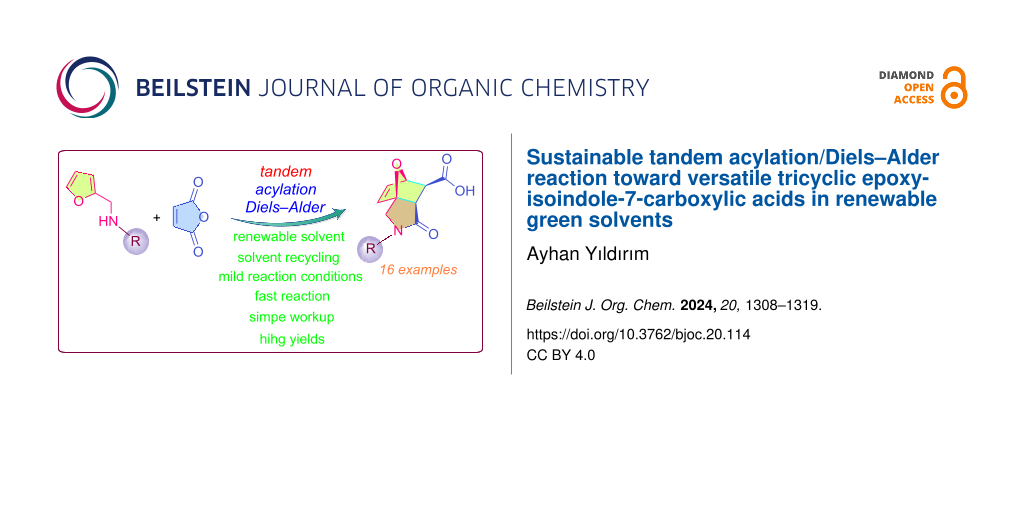
Abstract
Tandem Diels–Alder reactions are often used for the straightforward formation of complex natural compounds and the fused polycyclic systems contained in their precursors. In the second step of this reaction, regio- and stereochemically controlled intramolecular cyclization leads to the formation of versatile nitrogen-containing tricyclic systems. However, these useful organic transformations are usually carried out in highly toxic organic solvents such as benzene, toluene, chloroform, etc. Despite recent efforts by 'green chemists', synthetic chemists still use these traditional toxic organic solvents in many of their reactions, even though safer alternatives are available. However, in addition to the harmful effects of these petrochemical solvents on the environment, the prediction that their resources will run out in the near future has led 'green chemists' to explore solvents that can be derived from renewable resources and used effectively in various organic transformations. In this context, we have shown for the first time that the 100% atom-economical tandem Diels–Alder reaction between aminofuranes and maleic anhydride can be carried out successfully in vegetable oils and waxes. The reaction was successfully carried out in sunflower seed oil, olive oil, oleic acid and lauryl myristate under mild reaction conditions. A series of epoxyisoindole-7-carboxylic acid and bisepoxyisoindole-7-carboxylic acids were obtained in good yields after a practical isolation procedure. The results obtained in this study demonstrate the potential of vegetable oils and their renewable materials to provide a reaction medium that is more sustainable than conventional organic solvents in cascade Diels–Alder reactions and can be used repeatedly without significant degradation. These materials also allow the reaction to be completed in less time, with less energy consumption and higher yields.
Graphical Abstract
Introduction
For many years, fossil fuels have provided the chemicals needed by many industries, particularly the chemical and pharmaceutical industries [1]. It is well known that the processing of these resources and the steps involved in converting them into various chemical compounds are among the main sources of serious environmental problems [2]. However, the risk of depletion of these resources in the near future has led researchers to research and develop safer, environmentally friendly and renewable resources [3-5]. Biomass is an important and more economical renewable source of hydrocarbons and a variety of intermediates that are needed by a wide range of industries [6-14].
Worldwide, vegetable oils are mainly produced for food and feed purposes, but a small number of specific oils are also produced as a source of materials for industrial applications [15,16]. These oils are of increasing interest for the production of a wide range of polymeric materials [17-23], drug delivery systems [24-29], less toxic anticancer drugs [30-33] and intermediates suitable for various organic transformations [14,34-38]. In addition to their unique properties, vegetable oils are known to be useful as green solvents in many applications [39-42]. The evaluation of vegetable oils as alternative solvents in organic synthesis is very limited [43-47].
Synthetic chemists are still trying to make the conditions for Diels–Alder reactions more environmentally friendly [48-58]. However, the number and variety of substrates in these studies is often limited, deep eutectic solvents are required and it is difficult to achieve both satisfactory product yields and the desired stereoselectivity [59-61]. The viscous nature of the aminofuranes usually requires the use of toxic solvents such as toluene, and in some studies, solvents such as DMSO, which are difficult to remove from the reaction medium [62-64]. Although solvent effects are of little importance in intramolecular Diels–Alder reactions, the effects of solvents such as glycerol, polyethylene glycol, organic carbonates, deep eutectic solvents, supercritical CO2 and H2O have recently been extensively studied [65-67]. Recently, attention has also been drawn to photoinduced oxidative [4 + 2] annulation reactions [68-70].
This study was the first to evaluate the performances of sunflower seed oil (SSO), olive oil (OO), oleic acid (OA) and lauryl myristate (LM) as green solvents in the well-known intramolecular Diels–Alder furan (IMDAF) reaction between aminofuranes and maleic anhydride. This is a useful approach for the total synthesis of complex natural compounds, epoxyisoindole skeleton-based precursors for various polycyclic compounds, and bio-based homo- and copolymers [71-98]. Therefore, in the present study, we report for the first time a milder, greener strategy to prepare a series of epoxyisoindolinones from aminofuranes and maleic anhydride via cascade amidation and stereocontrolled IMDAF reaction in bio-based solvents as an alternative reaction medium.
Results and Discussion
In this study, the course of the reaction was first investigated in aqueous micellar media using cetyltrimethylammonium bromide (CTAB), a cationic surfactant. The aminofuran and maleic anhydride shown in Table 1 were reacted with 10% CTAB in the same molar ratios. However, contrary to what some previous studies have reported, hydrophobic packing did not accelerate the reaction and there was no transformation in the reaction within 10 h at room temperature [99-106]. This result suggests that it would be difficult to carry out the relevant IMDAF reactions in water due to the remarkable lack of water solubility of the highly hydrophobic aminofurans selected in this study. Therefore, the decision was made to perform these reactions using vegetable oil as an alternative green solvent system. For this purpose, SSO, OO, OA and LM, which can act as potential solvents, were evaluated to determine the optimum conditions for the reaction (Table 1). Some physical parameters of these materials used as reaction media are listed in Table S1 (Supporting Information File 1). The lauryl myristate used as reaction medium in this study was synthesized in our laboratory according to a previously published procedure [107].
Table 1: Optimization of IMDAF cycloaddition using different solvents.a
|
|
||||
| Entry | Solvent | Temperature (°C) | Time (h) | Yield (%)b |
| 1 | SSO | rt | 3 | 89 |
| 2 | SSO | rt | 6 | 91 |
| 3 | SSO | rt | 15 | 90 |
| 4 | SSO | 50 | 0.5 | 96 |
| 5 | SSO | 50 | 1 | 91 |
| 6 | OO | 50 | 0.5 | 96 |
| 7 | OA | 50 | 0.5 | 99 |
| 8 | benzene | rt | 48 | 89 |
| 9 | LM | 50 | 0.5 | 99 |
aReaction conditions: N-benzyl-1-(furan-2-yl)methanamine (1a, 1.07 mmol), maleic anhydride (1.07 mmol), solvent: SSO, OO or OA (2 mL) or LM (1 g). bIsolated yields.
The starting aminofuranes were easily prepared by reduction of Schiff bases (formed by condensation of the corresponding mono- or bis-amines with furfural) with sodium borohydride in methanol [83,108-111]. In order to determine the optimum reaction conditions, a series of reactions between N-benzyl-1-(furan-2-yl)methanamine (1a) and maleic anhydride were carried out and the results obtained are shown in Table 1. As can be seen from the table, SSO, OO, OA and LM are all excellent solvents for the IMDAF reaction and allow the formation of the corresponding addition product in good yields in a very short time and under mild reaction conditions. In particular, in OA and LM, the corresponding reaction takes place in quantitative terms (Table 1, entries 7 and 9). SSO is known to be rich in unsaturated fatty acids, can remain in liquid form over a wide temperature range, is economical and is at the forefront of production and use in various sectors worldwide [112]. In addition, despite its high unsaturation content, SSO has very good thermal stability compared to OO, even at temperatures above 150 °C under normal atmospheric conditions [113]. Both diene and dienophile systems are readily soluble in the reaction medium of interest under the conditions studied, in contrast to aqueous media. The non-polar aprotic nature of vegetable oils and wax esters makes them inert to many reagents, as in this study. The reaction was completed in a much shorter time (Table 1, entry 4) when the temperature of the reaction medium was increased to 50 °C.
According to X-ray and Raman spectroscopy techniques, triglyceride molecules in liquid form are arranged in a dynamic chain-like conformation [114-116]. As the temperature of the reaction medium increases, the size of these lamellar units decreases, increasing the ability to dissolve Diels–Alder components and intermediates. It should be noted that the hydrophobicity of triglycerides is much higher than that of aromatic solvents such as benzene and toluene. For comparison with vegetable oils, the reaction was also carried out in a conventional organic solvent such as benzene, and the yield of the corresponding product is given in Table 1, entry 8. The epoxyisoindolinone product 2a formed by tandem intramolecular addition was easily separated from the reaction medium by precipitation with a hexane–ether mixture and was found to be of sufficient purity for spectroscopic analysis. If desired, the product can be crystallized from an EtOAc–MeOH mixture for further purification. On the other hand, compound 2a has also been previously investigated for its potential covalent prolyl oligopeptidase inhibitory activity in human cells [117]. The solvent mixture used to precipitate the product was removed from the filtrate using a rotary evaporator and it was found that the used bio-based solvents could be recovered in a near quantitative amount for reuse. The recovered SSO solvent was reused as the reaction medium seven times under optimum conditions and the results for the cycloadduct 2k are shown in Figure 1. As shown in the figure, there is no significant decrease in product yield. The reaction conditions and isolation method used in the literature procedures for the previous preparation of compound 2a are given in Table S2 (Supporting Information File 1). As can be seen from the table, most of these methods use toxic and volatile organic solvents, and the duration of the associated intramolecular exo-[4 + 2] reaction is quite long. In the scope of this study, the structures and synthesis scheme of various epoxyisoindole-7-carboxylic acids synthesized using the reaction conditions given in Table 1, entry 4 are given in Table 2 and Scheme 1, respectively.
Figure 1: Reaction yields after seven uses of SSO and average recovery of the oil.
Figure 1: Reaction yields after seven uses of SSO and average recovery of the oil.
Scheme 1: Synthesis of epoxyisoindole-7-carboxylic acids 2a–m and 2n–p.
Scheme 1: Synthesis of epoxyisoindole-7-carboxylic acids 2a–m and 2n–p.
Table 2: Synthesized tricyclic epoxyisoindole-7-carboxylic acidsa.
| Compound | Structure | Yield (%) SSO | Yield (%) OO | Yield (%) OA | Yield (%) LM |
| 2a |
|
96 | 96 | 99 | 99 |
| 2b |
|
93 | 92 | 98 | 97 |
| 2c |
|
94 | 94 | 99 | 98 |
| 2d |
|
93 | 94 | 98 | 98 |
| 2e |
|
95 | 92 | 98 | 92 |
| 2f |
|
96 | 93 | 97 | 96 |
| 2g |
|
95 | 94 | 97 | 95 |
| 2h |
|
95 | 92 | 96 | 94 |
| 2i |
|
96 | 93 | 97 | 95 |
| 2j |
|
97 | 92 | 98 | 96 |
| 2k |
|
99 | 97 | 99 | 99 |
| 2l |
|
93 | 96 | 97 | 97 |
| 2m |
|
94 | 95 | 96 | 97 |
| 2n |
|
98 | 96 | 97 | 97 |
| 2o |
|
97 | 95 | 96 | 97 |
| 2p |
|
98 | 97 | 99 | 98 |
aIsolated yields.
In general, IMDAF reactions proceed with high stereoselectivity to form the major exo addition product, in contrast to conventional Diels–Alder reactions [95,118-124]. On the other hand, the initially exo carboxylic acid substituent in the oxanorbornene ring is converted to its endo isomer by epimerization upon heating the cycloadduct in a mixture of pyridine–glacial acetic acid (1:1). In the process developed in this study, isolated double bonds in the fatty acid chains in sunflower oil do not undergo a side ene reaction with maleic anhydride, which requires high temperatures and/or metal catalysts, since the IMDAF reaction can be performed at 50 ºC or room temperature (Scheme 2) [125,126]. The olefin moiety in N-furfuryl-N-benzylmaleamic acid, formed by acylation of N-benzyl-N-furfurylamine with maleic anhydride, adopts a favorable conformational structure thanks to two activating electron-withdrawing groups and participates in the formation of the corresponding IMDAF product in oil medium rapidly and in good yields. The ring stereochemistry (exo- or endo-geometry) of the product, epoxyisoindole-7-carboxylic acid, is easily determined by 1H NMR by evaluating the coupling constants of the protons Ha, Hb and Hc (Figure 2) [119,127,128].
Scheme 2: Possible side reactions of unsaturated fatty acids with maleic anhydride.
Scheme 2: Possible side reactions of unsaturated fatty acids with maleic anhydride.
Figure 2: Coupling constants of selected protons in compound 2a and its optimized geometric structure.
Figure 2: Coupling constants of selected protons in compound 2a and its optimized geometric structure.
The values of the coupling constants shown in Figure 2 are in good agreement with those reported for this compound in the literature. In all synthesized compounds, Ha and Hb protons are only in endo conformation and therefore the amide and carboxylic acid functional groups are arranged in exo conformation. The fact that the flexible 2n and 2o compounds in the bis structure are in equilibrium with maleamic acid precursors (Scheme 3) complicates the interpretation of their 1H and 13C NMR spectra. The existence of these precursors is clearly demonstrated by both their 1H and 13C NMR spectra (Supporting Information File 1). It should be noted that in the case of compound 2p, unlike the other two compounds, the equilibrium is largely in favor of the product of interest and the NMR spectra confirm this.
Scheme 3: Equilibrium of 2n–p with maleamic acid precursors.
Scheme 3: Equilibrium of 2n–p with maleamic acid precursors.
From the mechanistic point of view of the IMDAF reaction, the presence of both s-cis and s-trans conformations of maleamic acid intermediates in the reaction medium has been previously demonstrated by 1H NMR studies [129]. The elongation of the alkyl chain attached to the N atom facilitates the formation of the epoxyisoindole product by favoring the s-cis conformation due to steric factors (Thorpe–Ingold effect) [130,131].
Briefly, in this type of reaction, the acylation is followed by an intramolecular Diels–Alder reaction and the amide intermediate (a sample compound has been previously isolated and fully characterized as a result of detailed studies) is in equilibrium with the final product at room temperature in polar solvents such as DMSO (Figure 3, path I) [132]. Similarly, Tomberg et al., note that in such reactions, amide is formed rapidly by opening the maleic anhydride ring first, followed by a slower cyclization [133]. On the other hand, according to the results of some DFT calculations, Naguib et al., state that [4 + 2] cycloaddition between maleic anhydride and secondary amine occurs first, followed by the corresponding amidation (Figure 3, path II) [110].
Figure 3: Possible mechanism for the IMDAF reaction.
Figure 3: Possible mechanism for the IMDAF reaction.
Although IMDAF reactions involving furan systems bearing amide functionality are generally reversible [134], the formation of the addition products in high yields in this study indicates that the retro-IMDAF reaction does not occur under the reaction conditions developed. As seen in Figure 4b, the different sigma bond lengths formed in the exo transition state (calculations which were performed in Gaussian 16 Rev B.01 (Gaussian, Inc., Wallingford, CT, USA)) [135] indicate that the IMDAF reaction proceeds through an asynchronous process (the same is true for the endo transition state). Accordingly, the length of the σ bond formed near the amide functional group was calculated to be 2.012 Å, while the length of the bond formed on the far side was calculated to be 2.160 Å. These bond lengths support path I, which is a more valid pathway in the reaction mechanism. As can be seen in Figure 4, the energies of both the exo transition state and the exo product are lower than those of the endo, which also supports the experimental results.
![[1860-5397-20-114-4]](/bjoc/content/figures/1860-5397-20-114-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: IRC calculations of a) endo-, b) exo-transition structures and products for compound 2a (semi-empirical method, PM6) [136].
Figure 4: IRC calculations of a) endo-, b) exo-transition structures and products for compound 2a (semi-empir...
Conclusion
Vegetable oils, which are readily available from a large number and variety of natural sources around the world, fatty acids and wax esters, can be excellent alternative reaction media to existing conventional and green solvents for IMDAF reactions. This study reveals the first utilization of these materials as biocompatible solvents for IMDAF reactions. The corresponding tricyclic epoxyisoindole-7-carboxylic acids were obtained in less time and in higher yields than in reactions carried out in conventional toxic and volatile organic solvents. A challenge in the use of vegetable oils as solvents in organic transformations is the purification of the synthesized product, since these oils are non-volatile and non-polar. However, in this study, the choice of these materials as green solvents allows convenient isolation of the cycloadducts by precipitation followed by filtration with hexane or diethyl ether. Thus, IMDAF reactions, which are a useful strategy for the preparation of many natural compounds and various organic intermediates, can be safely carried out under more environmentally friendly conditions.
Experimental
All reagents and solvents were purchased from Merck (Merck, Darmstadt, Germany), Sigma-Aldrich (St. Louis, MO), or Acros Organics (Thermo Fisher Scientific, Geel, Belgium) and used without further purification. Thin-layer chromatography was performed using silica gel (60 F254, Merck, Darmstadt, Germany) plates. Melting points were recorded by Büchi melting point B-540 apparatus (Büchi Labortechnik AG in Flawil, Switzerland). The IR spectra were measured by Spectrum Two FT-IR spectrometer (PerkinElmer, Massachusetts, USA). The NMR spectra were measured using Bruker Ultrashield Plus Biospin 400 MHz NMR spectrometer and A600a Agilent DD2 600 MHz NMR spectrometer (Santa Clara, California, USA) and chloroform-d (CDCl3) or hexadeuterodimethyl sulfoxide (DMSO-d6) as a solvent. Chemical shifts (δ) are reported in ppm and J values in Hertz. The elemental analyses were performed using an LECO CHNS-932 elemental analyzer (Saint Joseph, MI, USA).
Representative procedure for the IMDAF reaction to afford 2a
In a 50 mL round bottom flask, N-benzyl-1-(furan-2-yl)methanamine (1a, 0.30 g, 1.60 mmol) was dissolved in 2 mL of SSO and heated in an oil bath to 50 °C. Then, into the observed solution, maleic anhydride (0.16 g, 1.63 mmol) was added and the resulted mixture was heated at 50 °C for 30 min. Thereafter, the reaction mixture was cooled to room temperature and the desired product, 2a was precipitated after trituration with hexane. The obtained solid product was isolated by filtration under vacuum, washed with ether–hexane solvent mixture and dried at room temperature. If desired for further purification the solid product can be crystallized from EtOAc/MeOH solvent mixture. Beige crystalline solid, mp 171–172 °C (EtOAc/MeOH); 1H NMR (600 MHz, CDCl3) δ 7.33 (t, J = 7.6 Hz, 2H, Ar), 7.28 (d, J = 7.4 Hz, 1H, Ar), 7.24 (t, J = 7.4 Hz, 2H, Ar), 6.42–6.39 (m, 2H, Hf, Hg), 5.25 (d, J = 1.6 Hz, 1H, Hc), 4.63 (d, J = 15.1 Hz, 1H, Hh), 4.42 (d, J = 15.1 Hz, 1H, Hi), 3.83 (d, J = 12 Hz, 1H, Hd), 3.65 (d, J = 12 Hz, 1H, He), 2.96 (d, J = 9.1 Hz, 1H, Ha), 2.84 (d, J = 9.1 Hz, 1H, Hb); 13C NMR (150 MHz, CDCl3) δ 173.10, 172.51, 137.22, 135.20, 134.83, 128.92, 127.98, 127.87, 88.79, 82.36, 50.72, 48.53, 47.03, 45.86; Anal calcd for C16H15NO4 (285.30): C, 67.36; H, 5.30; N, 4.91; found: C, 67.30; H, 5.26; N, 4.86.
Supporting Information
| Supporting Information File 1: Tables S1 and S2, Cartesian coordinates of the optimized structures and copies of NMR spectra. | ||
| Format: PDF | Size: 2.3 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Tickner, J.; Geiser, K.; Baima, S. Environment 2021, 63, 4–15. doi:10.1080/00139157.2021.1979857
Return to citation in text: [1] -
Ragothaman, A.; Anderson, W. A. Environments 2017, 4, 66. doi:10.3390/environments4030066
Return to citation in text: [1] -
Tenhumberg, N.; Büttner, H.; Schäffner, B.; Kruse, D.; Blumenstein, M.; Werner, T. Green Chem. 2016, 18, 3775–3788. doi:10.1039/c6gc00671j
Return to citation in text: [1] -
Mori, R. RSC Sustainability 2023, 1, 179–212. doi:10.1039/d2su00014h
Return to citation in text: [1] -
Lakshmi, D. D.; Yogita; Srinivasa Rao, B.; Lingaiah, N. Sustainable Energy Fuels 2024, 8, 43–53. doi:10.1039/d3se01096a
Return to citation in text: [1] -
Serrano-Ruiz, J. C.; Luque, R.; Sepúlveda-Escribano, A. Chem. Soc. Rev. 2011, 40, 5266–5281. doi:10.1039/c1cs15131b
Return to citation in text: [1] -
Fraile, J. M.; García, J. I.; Herrerías, C. I.; Pires, E. Synthesis 2017, 49, 1444–1460. doi:10.1055/s-0036-1588699
Return to citation in text: [1] -
Lucas, F. W. S.; Grim, R. G.; Tacey, S. A.; Downes, C. A.; Hasse, J.; Roman, A. M.; Farberow, C. A.; Schaidle, J. A.; Holewinski, A. ACS Energy Lett. 2021, 6, 1205–1270. doi:10.1021/acsenergylett.0c02692
Return to citation in text: [1] -
Campos da Paixão, I.; Cardozo, J. C.; Sales Monteiro, M. K.; Gondim, A. D.; Cavalcanti, L. N.; Fabiano de Santana Souza, D.; Martínez-Huitle, C. A.; Vieira dos Santos, E. RSC Adv. 2023, 13, 35755–35765. doi:10.1039/d3ra05772k
Return to citation in text: [1] -
Gao, X.; Tong, X.; Liu, R.; Zhang, Y. Catal. Sci. Technol. 2023, 13, 6132–6136. doi:10.1039/d3cy01152f
Return to citation in text: [1] -
Abu-Omar, M. M.; Ford, P. C. RSC Sustainability 2023, 1, 1686–1703. doi:10.1039/d3su00117b
Return to citation in text: [1] -
Yuan, K.; Zhao, W.; Zhou, Y.; Zhang, X.; Chen, S.; Chen, J.; Liu, C.; Xiong, W. ACS Sustainable Chem. Eng. 2023, 11, 17769–17777. doi:10.1021/acssuschemeng.3c05865
Return to citation in text: [1] -
Biermann, U.; Bornscheuer, U.; Meier, M. A. R.; Metzger, J. O.; Schäfer, H. J. Angew. Chem., Int. Ed. 2011, 50, 3854–3871. doi:10.1002/anie.201002767
Return to citation in text: [1] -
Len, C.; Duhan, V.; Ouyang, W.; Nguyen, R.; Lochab, B. Front. Chem. (Lausanne, Switz.) 2023, 11, 1306182. doi:10.3389/fchem.2023.1306182
Return to citation in text: [1] [2] -
Lanero, F.; Bresolin, B. M.; Scettri, A.; Nogarole, M.; Schievano, E.; Mammi, S.; Saielli, G.; Famengo, A.; Semenzato, A.; Tafuro, G.; Sgarbossa, P.; Bertani, R. Molecules 2022, 27, 8142. doi:10.3390/molecules27238142
Return to citation in text: [1] -
Choe, Y.-A.; Kim, S.-I.; Ju, K.-S. Chem. Phys. Lett. 2022, 805, 139934. doi:10.1016/j.cplett.2022.139934
Return to citation in text: [1] -
Zhang, C.; Madbouly, S. A.; Kessler, M. R. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. doi:10.1021/am5071333
Return to citation in text: [1] -
Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. ACS Sustainable Chem. Eng. 2017, 5, 7365–7373. doi:10.1021/acssuschemeng.7b01672
Return to citation in text: [1] -
Bétron, C.; Cassagnau, P.; Bounor-Legaré, V. Mater. Chem. Phys. 2018, 211, 361–374. doi:10.1016/j.matchemphys.2018.02.038
Return to citation in text: [1] -
Smith, A. D.; Tennyson, A. G.; Smith, R. C. Sustainable Chem. 2020, 1, 209–237. doi:10.3390/suschem1030015
Return to citation in text: [1] -
Kirianchuk, V.; Demchuk, Z.; Polunin, Y.; Kohut, A.; Voronov, S.; Voronov, A. Molecules 2022, 27, 932. doi:10.3390/molecules27030932
Return to citation in text: [1] -
Gaglieri, C.; Alarcon, R. T.; de Moura, A.; Bannach, G. Curr. Res. Green Sustainable Chem. 2022, 5, 100343. doi:10.1016/j.crgsc.2022.100343
Return to citation in text: [1] -
Stavila, E.; Yuliati, F.; Adharis, A.; Laksmono, J. A.; Iqbal, M. RSC Adv. 2023, 13, 14747–14775. doi:10.1039/d3ra01913f
Return to citation in text: [1] -
Gregoritza, M.; Brandl, F. P. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. doi:10.1016/j.ejpb.2015.06.007
Return to citation in text: [1] -
Akbari Javar, R.; Bin Noordin, M. I.; Khoobi, M.; Ghaedi, A. J. Inorg. Organomet. Polym. 2020, 30, 2520–2532. doi:10.1007/s10904-020-01512-x
Return to citation in text: [1] -
Fattahi, N.; Shahbazi, M.-A.; Maleki, A.; Hamidi, M.; Ramazani, A.; Santos, H. A. J. Controlled Release 2020, 326, 556–598. doi:10.1016/j.jconrel.2020.07.012
Return to citation in text: [1] -
Large, D. E.; Abdelmessih, R. G.; Fink, E. A.; Auguste, D. T. Adv. Drug Delivery Rev. 2021, 176, 113851. doi:10.1016/j.addr.2021.113851
Return to citation in text: [1] -
Wang, H.; Ullah, A. Polymers (Basel, Switz.) 2022, 14, 3436. doi:10.3390/polym14173436
Return to citation in text: [1] -
Huang, L.; Yang, J.; Wang, T.; Gao, J.; Xu, D. J. Nanobiotechnol. 2022, 20, 49. doi:10.1186/s12951-022-01257-4
Return to citation in text: [1] -
Zhang, C.-h.; Li, X.-g.; Gao, Y.-g.; Zhang, L.-x.; Fu, X.-q. Chem. Res. Chin. Univ. 2007, 23, 176–182. doi:10.1016/s1005-9040(07)60037-3
Return to citation in text: [1] -
Chhikara, B. S.; St. Jean, N.; Mandal, D.; Kumar, A.; Parang, K. Eur. J. Med. Chem. 2011, 46, 2037–2042. doi:10.1016/j.ejmech.2011.02.056
Return to citation in text: [1] -
Mielczarek-Puta, M.; Struga, M.; Roszkowski, P. Med. Chem. Res. 2019, 28, 2153–2164. doi:10.1007/s00044-019-02443-0
Return to citation in text: [1] -
Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Eur. J. Pharmacol. 2020, 871, 172937. doi:10.1016/j.ejphar.2020.172937
Return to citation in text: [1] -
Soutelo-Maria, A.; Dubois, J.-L.; Couturier, J.-L.; Cravotto, G. Catalysts 2018, 8, 464. doi:10.3390/catal8100464
Return to citation in text: [1] -
Saito, S.; Nanba, Y.; Morita, M.; Kobayashi, Y. Synlett 2019, 30, 1085–1089. doi:10.1055/s-0037-1611809
Return to citation in text: [1] -
Hinzmann, A.; Druhmann, S. S.; Gröger, H. Sustainable Chem. 2020, 1, 275–289. doi:10.3390/suschem1030018
Return to citation in text: [1] -
Bertolini, V.; Pallavicini, M.; Tibhe, G.; Roda, G.; Arnoldi, S.; Monguzzi, L.; Zoccola, M.; Di Nardo, G.; Gilardi, G.; Bolchi, C. ACS Omega 2021, 6, 31901–31906. doi:10.1021/acsomega.1c04640
Return to citation in text: [1] -
Biermann, U.; Bornscheuer, U. T.; Feussner, I.; Meier, M. A. R.; Metzger, J. O. Angew. Chem., Int. Ed. 2021, 60, 20144–20165. doi:10.1002/anie.202100778
Return to citation in text: [1] -
Chang, S. H.; Teng, T. T.; Ismail, N. J. Hazard. Mater. 2010, 181, 868–872. doi:10.1016/j.jhazmat.2010.05.093
Return to citation in text: [1] -
Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Molecules 2017, 22, 1474. doi:10.3390/molecules22091474
Return to citation in text: [1] -
Portillo‐López, R.; Morales‐Contreras, B. E.; Lozano‐Guzmán, E.; Basilio‐Heredia, J.; Muy‐Rangel, M. D.; Ochoa‐Martínez, L. A.; Rosas‐Flores, W.; Morales‐Castro, J. J. Food Sci. 2021, 86, 3122–3136. doi:10.1111/1750-3841.15815
Return to citation in text: [1] -
Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Ind. Crops Prod. 2022, 175, 114257. doi:10.1016/j.indcrop.2021.114257
Return to citation in text: [1] -
Menges, N.; Şahin, E. ACS Sustainable Chem. Eng. 2014, 2, 226–230. doi:10.1021/sc400281h
Return to citation in text: [1] -
Ishizuka, F.; Stenzel, M. H.; Zetterlund, P. B. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 831–839. doi:10.1002/pola.28958
Return to citation in text: [1] -
Noppawan, P.; Sangon, S.; Supanchaiyamat, N.; Hunt, A. J. Green Chem. 2021, 23, 5766–5774. doi:10.1039/d1gc00872b
Return to citation in text: [1] -
Gevorgyan, A.; Hopmann, K. H.; Bayer, A. Green Chem. 2021, 23, 7219–7227. doi:10.1039/d1gc02311j
Return to citation in text: [1] -
Gevorgyan, A.; Hopmann, K. H.; Bayer, A. Organometallics 2022, 41, 1777–1785. doi:10.1021/acs.organomet.1c00517
Return to citation in text: [1] -
Xiao, Y.; Malhotra, S. V. Tetrahedron Lett. 2004, 45, 8339–8342. doi:10.1016/j.tetlet.2004.09.070
Return to citation in text: [1] -
Cott, D. J.; Ziegler, K. J.; Owens, V. P.; Glennon, J. D.; Graham, A. E.; Holmes, J. D. Green Chem. 2005, 7, 105–110. doi:10.1039/b408327j
Return to citation in text: [1] -
Reinhardt, D.; Ilgen, F.; Kralisch, D.; König, B.; Kreisel, G. Green Chem. 2008, 10, 1170–1181. doi:10.1039/b807379a
Return to citation in text: [1] -
Huertas, D.; Florscher, M.; Dragojlovic, V. Green Chem. 2009, 11, 91–95. doi:10.1039/b813485e
Return to citation in text: [1] -
Chiappe, C.; Malvaldi, M.; Pomelli, C. S. Green Chem. 2010, 12, 1330–1339. doi:10.1039/c0gc00074d
Return to citation in text: [1] -
Mirgane, N.; Kotwal, S.; Karnik, A. Cent. Eur. J. Chem. 2010, 8, 356–360. doi:10.2478/s11532-009-0136-6
Return to citation in text: [1] -
Seo, J.-M.; Baek, J.-B. Chem. Commun. 2014, 50, 14651–14653. doi:10.1039/c4cc07173e
Return to citation in text: [1] -
Donatoni, M. C.; Junior, G. A. B.; de Oliveira, K. T.; Ando, R. A.; Brocksom, T. J.; Dos Santos, A. A. Tetrahedron 2014, 70, 3231–3238. doi:10.1016/j.tet.2014.02.017
Return to citation in text: [1] -
Parmar, N. J.; Parmar, B. D.; Sutariya, T. R.; Kant, R.; Gupta, V. K. Tetrahedron Lett. 2014, 55, 6060–6064. doi:10.1016/j.tetlet.2014.09.026
Return to citation in text: [1] -
Grosso, C.; Brigas, A.; de los Santos, J. M.; Palacios, F.; Lemos, A.; Pinho e Melo, T. M. V. D. Monatsh. Chem. 2019, 150, 1275–1288. doi:10.1007/s00706-019-02421-7
Return to citation in text: [1] -
Rammohan, A.; Krinochkin, A. P.; Khasanov, A. F.; Kopchuk, D. S.; Zyryanov, G. V. Top. Curr. Chem. 2022, 380, 43. doi:10.1007/s41061-022-00398-2
Return to citation in text: [1] -
Mance, A. D.; Jakopčić, K. Mol. Diversity 2005, 9, 229–231. doi:10.1007/s11030-005-3434-8
Return to citation in text: [1] -
Marullo, S.; Meli, A.; D’Anna, F. ACS Sustainable Chem. Eng. 2020, 8, 4889–4899. doi:10.1021/acssuschemeng.0c00193
Return to citation in text: [1] -
Kumar, A.; Pawar, S. S. Sci. China: Chem. 2012, 55, 1633–1637. doi:10.1007/s11426-012-4684-9
Return to citation in text: [1] -
Firth, J. D.; Craven, P. G. E.; Lilburn, M.; Pahl, A.; Marsden, S. P.; Nelson, A. Chem. Commun. 2016, 52, 9837–9840. doi:10.1039/c6cc04662b
Return to citation in text: [1] -
Blanpain, A.; Clark, J. H.; Farmer, T. J.; Guo, Y.; Ingram, I. D. V.; Kendrick, J. E.; Lawrenson, S. B.; North, M.; Rodgers, G.; Whitwood, A. C. ChemSusChem 2019, 12, 2393–2401. doi:10.1002/cssc.201900748
Return to citation in text: [1] -
Xu, F.; Li, Z.; Zhang, L.-L.; Liu, S.; Li, H.; Liao, Y.; Yang, S. Green Chem. 2023, 25, 3297–3305. doi:10.1039/d2gc04786a
Return to citation in text: [1] -
Jung, M. E.; Gervay, J. J. Am. Chem. Soc. 1989, 111, 5469–5470. doi:10.1021/ja00196a065
Return to citation in text: [1] -
Jung, M. E.; Gervay, J. J. Am. Chem. Soc. 1991, 113, 224–232. doi:10.1021/ja00001a032
Return to citation in text: [1] -
Soares, M. I. L.; Cardoso, A. L.; Pinho e Melo, T. M. V. D. Molecules 2022, 27, 1304. doi:10.3390/molecules27041304
Return to citation in text: [1] -
Wang, L.; Wu, F.; Chen, J.; Nicewicz, D. A.; Huang, Y. Angew. Chem., Int. Ed. 2017, 56, 6896–6900. doi:10.1002/anie.201702940
Return to citation in text: [1] -
Hu, X.; Zhang, G.; Bu, F.; Lei, A. Angew. Chem., Int. Ed. 2018, 57, 1286–1290. doi:10.1002/anie.201711359
Return to citation in text: [1] -
Zhang, G.; Lin, Y.; Luo, X.; Hu, X.; Chen, C.; Lei, A. Nat. Commun. 2018, 9, 1225. doi:10.1038/s41467-018-03534-z
Return to citation in text: [1] -
Hudlicky, T.; Butora, G.; Fearnley, S. P.; Gum, A. G.; Persichini, P. J.; Stabile, M. R.; Merola, J. S. J. Chem. Soc., Perkin Trans. 1 1995, 2393–2398. doi:10.1039/p19950002393
Return to citation in text: [1] -
Mahmood, S. Y.; Lallemand, M.-C.; Sader-Bakaouni, L.; Charton, O.; Vérité, P.; Dufat, H.; Tillequin, F. Tetrahedron 2004, 60, 5105–5110. doi:10.1016/j.tet.2004.03.091
Return to citation in text: [1] -
Fokas, D.; Yu, L.; Baldino, C. M. Mol. Diversity 2005, 9, 81–89. doi:10.1007/s11030-005-1292-z
Return to citation in text: [1] -
Takao, K.-i.; Munakata, R.; Tadano, K.-i. Chem. Rev. 2005, 105, 4779–4807. doi:10.1021/cr040632u
Return to citation in text: [1] -
Zubkov, F. I.; Ershova, J. D.; Orlova, A. A.; Zaytsev, V. P.; Nikitina, E. V.; Peregudov, A. S.; Gurbanov, A. V.; Borisov, R. S.; Khrustalev, V. N.; Maharramov, A. M.; Varlamov, A. V. Tetrahedron 2009, 65, 3789–3803. doi:10.1016/j.tet.2009.02.024
Return to citation in text: [1] -
Zubkov, F. I.; Ershova, J. D.; Zaytsev, V. P.; Obushak, M. D.; Matiychuk, V. S.; Sokolova, E. A.; Khrustalev, V. N.; Varlamov, A. V. Tetrahedron Lett. 2010, 51, 6822–6824. doi:10.1016/j.tetlet.2010.10.046
Return to citation in text: [1] -
Leverett, C. A.; Li, G.; France, S.; Padwa, A. J. Org. Chem. 2016, 81, 10193–10203. doi:10.1021/acs.joc.6b00771
Return to citation in text: [1] -
Gulbrandsen, H. S.; Serigstad, H.; Lovell Read, M.; Joos, I.; Gundersen, L.-L. Eur. J. Org. Chem. 2019, 6044–6052. doi:10.1002/ejoc.201901000
Return to citation in text: [1] -
Zubkov, F. I.; Boltukhina, E. V.; Turchin, K. F.; Varlamov, A. V. Tetrahedron 2004, 60, 8455–8463. doi:10.1016/j.tet.2004.07.008
Return to citation in text: [1] -
Zubkov, F. I.; Nikitina, E. V.; Turchin, K. F.; Aleksandrov, G. G.; Safronova, A. A.; Borisov, R. S.; Varlamov, A. V. J. Org. Chem. 2004, 69, 432–438. doi:10.1021/jo0353684
Return to citation in text: [1] -
Zubkov, F. I.; Boltukhina, E. V.; Turchin, K. F.; Borisov, R. S.; Varlamov, A. V. Tetrahedron 2005, 61, 4099–4113. doi:10.1016/j.tet.2005.02.017
Return to citation in text: [1] -
Zubkov, F. I.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Rev. 2005, 74, 639–669. doi:10.1070/rc2005v074n07abeh001180
Return to citation in text: [1] -
Zubkov, F. I.; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. Tetrahedron 2011, 67, 9148–9163. doi:10.1016/j.tet.2011.09.099
Return to citation in text: [1] [2] -
Zubkov, F. I.; Zaytsev, V. P.; Airiyan, I. K.; Golubev, V. D.; Puzikova, E. S.; Sorokina, E. A.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Bull. 2012, 61, 600–605. doi:10.1007/s11172-012-0087-5
Return to citation in text: [1] -
Zaytsev, V. P.; Zubkov, F. I.; Mertsalov, D. F.; Orlova, D. N.; Sorokina, E. A.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Bull. 2015, 64, 112–126. doi:10.1007/s11172-015-0829-2
Return to citation in text: [1] -
Read, M. L.; Krapp, A.; Miranda, P. O.; Gundersen, L.-L. Tetrahedron 2012, 68, 1869–1885. doi:10.1016/j.tet.2011.12.079
Return to citation in text: [1] -
Zubkov, F. I.; Nikitina, E. V.; Galeev, T. R.; Zaytsev, V. P.; Khrustalev, V. N.; Novikov, R. A.; Orlova, D. N.; Varlamov, A. V. Tetrahedron 2014, 70, 1659–1690. doi:10.1016/j.tet.2014.01.008
Return to citation in text: [1] -
Zubkov, F. I.; Orlova, D. N.; Zaytsev, V. P.; Voronov, A. A.; Nikitina, E. V.; Khrustalev, V. N.; Novikov, R. A.; Krasavin, M.; Varlamov, A. V. Curr. Org. Synth. 2017, 14, 733–746. doi:10.2174/1570179414666161116123221
Return to citation in text: [1] -
Nadirova, M. A.; Khanova, A. V.; Zubkov, F. I.; Mertsalov, D. F.; Kolesnik, I. A.; Petkevich, S. K.; Potkin, V. I.; Shetnev, A. A.; Presnukhina, S. I.; Sinelshchikova, A. A.; Grigoriev, M. S.; Zaytsev, V. P. Tetrahedron 2021, 85, 132032. doi:10.1016/j.tet.2021.132032
Return to citation in text: [1] -
Gulbrandsen, H. S.; Alfaro, J. L. D.; Read, M. L.; Gundersen, L.-L. Eur. J. Org. Chem. 2017, 2305–2311. doi:10.1002/ejoc.201700180
Return to citation in text: [1] -
Osano, M.; Jhaveri, D. P.; Wipf, P. Org. Lett. 2020, 22, 2215–2219. doi:10.1021/acs.orglett.0c00417
Return to citation in text: [1] -
Mertsalov, D. F.; Lovtsevich, L. V.; Shelukho, E. R.; Kolesnik, I. A.; Petkevich, S. K.; Potkin, V. I.; Zaytsev, V. P. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2021, 57, 1031–1036. doi:10.1007/s10593-021-03018-x
Return to citation in text: [1] -
Yarovaya, O. I.; Kovaleva, K. S.; Zaykovskaya, A. A.; Yashina, L. N.; Scherbakova, N. S.; Scherbakov, D. N.; Borisevich, S. S.; Zubkov, F. I.; Antonova, A. S.; Peshkov, R. Y.; Eltsov, I. V.; Pyankov, O. V.; Maksyutov, R. A.; Salakhutdinov, N. F. Bioorg. Med. Chem. Lett. 2021, 40, 127926. doi:10.1016/j.bmcl.2021.127926
Return to citation in text: [1] -
Golubev, P.; Pankova, A.; Krasavin, M. Tetrahedron Lett. 2019, 60, 1578–1581. doi:10.1016/j.tetlet.2019.05.018
Return to citation in text: [1] -
Sarang, P. S.; Yadav, A. A.; Patil, P. S.; Krishna, U. M.; Trivedi, G. K.; Salunkhe, M. M. Synthesis 2007, 1091–1095. doi:10.1055/s-2007-965950
Return to citation in text: [1] [2] -
Mir, A. A.; Mulwad, V. V.; Trivedi, G. K. J. Heterocycl. Chem. 2010, 47, 214–218. doi:10.1002/jhet.274
Return to citation in text: [1] -
Zubkov, F. I.; Airiyan, I. K.; Ershova, J. D.; Galeev, T. R.; Zaytsev, V. P.; Nikitina, E. V.; Varlamov, A. V. RSC Adv. 2012, 2, 4103–4109. doi:10.1039/c2ra20295f
Return to citation in text: [1] -
Zaytsev, V. P.; Mertsalov, D. F.; Trunova, A. M.; Khanova, A. V.; Nikitina, E. V.; Sinelshchikova, A. A.; Grigoriev, M. S. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2020, 56, 930–935. doi:10.1007/s10593-020-02752-y
Return to citation in text: [1] -
Sauer, J.; Sustmann, R. Angew. Chem., Int. Ed. Engl. 1980, 19, 779–807. doi:10.1002/anie.198007791
Return to citation in text: [1] -
Rideout, D. C.; Breslow, R. J. Am. Chem. Soc. 1980, 102, 7816–7817. doi:10.1021/ja00546a048
Return to citation in text: [1] -
Breslow, R.; Maitra, U.; Rideout, D. Tetrahedron Lett. 1983, 24, 1901–1904. doi:10.1016/s0040-4039(00)81801-8
Return to citation in text: [1] -
Blokzijl, W.; Blandamer, M. J.; Engberts, J. B. F. N. J. Am. Chem. Soc. 1991, 113, 4241–4246. doi:10.1021/ja00011a029
Return to citation in text: [1] -
Blokzijl, W.; Engberts, J. B. F. N. Angew. Chem., Int. Ed. Engl. 1993, 32, 1545–1579. doi:10.1002/anie.199315451
Return to citation in text: [1] -
Moulay, S.; Touati, A. C. R. Chim. 2010, 13, 1474–1511. doi:10.1016/j.crci.2010.05.025
Return to citation in text: [1] -
Otto, S.; Engberts, J. B. F. N.; Kwak, J. C. T. J. Am. Chem. Soc. 1998, 120, 9517–9525. doi:10.1021/ja9816537
Return to citation in text: [1] -
Rispens, T.; Engberts, J. B. F. N. J. Org. Chem. 2002, 67, 7369–7377. doi:10.1021/jo0260802
Return to citation in text: [1] -
Yıldırım, A.; Mudaber, S.; Öztürk, S. Eur. J. Lipid Sci. Technol. 2019, 121, 1800303. doi:10.1002/ejlt.201800303
Return to citation in text: [1] -
Rani, P. J.; Thirumaran, S. Eur. J. Med. Chem. 2013, 62, 139–147. doi:10.1016/j.ejmech.2012.12.047
Return to citation in text: [1] -
Selvaganapathi, P.; Thirumaran, S.; Ciattini, S. Appl. Organomet. Chem. 2019, 33, e5089. doi:10.1002/aoc.5089
Return to citation in text: [1] -
Naguib, M.; Rashed, A.; Keddie, D. J. J. Mater. Sci. 2021, 56, 8900–8909. doi:10.1007/s10853-021-05853-x
Return to citation in text: [1] [2] -
Li, F.; Xu, Y.; Xu, Y.; Wang, C.; Ma, J.; Chen, P.; Wang, L. Mol. Catal. 2022, 529, 112576. doi:10.1016/j.mcat.2022.112576
Return to citation in text: [1] -
Hill, K. Pure Appl. Chem. 2000, 72, 1255–1264. doi:10.1351/pac200072071255
Return to citation in text: [1] -
Alvarenga, B. R., Jr.; Xavier, F. A. N.; Soares, F. L. F.; Carneiro, R. L. Food Anal. Methods 2018, 11, 1969–1976. doi:10.1007/s12161-018-1160-y
Return to citation in text: [1] -
Hernqvist, L. Eur. J. Lipid Sci. Technol. 1984, 86, 297–300. doi:10.1002/lipi.19840860802
Return to citation in text: [1] -
Larsson, K. Eur. J. Lipid Sci. Technol. 1972, 74, 136–142. doi:10.1002/lipi.19720740302
Return to citation in text: [1] -
Hernqvist, L. Food Struct. 1990, 9, 39–44.
Return to citation in text: [1] -
De Cesco, S.; Deslandes, S.; Therrien, E.; Levan, D.; Cueto, M.; Schmidt, R.; Cantin, L.-D.; Mittermaier, A.; Juillerat-Jeanneret, L.; Moitessier, N. J. Med. Chem. 2012, 55, 6306–6315. doi:10.1021/jm3002839
Return to citation in text: [1] -
Takano, S.; Oshima, Y.; Ito, F.; Ogasawara, K. Yakugaku Zasshi 1980, 100, 1194–1202. doi:10.1248/yakushi1947.100.12_1194
Return to citation in text: [1] -
Varlamov, A. V.; Boltukhina, E. V.; Zubkov, F. I.; Sidorenko, N. V.; Chernyshev, A. I.; Grudinin, D. G. Chem. Heterocycl. Compd. 2004, 40, 22–28. doi:10.1023/b:cohc.0000023763.75894.63
Return to citation in text: [1] [2] -
Gordon, C. P.; Byrne, N.; McCluskey, A. Green Chem. 2010, 12, 1000–1006. doi:10.1039/b924835h
Return to citation in text: [1] -
Papeo, G.; Posteri, H.; Borghi, D.; Busel, A. A.; Caprera, F.; Casale, E.; Ciomei, M.; Cirla, A.; Corti, E.; D’Anello, M.; Fasolini, M.; Forte, B.; Galvani, A.; Isacchi, A.; Khvat, A.; Krasavin, M. Y.; Lupi, R.; Orsini, P.; Perego, R.; Pesenti, E.; Pezzetta, D.; Rainoldi, S.; Riccardi-Sirtori, F.; Scolaro, A.; Sola, F.; Zuccotto, F.; Felder, E. R.; Donati, D.; Montagnoli, A. J. Med. Chem. 2015, 58, 6875–6898. doi:10.1021/acs.jmedchem.5b00680
Return to citation in text: [1] -
Hizartzidis, L.; Gilbert, J.; Gordon, C. P.; Sakoff, J. A.; McCluskey, A. ChemMedChem 2019, 14, 1152–1161. doi:10.1002/cmdc.201900180
Return to citation in text: [1] -
Naguib, M.; Yassin, M. A. ACS Appl. Polym. Mater. 2022, 4, 2181–2188. doi:10.1021/acsapm.2c00158
Return to citation in text: [1] -
Bilović, D. Croat. Chem. Acta 1968, 40, 15–22.
Return to citation in text: [1] -
Candy, L.; Vaca‐Garcia, C.; Borredon, E. J. Am. Oil Chem. Soc. 2005, 82, 271–277. doi:10.1007/s11746-005-1066-5
Return to citation in text: [1] -
Eschig, S.; Philipp, C.; Salthammer, T. Eur. J. Lipid Sci. Technol. 2013, 115, 101–110. doi:10.1002/ejlt.201200056
Return to citation in text: [1] -
Nelson, W. L.; Allen, D. R. J. Heterocycl. Chem. 1972, 9, 561–568. doi:10.1002/jhet.5570090317
Return to citation in text: [1] -
Fischer, K.; Huenig, S. J. Org. Chem. 1987, 52, 564–569. doi:10.1021/jo00380a016
Return to citation in text: [1] -
Blokzijl, W.; Engberts, J. B. F. N. Enforced Hydrophobic Interactions and Hydrogen Bonding in the Acceleration of Diels–Alder Reactions in Water. In Structure and Reactivity in Aqueous Solution; Cramer, C. J.; Truhlar, D. G., Eds.; ACS Symposium Series 568; American Chemical Society: Washington, DC, USA, 1994; pp 303–317. doi:10.1021/bk-1994-0568.ch021
Return to citation in text: [1] -
Jung, M. E.; Piizzi, G. Chem. Rev. 2005, 105, 1735–1766. doi:10.1021/cr940337h
Return to citation in text: [1] -
Zubkov, F. I.; Golubev, V. D.; Zaytsev, V. P.; Bakhanovich, O. V.; Nikitina, E. V.; Khrustalev, V. N.; Aysin, R. R.; Timofeeva, T. V.; Novikov, R. A.; Varlamov, A. V. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2016, 52, 225–236. doi:10.1007/s10593-016-1868-9
Return to citation in text: [1] -
Brun, P.; Zylber, J.; Pepe, G.; Reboul, J.-P. Heterocycl. Commun. 1994, 1, 13–16. doi:10.1515/hc.1994.1.1.13
Return to citation in text: [1] -
Tomberg, A.; De Cesco, S.; Huot, M.; Moitessier, N. Tetrahedron Lett. 2015, 56, 6852–6856. doi:10.1016/j.tetlet.2015.10.086
Return to citation in text: [1] -
Nadirova, M. A.; Pokazeev, K. M.; Kolesnik, I. A.; Dorovatovskii, P. V.; Bumagin, N. A.; Potkin, V. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2019, 55, 729–738. doi:10.1007/s10593-019-02528-z
Return to citation in text: [1] -
Gaussian 16, Rev B.01; Gaussian, Inc.: Wallingford, CT, 2016.
Return to citation in text: [1] -
Stewart, J. J. P. J. Mol. Model. 2007, 13, 1173–1213. doi:10.1007/s00894-007-0233-4
Return to citation in text: [1]
| 129. | Blokzijl, W.; Engberts, J. B. F. N. Enforced Hydrophobic Interactions and Hydrogen Bonding in the Acceleration of Diels–Alder Reactions in Water. In Structure and Reactivity in Aqueous Solution; Cramer, C. J.; Truhlar, D. G., Eds.; ACS Symposium Series 568; American Chemical Society: Washington, DC, USA, 1994; pp 303–317. doi:10.1021/bk-1994-0568.ch021 |
| 130. | Jung, M. E.; Piizzi, G. Chem. Rev. 2005, 105, 1735–1766. doi:10.1021/cr940337h |
| 131. | Zubkov, F. I.; Golubev, V. D.; Zaytsev, V. P.; Bakhanovich, O. V.; Nikitina, E. V.; Khrustalev, V. N.; Aysin, R. R.; Timofeeva, T. V.; Novikov, R. A.; Varlamov, A. V. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2016, 52, 225–236. doi:10.1007/s10593-016-1868-9 |
| 132. | Brun, P.; Zylber, J.; Pepe, G.; Reboul, J.-P. Heterocycl. Commun. 1994, 1, 13–16. doi:10.1515/hc.1994.1.1.13 |
| 1. | Tickner, J.; Geiser, K.; Baima, S. Environment 2021, 63, 4–15. doi:10.1080/00139157.2021.1979857 |
| 15. | Lanero, F.; Bresolin, B. M.; Scettri, A.; Nogarole, M.; Schievano, E.; Mammi, S.; Saielli, G.; Famengo, A.; Semenzato, A.; Tafuro, G.; Sgarbossa, P.; Bertani, R. Molecules 2022, 27, 8142. doi:10.3390/molecules27238142 |
| 16. | Choe, Y.-A.; Kim, S.-I.; Ju, K.-S. Chem. Phys. Lett. 2022, 805, 139934. doi:10.1016/j.cplett.2022.139934 |
| 65. | Jung, M. E.; Gervay, J. J. Am. Chem. Soc. 1989, 111, 5469–5470. doi:10.1021/ja00196a065 |
| 66. | Jung, M. E.; Gervay, J. J. Am. Chem. Soc. 1991, 113, 224–232. doi:10.1021/ja00001a032 |
| 67. | Soares, M. I. L.; Cardoso, A. L.; Pinho e Melo, T. M. V. D. Molecules 2022, 27, 1304. doi:10.3390/molecules27041304 |
| 6. | Serrano-Ruiz, J. C.; Luque, R.; Sepúlveda-Escribano, A. Chem. Soc. Rev. 2011, 40, 5266–5281. doi:10.1039/c1cs15131b |
| 7. | Fraile, J. M.; García, J. I.; Herrerías, C. I.; Pires, E. Synthesis 2017, 49, 1444–1460. doi:10.1055/s-0036-1588699 |
| 8. | Lucas, F. W. S.; Grim, R. G.; Tacey, S. A.; Downes, C. A.; Hasse, J.; Roman, A. M.; Farberow, C. A.; Schaidle, J. A.; Holewinski, A. ACS Energy Lett. 2021, 6, 1205–1270. doi:10.1021/acsenergylett.0c02692 |
| 9. | Campos da Paixão, I.; Cardozo, J. C.; Sales Monteiro, M. K.; Gondim, A. D.; Cavalcanti, L. N.; Fabiano de Santana Souza, D.; Martínez-Huitle, C. A.; Vieira dos Santos, E. RSC Adv. 2023, 13, 35755–35765. doi:10.1039/d3ra05772k |
| 10. | Gao, X.; Tong, X.; Liu, R.; Zhang, Y. Catal. Sci. Technol. 2023, 13, 6132–6136. doi:10.1039/d3cy01152f |
| 11. | Abu-Omar, M. M.; Ford, P. C. RSC Sustainability 2023, 1, 1686–1703. doi:10.1039/d3su00117b |
| 12. | Yuan, K.; Zhao, W.; Zhou, Y.; Zhang, X.; Chen, S.; Chen, J.; Liu, C.; Xiong, W. ACS Sustainable Chem. Eng. 2023, 11, 17769–17777. doi:10.1021/acssuschemeng.3c05865 |
| 13. | Biermann, U.; Bornscheuer, U.; Meier, M. A. R.; Metzger, J. O.; Schäfer, H. J. Angew. Chem., Int. Ed. 2011, 50, 3854–3871. doi:10.1002/anie.201002767 |
| 14. | Len, C.; Duhan, V.; Ouyang, W.; Nguyen, R.; Lochab, B. Front. Chem. (Lausanne, Switz.) 2023, 11, 1306182. doi:10.3389/fchem.2023.1306182 |
| 68. | Wang, L.; Wu, F.; Chen, J.; Nicewicz, D. A.; Huang, Y. Angew. Chem., Int. Ed. 2017, 56, 6896–6900. doi:10.1002/anie.201702940 |
| 69. | Hu, X.; Zhang, G.; Bu, F.; Lei, A. Angew. Chem., Int. Ed. 2018, 57, 1286–1290. doi:10.1002/anie.201711359 |
| 70. | Zhang, G.; Lin, Y.; Luo, X.; Hu, X.; Chen, C.; Lei, A. Nat. Commun. 2018, 9, 1225. doi:10.1038/s41467-018-03534-z |
| 3. | Tenhumberg, N.; Büttner, H.; Schäffner, B.; Kruse, D.; Blumenstein, M.; Werner, T. Green Chem. 2016, 18, 3775–3788. doi:10.1039/c6gc00671j |
| 4. | Mori, R. RSC Sustainability 2023, 1, 179–212. doi:10.1039/d2su00014h |
| 5. | Lakshmi, D. D.; Yogita; Srinivasa Rao, B.; Lingaiah, N. Sustainable Energy Fuels 2024, 8, 43–53. doi:10.1039/d3se01096a |
| 59. | Mance, A. D.; Jakopčić, K. Mol. Diversity 2005, 9, 229–231. doi:10.1007/s11030-005-3434-8 |
| 60. | Marullo, S.; Meli, A.; D’Anna, F. ACS Sustainable Chem. Eng. 2020, 8, 4889–4899. doi:10.1021/acssuschemeng.0c00193 |
| 61. | Kumar, A.; Pawar, S. S. Sci. China: Chem. 2012, 55, 1633–1637. doi:10.1007/s11426-012-4684-9 |
| 136. | Stewart, J. J. P. J. Mol. Model. 2007, 13, 1173–1213. doi:10.1007/s00894-007-0233-4 |
| 2. | Ragothaman, A.; Anderson, W. A. Environments 2017, 4, 66. doi:10.3390/environments4030066 |
| 62. | Firth, J. D.; Craven, P. G. E.; Lilburn, M.; Pahl, A.; Marsden, S. P.; Nelson, A. Chem. Commun. 2016, 52, 9837–9840. doi:10.1039/c6cc04662b |
| 63. | Blanpain, A.; Clark, J. H.; Farmer, T. J.; Guo, Y.; Ingram, I. D. V.; Kendrick, J. E.; Lawrenson, S. B.; North, M.; Rodgers, G.; Whitwood, A. C. ChemSusChem 2019, 12, 2393–2401. doi:10.1002/cssc.201900748 |
| 64. | Xu, F.; Li, Z.; Zhang, L.-L.; Liu, S.; Li, H.; Liao, Y.; Yang, S. Green Chem. 2023, 25, 3297–3305. doi:10.1039/d2gc04786a |
| 14. | Len, C.; Duhan, V.; Ouyang, W.; Nguyen, R.; Lochab, B. Front. Chem. (Lausanne, Switz.) 2023, 11, 1306182. doi:10.3389/fchem.2023.1306182 |
| 34. | Soutelo-Maria, A.; Dubois, J.-L.; Couturier, J.-L.; Cravotto, G. Catalysts 2018, 8, 464. doi:10.3390/catal8100464 |
| 35. | Saito, S.; Nanba, Y.; Morita, M.; Kobayashi, Y. Synlett 2019, 30, 1085–1089. doi:10.1055/s-0037-1611809 |
| 36. | Hinzmann, A.; Druhmann, S. S.; Gröger, H. Sustainable Chem. 2020, 1, 275–289. doi:10.3390/suschem1030018 |
| 37. | Bertolini, V.; Pallavicini, M.; Tibhe, G.; Roda, G.; Arnoldi, S.; Monguzzi, L.; Zoccola, M.; Di Nardo, G.; Gilardi, G.; Bolchi, C. ACS Omega 2021, 6, 31901–31906. doi:10.1021/acsomega.1c04640 |
| 38. | Biermann, U.; Bornscheuer, U. T.; Feussner, I.; Meier, M. A. R.; Metzger, J. O. Angew. Chem., Int. Ed. 2021, 60, 20144–20165. doi:10.1002/anie.202100778 |
| 43. | Menges, N.; Şahin, E. ACS Sustainable Chem. Eng. 2014, 2, 226–230. doi:10.1021/sc400281h |
| 44. | Ishizuka, F.; Stenzel, M. H.; Zetterlund, P. B. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 831–839. doi:10.1002/pola.28958 |
| 45. | Noppawan, P.; Sangon, S.; Supanchaiyamat, N.; Hunt, A. J. Green Chem. 2021, 23, 5766–5774. doi:10.1039/d1gc00872b |
| 46. | Gevorgyan, A.; Hopmann, K. H.; Bayer, A. Green Chem. 2021, 23, 7219–7227. doi:10.1039/d1gc02311j |
| 47. | Gevorgyan, A.; Hopmann, K. H.; Bayer, A. Organometallics 2022, 41, 1777–1785. doi:10.1021/acs.organomet.1c00517 |
| 134. | Nadirova, M. A.; Pokazeev, K. M.; Kolesnik, I. A.; Dorovatovskii, P. V.; Bumagin, N. A.; Potkin, V. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2019, 55, 729–738. doi:10.1007/s10593-019-02528-z |
| 30. | Zhang, C.-h.; Li, X.-g.; Gao, Y.-g.; Zhang, L.-x.; Fu, X.-q. Chem. Res. Chin. Univ. 2007, 23, 176–182. doi:10.1016/s1005-9040(07)60037-3 |
| 31. | Chhikara, B. S.; St. Jean, N.; Mandal, D.; Kumar, A.; Parang, K. Eur. J. Med. Chem. 2011, 46, 2037–2042. doi:10.1016/j.ejmech.2011.02.056 |
| 32. | Mielczarek-Puta, M.; Struga, M.; Roszkowski, P. Med. Chem. Res. 2019, 28, 2153–2164. doi:10.1007/s00044-019-02443-0 |
| 33. | Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Eur. J. Pharmacol. 2020, 871, 172937. doi:10.1016/j.ejphar.2020.172937 |
| 48. | Xiao, Y.; Malhotra, S. V. Tetrahedron Lett. 2004, 45, 8339–8342. doi:10.1016/j.tetlet.2004.09.070 |
| 49. | Cott, D. J.; Ziegler, K. J.; Owens, V. P.; Glennon, J. D.; Graham, A. E.; Holmes, J. D. Green Chem. 2005, 7, 105–110. doi:10.1039/b408327j |
| 50. | Reinhardt, D.; Ilgen, F.; Kralisch, D.; König, B.; Kreisel, G. Green Chem. 2008, 10, 1170–1181. doi:10.1039/b807379a |
| 51. | Huertas, D.; Florscher, M.; Dragojlovic, V. Green Chem. 2009, 11, 91–95. doi:10.1039/b813485e |
| 52. | Chiappe, C.; Malvaldi, M.; Pomelli, C. S. Green Chem. 2010, 12, 1330–1339. doi:10.1039/c0gc00074d |
| 53. | Mirgane, N.; Kotwal, S.; Karnik, A. Cent. Eur. J. Chem. 2010, 8, 356–360. doi:10.2478/s11532-009-0136-6 |
| 54. | Seo, J.-M.; Baek, J.-B. Chem. Commun. 2014, 50, 14651–14653. doi:10.1039/c4cc07173e |
| 55. | Donatoni, M. C.; Junior, G. A. B.; de Oliveira, K. T.; Ando, R. A.; Brocksom, T. J.; Dos Santos, A. A. Tetrahedron 2014, 70, 3231–3238. doi:10.1016/j.tet.2014.02.017 |
| 56. | Parmar, N. J.; Parmar, B. D.; Sutariya, T. R.; Kant, R.; Gupta, V. K. Tetrahedron Lett. 2014, 55, 6060–6064. doi:10.1016/j.tetlet.2014.09.026 |
| 57. | Grosso, C.; Brigas, A.; de los Santos, J. M.; Palacios, F.; Lemos, A.; Pinho e Melo, T. M. V. D. Monatsh. Chem. 2019, 150, 1275–1288. doi:10.1007/s00706-019-02421-7 |
| 58. | Rammohan, A.; Krinochkin, A. P.; Khasanov, A. F.; Kopchuk, D. S.; Zyryanov, G. V. Top. Curr. Chem. 2022, 380, 43. doi:10.1007/s41061-022-00398-2 |
| 24. | Gregoritza, M.; Brandl, F. P. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. doi:10.1016/j.ejpb.2015.06.007 |
| 25. | Akbari Javar, R.; Bin Noordin, M. I.; Khoobi, M.; Ghaedi, A. J. Inorg. Organomet. Polym. 2020, 30, 2520–2532. doi:10.1007/s10904-020-01512-x |
| 26. | Fattahi, N.; Shahbazi, M.-A.; Maleki, A.; Hamidi, M.; Ramazani, A.; Santos, H. A. J. Controlled Release 2020, 326, 556–598. doi:10.1016/j.jconrel.2020.07.012 |
| 27. | Large, D. E.; Abdelmessih, R. G.; Fink, E. A.; Auguste, D. T. Adv. Drug Delivery Rev. 2021, 176, 113851. doi:10.1016/j.addr.2021.113851 |
| 28. | Wang, H.; Ullah, A. Polymers (Basel, Switz.) 2022, 14, 3436. doi:10.3390/polym14173436 |
| 29. | Huang, L.; Yang, J.; Wang, T.; Gao, J.; Xu, D. J. Nanobiotechnol. 2022, 20, 49. doi:10.1186/s12951-022-01257-4 |
| 133. | Tomberg, A.; De Cesco, S.; Huot, M.; Moitessier, N. Tetrahedron Lett. 2015, 56, 6852–6856. doi:10.1016/j.tetlet.2015.10.086 |
| 17. | Zhang, C.; Madbouly, S. A.; Kessler, M. R. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. doi:10.1021/am5071333 |
| 18. | Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. ACS Sustainable Chem. Eng. 2017, 5, 7365–7373. doi:10.1021/acssuschemeng.7b01672 |
| 19. | Bétron, C.; Cassagnau, P.; Bounor-Legaré, V. Mater. Chem. Phys. 2018, 211, 361–374. doi:10.1016/j.matchemphys.2018.02.038 |
| 20. | Smith, A. D.; Tennyson, A. G.; Smith, R. C. Sustainable Chem. 2020, 1, 209–237. doi:10.3390/suschem1030015 |
| 21. | Kirianchuk, V.; Demchuk, Z.; Polunin, Y.; Kohut, A.; Voronov, S.; Voronov, A. Molecules 2022, 27, 932. doi:10.3390/molecules27030932 |
| 22. | Gaglieri, C.; Alarcon, R. T.; de Moura, A.; Bannach, G. Curr. Res. Green Sustainable Chem. 2022, 5, 100343. doi:10.1016/j.crgsc.2022.100343 |
| 23. | Stavila, E.; Yuliati, F.; Adharis, A.; Laksmono, J. A.; Iqbal, M. RSC Adv. 2023, 13, 14747–14775. doi:10.1039/d3ra01913f |
| 39. | Chang, S. H.; Teng, T. T.; Ismail, N. J. Hazard. Mater. 2010, 181, 868–872. doi:10.1016/j.jhazmat.2010.05.093 |
| 40. | Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Molecules 2017, 22, 1474. doi:10.3390/molecules22091474 |
| 41. | Portillo‐López, R.; Morales‐Contreras, B. E.; Lozano‐Guzmán, E.; Basilio‐Heredia, J.; Muy‐Rangel, M. D.; Ochoa‐Martínez, L. A.; Rosas‐Flores, W.; Morales‐Castro, J. J. Food Sci. 2021, 86, 3122–3136. doi:10.1111/1750-3841.15815 |
| 42. | Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Ind. Crops Prod. 2022, 175, 114257. doi:10.1016/j.indcrop.2021.114257 |
| 110. | Naguib, M.; Rashed, A.; Keddie, D. J. J. Mater. Sci. 2021, 56, 8900–8909. doi:10.1007/s10853-021-05853-x |
| 107. | Yıldırım, A.; Mudaber, S.; Öztürk, S. Eur. J. Lipid Sci. Technol. 2019, 121, 1800303. doi:10.1002/ejlt.201800303 |
| 71. | Hudlicky, T.; Butora, G.; Fearnley, S. P.; Gum, A. G.; Persichini, P. J.; Stabile, M. R.; Merola, J. S. J. Chem. Soc., Perkin Trans. 1 1995, 2393–2398. doi:10.1039/p19950002393 |
| 72. | Mahmood, S. Y.; Lallemand, M.-C.; Sader-Bakaouni, L.; Charton, O.; Vérité, P.; Dufat, H.; Tillequin, F. Tetrahedron 2004, 60, 5105–5110. doi:10.1016/j.tet.2004.03.091 |
| 73. | Fokas, D.; Yu, L.; Baldino, C. M. Mol. Diversity 2005, 9, 81–89. doi:10.1007/s11030-005-1292-z |
| 74. | Takao, K.-i.; Munakata, R.; Tadano, K.-i. Chem. Rev. 2005, 105, 4779–4807. doi:10.1021/cr040632u |
| 75. | Zubkov, F. I.; Ershova, J. D.; Orlova, A. A.; Zaytsev, V. P.; Nikitina, E. V.; Peregudov, A. S.; Gurbanov, A. V.; Borisov, R. S.; Khrustalev, V. N.; Maharramov, A. M.; Varlamov, A. V. Tetrahedron 2009, 65, 3789–3803. doi:10.1016/j.tet.2009.02.024 |
| 76. | Zubkov, F. I.; Ershova, J. D.; Zaytsev, V. P.; Obushak, M. D.; Matiychuk, V. S.; Sokolova, E. A.; Khrustalev, V. N.; Varlamov, A. V. Tetrahedron Lett. 2010, 51, 6822–6824. doi:10.1016/j.tetlet.2010.10.046 |
| 77. | Leverett, C. A.; Li, G.; France, S.; Padwa, A. J. Org. Chem. 2016, 81, 10193–10203. doi:10.1021/acs.joc.6b00771 |
| 78. | Gulbrandsen, H. S.; Serigstad, H.; Lovell Read, M.; Joos, I.; Gundersen, L.-L. Eur. J. Org. Chem. 2019, 6044–6052. doi:10.1002/ejoc.201901000 |
| 79. | Zubkov, F. I.; Boltukhina, E. V.; Turchin, K. F.; Varlamov, A. V. Tetrahedron 2004, 60, 8455–8463. doi:10.1016/j.tet.2004.07.008 |
| 80. | Zubkov, F. I.; Nikitina, E. V.; Turchin, K. F.; Aleksandrov, G. G.; Safronova, A. A.; Borisov, R. S.; Varlamov, A. V. J. Org. Chem. 2004, 69, 432–438. doi:10.1021/jo0353684 |
| 81. | Zubkov, F. I.; Boltukhina, E. V.; Turchin, K. F.; Borisov, R. S.; Varlamov, A. V. Tetrahedron 2005, 61, 4099–4113. doi:10.1016/j.tet.2005.02.017 |
| 82. | Zubkov, F. I.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Rev. 2005, 74, 639–669. doi:10.1070/rc2005v074n07abeh001180 |
| 83. | Zubkov, F. I.; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. Tetrahedron 2011, 67, 9148–9163. doi:10.1016/j.tet.2011.09.099 |
| 84. | Zubkov, F. I.; Zaytsev, V. P.; Airiyan, I. K.; Golubev, V. D.; Puzikova, E. S.; Sorokina, E. A.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Bull. 2012, 61, 600–605. doi:10.1007/s11172-012-0087-5 |
| 85. | Zaytsev, V. P.; Zubkov, F. I.; Mertsalov, D. F.; Orlova, D. N.; Sorokina, E. A.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Bull. 2015, 64, 112–126. doi:10.1007/s11172-015-0829-2 |
| 86. | Read, M. L.; Krapp, A.; Miranda, P. O.; Gundersen, L.-L. Tetrahedron 2012, 68, 1869–1885. doi:10.1016/j.tet.2011.12.079 |
| 87. | Zubkov, F. I.; Nikitina, E. V.; Galeev, T. R.; Zaytsev, V. P.; Khrustalev, V. N.; Novikov, R. A.; Orlova, D. N.; Varlamov, A. V. Tetrahedron 2014, 70, 1659–1690. doi:10.1016/j.tet.2014.01.008 |
| 88. | Zubkov, F. I.; Orlova, D. N.; Zaytsev, V. P.; Voronov, A. A.; Nikitina, E. V.; Khrustalev, V. N.; Novikov, R. A.; Krasavin, M.; Varlamov, A. V. Curr. Org. Synth. 2017, 14, 733–746. doi:10.2174/1570179414666161116123221 |
| 89. | Nadirova, M. A.; Khanova, A. V.; Zubkov, F. I.; Mertsalov, D. F.; Kolesnik, I. A.; Petkevich, S. K.; Potkin, V. I.; Shetnev, A. A.; Presnukhina, S. I.; Sinelshchikova, A. A.; Grigoriev, M. S.; Zaytsev, V. P. Tetrahedron 2021, 85, 132032. doi:10.1016/j.tet.2021.132032 |
| 90. | Gulbrandsen, H. S.; Alfaro, J. L. D.; Read, M. L.; Gundersen, L.-L. Eur. J. Org. Chem. 2017, 2305–2311. doi:10.1002/ejoc.201700180 |
| 91. | Osano, M.; Jhaveri, D. P.; Wipf, P. Org. Lett. 2020, 22, 2215–2219. doi:10.1021/acs.orglett.0c00417 |
| 92. | Mertsalov, D. F.; Lovtsevich, L. V.; Shelukho, E. R.; Kolesnik, I. A.; Petkevich, S. K.; Potkin, V. I.; Zaytsev, V. P. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2021, 57, 1031–1036. doi:10.1007/s10593-021-03018-x |
| 93. | Yarovaya, O. I.; Kovaleva, K. S.; Zaykovskaya, A. A.; Yashina, L. N.; Scherbakova, N. S.; Scherbakov, D. N.; Borisevich, S. S.; Zubkov, F. I.; Antonova, A. S.; Peshkov, R. Y.; Eltsov, I. V.; Pyankov, O. V.; Maksyutov, R. A.; Salakhutdinov, N. F. Bioorg. Med. Chem. Lett. 2021, 40, 127926. doi:10.1016/j.bmcl.2021.127926 |
| 94. | Golubev, P.; Pankova, A.; Krasavin, M. Tetrahedron Lett. 2019, 60, 1578–1581. doi:10.1016/j.tetlet.2019.05.018 |
| 95. | Sarang, P. S.; Yadav, A. A.; Patil, P. S.; Krishna, U. M.; Trivedi, G. K.; Salunkhe, M. M. Synthesis 2007, 1091–1095. doi:10.1055/s-2007-965950 |
| 96. | Mir, A. A.; Mulwad, V. V.; Trivedi, G. K. J. Heterocycl. Chem. 2010, 47, 214–218. doi:10.1002/jhet.274 |
| 97. | Zubkov, F. I.; Airiyan, I. K.; Ershova, J. D.; Galeev, T. R.; Zaytsev, V. P.; Nikitina, E. V.; Varlamov, A. V. RSC Adv. 2012, 2, 4103–4109. doi:10.1039/c2ra20295f |
| 98. | Zaytsev, V. P.; Mertsalov, D. F.; Trunova, A. M.; Khanova, A. V.; Nikitina, E. V.; Sinelshchikova, A. A.; Grigoriev, M. S. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2020, 56, 930–935. doi:10.1007/s10593-020-02752-y |
| 99. | Sauer, J.; Sustmann, R. Angew. Chem., Int. Ed. Engl. 1980, 19, 779–807. doi:10.1002/anie.198007791 |
| 100. | Rideout, D. C.; Breslow, R. J. Am. Chem. Soc. 1980, 102, 7816–7817. doi:10.1021/ja00546a048 |
| 101. | Breslow, R.; Maitra, U.; Rideout, D. Tetrahedron Lett. 1983, 24, 1901–1904. doi:10.1016/s0040-4039(00)81801-8 |
| 102. | Blokzijl, W.; Blandamer, M. J.; Engberts, J. B. F. N. J. Am. Chem. Soc. 1991, 113, 4241–4246. doi:10.1021/ja00011a029 |
| 103. | Blokzijl, W.; Engberts, J. B. F. N. Angew. Chem., Int. Ed. Engl. 1993, 32, 1545–1579. doi:10.1002/anie.199315451 |
| 104. | Moulay, S.; Touati, A. C. R. Chim. 2010, 13, 1474–1511. doi:10.1016/j.crci.2010.05.025 |
| 105. | Otto, S.; Engberts, J. B. F. N.; Kwak, J. C. T. J. Am. Chem. Soc. 1998, 120, 9517–9525. doi:10.1021/ja9816537 |
| 106. | Rispens, T.; Engberts, J. B. F. N. J. Org. Chem. 2002, 67, 7369–7377. doi:10.1021/jo0260802 |
| 125. | Candy, L.; Vaca‐Garcia, C.; Borredon, E. J. Am. Oil Chem. Soc. 2005, 82, 271–277. doi:10.1007/s11746-005-1066-5 |
| 126. | Eschig, S.; Philipp, C.; Salthammer, T. Eur. J. Lipid Sci. Technol. 2013, 115, 101–110. doi:10.1002/ejlt.201200056 |
| 119. | Varlamov, A. V.; Boltukhina, E. V.; Zubkov, F. I.; Sidorenko, N. V.; Chernyshev, A. I.; Grudinin, D. G. Chem. Heterocycl. Compd. 2004, 40, 22–28. doi:10.1023/b:cohc.0000023763.75894.63 |
| 127. | Nelson, W. L.; Allen, D. R. J. Heterocycl. Chem. 1972, 9, 561–568. doi:10.1002/jhet.5570090317 |
| 128. | Fischer, K.; Huenig, S. J. Org. Chem. 1987, 52, 564–569. doi:10.1021/jo00380a016 |
| 117. | De Cesco, S.; Deslandes, S.; Therrien, E.; Levan, D.; Cueto, M.; Schmidt, R.; Cantin, L.-D.; Mittermaier, A.; Juillerat-Jeanneret, L.; Moitessier, N. J. Med. Chem. 2012, 55, 6306–6315. doi:10.1021/jm3002839 |
| 95. | Sarang, P. S.; Yadav, A. A.; Patil, P. S.; Krishna, U. M.; Trivedi, G. K.; Salunkhe, M. M. Synthesis 2007, 1091–1095. doi:10.1055/s-2007-965950 |
| 118. | Takano, S.; Oshima, Y.; Ito, F.; Ogasawara, K. Yakugaku Zasshi 1980, 100, 1194–1202. doi:10.1248/yakushi1947.100.12_1194 |
| 119. | Varlamov, A. V.; Boltukhina, E. V.; Zubkov, F. I.; Sidorenko, N. V.; Chernyshev, A. I.; Grudinin, D. G. Chem. Heterocycl. Compd. 2004, 40, 22–28. doi:10.1023/b:cohc.0000023763.75894.63 |
| 120. | Gordon, C. P.; Byrne, N.; McCluskey, A. Green Chem. 2010, 12, 1000–1006. doi:10.1039/b924835h |
| 121. | Papeo, G.; Posteri, H.; Borghi, D.; Busel, A. A.; Caprera, F.; Casale, E.; Ciomei, M.; Cirla, A.; Corti, E.; D’Anello, M.; Fasolini, M.; Forte, B.; Galvani, A.; Isacchi, A.; Khvat, A.; Krasavin, M. Y.; Lupi, R.; Orsini, P.; Perego, R.; Pesenti, E.; Pezzetta, D.; Rainoldi, S.; Riccardi-Sirtori, F.; Scolaro, A.; Sola, F.; Zuccotto, F.; Felder, E. R.; Donati, D.; Montagnoli, A. J. Med. Chem. 2015, 58, 6875–6898. doi:10.1021/acs.jmedchem.5b00680 |
| 122. | Hizartzidis, L.; Gilbert, J.; Gordon, C. P.; Sakoff, J. A.; McCluskey, A. ChemMedChem 2019, 14, 1152–1161. doi:10.1002/cmdc.201900180 |
| 123. | Naguib, M.; Yassin, M. A. ACS Appl. Polym. Mater. 2022, 4, 2181–2188. doi:10.1021/acsapm.2c00158 |
| 124. | Bilović, D. Croat. Chem. Acta 1968, 40, 15–22. |
| 113. | Alvarenga, B. R., Jr.; Xavier, F. A. N.; Soares, F. L. F.; Carneiro, R. L. Food Anal. Methods 2018, 11, 1969–1976. doi:10.1007/s12161-018-1160-y |
| 114. | Hernqvist, L. Eur. J. Lipid Sci. Technol. 1984, 86, 297–300. doi:10.1002/lipi.19840860802 |
| 115. | Larsson, K. Eur. J. Lipid Sci. Technol. 1972, 74, 136–142. doi:10.1002/lipi.19720740302 |
| 116. | Hernqvist, L. Food Struct. 1990, 9, 39–44. |
| 83. | Zubkov, F. I.; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. Tetrahedron 2011, 67, 9148–9163. doi:10.1016/j.tet.2011.09.099 |
| 108. | Rani, P. J.; Thirumaran, S. Eur. J. Med. Chem. 2013, 62, 139–147. doi:10.1016/j.ejmech.2012.12.047 |
| 109. | Selvaganapathi, P.; Thirumaran, S.; Ciattini, S. Appl. Organomet. Chem. 2019, 33, e5089. doi:10.1002/aoc.5089 |
| 110. | Naguib, M.; Rashed, A.; Keddie, D. J. J. Mater. Sci. 2021, 56, 8900–8909. doi:10.1007/s10853-021-05853-x |
| 111. | Li, F.; Xu, Y.; Xu, Y.; Wang, C.; Ma, J.; Chen, P.; Wang, L. Mol. Catal. 2022, 529, 112576. doi:10.1016/j.mcat.2022.112576 |
© 2024 Yıldırım; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.