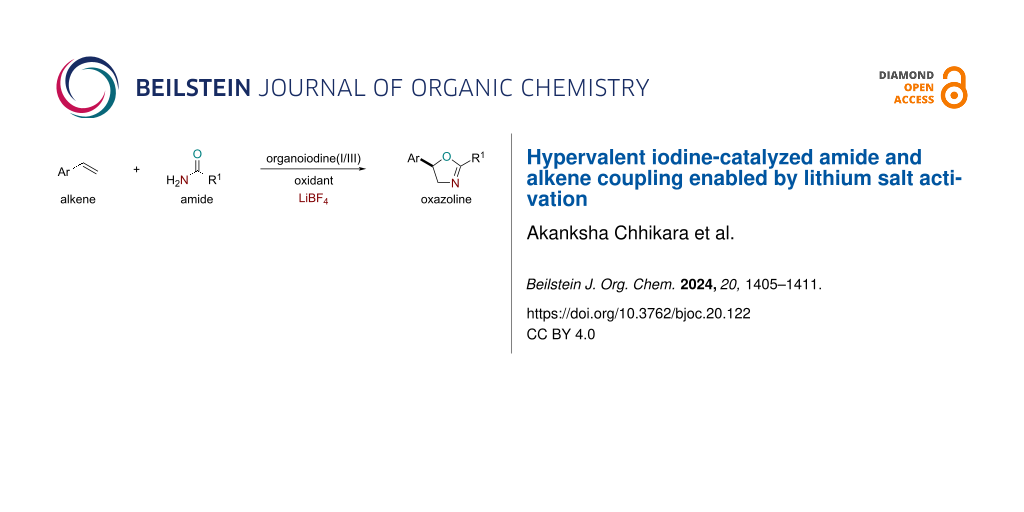
Abstract
Hypervalent iodine catalysis has been widely utilized in olefin functionalization reactions. Intermolecularly, the regioselective addition of two distinct nucleophiles across the olefin is a challenging process in hypervalent iodine catalysis. We introduce here a unique strategy using simple lithium salts for hypervalent iodine catalyst activation. The activated hypervalent iodine catalyst allows the intermolecular coupling of soft nucleophiles such as amides onto electronically activated olefins with high regioselectivity.
Graphical Abstract
Introduction
Hypervalent iodine(III) reagents, also known as λ3–iodanes, have been well established and used in organic synthesis for the past decades [1-5]. The pioneering works of Fuchigami and Fugita, Ochiai, Kita, and later the development of chiral hypervalent iodines by Wirth, Kita, Ishihara, Muñiz, and many others, have firmly established these reagents as useful catalysts for a wide variety of chemical transformations [6-17]. A number of features, including low toxicity, high stability, ease of handling, and versatile reactivity, etc. render these catalysts highly attractive for adoption in organic synthesis. In particular, the field of olefin difunctionalization, known for its rapid assembly of molecular complexity, has been a fertile ground for innovation for hypervalent iodine catalysis, which often involves the catalytic use of an iodoarene with stoichiometric oxidants such as MCPBA, Selectfluor, etc. [18-20]. Earlier and recent hypervalent iodine-catalyzed olefin halofunctionalizations by several groups have predicated on the use of intramolecular olefin substrates tethered with a nucleophile to avoid the lack of regiochemical additions (Scheme 1a) [21-28]. Intermolecular hypervalent iodine-catalyzed olefin difunctionalizations have been realized for olefin dihalogenation, dioxygenation and diamination reactions, where often the same type of nucleophiles were incorporated (Scheme 1b) [29-40]. Intermolecular hypervalent iodine catalysis with the regioselective additions of two distinct nucleophilic functionalities across an olefin, however, remains challenging with limited solutions [41-46]. Notably, an interesting work by Hashimoto has recently enabled the intermolecular addition of N-(fluorosulfonyl)-protected carbamates as oxyamination reagents across a variety of olefin structures [47]. This work engages the hypervalent iodine catalyst in an anionic ligand exchange with the substrate, which then partitions into an ion pair suitable for olefin activation, followed by the addition of the bifunctional anionic carbamate (Scheme 1c).
Scheme 1: Hypervalent iodine-catalyzed olefin difunctionalizations background.
Scheme 1: Hypervalent iodine-catalyzed olefin difunctionalizations background.
Our hypothesis here aims to directly access the reactivity of the cationic hypervalent iodine catalyst through an initial activation first, which we reason will then enable soft nucleophiles such as unadorned amides to readily participate in the ensuing olefin addition. In this regard, we wondered if the hypervalent iodine with difluoro ligands could undergo salt metathesis with lithium salts such as LiBF4 or LiPF6 to afford the more reactive cationic hypervalent iodine catalyst. The cationic hypervalent iodine catalyst could then activate the olefin to allow the addition of bifunctional nucleophiles such as an amide to achieve an overall olefin oxyamination process. We have previously reported a series of iodide-catalyzed processes, in which the electrophilicity of the halogen source could be modulated to render different classes of nucleophiles for additions onto olefins in various olefin difunctionalization reactions [48-52]. In particular, we demonstrated that addition of either a Lewis acid or a base could activate amides to couple with alkenes regioselectively to furnish their respective oxazoline regioisomer (Scheme 1d). Herein, we report that lithium salts such as LiBF4 or LiPF6, which are often used in lithium-ion batteries, can be used to activate hypervalent iodine catalysts to enable olefin oxyamination reactions with simple bifunctional amide nucleophiles (Scheme 1e).
Results and Discussion
Our studies here focused on the development of hypervalent iodine-catalyzed amide and alkene coupling reaction [53-55]. In this case, we started with styrene (1) and benzamide (2) as the standard substrates. Using iodotoluene A as the hypervalent iodine catalyst precursor, Selectfluor as the oxidant, and LiBF4 as the lithium salt for hypervalent iodine activation, we were gratified to observe the formation of the desired oxazoline 3 in 59% yield as the major regioisomer in nitromethane (MeNO2) solvent (Table 1, entry 1). To further improve the reaction efficiency, we screened several additional parameters including solvents and concentration. In these cases, we found that while both acetonitrile and MeNO2 (0.25 M) were suitable solvents, other solvents in general afforded no product formation (Table 1, entries 2–6). Lower catalyst loading and longer reaction time did not improve the overall reaction efficiency (Table 1, entries 6–8). Furthermore, we evaluated several salt additives containing different counterions, and found that LiBF4 was the optimal additive (Table 1, entries 7, 9, and 10). The optimal conditions were shown in entry 7 in Table 1, resulting in the formation of the desired oxazoline product 3 in 61% isolated yield. Control reactions in the absence of either the precatalyst or oxidant afforded no product formation (Table 1, entries 11 and 12). The control reaction in the absence of the lithium salt only afforded 8% of the oxazoline product 3 (Table 1, entry 13). These reactions validated the critical roles of each individual component to achieve an efficient reaction.
Table 1: Amide and alkene reaction optimization studies.
|
|
|||||
| entrya | precatalyst (mol %) | solvent (M) | additive (mol %) | yield (%)c | rr |
| 1 | A (20) | MeNO2 (0.5) | LiBF4 (100) | 59 | >95:5 |
| 2 | A (20) | MeCN (0.5) | LiBF4 (100) | 41 | 94:6 |
| 3 | A (20) | MeOH (0.5) | LiBF4 (100) | 0 | – |
| 4 | A (20) | DMF (0.5) | LiBF4 (100) | 0 | – |
| 5 | A (20) | MeNO2 (0.3) | LiBF4 (100) | 62 | >95:5 |
| 6b | A (20) | MeNO2 (0.25) | LiBF4 (100) | 64 | >95:5 |
| 7 | A (20) | MeNO2 (0.25) | LiBF4 (100) | 65 (61) | >95:5 |
| 8 | A (15) | MeNO2 (0.5) | LiBF4 (100) | 55 | >95:5 |
| 9 | A (20) | MeNO2 (0.5) | LiPF6 (100) | 56 | >95:5 |
| 10 | A (20) | MeNO2 (0.5) | AgBF4 (100) | 12 | >95:5 |
| 11 | – | MeNO2 (0.5) | LiBF4 (100) | 0 | – |
| 12d | A (20) | MeNO2 (0.5) | LiBF4 (100) | 0 | – |
| 13 | A (20) | MeNO2 (0.5) | – | 8 | >95:5 |
aOptimized conditions: styrene (1, 0.25 mmol), iodotoluene A (20 mol %), LiBF4 (100 mol %), Selectfluor (150 mol %), benzamide (2, 400 mol %), MeNO2 (0.25 M), rt, 16 h. Yields were determined by crude 1H NMR using 1,3-benzodioxole as the internal standard. bReaction time is 24 hours. cThe yield in parenthesis is isolated yield. dNo Selectfluor added.
To understand this coupling reaction better, we have also performed time studies to elucidate the effects of several key features in this reaction. First, we studied the iodoarene catalyst precursor and the lithium salt in terms of their effects on the overall reaction rate. In this case, we observed that the overall reaction proceeded faster with the more electron-rich iodoarene catalysts than electron-poor ones. Qualitatively, the electron-rich iodoarene catalysts are likely to be worse at activating the olefins than the electron-deficient hypervalent iodine catalysts. Therefore, the faster rate with the electron-rich catalyst precursor is because the electron-rich iodoarene catalyst precursors are more easily oxidized to the hypervalent iodine catalyst with difluoro ligands. Interestingly, the use of different lithium salts also impacted the overall reaction rate, with the reaction using the less coordinating LiAsF6 salt proceeding faster than LiPF6 and LiBF4. This time study suggested that the hypervalent iodine precatalyst with the less coordinating counterion is more reactive to activate the olefin. We also conducted kinetic studies on how the olefin and amide structures impacted the overall reaction rate. The more electron-rich olefins generally proceeded faster than the electron-poor ones, suggesting that a significant positive charge was likely built up on the olefin prior to the nucleophilic addition. On the other hand, the electronic nature of the para-substituted benzamides had little impact on the overall reaction rate as both electron-rich and electron-deficient benzamides proceeded with similar kinetic profiles. All the kinetic plots are shown in Figure 1.
![[1860-5397-20-122-1]](/bjoc/content/figures/1860-5397-20-122-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Time studies of the amide and alkene coupling. a) Iodoarene time studies: styrene (1), para-substituted iodoarenes, LiBF4, and benzamide (2). b) Li salt time studies: styrene (1), iodoanisole, Li salts, and benzamide (2). c) Alkene time studies: para-substituted styrenes, iodotoluene A, LiBF4, and benzamide (2). d) Benzamide time studies: styrene (1), iodotoluene A, LiBF4, and para-substituted benzamides.
Figure 1: Time studies of the amide and alkene coupling. a) Iodoarene time studies: styrene (1), para-substit...
With the optimized conditions and kinetic information in hand, we turned our attention to the amide substrate scope. In this case, both electron-rich and -deficient benzamides proceeded to the desired products in reasonable yields and high regioselectivities (Figure 2, products 4–7). Concurring with our kinetic data, the electronic nature of the amide bears little impact on the overall reaction rate and in this case, on the final yields as well. Similarly, ortho- and meta-substituted benzamides with halogen functionalities could also generate the desired oxazoline products with reasonable yields (Figure 2, products 8–10). Heteroaromatic amides could also furnish the oxazolines 11 and 12 with good efficiency. Naphthaleneamide also generated the desired product 13, albeit with slightly lower efficiency. Interestingly, o- and m-methyl-substituted benzamides provided a significant yield boost to provide the oxazoline structures 14 and 15. Finally, sterically encumbered tertiary amides participated in the reaction to afford the respective regioisomeric product 16.
Figure 2: Amide substrate scope studies. a) Standard conditions: styrene (0.25 mmol), iodotoluene (20 mol %), LiBF4 (100 mol %), Selectfluor (150 mol %), amide (400 mol %), MeNO2 (0.25 M), rt, 16 h. b) Iodoanisole (20 mol %).
Figure 2: Amide substrate scope studies. a) Standard conditions: styrene (0.25 mmol), iodotoluene (20 mol %),...
Encouraged by these results, we then turned our attention to explore the extent of alkene substrate scope using 3,4-dimethylbenzamide, which afforded the oxazoline product 17 in 70% yield (Figure 3). Based on this optimal amide structure, we examined various electronically activated olefins under the optimal reaction conditions. A number of styrenyl derivatives with para-substituted halogens, ester, and phthalimide proceeded smoothly with good yields and excellent regioselectivities to access the oxazoline products as single regioisomers (Figure 3, products 18–22). The o-bromo-substituted styrene also afforded the corresponding product 23. Furthermore, 1,1-di-substituted α-methylstyrene and α-phenylstyrene produced the respective oxazoline products with high regioselectivity and reasonable yields using iodoanisole as the catalyst precursor (Figure 3, products 24 and 25).
Figure 3: Alkene substrate scope studies. a) Standard conditions: alkene (0.25 mmol), iodotoluene (20 mol %), LiBF4 (100 mol %), Selectfluor (150 mol %), 3,4-dimethylbenzamide (400 mol %), MeNO2 (0.25 M), rt, 16 h. b) Iodoanisole (20 mol %), MeCN (0.25 M).
Figure 3: Alkene substrate scope studies. a) Standard conditions: alkene (0.25 mmol), iodotoluene (20 mol %),...
The proposed catalytic cycle (Figure 4) begins with iodotoluene A which is oxidized by Selectfluor salt into the difluorinated iodotoluene B. Then, LiBF4 can perform a salt metathesis with B to produce LiF along with the active hypervalent iodoarene catalyst C. The activated hypervalent iodine catalyst C can coordinate to the alkene to form complex D. The nucleophilic oxygen of the amide will attack in the internal position and subsequent cyclization will furnish the desired oxazoline.
Figure 4: Proposed catalytic cycle for the hypervalent iodine-catalyzed amide and alkene coupling.
Figure 4: Proposed catalytic cycle for the hypervalent iodine-catalyzed amide and alkene coupling.
Conclusion
We have developed a hypervalent iodine-catalyzed amide and alkene coupling reaction. This reaction protocol furnished useful oxazoline products and introduced the use of lithium salts to activate hypervalent iodine catalysts. This strategy rendered the participation of simple and unadorned amides as bifunctional nucleophiles to achieve olefin oxyamination reactions. Time studies of these reactions further unveiled interesting mechanistic features that will be useful for our future catalysis development and asymmetric reaction designs.
Supporting Information
| Supporting Information File 1: Spectral characterization of the products and kinetic studies. | ||
| Format: PDF | Size: 1.6 MB | Download |
Acknowledgements
We thank Dr. Yong W. Kim (University of Toledo) for NMR assistance. Mr. Babatunde Obadawo (University of Toledo) is acknowledged for collecting the high-resolution mass spectrometry data. We acknowledge Dr. Navdeep Kaur’s Ph. D. for her contributions in her Ph. D. thesis titled “Selective Conversion of Chemical Feedstock to O- and N-Containing Heterocycles”.
Funding
We thank the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM139156 for supporting this work. We thank the University of Toledo for an internal seed grant from the Summer Research Awards and Fellowship Programs for supporting our initial work.
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] -
Wirth, T. Top. Curr. Chem. 2003, 224, 1–4.
Return to citation in text: [1] -
Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] -
Ochiai, M.; Miyamoto, K. Eur. J. Org. Chem. 2008, 4229–4239. doi:10.1002/ejoc.200800416
Return to citation in text: [1] -
Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e
Return to citation in text: [1] -
Fuchigami, T.; Fujita, T. J. Org. Chem. 1994, 59, 7190–7192. doi:10.1021/jo00103a003
Return to citation in text: [1] -
Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J. Am. Chem. Soc. 2005, 127, 12244–12245. doi:10.1021/ja0542800
Return to citation in text: [1] -
Dohi, T.; Maruyama, A.; Yoshimura, M.; Morimoto, K.; Tohma, H.; Kita, Y. Angew. Chem., Int. Ed. 2005, 44, 6193–6196. doi:10.1002/anie.200501688
Return to citation in text: [1] -
Hirt, U. H.; Spingler, B.; Wirth, T. J. Org. Chem. 1998, 63, 7674–7679. doi:10.1021/jo980475x
Return to citation in text: [1] -
Dohi, T.; Maruyama, A.; Takenaga, N.; Senami, K.; Minamitsuji, Y.; Fujioka, H.; Caemmerer, S. B.; Kita, Y. Angew. Chem., Int. Ed. 2008, 47, 3787–3790. doi:10.1002/anie.200800464
Return to citation in text: [1] -
Farid, U.; Wirth, T. Angew. Chem., Int. Ed. 2012, 51, 3462–3465. doi:10.1002/anie.201107703
Return to citation in text: [1] -
Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352
Return to citation in text: [1] -
Guilbault, A.-A.; Basdevant, B.; Wanie, V.; Legault, C. Y. J. Org. Chem. 2012, 77, 11283–11295. doi:10.1021/jo302393u
Return to citation in text: [1] -
Quindeau, S.; Lyvinec, G.; Marguerit, M.; Bathany, K.; Ozanne-Beaudenon, A.; Buffeteau, T.; Cavagnat, D.; Chénedé, A. Angew. Chem., Int. Ed. 2009, 48, 4605–4609. doi:10.1002/anie.200901039
Return to citation in text: [1] -
Ishihara, K.; Muñiz, K. Iodine Catalysis in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527829569
Return to citation in text: [1] -
Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154
Return to citation in text: [1] -
Romero, R. M.; Wöste, T. H.; Muñiz, K. Chem. – Asian J. 2014, 9, 972–983. doi:10.1002/asia.201301637
Return to citation in text: [1] -
Lee, J. H.; Choi, S.; Hong, K. B. Molecules 2019, 24, 2634. doi:10.3390/molecules24142634
Return to citation in text: [1] -
Ngatimin, M.; Frey, R.; Levens, A.; Nakano, Y.; Kowalczyk, M.; Konstas, K.; Hutt, O. E.; Lupton, D. W. Org. Lett. 2013, 15, 5858–5861. doi:10.1021/ol4029308
Return to citation in text: [1] -
Braddock, D. C.; Cansell, G.; Hermitage, S. A. Chem. Commun. 2006, 2483–2485. doi:10.1039/b604130b
Return to citation in text: [1] -
Fabry, D. C.; Stodulski, M.; Hoerner, S.; Gulder, T. Chem. – Eur. J. 2012, 18, 10834–10838. doi:10.1002/chem.201201232
Return to citation in text: [1] -
Kong, W.; Feige, P.; de Haro, T.; Nevado, C. Angew. Chem., Int. Ed. 2013, 52, 2469–2473. doi:10.1002/anie.201208471
Return to citation in text: [1] -
Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754–2760. doi:10.1039/c3sc53107d
Return to citation in text: [1] -
Alhalib, A.; Kamouka, S.; Moran, W. J. Org. Lett. 2015, 17, 1453–1456. doi:10.1021/acs.orglett.5b00333
Return to citation in text: [1] -
Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 140, 4797–4802. doi:10.1021/jacs.8b02143
Return to citation in text: [1] -
Woerly, E. M.; Banik, S. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 13858–13861. doi:10.1021/jacs.6b09499
Return to citation in text: [1] -
Stodulski, M.; Goetzinger, A.; Kohlhepp, S. V.; Gulder, T. Chem. Commun. 2014, 50, 3435–3438. doi:10.1039/c3cc49850f
Return to citation in text: [1] -
Zhao, Z.; Jameel, I.; Murphy, G. K. Synthesis 2019, 51, 2648–2659. doi:10.1055/s-0037-1611562
Return to citation in text: [1] -
Molnár, I. G.; Gilmour, R. J. Am. Chem. Soc. 2016, 138, 5004–5007. doi:10.1021/jacs.6b01183
Return to citation in text: [1] -
Banik, S. M.; Medley, J. W.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 5000–5003. doi:10.1021/jacs.6b02391
Return to citation in text: [1] -
Yan, J.; Wang, H.; Yang, Z.; He, Y. Synlett 2009, 2669–2672. doi:10.1055/s-0029-1217977
Return to citation in text: [1] -
Zhong, W.; Liu, S.; Yang, J.; Meng, X.; Li, Z. Org. Lett. 2012, 14, 3336–3339. doi:10.1021/ol301311e
Return to citation in text: [1] -
Haubenreisser, S.; Wöste, T. H.; Martínez, C.; Ishihara, K.; Muñiz, K. Angew. Chem., Int. Ed. 2016, 55, 413–417. doi:10.1002/anie.201507180
Return to citation in text: [1] -
Gelis, C.; Dumoulin, A.; Bekkaye, M.; Neuville, L.; Masson, G. Org. Lett. 2017, 19, 278–281. doi:10.1021/acs.orglett.6b03631
Return to citation in text: [1] -
Kong, A.; Blakey, S. B. Synthesis 2012, 44, 1190–1198. doi:10.1055/s-0031-1290591
Return to citation in text: [1] -
Mizar, P.; Laverny, A.; El-Sherbini, M.; Farid, U.; Brown, M.; Malmedy, F.; Wirth, T. Chem. – Eur. J. 2014, 20, 9910–9913. doi:10.1002/chem.201403891
Return to citation in text: [1] -
Röben, C.; Souto, J. A.; González, Y.; Lishchynskyi, A.; Muñiz, K. Angew. Chem., Int. Ed. 2011, 50, 9478–9482. doi:10.1002/anie.201103077
Return to citation in text: [1] -
Muñiz, K.; Barreiro, L.; Romero, R. M.; Martínez, C. J. Am. Chem. Soc. 2017, 139, 4354–4357. doi:10.1021/jacs.7b01443
Return to citation in text: [1] -
Qurban, J.; Elsherbini, M.; Wirth, T. J. Org. Chem. 2017, 82, 11872–11876. doi:10.1021/acs.joc.7b01571
Return to citation in text: [1] -
Ulmer, A.; Stodulski, M.; Kohlhepp, S. V.; Patzelt, C.; Pöthig, A.; Bettray, W.; Gulder, T. Chem. – Eur. J. 2015, 21, 1444–1448. doi:10.1002/chem.201405888
Return to citation in text: [1] -
Li, M.; Yu, F.; Qi, X.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2016, 55, 13843–13848. doi:10.1002/anie.201607248
Return to citation in text: [1] -
Qi, X.; Yu, F.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2017, 56, 12692–12696. doi:10.1002/anie.201706401
Return to citation in text: [1] -
Yoshimura, A.; Middleton, K. R.; Todora, A. D.; Kastern, B. J.; Koski, S. R.; Maskaev, A. V.; Zhdankin, V. V. Org. Lett. 2013, 15, 4010–4013. doi:10.1021/ol401815n
Return to citation in text: [1] -
Xiang, C.; Li, T.; Yan, J. Synth. Commun. 2014, 44, 682–688. doi:10.1080/00397911.2013.834364
Return to citation in text: [1] -
Wata, C.; Hashimoto, T. J. Am. Chem. Soc. 2021, 143, 1745–1751. doi:10.1021/jacs.0c11440
Return to citation in text: [1] -
Gembreska, N. R.; Vogel, A. K.; Ziegelmeyer, E. C.; Cheng, E.; Wu, F.; Roberts, L. P.; Vesoulis, M. M.; Li, W. Synlett 2020, 32, 539–544. doi:10.1055/a-1277-8669
Return to citation in text: [1] -
Wu, F.; Stewart, S.; Ariyarathna, J. P.; Li, W. ACS Catal. 2018, 8, 1921–1925. doi:10.1021/acscatal.7b04060
Return to citation in text: [1] -
Wu, F.; Ariyarathna, J. P.; Alom, N.-E.; Kaur, N.; Li, W. Org. Lett. 2020, 22, 884–890. doi:10.1021/acs.orglett.9b04432
Return to citation in text: [1] -
Wu, F.; Alom, N.-E.; Ariyarathna, J. P.; Naß, J.; Li, W. Angew. Chem., Int. Ed. 2019, 58, 11676–11680. doi:10.1002/anie.201904662
Return to citation in text: [1] -
Wu, F.; Kaur, N.; Alom, N.-E.; Li, W. JACS Au 2021, 1, 734–741. doi:10.1021/jacsau.1c00103
Return to citation in text: [1] -
Gratia, S. S.; Vigneau, E. S.; Eltayeb, S.; Patel, K.; Meyerhoefer, T. J.; Kershaw, S.; Huang, V.; De Castro, M. Tetrahedron Lett. 2014, 55, 448–452. doi:10.1016/j.tetlet.2013.11.054
Return to citation in text: [1] -
Minakata, S.; Morino, Y.; Ide, T.; Oderaotoshi, Y.; Komatsu, M. Chem. Commun. 2007, 3279–3281. doi:10.1039/b706572h
Return to citation in text: [1] -
Mumford, E. M.; Hemric, B. N.; Denmark, S. E. J. Am. Chem. Soc. 2021, 143, 13408–13417. doi:10.1021/jacs.1c06750
Return to citation in text: [1]
| 1. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 2. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 3. | Wirth, T. Top. Curr. Chem. 2003, 224, 1–4. |
| 4. | Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689 |
| 5. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 29. | Stodulski, M.; Goetzinger, A.; Kohlhepp, S. V.; Gulder, T. Chem. Commun. 2014, 50, 3435–3438. doi:10.1039/c3cc49850f |
| 30. | Zhao, Z.; Jameel, I.; Murphy, G. K. Synthesis 2019, 51, 2648–2659. doi:10.1055/s-0037-1611562 |
| 31. | Molnár, I. G.; Gilmour, R. J. Am. Chem. Soc. 2016, 138, 5004–5007. doi:10.1021/jacs.6b01183 |
| 32. | Banik, S. M.; Medley, J. W.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 5000–5003. doi:10.1021/jacs.6b02391 |
| 33. | Yan, J.; Wang, H.; Yang, Z.; He, Y. Synlett 2009, 2669–2672. doi:10.1055/s-0029-1217977 |
| 34. | Zhong, W.; Liu, S.; Yang, J.; Meng, X.; Li, Z. Org. Lett. 2012, 14, 3336–3339. doi:10.1021/ol301311e |
| 35. | Haubenreisser, S.; Wöste, T. H.; Martínez, C.; Ishihara, K.; Muñiz, K. Angew. Chem., Int. Ed. 2016, 55, 413–417. doi:10.1002/anie.201507180 |
| 36. | Gelis, C.; Dumoulin, A.; Bekkaye, M.; Neuville, L.; Masson, G. Org. Lett. 2017, 19, 278–281. doi:10.1021/acs.orglett.6b03631 |
| 37. | Kong, A.; Blakey, S. B. Synthesis 2012, 44, 1190–1198. doi:10.1055/s-0031-1290591 |
| 38. | Mizar, P.; Laverny, A.; El-Sherbini, M.; Farid, U.; Brown, M.; Malmedy, F.; Wirth, T. Chem. – Eur. J. 2014, 20, 9910–9913. doi:10.1002/chem.201403891 |
| 39. | Röben, C.; Souto, J. A.; González, Y.; Lishchynskyi, A.; Muñiz, K. Angew. Chem., Int. Ed. 2011, 50, 9478–9482. doi:10.1002/anie.201103077 |
| 40. | Muñiz, K.; Barreiro, L.; Romero, R. M.; Martínez, C. J. Am. Chem. Soc. 2017, 139, 4354–4357. doi:10.1021/jacs.7b01443 |
| 21. | Ngatimin, M.; Frey, R.; Levens, A.; Nakano, Y.; Kowalczyk, M.; Konstas, K.; Hutt, O. E.; Lupton, D. W. Org. Lett. 2013, 15, 5858–5861. doi:10.1021/ol4029308 |
| 22. | Braddock, D. C.; Cansell, G.; Hermitage, S. A. Chem. Commun. 2006, 2483–2485. doi:10.1039/b604130b |
| 23. | Fabry, D. C.; Stodulski, M.; Hoerner, S.; Gulder, T. Chem. – Eur. J. 2012, 18, 10834–10838. doi:10.1002/chem.201201232 |
| 24. | Kong, W.; Feige, P.; de Haro, T.; Nevado, C. Angew. Chem., Int. Ed. 2013, 52, 2469–2473. doi:10.1002/anie.201208471 |
| 25. | Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754–2760. doi:10.1039/c3sc53107d |
| 26. | Alhalib, A.; Kamouka, S.; Moran, W. J. Org. Lett. 2015, 17, 1453–1456. doi:10.1021/acs.orglett.5b00333 |
| 27. | Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 140, 4797–4802. doi:10.1021/jacs.8b02143 |
| 28. | Woerly, E. M.; Banik, S. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 13858–13861. doi:10.1021/jacs.6b09499 |
| 18. | Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154 |
| 19. | Romero, R. M.; Wöste, T. H.; Muñiz, K. Chem. – Asian J. 2014, 9, 972–983. doi:10.1002/asia.201301637 |
| 20. | Lee, J. H.; Choi, S.; Hong, K. B. Molecules 2019, 24, 2634. doi:10.3390/molecules24142634 |
| 6. | Ochiai, M.; Miyamoto, K. Eur. J. Org. Chem. 2008, 4229–4239. doi:10.1002/ejoc.200800416 |
| 7. | Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e |
| 8. | Fuchigami, T.; Fujita, T. J. Org. Chem. 1994, 59, 7190–7192. doi:10.1021/jo00103a003 |
| 9. | Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J. Am. Chem. Soc. 2005, 127, 12244–12245. doi:10.1021/ja0542800 |
| 10. | Dohi, T.; Maruyama, A.; Yoshimura, M.; Morimoto, K.; Tohma, H.; Kita, Y. Angew. Chem., Int. Ed. 2005, 44, 6193–6196. doi:10.1002/anie.200501688 |
| 11. | Hirt, U. H.; Spingler, B.; Wirth, T. J. Org. Chem. 1998, 63, 7674–7679. doi:10.1021/jo980475x |
| 12. | Dohi, T.; Maruyama, A.; Takenaga, N.; Senami, K.; Minamitsuji, Y.; Fujioka, H.; Caemmerer, S. B.; Kita, Y. Angew. Chem., Int. Ed. 2008, 47, 3787–3790. doi:10.1002/anie.200800464 |
| 13. | Farid, U.; Wirth, T. Angew. Chem., Int. Ed. 2012, 51, 3462–3465. doi:10.1002/anie.201107703 |
| 14. | Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352 |
| 15. | Guilbault, A.-A.; Basdevant, B.; Wanie, V.; Legault, C. Y. J. Org. Chem. 2012, 77, 11283–11295. doi:10.1021/jo302393u |
| 16. | Quindeau, S.; Lyvinec, G.; Marguerit, M.; Bathany, K.; Ozanne-Beaudenon, A.; Buffeteau, T.; Cavagnat, D.; Chénedé, A. Angew. Chem., Int. Ed. 2009, 48, 4605–4609. doi:10.1002/anie.200901039 |
| 17. | Ishihara, K.; Muñiz, K. Iodine Catalysis in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527829569 |
| 53. | Gratia, S. S.; Vigneau, E. S.; Eltayeb, S.; Patel, K.; Meyerhoefer, T. J.; Kershaw, S.; Huang, V.; De Castro, M. Tetrahedron Lett. 2014, 55, 448–452. doi:10.1016/j.tetlet.2013.11.054 |
| 54. | Minakata, S.; Morino, Y.; Ide, T.; Oderaotoshi, Y.; Komatsu, M. Chem. Commun. 2007, 3279–3281. doi:10.1039/b706572h |
| 55. | Mumford, E. M.; Hemric, B. N.; Denmark, S. E. J. Am. Chem. Soc. 2021, 143, 13408–13417. doi:10.1021/jacs.1c06750 |
| 48. | Gembreska, N. R.; Vogel, A. K.; Ziegelmeyer, E. C.; Cheng, E.; Wu, F.; Roberts, L. P.; Vesoulis, M. M.; Li, W. Synlett 2020, 32, 539–544. doi:10.1055/a-1277-8669 |
| 49. | Wu, F.; Stewart, S.; Ariyarathna, J. P.; Li, W. ACS Catal. 2018, 8, 1921–1925. doi:10.1021/acscatal.7b04060 |
| 50. | Wu, F.; Ariyarathna, J. P.; Alom, N.-E.; Kaur, N.; Li, W. Org. Lett. 2020, 22, 884–890. doi:10.1021/acs.orglett.9b04432 |
| 51. | Wu, F.; Alom, N.-E.; Ariyarathna, J. P.; Naß, J.; Li, W. Angew. Chem., Int. Ed. 2019, 58, 11676–11680. doi:10.1002/anie.201904662 |
| 52. | Wu, F.; Kaur, N.; Alom, N.-E.; Li, W. JACS Au 2021, 1, 734–741. doi:10.1021/jacsau.1c00103 |
| 47. | Wata, C.; Hashimoto, T. J. Am. Chem. Soc. 2021, 143, 1745–1751. doi:10.1021/jacs.0c11440 |
| 41. | Qurban, J.; Elsherbini, M.; Wirth, T. J. Org. Chem. 2017, 82, 11872–11876. doi:10.1021/acs.joc.7b01571 |
| 42. | Ulmer, A.; Stodulski, M.; Kohlhepp, S. V.; Patzelt, C.; Pöthig, A.; Bettray, W.; Gulder, T. Chem. – Eur. J. 2015, 21, 1444–1448. doi:10.1002/chem.201405888 |
| 43. | Li, M.; Yu, F.; Qi, X.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2016, 55, 13843–13848. doi:10.1002/anie.201607248 |
| 44. | Qi, X.; Yu, F.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2017, 56, 12692–12696. doi:10.1002/anie.201706401 |
| 45. | Yoshimura, A.; Middleton, K. R.; Todora, A. D.; Kastern, B. J.; Koski, S. R.; Maskaev, A. V.; Zhdankin, V. V. Org. Lett. 2013, 15, 4010–4013. doi:10.1021/ol401815n |
| 46. | Xiang, C.; Li, T.; Yan, J. Synth. Commun. 2014, 44, 682–688. doi:10.1080/00397911.2013.834364 |
© 2024 Chhikara et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.