | Temp. (°C) | Time (h) | Yield (%)b |
| 1 | – | MeCN | 80 | 12 | n.d. |
| 2 | – | H2O | 80 | 12 | trace |
| 3 | pyridine (2) | MeCN | 80 | 12 | 90 |
| 4 | pyridine (2) | MeCN | 80 | 1 | 96 |
| 5 | pyridine (2) | MeCN | 80 | 0.5 | 96 |
| 6 | pyridine (2) | MeCN | 60 | 1 | 40 |
| 7 | pyridine (1) | MeCN | 80 | 1 | 80 |
| 8 | Et3N (2) | MeCN | 80 | 1 | 60 |
| 9 | DBU (2) | MeCN | 80 | 1 | 92 |
| 10 | DABCO (2) | MeCN | 80 | 1 | 90 |
| 11 | K2CO3 (2) | MeCN | 80 | 1 | 75 |
| 12 | pyridine (2) | DCE | 80 | 1 | 89 |
| 13 | pyridine (2) | H2O | 80 | 1 | 20 |
aReaction conditions: 1a (0.25 mmol), K2S2O8 (0.5 mmol), additive (0.5 mmol) in solvent (1 mL) at 80 °C for the specified period of time. n.d. = not detected. bIsolated yield.
With the optimized reaction conditions in hand (Table 1, entry 5), we further investigated the substrate scope for the above transformation (Scheme 2). A limited variety of N-(arylsulfonyl)benzylamines 1a–m carrying substitutions on the aromatic rings was examined. Firstly, N-(arylsulfonyl)benzylamines having substitution(s) on one or both rings delivered the N-arylsulfonylimines 2a–h in 80–96% yield. The presence of a disubstitution in 1i gave product 2i in 78% yield. Replacing phenyl with naphthyl in N-(arylsulfonyl)benzylamines 1j and 1k resulted in the formation of N-arylsulfonylimines 2j and 2k also in very good yield (82–84%). Interestingly, when the arylsulfonyl group was replaced by methylsulfonyl, as in substrate 1l, the desired N-sulfonylimine 2l was obtained in 90% yield under the optimized reaction conditions. However, an attempted synthesis of N-arylsulfonylketimines was unsuccessful. Thus, N-(arylsulfonyl)benzylamine 1m having a phenyl substituent at the benzylic position gave benzophenone in 80% yield with a trace of N-benzenesulfonylketimine 2m under the optimized reaction conditions. Likewise, N-(arylsulfonyl)benzylamine 1n having a methyl group present at the benzylic position gave product 2n only in a trace quantity. To demonstrate further the scalability of the developed protocol, we carried out a gram-scale synthesis of 2a from 1a under the optimized reaction conditions. A complete conversion of substrate 1a was observed within 2 h under the optimized reaction conditions giving the product with an isolated yield of 92%.
Scheme 2: Substrate scope for the synthesis of N-arylsulfonylimines. Reaction conditions: 1a (0.25 mmol), K2S2O8 (0.5 mmol), pyridine (0.5 mmol) in MeCN (1 mL) at 80 °C for 0.5 h. Yields refer to isolated compounds. aGram-scale synthesis (1a, 5 mmol).
Scheme 2: Substrate scope for the synthesis of N-arylsulfonylimines. Reaction conditions: 1a (0.25 mmol), K2S2...
Furthermore, to demonstrate the synthetic utility of the developed protocol, a tandem “one-pot” synthesis of N-heterocycles was successfully executed (Scheme 3). Thus, exposition of substrates 1 under the optimized reaction conditions followed by the addition of ortho-substituted anilines 3 and K2S2O8 (1 equiv) and heating the reaction mixture at 80 °C for 2 h furnished the desired N-heterocycles 4. Thus, treatment of substrate 1a under the standard conditions, followed by reaction of the intermediate N-benzenesulfonylimine 2a with 2-aminobenzamide in one-pot gave 2-phenylquinazolin-4(3H)-one (4a) in 86% yield. Similarly, the reaction of the intermediate product 2c and 2-aminobenzamide gave 2-(p-tolyl)quinazolin-4(3H)-one (4b) in 85% yield.
Scheme 3: Tandem “one-pot” synthesis of N-heterocycles. Reaction conditions: 1a (0.25 mmol), K2S2O8 (0.5 mmol), and pyridine (0.5 mmol) in MeCN (1mL) at 80 °C for 0.5 h followed by the addition of 1 equiv of K2S2O8 and the corresponding ortho-substituted anilines 3 (1.2 equiv) and stirring at 80 °C for 2 h. Yields correspond to isolated products.
Scheme 3: Tandem “one-pot” synthesis of N-heterocycles. Reaction conditions: 1a (0.25 mmol), K2S2O8 (0.5 mmol...
Furthermore, when various other ortho-substituted aniline derivatives such as 2-aminobenzylamine, 2-aminothiophenol, and o-phenylenediamine are reacted with imine 2a in a similar manner, the corresponding N-heterocycles 4c–f were obtained in good to moderate yield. However, the reaction with 2-aminophenol did not give the corresponding cyclized product 4g. This could be possibly due to the poor nucleophilicity of the ortho-OH group in 2-aminophenol thereby restricting the intramolecular nucleophilic addition and as a result the corresponding cyclized product is not formed. The synthesis of these nitrogen heterocycles signifies the innate ability of in situ-generated N-arylsulfonylimines in a variety of reactions with various ortho-substituted anilines without the need for pre-isolation or purification.
Next, in order to determine whether the reaction proceeds via a radical pathway, we performed a control experiment. When substrate 1a was treated with the radical scavenger TEMPO under the optimized reaction conditions, the formation of product 2a was completely suppressed (Scheme 4). This confirms that the reaction proceeds via a radical pathway.
Based on the literature [15,16], our previous experience [14,17,18], and current understanding, a plausible mechanism for the benzylic oxidation is depicted in Scheme 5. Initially, a sulfate radical anion (SO4·−) is generated by homolytic cleavage of the peroxy linkage under heating conditions [17]. The hydrogen atom is abstracted from the benzylic position of 1 by SO4·−, generating benzylic radical 1aa [14-16]. A single electron transfer (SET) could subsequently occur from 1aa to form the reactive species 1ab. Finally, the base abstracts the activated NH proton to produce imine 2. The dual role of SO4·− involving HAT and SET is proposed in this plausible mechanism, which requires further investigation.
Scheme 5: Plausible mechanism for the K2S2O8-induced oxidation of N-(arylsulfonyl)benzylamines.
Scheme 5: Plausible mechanism for the K2S2O8-induced oxidation of N-(arylsulfonyl)benzylamines.
Similarly, a plausible mechanism for the one-pot synthesis of N-heterocycles is shown in Scheme 6. Initially, the N-arylsulfonylimine 2, generated in situ from the corresponding N-(arylsulfonyl)benzylamine 1, undergoes transimination with the ortho-substituted aniline 3 to form imine 3ab via 3aa. Subsequent intramolecular nucleophilic addition in imine 3ab produces intermediate 3ac, which upon oxidation delivers the desired N-heterocycle 4.
Scheme 6: Plausible mechanism for one-pot synthesis of N-heterocycles.
Scheme 6: Plausible mechanism for one-pot synthesis of N-heterocycles.
Conclusion
In conclusion, we have developed a complementary approach to the currently available methods for the oxidation of N-(arylsulfonyl)benzylamines to N-arylsulfonylimines using K2S2O8 and pyridine as a base. While N-arylimines are difficult to prepare by the oxidation of N-aryl(benzyl)amines using K2S2O8, N-arylsulfonylimines are successfully prepared and are quite stable under the oxidative conditions. In addition, we demonstrated a “one-pot” tandem synthesis of pharmaceutically relevant N-heterocycles through the reaction of in situ-generated N-arylsulfonylimines with ortho-substituted anilines. The key features including the use of a green oxidant, a short reaction time, chromatography-free isolation, and scalability mark a distinction from the contemporary methods. Although we propose a dual role for SO4·− involving both hydrogen atom abstraction (HAT) and single electron transfer (SET), further investigation of the mechanism would enrich our understanding of persulfate-mediated oxidative reactions.
Supporting Information
| Supporting Information File 1: General procedures, product characterization, and copies of 1H NMR and 13C NMR spectra of all compounds. | ||
| Format: PDF | Size: 5.6 MB | Download |
References
-
Belowich, M. E.; Stoddart, J. F. Chem. Soc. Rev. 2012, 41, 2003–2024. doi:10.1039/c2cs15305j
Return to citation in text: [1] -
Weinreb, S. M. Top. Curr. Chem. 1997, 190, 131–184. doi:10.1007/bfb0119248
Return to citation in text: [1] -
Hopkins, M. D.; Scott, K. A.; DeMier, B. C.; Morgan, H. R.; Macgruder, J. A.; Lamar, A. A. Org. Biomol. Chem. 2017, 15, 9209–9216. doi:10.1039/c7ob02120h
Return to citation in text: [1] [2] [3] [4] -
Laha, J. K.; Satyanarayana Tummalapalli, K. S.; Jethava, K. P. Org. Biomol. Chem. 2016, 14, 2473–2479. doi:10.1039/c5ob02670a
Return to citation in text: [1] -
Jennings, W. B.; Lovely, C. J. Tetrahedron 1991, 47, 5561–5568. doi:10.1016/s0040-4020(01)80987-x
Return to citation in text: [1] -
Verrier, C.; Carret, S.; Poisson, J.-F. ACS Sustainable Chem. Eng. 2018, 6, 8563–8569. doi:10.1021/acssuschemeng.8b00864
Return to citation in text: [1] -
Boger, D. L.; Corbett, W. L. J. Org. Chem. 1992, 57, 4777–4780. doi:10.1021/jo00043a047
Return to citation in text: [1] -
Liu, Y.; Lin, L.; Han, Y.; Liu, Y. Chin. J. Org. Chem. 2020, 40, 4216. doi:10.6023/cjoc202004053
and references cited therein.
Return to citation in text: [1] -
Smith, E. M. Preparation of sulfonyl imine compounds. Australian Pat. Appl. AU7206601A, Feb 5, 2002.
Return to citation in text: [1] -
Fan, R.; Pu, D.; Wen, F.; Wu, J. J. Org. Chem. 2007, 72, 8994–8997. doi:10.1021/jo7016982
Return to citation in text: [1] -
Moriyama, K.; Kuramochi, M.; Fujii, K.; Morita, T.; Togo, H. Angew. Chem. 2016, 128, 14766–14771. doi:10.1002/ange.201607223
Return to citation in text: [1] -
Wang, J.; Yi, W.-J. Molecules 2019, 24, 3771. doi:10.3390/molecules24203771
Return to citation in text: [1] -
Wang, J.-R.; Fu, Y.; Zhang, B.-B.; Cui, X.; Liu, L.; Guo, Q.-X. Tetrahedron Lett. 2006, 47, 8293–8297. doi:10.1016/j.tetlet.2006.09.088
Return to citation in text: [1] -
Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872
Return to citation in text: [1] [2] [3] [4] [5] -
Ueda, M.; Kamikawa, K.; Fukuyama, T.; Wang, Y.-T.; Wu, Y.-K.; Ryu, I. Angew. Chem. 2021, 133, 3587–3592. doi:10.1002/ange.202011992
Return to citation in text: [1] [2] -
Zhang, H.; Wang, S.; Wang, X.; Wang, P.; Yi, H.; Zhang, H.; Lei, A. Green Chem. 2022, 24, 147–151. doi:10.1039/d1gc03896f
Return to citation in text: [1] [2] -
Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085–5144. doi:10.1021/acscatal.8b00743
Return to citation in text: [1] [2] -
Gupta, P.; Laha, J. K. Development of Synthetic Approaches to Biaryl Sultams via C–H Functionalization. Handbook of CH-Functionalization; Wiley-VCH: Weinheim, Germany, 2002; pp 1–24. doi:10.1002/9783527834242.chf0117
Return to citation in text: [1]
| 14. | Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872 |
| 15. | Ueda, M.; Kamikawa, K.; Fukuyama, T.; Wang, Y.-T.; Wu, Y.-K.; Ryu, I. Angew. Chem. 2021, 133, 3587–3592. doi:10.1002/ange.202011992 |
| 16. | Zhang, H.; Wang, S.; Wang, X.; Wang, P.; Yi, H.; Zhang, H.; Lei, A. Green Chem. 2022, 24, 147–151. doi:10.1039/d1gc03896f |
| 14. | Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872 |
| 17. | Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085–5144. doi:10.1021/acscatal.8b00743 |
| 18. | Gupta, P.; Laha, J. K. Development of Synthetic Approaches to Biaryl Sultams via C–H Functionalization. Handbook of CH-Functionalization; Wiley-VCH: Weinheim, Germany, 2002; pp 1–24. doi:10.1002/9783527834242.chf0117 |
| 17. | Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085–5144. doi:10.1021/acscatal.8b00743 |
| 1. | Belowich, M. E.; Stoddart, J. F. Chem. Soc. Rev. 2012, 41, 2003–2024. doi:10.1039/c2cs15305j |
| 3. | Hopkins, M. D.; Scott, K. A.; DeMier, B. C.; Morgan, H. R.; Macgruder, J. A.; Lamar, A. A. Org. Biomol. Chem. 2017, 15, 9209–9216. doi:10.1039/c7ob02120h |
| 5. | Jennings, W. B.; Lovely, C. J. Tetrahedron 1991, 47, 5561–5568. doi:10.1016/s0040-4020(01)80987-x |
| 6. | Verrier, C.; Carret, S.; Poisson, J.-F. ACS Sustainable Chem. Eng. 2018, 6, 8563–8569. doi:10.1021/acssuschemeng.8b00864 |
| 7. | Boger, D. L.; Corbett, W. L. J. Org. Chem. 1992, 57, 4777–4780. doi:10.1021/jo00043a047 |
| 8. |
Liu, Y.; Lin, L.; Han, Y.; Liu, Y. Chin. J. Org. Chem. 2020, 40, 4216. doi:10.6023/cjoc202004053
and references cited therein. |
| 14. | Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872 |
| 4. | Laha, J. K.; Satyanarayana Tummalapalli, K. S.; Jethava, K. P. Org. Biomol. Chem. 2016, 14, 2473–2479. doi:10.1039/c5ob02670a |
| 15. | Ueda, M.; Kamikawa, K.; Fukuyama, T.; Wang, Y.-T.; Wu, Y.-K.; Ryu, I. Angew. Chem. 2021, 133, 3587–3592. doi:10.1002/ange.202011992 |
| 16. | Zhang, H.; Wang, S.; Wang, X.; Wang, P.; Yi, H.; Zhang, H.; Lei, A. Green Chem. 2022, 24, 147–151. doi:10.1039/d1gc03896f |
| 3. | Hopkins, M. D.; Scott, K. A.; DeMier, B. C.; Morgan, H. R.; Macgruder, J. A.; Lamar, A. A. Org. Biomol. Chem. 2017, 15, 9209–9216. doi:10.1039/c7ob02120h |
| 14. | Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872 |
| 14. | Laha, J. K.; Tummalapalli, K. S. S.; Nair, A.; Patel, N. J. Org. Chem. 2015, 80, 11351–11359. doi:10.1021/acs.joc.5b01872 |
| 10. | Fan, R.; Pu, D.; Wen, F.; Wu, J. J. Org. Chem. 2007, 72, 8994–8997. doi:10.1021/jo7016982 |
| 9. | Smith, E. M. Preparation of sulfonyl imine compounds. Australian Pat. Appl. AU7206601A, Feb 5, 2002. |
| 13. | Wang, J.-R.; Fu, Y.; Zhang, B.-B.; Cui, X.; Liu, L.; Guo, Q.-X. Tetrahedron Lett. 2006, 47, 8293–8297. doi:10.1016/j.tetlet.2006.09.088 |
| 3. | Hopkins, M. D.; Scott, K. A.; DeMier, B. C.; Morgan, H. R.; Macgruder, J. A.; Lamar, A. A. Org. Biomol. Chem. 2017, 15, 9209–9216. doi:10.1039/c7ob02120h |
| 3. | Hopkins, M. D.; Scott, K. A.; DeMier, B. C.; Morgan, H. R.; Macgruder, J. A.; Lamar, A. A. Org. Biomol. Chem. 2017, 15, 9209–9216. doi:10.1039/c7ob02120h |
| 11. | Moriyama, K.; Kuramochi, M.; Fujii, K.; Morita, T.; Togo, H. Angew. Chem. 2016, 128, 14766–14771. doi:10.1002/ange.201607223 |
© 2023 Laha et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.