Abstract
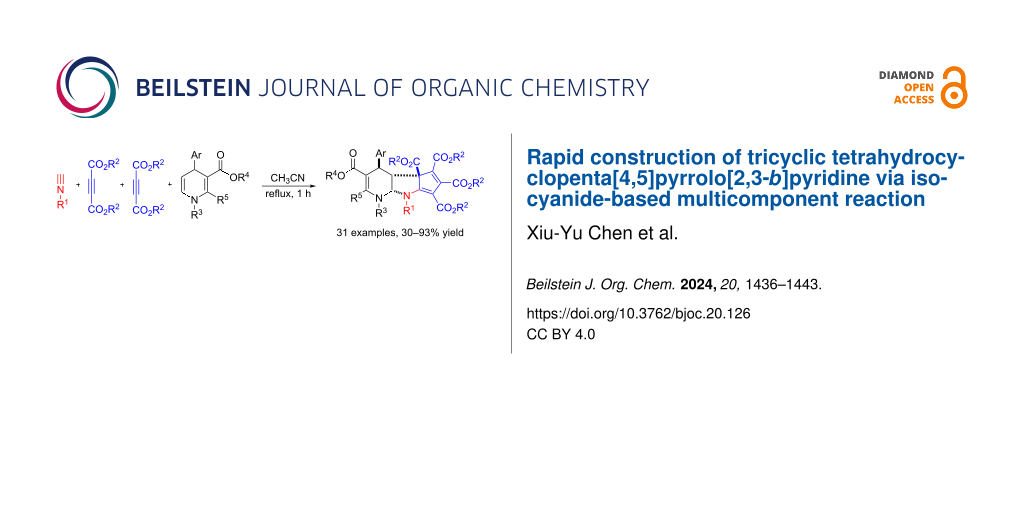
An efficient protocol for the synthesis of polyfunctionalized tetrahydrocyclopenta[4,5]pyrrolo[2,3-b]pyridine-3,4b,5,6,7(1H)-pentacarboxylates was developed by a three-component reaction. In the absence of any catalyst, the three-component reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 5,6-unsubstituted 1,4-dihydropyridines in refluxing acetonitrile afforded polyfunctionalized tetrahydrocyclopenta[4,5]pyrrolo[2,3-b]pyridine-3,4b,5,6,7(1H)-pentacarboxylates in high yields and with high diastereoselectivity. The reaction was finished by in situ generation of activated 5-(alkylimino)cyclopenta-1,3-dienes from addition of alkyl isocyanide to two molecules of but-2-ynedioates and sequential formal [3 + 2] cycloaddition reaction with 5,6-unsubstituted 1,4-dihydropyridine.
Graphical Abstract
Introduction
Isocyanide is a unique and attractive functional group in organic chemistry. The carbon atom of isocyanide has both a lone electron pair and empty orbitals, so it has outstanding electrophilic and nucleophilic reactivity. At the same time, isocyanide also has good coordination ability to coordinate with metals to form diverse metal complexes [1-3]. Therefore, isocyanides have been known as indispensable building blocks in modern organic chemistry. Many isocyanide-based carbon–carbon and carbon–heteroatom bond forming reactions have been developed in fascinating ways over the past decades [4-6]. The famous multicomponent reactions such as Passerini reaction, Ugi reaction, Orru reaction and Van Leusen reaction, in which isocyanides were employed as key substrates have become the most powerful tools for rapid construction of various nitrogen-containing organic compounds [7-14]. On the other hand, the reactive Huisgen’ 1,4-dipoles can be in situ generated by addition reaction of isocyanides to electron-deficient alkynes, which were sequentially trapped by various electrophiles and nucleophiles to give versatile acyclic and heterocyclic compounds [15-26]. In recent years, many multicomponent reactions based on alkyl isocyanides, electron-deficient alkynes and other reagents have been successfully developed for the synthesis of various carbocyclic and heterocyclic compounds [27-35].
The 5,6-unsubstituted 1,4-dihydropyridine is one of special kinds of 1,4-dihydropyridines. It can act as an activated enamino unit and electron-rich dienophile to take part in some synthetic reactions [36-39]. In recent years, 5,6-unsubstituted 1,4-dihydropyridines have been recognized as the reactive electron-rich dienophiles, which proceeded several Povarov reactions with various 1-aza-1,3-butadienes [40-44]. Recently, we have found that the three-component reaction of isoquinolines, dialkyl but-2-ynediaotes and 5,6-unsubstituted 1,4-dihydropyridines afforded functionalized isoquinolino[1,2-f][1,6]naphthyridines in good yields and with high diastereoselectivity via a [4 + 2] cycloaddition process [45]. Very recently, we also found that base-promoted [4 + 2] cycloaddition of salicyl N-tosylimines and 5,6-unsubstituted 1,4-dihydropyridines resulted in novel tetrahydrochromeno[3,2-b]pyridine derivatives in satisfactory yields [46]. Inspired by these efficient synthetic protocols and in continuation of our aim to develop isocyanide-based multicomponent reactions for construction of diverse nitrogen-containing heterocyclic compounds [47-58], herein we wish to report the mutlicomponent reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 5,6-unsubstituted dihydropyridines for the efficient synthesis of polyfunctionalized tetrahydrocyclopenta[4,5]pyrrolo[2,3-b]pyridine-3,4b,5,6,7(1H)-pentacarboxylates.
Results and Discussion
Initially, the reaction conditions were examined by employing cyclohexyl isocyanide (1a), dimethyl but-2-ynedioate (2a) and 5,6-unsubstituted dihydropyridine 3a as standard reaction. The main results are summarized in Table 1. The expected product was not observed when the three-component reaction was carried out in methanol, ethanol or tetrahydrofuran at room temperature (Table 1, entries 1–3). The reaction in toluene, methylene dichloride or acetonitrile at room temperature afforded an unexpected tricyclic compound 4a in 12–18% yields (Table 1, entries 4–6). 1H NMR spectra clearly indicated that two molecules of dimethyl but-2-ynedioates took part in the reaction. The yields of the product 4a slightly increased to 29–45% yields when the reaction was carried out at elevated temperature in toluene, methylene dichloride or acetonitrile (Table 1, entries 7–10). When the reaction was carried out in refluxing acetonitrile, the tricyclic compound 4a can be obtained in 47% yield (Table 1, entry 11). Then, the stoichiometry of dimethyl but-2-ynedioate was examined (Table 1, entries 12–15). The highest yield of 4a (89%) was obtained by employing five equiv of dimethyl but-2-ynedioate in the reaction (Table 1, entry 15). It can be found that the reaction can be finished in less than one hour. In the presence of DABCO as base catalyst, the yield of 4a decreased to 27% (Table 1, entry 16). Other common bases such as Et3N and DMAP were also employed in the reaction, they did no gave the product 4a in higher yields than that in the absence of any base, which showed that the reaction does not need any base promotor (Table 1, entry 17 and 18). It was also found that the yield of product 4a cannot be increased when the reaction time was prolonged to three hours (Table 1, entry 19). Thus, the optimized reaction conditions for this multicomponent reaction were successfully established.
Table 1: Optimizing reaction conditionsa.
|
|
||||||
| Entry | Base |
Ratio of
1a/2a/3a |
Solvent | Temp (°C) | Time (h) | Yield (%)b |
| 1 | 1.5:3:1 | MeOH | rt | 6 | – | |
| 2 | 1.5:3:1 | EtOH | rt | 6 | – | |
| 3 | 1.5:3:1 | THF | rt | 6 | – | |
| 4 | 1.5:3:1 | PhMe | rt | 1 | 12 | |
| 5 | 1.5:3:1 | CH2Cl2 | rt | 1 | 13 | |
| 6 | 1.5:3:1 | MeCN | rt | 1 | 18 | |
| 7 | 1.5:3:1 | CH2Cl2 | reflux | 1 | 29 | |
| 8 | 1.5:3:1 | PhMe | reflux | 1 | 45 | |
| 9 | 1.5:3:1 | MeCN | 40 °C | 1 | 27 | |
| 10 | 1.5:3:1 | MeCN | 60 °C | 1 | 40 | |
| 11 | 1.5:3:1 | MeCN | reflux | 1 | 47 | |
| 12 | 1:2:1 | MeCN | reflux | 1 | 52 | |
| 13 | 1:3:1 | MeCN | reflux | 1 | 62 | |
| 14 | 1:4:1 | MeCN | reflux | 1 | 71 | |
| 15 | 1:5:1 | MeCN | reflux | 1 | 89 | |
| 16 | DABCO | 1:5:1 | MeCN | reflux | 1 | 27 |
| 17 | Et3N | 1:5:1 | MeCN | reflux | 1 | 70 |
| 18 | DMAP | 1:5:1 | MeCN | reflux | 1 | 39 |
| 19 | 1:5:1 | MeCN | reflux | 3 | 87 | |
aReaction conditions: cyclohexyl isocyanide (0.1 mmol), dialkyl but-2-ynedioate, 1,4-dihydropyridine, acetonitrile (5.0 mL); bisolated yields.
Under the optimized reaction conditions, the scope of the reaction was developed by using various substrates. The results are summarized in Table 2. At first, several alkyl isocyanides such as cyclohexyl, tert-butyl and benzyl isocyanide have been successfully employed in the reaction. Dimethyl but-2-ynedioate usually gave the expected tricyclic products in good yields. However, the reaction with diethyl but-2-ynedioate afforded products 4p, 4r and 4t in moderate to lower yields. The 5,6- unsubstituted dihydropyridines with various substituents showed marginal effects on the yields. These results clearly showed that this reaction has a wide scope of substrates. The obtained compounds 4a–t have four chiral carbon atoms. The multicomponent reaction might result in several diastereomers. On the basis of TLC analysis and 1H NMR spectra of the crude products, only one relative stereochemistry was produced in the reaction, while the other diastereomers were not detected. In order to elucidate the relative configuration of the obtained compounds, the molecular structure of the compound 4a was determined by single crystal X-ray diffraction (Figure 1). From Figure 1, it can be seen that the fused dihydropyridine ring connects with the pyrrolidine ring in cis-position. The 4-aryl group exists on the trans-position to the 2,3-pyrrolidine ring. The methoxycarbonyl group in the ring of the cyclopentadiene stretches to the cis-position of the 4-aryl group in the dihydropyridine ring. Thus, it can be assigned that all tricyclic compounds have this kind of relative configuration on the basis of NMR spectra and single crystal structure.
Table 2: The synthesis of the tricyclic compounds 4a–ta.
|
|
|||||||
| Entry | Compound | R1 | R2 | Ar | R3 | R4 | Yield (%)b |
| 1 | 4a | cyclohexyl | CH3 | o-CH3OC6H4 | Bn | CH3 | 89 |
| 2 | 4b | cyclohexyl | CH3 | p-NO2C6H4 | Bn | CH3 | 84 |
| 3 | 4c | cyclohexyl | CH3 | p-CH3C6H4 | Bn | CH3 | 81 |
| 4 | 4d | cyclohexyl | CH3 | o-CH3OC6H4 | p-CH3OC6H4CH2 | CH3 | 84 |
| 5 | 4e | cyclohexyl | CH3 | C6H5 | p-ClC6H4CH2 | CH3 | 82 |
| 6 | 4f | cyclohexyl | CH3 | C6H5 | Bn | CH3 | 90 |
| 7 | 4g | cyclohexyl | CH3 | C6H5 | o-CH3C6H4CH2 | CH3 | 81 |
| 8 | 4h | cyclohexyl | CH3 | C6H5 | o-ClC6H4CH2 | CH3 | 80 |
| 9 | 4i | cyclohexyl | CH3 | C6H5 | m-CH3OC6H4CH2 | CH3 | 80 |
| 10 | 4j | cyclohexyl | CH3 | C6H5 | p-CH3OC6H4 | CH3 | 87 |
| 11 | 4k | cyclohexyl | CH3 | C6H5 | p-BrC6H4 | CH3 | 93 |
| 12 | 4l | cyclohexyl | CH3 | C6H5 | m-ClC6H4 | CH3 | 92 |
| 13 | 4m | cyclohexyl | CH3 | o-CH3OC6H4 | o-CH3C6H4 | CH3 | 93 |
| 14 | 4n | cyclohexyl | CH3 | C6H5 | n-Bu | CH3 | 80 |
| 15 | 4o | cyclohexyl | CH3 | C6H5 | Bn | CH2CH3 | 79 |
| 16 | 4p | cyclohexyl | CH2CH3 | C6H5 | Bn | CH3 | 72 |
| 17 | 4q | t-Bu | CH3 | C6H5 | Bn | CH3 | 66 |
| 18 | 4r | t-Bu | CH2CH3 | C6H5 | Bn | CH3 | 58 |
| 19 | 4s | Bn | CH3 | C6H5 | Bn | CH3 | 54 |
| 20 | 4t | Bn | CH2CH3 | C6H5 | Bn | CH3 | 30 |
aReaction conditions: cyclohexyl isocyanide (0.1 mmol), dialkyl but-2-ynedioate (0.5 mmol), 1,4-dihydropyridine (0.1 mmol), acetonitrile (5.0 mL), reflux, 1 h; bisolated yields.
![[1860-5397-20-126-1]](/bjoc/content/figures/1860-5397-20-126-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of compound 4a.
Figure 1: Molecular structure of compound 4a.
In order to develop the scope of the reaction, another kind of 5,6-unsubstituted 1,4-dihydropyridines 5 were also employed in the reaction, which were previously prepared from the three-component reaction of methyl propiolate, cinnamaldehyde and arylamines. The results are summarized in Table 3. It should be pointed out that TLC analysis and 1H NMR spectra of the crude products usually indicated that only one diastereomer was predominately produced in the reaction even though there are four chiral carbon atoms in the products. It can be found that all reactions proceeded smoothly to give the expected polycyclic compounds 6a–k in satisfactory yields. The substituents on the three components showed very little effect on the yields. These results showed that this reaction can be performed with a wide variety of substrates. The molecular structure of the compound 6g was determined by single crystal X-ray diffraction method (Figure 2). The o-methoxyphenyl group exists on the trans-position of the fused pyrrolidine unit. The methoxycarbonyl group also exists on the cis-position of the o-methoxyphenyl group. Therefore, compound 6g has the same relative configuration to that of the above mentioned product 3a, which also indicated that this reaction has same steric controlling effect.
Table 3: The synthesis of the tricyclic compounds 6a–ka.
|
|
||||||
| Entry | Compd | R1 | R2 | Ar | R3 | Yield (%)b |
| 1 | 6a | cyclohexyl | CH3 | C6H5 | Bn | 92 |
| 2 | 6b | cyclohexyl | CH3 | C6H5 | m-CH3OC6H4CH2 | 82 |
| 3 | 6c | cyclohexyl | CH3 | C6H5 | o-CH3C6H4CH2 | 83 |
| 4 | 6d | cyclohexyl | CH3 | C6H5 | p-CH3C6H4 | 83 |
| 5 | 6e | cyclohexyl | CH3 | C6H5 | m-ClC6H4 | 84 |
| 6 | 6f | cyclohexyl | CH3 | C6H5 | o-CH3C6H4 | 81 |
| 7 | 6g | cyclohexyl | CH3 | o-CH3OC6H4 | p-BrC6H4 | 88 |
| 8 | 6h | cyclohexyl | CH3 | p-NO2C6H4 | p-BrC6H4 | 82 |
| 9 | 6i | cyclohexyl | CH2CH3 | C6H5 | Bn | 77 |
| 10 | 6j | t-Bu | CH3 | C6H5 | Bn | 70 |
| 11 | 6k | Bn | CH3 | C6H5 | Bn | 58 |
aReaction conditions: cyclohexyl isocyanide (0.1 mmol), dialkyl but-2-ynedioate (0.5 mmol), 1,4-dihydropyridine (0.1 mmol), acetonitrile (5.0 mL), reflux, 1 h; bIsolated yields.
![[1860-5397-20-126-2]](/bjoc/content/figures/1860-5397-20-126-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of compound 6g.
Figure 2: Molecular structure of compound 6g.
A plausible reaction mechanism is proposed in Scheme 1 to explain the formation of the polycyclic compounds. At first, the nucleophilic addition of alkyl isocyanide to dialkyl but-2-ynedioate afforded the expected Huisgen’s 1,4-dipolar intermediate A. Secondly, the sequential addition of the Huisgen’s 1,4-dipole A to the second molecular dialkyl but-2-ynedioate resulted in a 1,5-dipolar intermediate B. Thirdly, the intramolecular coupling of the positive charge and the negative charge in intermediate B resulted in the formation of polysubstituted 5-(alkylimino)cyclopenta-1,3-diene intermediate C, which has been described in several papers about the reaction of alkyl isocyanides and electron-deficient alkynes [59-63]. The in situ generated cyclic intermediate C has a resonance hybrid C’. Then, the further nucleophilic addition of the electron-rich enamino unit to 5-(alkylimino)cyclopenta-1,3-diene intermediate C gave intermediate D. At last, the coupling of the iminium cation with the amide anion in intermediate D afforded the final product 4 or 6. On the other hand, the final product 4 or 6 might be directly produced by dipolar cycloaddition reaction of 5-(alkylimino)cyclopenta-1,3-diene intermediate C with 5,6-unsaturated dihydropyridine. In consideration of the high diastereoselectivity of the reaction, the concerted addition process is much more likely. However, it is difficult to distinguish between these two reaction processes at present.
Conclusion
In summary, we investigated the three-component reaction of alkyl isocyanides, dialkyl but-2-ynedioates and 5,6-unsubstituted 1,4-dihydropyridines in refluxing acetonitrile. This reaction provided an efficient synthetic protocol for the polyfunctionalized tetrahydrocyclopenta[4,5]pyrrolo[2,3-b]pyridine-3,4b,5,6,7(1H)-pentacarboxylates in high yields and with high diastereoselectivity. A novel example of an activated intermediate derived from the reaction of alkyl isocyanide and two molecules of but-2-ynedioate was successfully explored in the reaction. This reaction has the advantages of using readily available reagents, simple reaction conditions, high atomic convergence and atomic economy, which might be found potential applications in heterocyclic chemistry.
Experimental
General procedure for the multicomponent reaction
To a round flask was added alkyl isocyanide (0.1 mmol), dialkyl but-2-ynedioate (0.5 mmol), 5,6-unsubstitued 1,4-dihydropyridine (0.1 mmol) and acetonitrile (5.0 mL). The solution was stirred at reflux temperature for nearly one hour. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to column chromatography with a mixture of ethyl acetate and petroleum ether (v/v = 1:4) as eluent to give the pure product for analysis.
Pentamethyl rel-(4R,4aR,4bS,8aS)-1-benzyl-8-cyclohexyl-4-(2-methoxyphenyl)-2-methyl-4,4a,8,8a-tetrahydrocyclopenta[4,5]pyrrolo[2,3-b]pyridine-3,4b,5,6,7(1H)-pentacarboxylate (4a): yellow solid, 89%, mp 209–211 °C; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.0 Hz, 1H, ArH), 7.47–7.44 (m, 3H, ArH), 7.41–7.38 (m, 1H, ArH), 7.02–6.97 (m, 1H, ArH), 6.68–6.62 (m, 3H, ArH), 5.76 (d, J = 3.6 Hz, 1H, CH), 4.96–4.90 (m, 1H, CH), 4.84 (d, J = 16.0 Hz, 1H, CH2), 4.36 (d, J = 15.6 Hz, 1H, CH2), 3.95 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 3.65–3.63 (m, 1H, CH), 3.61 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 3.38 (d, J = 8.4 Hz, 1H, CH), 3.21 (s, 3H, OCH3), 2.96 (s, 3H, OCH3), 2.28 (s, 3H, CH3), 1.89–1.86 (m, 2H, CH2), 1.80–1.68 (m, 4H, CH2), 1.55–1.33 (m, 3H, CH2), 1.20–1.13 (m, 1H, CH2) ppm; 13C NMR (100 MHz, CDCl3) δ 170.1, 168.3, 167.8, 166.5, 162.5, 161.9, 157.2, 157.0, 144.7, 137.6, 131.4, 130.9, 129.1, 128.0, 127.9, 127.1, 112.0, 113.6, 111.0, 110.2, 98.9, 84.0, 72.2, 58.2, 56.7, 55.2, 53.3, 52.2, 51.3, 50.4, 50.2, 32.4, 31.5, 31.2, 26.2, 25.7, 25.6, 18.2 ppm; IR (KBr) ν: 3435, 2931, 2862, 2360, 1737, 1698, 1587, 1547, 1435, 1385, 1335, 1251, 1204, 1125, 1092, 1001, 977, 895, 853, 792 cm−1; HRESIMS (m/z): [M + Na]+ calcd. for C41H46NaN2O11, 765.2994; found, 765.2993.
Supporting Information
The crystallographic data of the compounds 4a (CCDC 2346135) and 6g (CCDC 2346136) have been deposited at the Cambridge Crystallographic Database Center (http://www.ccdc.cam.ac.uk).
| Supporting Information File 1: Characterization data and 1H NMR, 13C NMR, and HRMS spectra of compounds. | ||
| Format: PDF | Size: 6.1 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Zhu, J. Eur. J. Org. Chem. 2003, 1133–1144. doi:10.1002/ejoc.200390167
Return to citation in text: [1] -
Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723
Return to citation in text: [1] -
Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235–5331. doi:10.1021/cr900411f
Return to citation in text: [1] -
Dömling, A.; Huang, Y. Synthesis 2010, 2859–2883. doi:10.1055/s-0030-1257906
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u
Return to citation in text: [1] -
Wang, W.; Dömling, A. J. Comb. Chem. 2009, 11, 403–409. doi:10.1021/cc9000136
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a
Return to citation in text: [1] -
Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2014, 43, 4633–4657. doi:10.1039/c3cs60015g
Return to citation in text: [1] -
Wan, J.-P.; Liu, Y. RSC Adv. 2012, 2, 9763–9777. doi:10.1039/c2ra21406g
Return to citation in text: [1] -
Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c
Return to citation in text: [1] -
Khan, I.; Zaib, S.; Ibrar, A. Org. Chem. Front. 2020, 7, 3734–3791. doi:10.1039/d0qo00698j
Return to citation in text: [1] -
Safaei, H. R.; Dehbozorgi, F. RSC Adv. 2016, 6, 26783–26790. doi:10.1039/c5ra22293a
Return to citation in text: [1] -
Wang, H.-Y.; Bao, M.; Jiang, B.; Li, L. RSC Adv. 2016, 6, 6459–6466. doi:10.1039/c5ra25408f
Return to citation in text: [1] -
Rostamnia, S. RSC Adv. 2015, 5, 97044–97065. doi:10.1039/c5ra20455k
Return to citation in text: [1] -
Gao, Q.; Hao, W.-J.; Liu, F.; Tu, S.-J.; Wang, S.-L.; Li, G.; Jiang, B. Chem. Commun. 2016, 52, 900–903. doi:10.1039/c5cc08071a
Return to citation in text: [1] -
Terzidis, M. A.; Zarganes-Tzitzikas, T.; Tsimenidis, C.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A.; Kostakis, G. E. J. Org. Chem. 2012, 77, 9018–9028. doi:10.1021/jo3014947
Return to citation in text: [1] -
Fu, W.; Dong, L.; Shi, J.; Tong, B.; Cai, Z.; Zhi, J.; Dong, Y. Polym. Chem. 2018, 9, 5543–5550. doi:10.1039/c8py01336e
Return to citation in text: [1] -
Yavari, I.; Sheikhi, S.; Taheri, Z. Mol. Diversity 2024, 28, 143–157. doi:10.1007/s11030-023-10635-5
Return to citation in text: [1] -
Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013
Return to citation in text: [1] -
Nazeri, M. T.; Ahmadi, M.; Ghasemi, M.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2023, 21, 4095–4108. doi:10.1039/d3ob00250k
Return to citation in text: [1] -
Yavari, I.; Ravaghi, P.; Safaei, M. Synthesis 2022, 54, 1870–1876. doi:10.1055/s-0041-1737817
Return to citation in text: [1] -
Li, Y.; Kopcha, W. P.; Emge, T. J.; Sun, Y.; Zhang, J. Org. Lett. 2021, 23, 8867–8872. doi:10.1021/acs.orglett.1c03371
Return to citation in text: [1] -
Bankura, A.; Saha, J.; Maity, R.; Das, I. Adv. Synth. Catal. 2021, 363, 1014–1021. doi:10.1002/adsc.202001376
Return to citation in text: [1] -
Nazeri, M. T.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2021, 19, 3722–3734. doi:10.1039/d0ob02339f
Return to citation in text: [1] -
Mohlala, R. L.; Coyanis, E. M.; Fernandes, M. A.; Bode, M. L. Tetrahedron Lett. 2020, 61, 151796. doi:10.1016/j.tetlet.2020.151796
Return to citation in text: [1] -
Wang, C.-C.; Ma, Z.-W.; Qu, Y.-L.; Liu, Z.-J.; Chen, X.-P.; Zhou, J.; Chen, Y.-J. Chem. – Asian J. 2020, 15, 560–563. doi:10.1002/asia.201901780
Return to citation in text: [1] -
Nazeri, M. T.; Mohammadian, R.; Farhid, H.; Shaabani, A.; Notash, B. Tetrahedron Lett. 2020, 61, 151408. doi:10.1016/j.tetlet.2019.151408
Return to citation in text: [1] -
Alizadeh, A.; Amir Ashjaee Asalemi, K.; Halvagar, M. Synthesis 2019, 51, 2936–2944. doi:10.1055/s-0037-1612426
Return to citation in text: [1] -
Krishna Swaroop, D.; Ravi Kumar, N.; Reddy, N. S.; Punna, N.; Jagadeesh Babu, N.; Narsaiah, B. Eur. J. Org. Chem. 2019, 3654–3657. doi:10.1002/ejoc.201900482
Return to citation in text: [1] -
Li, Z.; Huo, T.; Li, L.; Feng, S.; Wang, Q.; Zhang, Z.; Pang, S.; Zhang, Z.; Wang, P.; Zhang, Z. Org. Lett. 2018, 20, 7762–7766. doi:10.1021/acs.orglett.8b03115
Return to citation in text: [1] -
Sabouri, N.; Mahdavinia, G. H.; Notash, B. Chin. Chem. Lett. 2016, 27, 1040–1043. doi:10.1016/j.cclet.2016.03.015
Return to citation in text: [1] -
Song, P.; Zhao, L.; Ji, S. Chin. J. Chem. 2014, 32, 381–386. doi:10.1002/cjoc.201400155
Return to citation in text: [1] -
Dimitriadou, E.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A.; Hadjipavlou-Litina, D. J.; Kontogiorgis, C.; Kostakis, G. E. Tetrahedron 2014, 70, 2938–2943. doi:10.1016/j.tet.2014.03.031
Return to citation in text: [1] -
Emtiazi, H.; Amrollahi, M. A. Helv. Chim. Acta 2014, 97, 1422–1426. doi:10.1002/hlca.201400002
Return to citation in text: [1] -
Ghandi, M.; Ghomi, A.-T.; Kubicki, M. J. Org. Chem. 2013, 78, 2611–2616. doi:10.1021/jo302790y
Return to citation in text: [1] -
Lavilla, R.; Carranco, I.; Díaz, J. L.; Bernabeu, M. C.; de la Rosa, G. Mol. Diversity 2003, 6, 171–175. doi:10.1023/b:modi.0000006756.83821.80
Return to citation in text: [1] -
Vicente-García, E.; Catti, F.; Ramón, R.; Lavilla, R. Org. Lett. 2010, 12, 860–863. doi:10.1021/ol902913j
Return to citation in text: [1] -
Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343
Return to citation in text: [1] -
Basu, S.; Chatterjee, S.; Ray, S.; Maity, S.; Ghosh, P.; Bhaumik, A.; Mukhopadhyay, C. Beilstein J. Org. Chem. 2022, 18, 133–142. doi:10.3762/bjoc.18.14
Return to citation in text: [1] -
Lavilla, R.; Bernabeu, M. C.; Carranco, I.; Díaz, J. L. Org. Lett. 2003, 5, 717–720. doi:10.1021/ol027545d
Return to citation in text: [1] -
Carranco, I.; Díaz, J. L.; Jiménez, O.; Vendrell, M.; Albericio, F.; Royo, M.; Lavilla, R. J. Comb. Chem. 2005, 7, 33–41. doi:10.1021/cc049877a
Return to citation in text: [1] -
Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b
Return to citation in text: [1] -
Islam, K.; Das, D. K.; Akram, E.; Khan, A. T. Synthesis 2015, 47, 2745–2755. doi:10.1055/s-0034-1380431
Return to citation in text: [1] -
Chen, X.-Y.; Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 982–990. doi:10.3762/bjoc.19.73
Return to citation in text: [1] -
Liu, X.; Wang, D.; Sun, J.; Yan, C.-G. J. Mol. Struct. 2024, 1304, 137684. doi:10.1016/j.molstruc.2024.137684
Return to citation in text: [1] -
Ma, W.; Han, Y.; Sun, J.; Yan, C. Chin. J. Org. Chem. 2021, 41, 3180–3191. doi:10.6023/cjoc202103034
Return to citation in text: [1] -
He, Y.-W.; Ma, W.-Q.; Han, Y.; Sun, J.; Yan, C.-G. J. Org. Chem. 2023, 88, 14911–14927. doi:10.1021/acs.joc.3c01246
Return to citation in text: [1] -
Liu, D.; Sun, J.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 17181–17196. doi:10.1021/acs.joc.3c02047
Return to citation in text: [1] -
Zhu, L.-Y.; Sun, J.; Liu, D.; Yan, C.-G. Org. Biomol. Chem. 2023, 21, 9392–9397. doi:10.1039/d3ob01560b
Return to citation in text: [1] -
Liu, X.; Shi, W.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 1923–1932. doi:10.3762/bjoc.19.143
Return to citation in text: [1] -
Xiao, Z.; Xu, F.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 1234–1242. doi:10.3762/bjoc.19.91
Return to citation in text: [1] -
Sun, J.; Liu, X.; Sun, Q.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 11562–11580. doi:10.1021/acs.joc.3c00887
Return to citation in text: [1] -
Huang, L.; Han, Y.; Sun, J.; Sun, Q.; Yan, C.-G. Org. Biomol. Chem. 2023, 21, 6028–6033. doi:10.1039/d3ob00769c
Return to citation in text: [1] -
Liu, D.; Sun, J.; Sun, Q.; Yan, C.-G. Org. Chem. Front. 2023, 10, 540–547. doi:10.1039/d2qo01771g
Return to citation in text: [1] -
Sun, J.; Yan, C.; Sun, Q.; Han, Y.; Yan, C.-G. New J. Chem. 2023, 47, 6694–6699. doi:10.1039/d3nj00013c
Return to citation in text: [1] -
Zhan, S.-C.; Sun, J.; Sun, Q.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 5440–5456. doi:10.1021/acs.joc.2c03094
Return to citation in text: [1] -
Wang, D.; Tang, T.; Sun, J.; Han, Y.; Yan, C.-G. Org. Lett. 2024, 26, 4117–4121. doi:10.1021/acs.orglett.4c01236
Return to citation in text: [1] -
Winterfeldt, E.; Schumann, D.; Dillinger, H.-J. Chem. Ber. 1969, 102, 1656–1664. doi:10.1002/cber.19691020530
Return to citation in text: [1] -
Gougoutas, J. Z.; Saenger, W. J. Org. Chem. 1971, 36, 3632–3633. doi:10.1021/jo00822a041
Return to citation in text: [1] -
Junjappa, H.; Saxena, M. K.; Ramaiah, D.; Loharay, B. B.; Rath, N. P.; George, M. V. J. Org. Chem. 1998, 63, 9801–9805. doi:10.1021/jo981427f
Return to citation in text: [1] -
Cheng, F.-Y.; Sung, K.; Lee, G.-H.; Wang, Y. J. Chin. Chem. Soc. 2000, 47, 1295–1298. doi:10.1002/jccs.200000180
Return to citation in text: [1] -
Zhao, L.-L.; Wang, S.-Y.; Xu, X.-P.; Ji, S.-J. Chem. Commun. 2013, 49, 2569–2571. doi:10.1039/c3cc38526d
Return to citation in text: [1]
| 1. | Zhu, J. Eur. J. Org. Chem. 2003, 1133–1144. doi:10.1002/ejoc.200390167 |
| 2. | Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723 |
| 3. | Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235–5331. doi:10.1021/cr900411f |
| 27. | Wang, C.-C.; Ma, Z.-W.; Qu, Y.-L.; Liu, Z.-J.; Chen, X.-P.; Zhou, J.; Chen, Y.-J. Chem. – Asian J. 2020, 15, 560–563. doi:10.1002/asia.201901780 |
| 28. | Nazeri, M. T.; Mohammadian, R.; Farhid, H.; Shaabani, A.; Notash, B. Tetrahedron Lett. 2020, 61, 151408. doi:10.1016/j.tetlet.2019.151408 |
| 29. | Alizadeh, A.; Amir Ashjaee Asalemi, K.; Halvagar, M. Synthesis 2019, 51, 2936–2944. doi:10.1055/s-0037-1612426 |
| 30. | Krishna Swaroop, D.; Ravi Kumar, N.; Reddy, N. S.; Punna, N.; Jagadeesh Babu, N.; Narsaiah, B. Eur. J. Org. Chem. 2019, 3654–3657. doi:10.1002/ejoc.201900482 |
| 31. | Li, Z.; Huo, T.; Li, L.; Feng, S.; Wang, Q.; Zhang, Z.; Pang, S.; Zhang, Z.; Wang, P.; Zhang, Z. Org. Lett. 2018, 20, 7762–7766. doi:10.1021/acs.orglett.8b03115 |
| 32. | Sabouri, N.; Mahdavinia, G. H.; Notash, B. Chin. Chem. Lett. 2016, 27, 1040–1043. doi:10.1016/j.cclet.2016.03.015 |
| 33. | Song, P.; Zhao, L.; Ji, S. Chin. J. Chem. 2014, 32, 381–386. doi:10.1002/cjoc.201400155 |
| 34. | Dimitriadou, E.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A.; Hadjipavlou-Litina, D. J.; Kontogiorgis, C.; Kostakis, G. E. Tetrahedron 2014, 70, 2938–2943. doi:10.1016/j.tet.2014.03.031 |
| 35. | Emtiazi, H.; Amrollahi, M. A. Helv. Chim. Acta 2014, 97, 1422–1426. doi:10.1002/hlca.201400002 |
| 15. | Rostamnia, S. RSC Adv. 2015, 5, 97044–97065. doi:10.1039/c5ra20455k |
| 16. | Gao, Q.; Hao, W.-J.; Liu, F.; Tu, S.-J.; Wang, S.-L.; Li, G.; Jiang, B. Chem. Commun. 2016, 52, 900–903. doi:10.1039/c5cc08071a |
| 17. | Terzidis, M. A.; Zarganes-Tzitzikas, T.; Tsimenidis, C.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A.; Kostakis, G. E. J. Org. Chem. 2012, 77, 9018–9028. doi:10.1021/jo3014947 |
| 18. | Fu, W.; Dong, L.; Shi, J.; Tong, B.; Cai, Z.; Zhi, J.; Dong, Y. Polym. Chem. 2018, 9, 5543–5550. doi:10.1039/c8py01336e |
| 19. | Yavari, I.; Sheikhi, S.; Taheri, Z. Mol. Diversity 2024, 28, 143–157. doi:10.1007/s11030-023-10635-5 |
| 20. | Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013 |
| 21. | Nazeri, M. T.; Ahmadi, M.; Ghasemi, M.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2023, 21, 4095–4108. doi:10.1039/d3ob00250k |
| 22. | Yavari, I.; Ravaghi, P.; Safaei, M. Synthesis 2022, 54, 1870–1876. doi:10.1055/s-0041-1737817 |
| 23. | Li, Y.; Kopcha, W. P.; Emge, T. J.; Sun, Y.; Zhang, J. Org. Lett. 2021, 23, 8867–8872. doi:10.1021/acs.orglett.1c03371 |
| 24. | Bankura, A.; Saha, J.; Maity, R.; Das, I. Adv. Synth. Catal. 2021, 363, 1014–1021. doi:10.1002/adsc.202001376 |
| 25. | Nazeri, M. T.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2021, 19, 3722–3734. doi:10.1039/d0ob02339f |
| 26. | Mohlala, R. L.; Coyanis, E. M.; Fernandes, M. A.; Bode, M. L. Tetrahedron Lett. 2020, 61, 151796. doi:10.1016/j.tetlet.2020.151796 |
| 7. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 8. | Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a |
| 9. | Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2014, 43, 4633–4657. doi:10.1039/c3cs60015g |
| 10. | Wan, J.-P.; Liu, Y. RSC Adv. 2012, 2, 9763–9777. doi:10.1039/c2ra21406g |
| 11. | Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c |
| 12. | Khan, I.; Zaib, S.; Ibrar, A. Org. Chem. Front. 2020, 7, 3734–3791. doi:10.1039/d0qo00698j |
| 13. | Safaei, H. R.; Dehbozorgi, F. RSC Adv. 2016, 6, 26783–26790. doi:10.1039/c5ra22293a |
| 14. | Wang, H.-Y.; Bao, M.; Jiang, B.; Li, L. RSC Adv. 2016, 6, 6459–6466. doi:10.1039/c5ra25408f |
| 4. | Dömling, A.; Huang, Y. Synthesis 2010, 2859–2883. doi:10.1055/s-0030-1257906 |
| 5. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u |
| 6. | Wang, W.; Dömling, A. J. Comb. Chem. 2009, 11, 403–409. doi:10.1021/cc9000136 |
| 46. | Liu, X.; Wang, D.; Sun, J.; Yan, C.-G. J. Mol. Struct. 2024, 1304, 137684. doi:10.1016/j.molstruc.2024.137684 |
| 59. | Winterfeldt, E.; Schumann, D.; Dillinger, H.-J. Chem. Ber. 1969, 102, 1656–1664. doi:10.1002/cber.19691020530 |
| 60. | Gougoutas, J. Z.; Saenger, W. J. Org. Chem. 1971, 36, 3632–3633. doi:10.1021/jo00822a041 |
| 61. | Junjappa, H.; Saxena, M. K.; Ramaiah, D.; Loharay, B. B.; Rath, N. P.; George, M. V. J. Org. Chem. 1998, 63, 9801–9805. doi:10.1021/jo981427f |
| 62. | Cheng, F.-Y.; Sung, K.; Lee, G.-H.; Wang, Y. J. Chin. Chem. Soc. 2000, 47, 1295–1298. doi:10.1002/jccs.200000180 |
| 63. | Zhao, L.-L.; Wang, S.-Y.; Xu, X.-P.; Ji, S.-J. Chem. Commun. 2013, 49, 2569–2571. doi:10.1039/c3cc38526d |
| 45. | Chen, X.-Y.; Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 982–990. doi:10.3762/bjoc.19.73 |
| 40. | Basu, S.; Chatterjee, S.; Ray, S.; Maity, S.; Ghosh, P.; Bhaumik, A.; Mukhopadhyay, C. Beilstein J. Org. Chem. 2022, 18, 133–142. doi:10.3762/bjoc.18.14 |
| 41. | Lavilla, R.; Bernabeu, M. C.; Carranco, I.; Díaz, J. L. Org. Lett. 2003, 5, 717–720. doi:10.1021/ol027545d |
| 42. | Carranco, I.; Díaz, J. L.; Jiménez, O.; Vendrell, M.; Albericio, F.; Royo, M.; Lavilla, R. J. Comb. Chem. 2005, 7, 33–41. doi:10.1021/cc049877a |
| 43. | Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b |
| 44. | Islam, K.; Das, D. K.; Akram, E.; Khan, A. T. Synthesis 2015, 47, 2745–2755. doi:10.1055/s-0034-1380431 |
| 36. | Ghandi, M.; Ghomi, A.-T.; Kubicki, M. J. Org. Chem. 2013, 78, 2611–2616. doi:10.1021/jo302790y |
| 37. | Lavilla, R.; Carranco, I.; Díaz, J. L.; Bernabeu, M. C.; de la Rosa, G. Mol. Diversity 2003, 6, 171–175. doi:10.1023/b:modi.0000006756.83821.80 |
| 38. | Vicente-García, E.; Catti, F.; Ramón, R.; Lavilla, R. Org. Lett. 2010, 12, 860–863. doi:10.1021/ol902913j |
| 39. | Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343 |
| 47. | Ma, W.; Han, Y.; Sun, J.; Yan, C. Chin. J. Org. Chem. 2021, 41, 3180–3191. doi:10.6023/cjoc202103034 |
| 48. | He, Y.-W.; Ma, W.-Q.; Han, Y.; Sun, J.; Yan, C.-G. J. Org. Chem. 2023, 88, 14911–14927. doi:10.1021/acs.joc.3c01246 |
| 49. | Liu, D.; Sun, J.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 17181–17196. doi:10.1021/acs.joc.3c02047 |
| 50. | Zhu, L.-Y.; Sun, J.; Liu, D.; Yan, C.-G. Org. Biomol. Chem. 2023, 21, 9392–9397. doi:10.1039/d3ob01560b |
| 51. | Liu, X.; Shi, W.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 1923–1932. doi:10.3762/bjoc.19.143 |
| 52. | Xiao, Z.; Xu, F.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2023, 19, 1234–1242. doi:10.3762/bjoc.19.91 |
| 53. | Sun, J.; Liu, X.; Sun, Q.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 11562–11580. doi:10.1021/acs.joc.3c00887 |
| 54. | Huang, L.; Han, Y.; Sun, J.; Sun, Q.; Yan, C.-G. Org. Biomol. Chem. 2023, 21, 6028–6033. doi:10.1039/d3ob00769c |
| 55. | Liu, D.; Sun, J.; Sun, Q.; Yan, C.-G. Org. Chem. Front. 2023, 10, 540–547. doi:10.1039/d2qo01771g |
| 56. | Sun, J.; Yan, C.; Sun, Q.; Han, Y.; Yan, C.-G. New J. Chem. 2023, 47, 6694–6699. doi:10.1039/d3nj00013c |
| 57. | Zhan, S.-C.; Sun, J.; Sun, Q.; Han, Y.; Yan, C.-G. J. Org. Chem. 2023, 88, 5440–5456. doi:10.1021/acs.joc.2c03094 |
| 58. | Wang, D.; Tang, T.; Sun, J.; Han, Y.; Yan, C.-G. Org. Lett. 2024, 26, 4117–4121. doi:10.1021/acs.orglett.4c01236 |
© 2024 Chen et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.