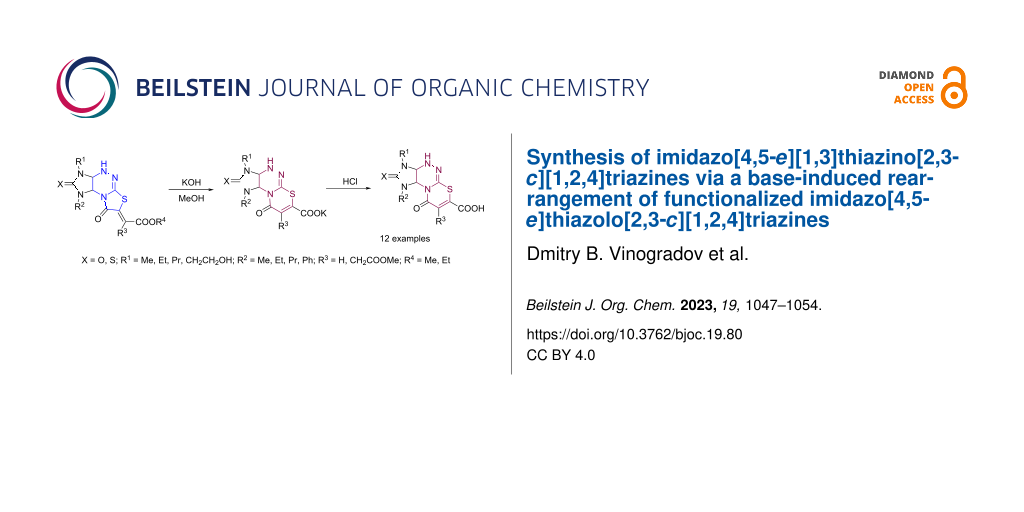
Abstract
A series of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines was synthesized via a cascade sequence of hydrolysis and skeletal rearrangement of imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazin-7(8H)-ylidene)acetic acid esters in methanol upon treatment with excess KOH. Imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazin-6(7H)-ylidene)acetic acid esters are also suitable substrates for the reaction. In this case hydrolysis and thiazole ring expansion were accompanied with the change of the thiazolotriazine junction type from thiazolo[3,2-b][1,2,4]triazine to thiazino[2,3-c][1,2,4]triazine.
Graphical Abstract
Introduction
Nitrogen- and sulfur-containing heterocyclic compounds are widely represented in nature and used for the synthesis of biologically active substances. Among the 1,3-thiazine derivatives, promising compounds as antimicrobial and antiviral drugs (PD404182) [1-4], sedative [5] and antitumor agents [6-8], as well as fungicides [9,10] and insecticides [11] have been found (Figure 1). The 1,3-thiazine heterocyclic system is comprised in some natural phytoalexins (cyclobrassinin, sinalbins A and B, rutalexin, and others) [12] and 7-aminocephalosporanic acid (7-ACA), which is a key fragment of broad-spectrum cephalosporin antibiotics [13,14].
Figure 1: Examples of natural and synthetic bioactive 1,3-thiazine and imidazothiazolotriazine derivatives with high antiproliferative activity.
Figure 1: Examples of natural and synthetic bioactive 1,3-thiazine and imidazothiazolotriazine derivatives wi...
Condensed 1,2,4-triazines attract attention of researchers due to their diverse biological activities [15] and also their application as starting materials for the constructing of new heterocyclic systems [16,17]. Recent studies of the antitumor activity of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazines revealed a number of compounds with a high antiproliferative effect towards a large number of human cancer cell lines (Figure 1) [18,19]. Therefore, the synthesis of new imidazothiazolotriazines and closely related hybrid compounds including fragments of 1,3-thiazine and imidazo-1,2,4-triazine is still highly relevant.
Earlier we have demonstrated that imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines and their derivatives functionalized at position 6 are capable of undergoing skeletal rearrangements and transformations of the heterocyclic system proceeding by an ANRORC mechanism under the action of KOH in methanol. Thus, 6-oxindolylideneimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines are transformed into substituted 2-oxoquinoline-4-carboxylates in the presence of excess KOH [20] while their 6-arylmethylidene derivatives undergo rearrangement into the corresponding isomeric derivatives of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine [18,21] (Scheme 1A).
Scheme 1: Base-induced transformations and rearrangements of functionalized imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine derivatives into new heterocyclic systems.
Scheme 1: Base-induced transformations and rearrangements of functionalized imidazo[4,5-e]thiazolo[3,2-b]-1,2...
In the present study, we report a new base-induced recyclization of functionalized imidazothiazolotriazines 1 and 2 resulting in derivatives of the new heterocyclic system, namely imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 3 (Scheme 1B).
Results and Discussion
In a continuation of our studies [22] aimed at the synthesis of functionalized imidazothiazolotriazine derivatives, we attempted to hydrolyze the ester group of imidazothiazolotriazines 1 and to prepare the corresponding carboxylic acids 4 using an aqueous KOH solution. Heating esters 1a,b in an aqueous solution of KOH and subsequent addition of hydrochloric acid led to the corresponding acids 4a,b as the main products. Acids 4a,b were isolated from the mixtures in 17 and 38% yield, respectively. However, we also detected by 1H NMR spectroscopy the formation of other minor products, presumably derivatives of a new heterocyclic system, imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 5a,b (Scheme 2).
Scheme 2: Alkaline hydrolysis of esters 1a,b. aDetermined by 1H NMR spectroscopy; bisolated yields.
Scheme 2: Alkaline hydrolysis of esters 1a,b. aDetermined by 1H NMR spectroscopy; bisolated yields.
To prepare the new compounds 5, the solvent, amount of KOH, reaction time, and temperature were varied. Increasing the amount of KOH and reaction time led to an increase in the yield of the potassium salts 3a,b even at room temperature. It was found that stirring esters 1a–i in methanol in the presence of 2.5 equivalents of an aqueous solution of KOH provided selective formation of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 3a–i as a result of ester group hydrolysis and thiazolidine ring expansion to the corresponding thiazine (Scheme 3). The potassium salts 3a,b were isolated in 81 and 63% yield, respectively. Compounds 3c–i were used in further transformations without isolation.
Scheme 3: Synthesis of potassium imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylates.
Scheme 3: Synthesis of potassium imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylates.
1H NMR reaction monitoring showed that compound 1d under conditions of excess of KOH in methanol undergoes alkaline hydrolysis along with transesterification of the ester group to give the ring-opened form 6d (Scheme 4), the maximum concentration of which was observed approximately 30 minutes after the start of the reaction. After an hour, the signals of the starting imidazothiazolotriazine 1d disappeared, and after 2–4 hours of reaction, only signals of the target product 3d were observed in the 1H NMR spectrum (Figure 2).
Scheme 4: Plausible rearrangement mechanism of imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine 1d into imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine 3d.
Scheme 4: Plausible rearrangement mechanism of imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine 1d into imidazo[4...
![[1860-5397-19-80-2]](/bjoc/content/figures/1860-5397-19-80-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: 1H NMR spectra of the starting compound 1d (a) and the reaction mixture after 1.5 (b) and 4 (c) hours in DMSO-d6 (the colored signals correspond to the protons shown in red in Scheme 4).
Figure 2: 1H NMR spectra of the starting compound 1d (a) and the reaction mixture after 1.5 (b) and 4 (c) hou...
Compounds 3a–d,j were prepared from imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines 2a-d,j of linear structure under similar conditions (Scheme 5). The isolated yield of the potassium salt 3b (65%) corresponded to the yield of the product obtained from the isomeric structure 1b of the angular type (63%), while the yield of compound 3a (67%) in the analogous reaction was inferior to that of the transformation of structure 1a (81%). Salt 3j was isolated in 44% yield. The absence of signals of the starting or intermediate compounds in the 1H NMR spectrum of the reaction mixture (for 3c,d) also indicates the complete conversion of esters 2c,d to the potassium salts 3c,d after 4 hours of reaction.
Scheme 5: Synthetic approaches to imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 3a–d,j.
Scheme 5: Synthetic approaches to imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 3a–d,j.
A one-pot method for the synthesis of 1,3-dimethylimidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine 3a was exemplified by successive reactions of imidazo[4,5-e][1,2,4]triazine 7 with diethyl acetylenedicarboxylate and excess KOH.
Acidification of aqueous solutions of potassium salts 3a,b,j or the reaction masses containing potassium salts 3c–i in methanol (obtained from 1c–i) with hydrochloric acid led to the formation of the corresponding 1,3-dialkyl-2,9-dioxoimidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5a–j in 47–96% yields (Scheme 6).
Scheme 6: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5a–j.
Scheme 6: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5a–j.
The developed method is also applicable to substrates 8a–c [23] substituted in the methylidene fragment. Thus, compounds 8a–c were converted into the corresponding potassium salts 3k–m under the same conditions (MeOH, room temperature, 1–24 h) (Scheme 7). However, acidification of aqueous solutions of the salts 3k–m with excess hydrochloric acid and further evaporation of the solvent at 40 °C led to decomposition products, two of which were isolated and characterized by NMR spectroscopy including 2D experiments and HRMS data. The target acids 5k,m were obtained using equivalent amounts of HCl at room temperature. however, acid 5l underwent partial transformations even under these conditions and was not isolated as individual substance.
Scheme 7: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5k,m.
Scheme 7: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5k,m.
We assumed the following mechanism for the formation of products 9 (Scheme 8). Redistribution of the electron density in the acid molecule 5 after protonation of the carbonyl group in the thiazine ring leads to the cleavage of the triazine C–N bond. Further proton transfer gives product 9.
Scheme 8: Plausible path for the formation of products 9.
Scheme 8: Plausible path for the formation of products 9.
The structures of the synthesized compounds 3a,b,j and 5a–k,m were confirmed by IR, 1H and 13C NMR spectroscopy, and high-resolution mass spectrometry. the potassium salts 3c–i,k,m were characterized by their 1H and 13C NMR spectra.
In the 1H NMR spectra, the doublets of the bridging hydrogen atom C(9a)H in compound 4 and C(10a)H in compound 5 are characteristic signals which allow to attribute the synthesized compounds to one of the two heterocyclic systems, i.e., imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine and imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine. Thus, the signals of the corresponding protons for isomeric acids 4a and 5a appeared at δ 5.59 and 6.23 ppm, respectively, that is obviously due to a deshielding effect of the carbonyl group of the products 4a and 5a as well as its closer location in structures 5 (Figure 3).
Figure 3: 1H NMR spectra of compounds 4a and 5a in DMSO-d6 in the region of 4.3–9.0 ppm.
Figure 3: 1H NMR spectra of compounds 4a and 5a in DMSO-d6 in the region of 4.3–9.0 ppm.
In the downfield region of the 13C NMR spectra registered without proton decoupling for isomeric acids 4a and 5a, the carbon atom doublets of the carboxyl groups, carbonyl groups of thiazole (for 4a) or thiazine (for 5a) cycles, as well as multiplets of carbonyl groups of the urea fragment are observed (Figure 4). Values of spin–spin interaction constants 3JCH equal to 5.3–6.0 Hz indicate the cis-orientation of the vinyl proton and the carbonyl (for 4a, blue) or the carboxyl group (for 5a, red) relative to the double bond [24-26]. The values of spin–spin interaction constants of other doublets (2JCH = 1.3–1.5 Hz) indicate the position of the carboxyl (for 4a, red) or carbonyl (for 5a, blue) groups through two bonds relative to the olefinic proton.
Figure 4: 13C NMR GATED spectra of compounds 4a and 5a in DMSO-d6 in the region of 156.0–168.0 ppm.
Figure 4: 13C NMR GATED spectra of compounds 4a and 5a in DMSO-d6 in the region of 156.0–168.0 ppm.
The structure of compound 5a was additionally confirmed by X-ray structural analysis data (Figure 5).
![[1860-5397-19-80-5]](/bjoc/content/figures/1860-5397-19-80-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: General view of 5a in the crystal in thermal ellipsoid representation (p = 80%).
Figure 5: General view of 5a in the crystal in thermal ellipsoid representation (p = 80%).
Conclusion
In summary, routes for the selective formation of various derivatives of the new heterocyclic system, imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine, were found during the cascade processes of alkaline hydrolysis of the ester group in functionalized derivatives of imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine or imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine and the expansion of the thiazolidine ring to a thiazine core. The methodology proved to be effective for the preparation of a series of target compounds with different substituents in the tricyclic fragment.
References
-
Birck, M. R.; Holler, T. P.; Woodard, R. W. J. Am. Chem. Soc. 2000, 122, 9334–9335. doi:10.1021/ja002142z
Return to citation in text: [1] -
Chockalingam, K.; Simeon, R. L.; Rice, C. M.; Chen, Z. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 3764–3769. doi:10.1073/pnas.0915117107
Return to citation in text: [1] -
Chamoun, A. M.; Chockalingam, K.; Bobardt, M.; Simeon, R.; Chang, J.; Gallay, P.; Chen, Z. Antimicrob. Agents Chemother. 2012, 56, 672–681. doi:10.1128/aac.05722-11
Return to citation in text: [1] -
Mizuhara, T.; Oishi, S.; Ohno, H.; Shimura, K.; Matsuoka, M.; Fujii, N. Bioorg. Med. Chem. 2012, 20, 6434–6441. doi:10.1016/j.bmc.2012.08.030
Return to citation in text: [1] -
Lombardino, J. G.; McLamore, W. M.; Lanbach, G. D. Derivatives of thiabenzopyrrocoline, thiabenzopyridocoline and thiazepine. U.S. Patent US2985649, May 23, 1961.
Return to citation in text: [1] -
Elkot, H. A.; Ragab, I.; Saleh, N. M.; Amin, M. N.; Al-Rashood, S. T.; El-Messery, S. M.; Hassan, G. S. Chem.-Biol. Interact. 2021, 344, 109530. doi:10.1016/j.cbi.2021.109530
Return to citation in text: [1] -
Fan, X.; Deng, J.; Shi, T.; Wen, H.; Li, J.; Liang, Z.; Lei, F.; Liu, D.; Zhang, H.; Liang, Y.; Hao, X.; Wang, Z. Bioorg. Chem. 2021, 114, 105154. doi:10.1016/j.bioorg.2021.105154
Return to citation in text: [1] -
Ferreira, M.; Assunção, L. S.; Filippin-Monteiro, F. B.; Creczynski-Pasa, T. B.; Sá, M. M. Eur. J. Med. Chem. 2013, 70, 411–418. doi:10.1016/j.ejmech.2013.10.017
Return to citation in text: [1] -
Vicentini, C. B.; Forlani, G.; Manfrini, M.; Romagnoli, C.; Mares, D. J. Agric. Food Chem. 2002, 50, 4839–4845. doi:10.1021/jf0202436
Return to citation in text: [1] -
Mares, D.; Romagnoli, C.; Andreotti, E.; Forlani, G.; Guccione, S.; Vicentini, C. B. Mycol. Res. 2006, 110, 686–696. doi:10.1016/j.mycres.2006.03.006
Return to citation in text: [1] -
Melnikov, N. N.; Grapov, A. F.; Razvodovskaya, L. V.; Englin, V. M.; Negrebetskii, V. V.; Bogelfer, L. Y.; Ivanova, G. B.; Orlova, V. I.; Zhuravleva, V. I. Phosphoryl 2-alkylaminodihydro-1,3-tiazines possessing insecticide activity. SU Pat. Appl. SU1157831A1, Dec 30, 1994.
Return to citation in text: [1] -
Pedras, M. S. C.; Abdoli, A. RSC Adv. 2017, 7, 23633–23646. doi:10.1039/c7ra01574g
Return to citation in text: [1] -
Prescott, J. F. Beta-lactam Antibiotics: Cephalosporins. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S.; Prescott, J. F.; Dowling, P. M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp 153–173. doi:10.1002/9781118675014.ch9
Return to citation in text: [1] -
Lima, L. M.; da Silva, B. N. M.; Barbosa, G.; Barreiro, E. J. Eur. J. Med. Chem. 2020, 208, 112829. doi:10.1016/j.ejmech.2020.112829
Return to citation in text: [1] -
Rusinov, V. L.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull. 2018, 67, 573–599. doi:10.1007/s11172-018-2113-8
Return to citation in text: [1] -
El Ashry, E. S. H.; Rashed, N.; Taha, M.; Ramadan, E. Adv. Heterocycl. Chem. 1994, 59, 39–177. doi:10.1016/s0065-2725(08)60007-0
Return to citation in text: [1] -
Gazieva, G. A.; Karpova, T. B.; Nechaeva, T. V.; Kravchenko, A. N. Russ. Chem. Bull. 2016, 65, 2172–2182. doi:10.1007/s11172-016-1565-y
Return to citation in text: [1] -
Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271–12285. doi:10.1039/d1nj02163j
Return to citation in text: [1] [2] -
Izmest’ev, A. N.; Anikina, L. V.; Zanin, I. E.; Kolotyrkina, N. G.; Izmalkova, E. S.; Kravchenko, A. N.; Gazieva, G. A. New J. Chem. 2022, 46, 11632–11647. doi:10.1039/d2nj01454h
Return to citation in text: [1] -
Izmest'ev, A. N.; Kravchenko, A. N.; Gazieva, G. A. Org. Biomol. Chem. 2023, 21, 1827–1834. doi:10.1039/d2ob02242g
Return to citation in text: [1] -
Izmest'ev, A. N.; Kim, N. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N.; Gazieva, G. A. ChemistrySelect 2019, 4, 10483–10487. doi:10.1002/slct.201902461
Return to citation in text: [1] -
Izmest’ev, A. N.; Vinogradov, D. B.; Kolotyrkina, N. G.; Kravchenko, A. N.; Gazieva, G. A. Beilstein J. Org. Chem. 2021, 17, 1141–1148. doi:10.3762/bjoc.17.87
Return to citation in text: [1] -
Izmest'ev, A. N.; Motornov, V. A.; Vinogradov, D. B.; Ioffe, S. L.; Kravchenko, A. N.; Gazieva, G. A. Org. Chem. Front. 2022, 9, 4998–5004. doi:10.1039/d2qo00911k
Return to citation in text: [1] -
Vögeli, U.; von Philipsborn, W.; Nagarajan, K.; Nair, M. D. Helv. Chim. Acta 1978, 61, 607–617. doi:10.1002/hlca.19780610207
Return to citation in text: [1] -
Kingsbury, C. A.; Draney, D.; Sopchik, A.; Rissler, W.; Durham, D. J. Org. Chem. 1976, 41, 3863–3868. doi:10.1021/jo00886a020
Return to citation in text: [1] -
Belskaya, N. P.; Lugovik, K. I.; Ivina, A. D.; Bakulev, V. A.; Fan, Z. J. Chem. Heterocycl. Compd. 2014, 50, 888–900. doi:10.1007/s10593-014-1543-y
Return to citation in text: [1]
| 1. | Birck, M. R.; Holler, T. P.; Woodard, R. W. J. Am. Chem. Soc. 2000, 122, 9334–9335. doi:10.1021/ja002142z |
| 2. | Chockalingam, K.; Simeon, R. L.; Rice, C. M.; Chen, Z. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 3764–3769. doi:10.1073/pnas.0915117107 |
| 3. | Chamoun, A. M.; Chockalingam, K.; Bobardt, M.; Simeon, R.; Chang, J.; Gallay, P.; Chen, Z. Antimicrob. Agents Chemother. 2012, 56, 672–681. doi:10.1128/aac.05722-11 |
| 4. | Mizuhara, T.; Oishi, S.; Ohno, H.; Shimura, K.; Matsuoka, M.; Fujii, N. Bioorg. Med. Chem. 2012, 20, 6434–6441. doi:10.1016/j.bmc.2012.08.030 |
| 11. | Melnikov, N. N.; Grapov, A. F.; Razvodovskaya, L. V.; Englin, V. M.; Negrebetskii, V. V.; Bogelfer, L. Y.; Ivanova, G. B.; Orlova, V. I.; Zhuravleva, V. I. Phosphoryl 2-alkylaminodihydro-1,3-tiazines possessing insecticide activity. SU Pat. Appl. SU1157831A1, Dec 30, 1994. |
| 24. | Vögeli, U.; von Philipsborn, W.; Nagarajan, K.; Nair, M. D. Helv. Chim. Acta 1978, 61, 607–617. doi:10.1002/hlca.19780610207 |
| 25. | Kingsbury, C. A.; Draney, D.; Sopchik, A.; Rissler, W.; Durham, D. J. Org. Chem. 1976, 41, 3863–3868. doi:10.1021/jo00886a020 |
| 26. | Belskaya, N. P.; Lugovik, K. I.; Ivina, A. D.; Bakulev, V. A.; Fan, Z. J. Chem. Heterocycl. Compd. 2014, 50, 888–900. doi:10.1007/s10593-014-1543-y |
| 9. | Vicentini, C. B.; Forlani, G.; Manfrini, M.; Romagnoli, C.; Mares, D. J. Agric. Food Chem. 2002, 50, 4839–4845. doi:10.1021/jf0202436 |
| 10. | Mares, D.; Romagnoli, C.; Andreotti, E.; Forlani, G.; Guccione, S.; Vicentini, C. B. Mycol. Res. 2006, 110, 686–696. doi:10.1016/j.mycres.2006.03.006 |
| 6. | Elkot, H. A.; Ragab, I.; Saleh, N. M.; Amin, M. N.; Al-Rashood, S. T.; El-Messery, S. M.; Hassan, G. S. Chem.-Biol. Interact. 2021, 344, 109530. doi:10.1016/j.cbi.2021.109530 |
| 7. | Fan, X.; Deng, J.; Shi, T.; Wen, H.; Li, J.; Liang, Z.; Lei, F.; Liu, D.; Zhang, H.; Liang, Y.; Hao, X.; Wang, Z. Bioorg. Chem. 2021, 114, 105154. doi:10.1016/j.bioorg.2021.105154 |
| 8. | Ferreira, M.; Assunção, L. S.; Filippin-Monteiro, F. B.; Creczynski-Pasa, T. B.; Sá, M. M. Eur. J. Med. Chem. 2013, 70, 411–418. doi:10.1016/j.ejmech.2013.10.017 |
| 22. | Izmest’ev, A. N.; Vinogradov, D. B.; Kolotyrkina, N. G.; Kravchenko, A. N.; Gazieva, G. A. Beilstein J. Org. Chem. 2021, 17, 1141–1148. doi:10.3762/bjoc.17.87 |
| 5. | Lombardino, J. G.; McLamore, W. M.; Lanbach, G. D. Derivatives of thiabenzopyrrocoline, thiabenzopyridocoline and thiazepine. U.S. Patent US2985649, May 23, 1961. |
| 23. | Izmest'ev, A. N.; Motornov, V. A.; Vinogradov, D. B.; Ioffe, S. L.; Kravchenko, A. N.; Gazieva, G. A. Org. Chem. Front. 2022, 9, 4998–5004. doi:10.1039/d2qo00911k |
| 16. | El Ashry, E. S. H.; Rashed, N.; Taha, M.; Ramadan, E. Adv. Heterocycl. Chem. 1994, 59, 39–177. doi:10.1016/s0065-2725(08)60007-0 |
| 17. | Gazieva, G. A.; Karpova, T. B.; Nechaeva, T. V.; Kravchenko, A. N. Russ. Chem. Bull. 2016, 65, 2172–2182. doi:10.1007/s11172-016-1565-y |
| 20. | Izmest'ev, A. N.; Kravchenko, A. N.; Gazieva, G. A. Org. Biomol. Chem. 2023, 21, 1827–1834. doi:10.1039/d2ob02242g |
| 15. | Rusinov, V. L.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull. 2018, 67, 573–599. doi:10.1007/s11172-018-2113-8 |
| 18. | Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271–12285. doi:10.1039/d1nj02163j |
| 21. | Izmest'ev, A. N.; Kim, N. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N.; Gazieva, G. A. ChemistrySelect 2019, 4, 10483–10487. doi:10.1002/slct.201902461 |
| 13. | Prescott, J. F. Beta-lactam Antibiotics: Cephalosporins. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S.; Prescott, J. F.; Dowling, P. M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp 153–173. doi:10.1002/9781118675014.ch9 |
| 14. | Lima, L. M.; da Silva, B. N. M.; Barbosa, G.; Barreiro, E. J. Eur. J. Med. Chem. 2020, 208, 112829. doi:10.1016/j.ejmech.2020.112829 |
| 12. | Pedras, M. S. C.; Abdoli, A. RSC Adv. 2017, 7, 23633–23646. doi:10.1039/c7ra01574g |
| 18. | Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271–12285. doi:10.1039/d1nj02163j |
| 19. | Izmest’ev, A. N.; Anikina, L. V.; Zanin, I. E.; Kolotyrkina, N. G.; Izmalkova, E. S.; Kravchenko, A. N.; Gazieva, G. A. New J. Chem. 2022, 46, 11632–11647. doi:10.1039/d2nj01454h |
© 2023 Vinogradov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.