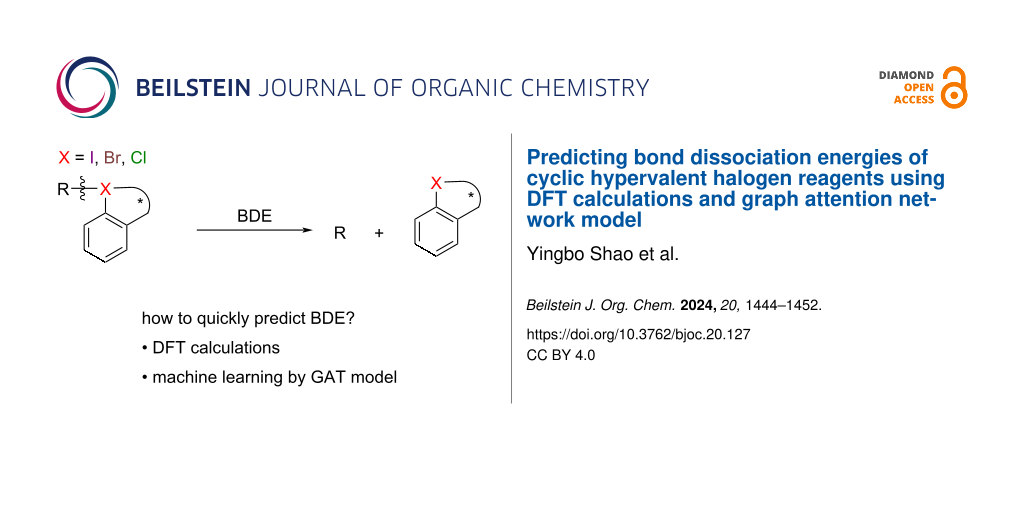
Abstract
Although hypervalent iodine(III) reagents have become staples in organic chemistry, the exploration of their isoelectronic counterparts, namely hypervalent bromine(III) and chlorine(III) reagents, has been relatively limited, partly due to challenges in synthesizing and stabilizing these compounds. In this study, we conduct a thorough examination of both homolytic and heterolytic bond dissociation energies (BDEs) critical for assessing the chemical stability and functional group transfer capability of cyclic hypervalent halogen compounds using density functional theory (DFT) analysis. A moderate linear correlation was observed between the homolytic BDEs across different halogen centers, while a strong linear correlation was noted among the heterolytic BDEs across these centers. Furthermore, we developed a predictive model for both homolytic and heterolytic BDEs of cyclic hypervalent halogen compounds using machine learning algorithms. The results of this study could aid in estimating the chemical stability and functional group transfer capabilities of hypervalent bromine(III) and chlorine(III) reagents, thereby facilitating their development.
Graphical Abstract
Introduction
Hypervalent iodine reagents are increasingly gaining attention in the fields of organic synthesis and catalysis due to their environmental benefits, accessibility, and cost-efficiency [1-11]. Over the last three decades, a series of cyclic hypervalent iodine(III) reagents has been developed [12-17] (Figure 1), including the well-known Zhdankin reagents [13] and Togni reagents [14]. These reagents are popularly used as electrophilic group transfer reagents [18,19] in a variety of reactions, such as C–H functionalization [20-22], unsaturated alkane addition [23,24], and cyclization [25,26].
Figure 1: Examples of cyclic hypervalent halogen reagents.
Figure 1: Examples of cyclic hypervalent halogen reagents.
Despite the rapid development of hypervalent iodine(III) reagents, the exploration of isoelectronic hypervalent bromine(III) and chlorine(III) reagents has been comparatively limited despite their demonstrated potential for unique applications [27-30]. For example, hypervalent bromine(III) reagents enable C–H amination and alkene aziridination reactions without the need for additional Lewis acid activation [31-33]. However, challenges in the synthesis and stabilization of cyclic hypervalent bromine and chlorine reagents have impeded their development relative to their iodine(III) analogs [27-30]. Cyclic hypervalent bromine(III) reagents were pioneered by Miyamoto [32] and have since been developed to a certain extent [33]. Biphenyl hypervalent bromine(III) reagents [34-39] have been synthesized by Yoshida and Wencel-Delord (Figure 1). Cyclic hypervalent chlorine(III) reagents with similar skeletal structures have not been reported yet, and only biphenyl hypervalent chlorine(III) reagents [40-42] and cyclic diaryliodonium salts [43] have been synthesized.
Previous investigations [44-47] have highlighted the critical role of bond dissociation energy (BDE) in understanding the group transfer capabilities and chemical stability of hypervalent iodine(III) reagents. In this context, detailed knowledge of the BDE of hypervalent bromine(III) and chlorine(III) reagents is especially crucial for designing novel reagents. Yet, the BDE values of hypervalent bromine(III) and chlorine(III) reagents remain largely elusive, hampering the design and synthesis of novel reagents.
In recent years, machine learning has emerged as a promising and cost-effective alternative to traditional DFT calculations for predicting key properties of organic molecules such as BDE, nucleophilicity, and electrophilicity [48-60]. Recently, applications of the Elastic Net model with Avalon fingerprints [55] and the deployment of artificial neural network (ANN) models [57] with the Mordred cheminformatics package have demonstrated considerable success in predicting the BDEs of hypervalent iodine(III) reagents. However, previous studies have been limited to the prediction of hypervalent iodine(III) reagents. Driven by their proven effectiveness and our ongoing interest in hypervalent halogen chemistry [61-72], we are motivated to develop a machine learning model for a broader array of cyclic hypervalent halogen reagents, thereby integrating different halogen centers and making it easier to predict the group transfer capacity and chemical stability of different cyclic hypervalent halogen reagents.
Results and Discussion
We selected five different skeletons and twenty common transfer groups for combination (Figure 2) and calculated their BDEs. Referring to the previous computational studies of hypervalent iodine [61-76] and the computational database of organic species by Paton and co-workers [77], geometry optimizations and single point energy calculations for homolytic BDEs are both performed using M06-2X/def2-TZVPP [78-80] in the gas phase at 298.15 K by Gaussian 16 [81]. Frequency calculations confirmed that optimized structures are minima (no imaginary frequency). The accuracy of computational BDEs of halides using M06-2X/def2-TZVPP is also evaluated and compared to experimental BDEs, demonstrating the reliability of the method (see Supporting Information File 1).
Figure 2: Common cyclic hypervalent halogen skeletons and transfer groups.
Figure 2: Common cyclic hypervalent halogen skeletons and transfer groups.
The computational homolytic BDEs are presented in Table 1. From the perspective of halogen centers, hypervalent iodine(III) reagents exhibit the highest homolytic BDEs, followed by hypervalent bromine(III) reagents, while hypervalent chlorine(III) reagents have the lowest. Generally, the homolytic BDEs of cyclic hypervalent iodine(III) reagents are above 30.0 kcal/mol, consistent with their good chemical stability. The homolytic BDEs of some cyclic hypervalent bromine(III) and most cyclic hypervalent chlorine(III) reagents are below 20 kcal/mol, implying these reagents should be too reactive to be isolated. From the perspective of transfer groups, the homolytic BDEs of groups with strong trans effects [82-84] such as -F, -CCH, -CN, -OCF3, -OTf, -OTs are elevated, while those of -N3, -NH2, -SCF3, etc. are smaller. These results are consistent with our previous studies on the group transfer ability of hypervalent iodine(III) reagents [44]. According to the calculation results, skeleton 5 may be a better candidate for synthesizing cyclic hypervalent bromine(III) and chlorine(III) reagents. The groups with strong trans effects, such as -F, -CCH, -CN, -OTf, can help stabilize cyclic hypervalent bromine(III) and chlorine(III) reagents.
Table 1: Computational homolytic BDEs (kcal/mol) of cyclic hypervalent halogen reagents.
|
|
||||||||||||||||
| 1-X | 2-X | 3-X | 4-X | 5-X | ||||||||||||
| R |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
|
| a | F | 78.6 | 50.5 | 30.5 | 81.9 | 56.6 | 35.5 | 80.8 | 54.3 | 33.3 | 80.6 | 61.4 | 29.4 | 84.0 | 62.0 | 42.9 |
| b | Cl | 55.3 | 29.4 | 10.1 | 58.1 | 35.6 | 14.0 | 57.4 | 33.1 | 12.1 | 57.1 | 40.9 | 10.7 | 60.1 | 40.3 | 21.3 |
| c | Br | 44.7 | 19.8 | 0.2 | 47.3 | 24.9 | 4.3 | 46.7 | 23.0 | 2.4 | 46.5 | 31.2 | 2.0 | 49.3 | 30.2 | 11.4 |
| d | CH3 | 33.2 | 13.4 | −0.3 | 42.4 | 28.7 | 16.5 | 42.8 | 29.6 | 20.0 | 49.1 | 49.3 | 40.0 | 39.9 | 28.9 | 22.2 |
| e | CF3 | 33.5 | 13.5 | −0.8 | 39.0 | 24.5 | 11.8 | 38.5 | 23.6 | 12.5 | 41.2 | 39.0 | 25.1 | 37.2 | 24.5 | 12.6 |
| f | CHCH2 | 40.4 | 21.4 | 8.7 | 49.6 | 36.5 | 25.8 | 49.6 | 36.8 | 28.1 | 56.0 | 58.2 | 47.1 | 46.8 | 36.1 | 27.0 |
| g | CCH | 66.1 | 42.0 | 24.5 | 73.1 | 53.9 | 38.2 | 72.6 | 53.0 | 39.2 | 76.3 | 68.5 | 48.5 | 71.1 | 53.8 | 39.0 |
| h | CN | 68.8 | 43.6 | 24.2 | 72.6 | 51.6 | 33.4 | 71.6 | 49.7 | 32.7 | 72.8 | 61.6 | 37.9 | 71.9 | 53.0 | 34.8 |
| i | N3 | 32.1 | 7.6 | −11.2 | 35.8 | 14.6 | −4.5 | 34.8 | 12.3 | −6.3 | 36.3 | 23.3 | −4.8 | 36.5 | 17.9 | 0.1 |
| j | NH2 | 37.7 | 12.6 | −4.3 | 45.5 | 25.1 | 9.6 | 45.0 | 24.1 | 8.3 | 49.4 | 40.2 | 16.7 | 44.5 | 27.5 | 11.7 |
| k | NHAc | 47.6 | 22.0 | 2.6 | 53.4 | 31.6 | 13.0 | 52.7 | 30.0 | 12.2 | 55.8 | 43.4 | 18.8 | 53.0 | 33.7 | 15.7 |
| l | OH | 53.0 | 26.4 | 6.5 | 58.7 | 35.7 | 15.9 | 57.7 | 33.5 | 14.1 | 60.1 | 44.9 | 16.3 | 58.7 | 38.3 | 19.5 |
| m | OCH3 | 40.7 | 15.3 | −3.5 | 46.2 | 24.6 | 6.1 | 45.2 | 22.4 | 4.3 | 47.9 | 34.5 | 7.7 | 46.2 | 26.9 | 9.1 |
| n | OCF3 | 62.9 | 37.0 | 18.4 | 64.4 | 40.5 | 19.8 | 63.5 | 38.1 | 17.4 | 62.7 | 45.0 | 13.3 | 67.0 | 46.6 | 28.6 |
| o | OCOCH3 | 54.6 | 27.4 | 7.5 | 58.1 | 33.7 | 12.3 | 57.5 | 31.1 | 9.9 | 57.6 | 39.3 | 8.6 | 59.1 | 37.1 | 17.4 |
| p | OCOCF3 | 62.3 | 36.1 | 17.4 | 63.5 | 39.0 | 18.0 | 62.7 | 36.7 | 15.8 | 61.2 | 43.4 | 11.3 | 65.9 | 45.1 | 27.0 |
| q | OCOPh | 55.8 | 28.5 | 8.7 | 58.9 | 33.8 | 12.4 | 58.4 | 31.8 | 10.4 | 58.4 | 40.5 | 9.0 | 60.1 | 38.0 | 19.1 |
| r | OTf | 66.3 | 41.9 | 25.8 | 65.1 | 41.3 | 21.3 | 64.3 | 39.0 | 18.7 | 62.0 | 43.6 | 11.1 | 69.8 | 50.8 | 35.4 |
| s | OTs | 61.8 | 36.5 | 18.4 | 62.4 | 38.4 | 18.3 | 62.3 | 36.2 | 15.5 | 61.0 | 43.0 | 12.9 | 65.8 | 45.8 | 28.9 |
| t | SCF3 | 40.1 | 16.1 | −3.1 | 44.6 | 23.3 | 4.0 | 43.2 | 21.6 | 3.0 | 44.9 | 33.7 | 14.0 | 44.2 | 26.2 | 7.9 |
In addition, we also calculated the heterolytic BDEs of cyclic hypervalent halogen reagents [46,47] to comprehensively examine the strength of chemical bonds (Table 2). Geometry optimizations and single point energy calculations for heterolytic BDEs are performed using M06-2X/def2-TZVPP in the SMD (acetonitrile) Implicit solvent model at 298.15 K. Due to the instability of some transfer group cations, such as +OCH3, +OCF3, +OCOCF3, +OCOPh, +OTf and +SCF3, it is difficult for us to investigate their heterolytic BDEs. From Table 2, it can be seen that, except for CF3 and CHCH2, all other transfer groups exhibit high heterolytic BDEs with hypervalent halogen centers.
Table 2: Computational heterolytic BDEs (kcal/mol) of cyclic hypervalent halogen reagents.
|
|
||||||||||||||||
| 1-X | 2-X | 3-X | 4-X | 5-X | ||||||||||||
| R |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
X =
I |
X =
Br |
X =
Cl |
|
| a | F | 375.2 | 346.9 | 327.5 | 354.4 | 322.1 | 301.1 | 345.0 | 309.7 | 290.0 | 324.1 | 286.4 | 263.3 | 353.0 | 321.5 | 303.1 |
| b | Cl | 239.5 | 214.1 | 195.2 | 217.7 | 188.5 | 166.9 | 208.4 | 176.3 | 156.5 | 187.6 | 154.8 | 134.6 | 216.4 | 188.0 | 170.2 |
| c | Br | 212.7 | 188.4 | 169.0 | 190.8 | 163.3 | 142.0 | 181.6 | 151.3 | 131.9 | 160.7 | 130.9 | 111.9 | 189.5 | 162.2 | 143.8 |
| d | CH3 | 83.8 | 66.1 | 55.6 | 74.0 | 57.4 | 47.9 | 67.9 | 51.4 | 47.8 | 58.6 | 44.5 | 43.0 | 66.2 | 48.7 | 43.4 |
| e | CF3 | 77.5 | 56.5 | 42.1 | 61.7 | 40.4 | 29.3 | 53.7 | 30.9 | 24.2 | 37.8 | 19.8 | 18.9 | 55.2 | 32.0 | 20.9 |
| f | CHCH2 | 71.2 | 54.2 | 43.9 | 60.3 | 44.9 | 37.7 | 54.2 | 38.4 | 34.9 | 44.0 | 28.8 | 29.3 | 53.0 | 35.9 | 30.7 |
| g | CCH | 231.7 | 207.2 | 189.8 | 216.5 | 192.5 | 178.7 | 208.7 | 183.3 | 174.1 | 194.4 | 171.4 | 163.5 | 210.1 | 183.8 | 171.0 |
| h | CN | 254.7 | 227.8 | 207.7 | 235.6 | 206.1 | 189.3 | 226.6 | 194.4 | 181.2 | 207.8 | 177.1 | 166.7 | 231.0 | 200.5 | 181.2 |
| i | N3 | 159.6 | 134.1 | 115.4 | 139.5 | 111.3 | 92.2 | 130.4 | 99.5 | 83.4 | 111.6 | 81.9 | 68.2 | 136.6 | 107.7 | 89.5 |
| j | NH2 | 145.5 | 121.4 | 103.8 | 133.0 | 108.4 | 94.0 | 124.9 | 99.2 | 89.9 | 111.7 | 87.7 | 79.7 | 125.5 | 99.9 | 86.9 |
| k | NHAc | 113.6 | 87.8 | 68.3 | 97.6 | 71.5 | 55.2 | 89.3 | 61.4 | 49.0 | 73.5 | 47.9 | 37.6 | 92.2 | 64.2 | 47.2 |
| l | OH | 244.1 | 217.6 | 197.1 | 227.3 | 199.1 | 179.6 | 218.4 | 187.7 | 170.9 | 200.9 | 170.3 | 154.0 | 222.9 | 193.6 | 174.0 |
| m | OCH3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| n | OCF3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| o | OCOCH3 | 149.1 | 122.7 | 102.9 | 128.7 | 100.4 | 78.1 | 119.6 | 87.4 | 67.7 | 99.4 | 67.8 | 47.0 | 127.0 | 97.5 | 77.2 |
| p | OCOCF3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| q | OCOPh | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| r | OTf | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| s | OTs | 104.8 | 79.7 | 62.4 | 80.2 | 50.0 | 29.1 | 70.9 | 37.0 | 18.5 | 47.8 | 13.2 | -7.6 | / | 52.5 | 37.9 |
| t | SCF3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
To elucidate the relationships between halogen centers and their corresponding homolytic BDEs, the homolytic BDEs of cyclic hypervalent halogen reagents were plotted against those of reagents with different halogen centers, giving moderate linear relationships (Figure 3a). For heterolytic BDEs, we found a strong linear relationship between different halogen centers, as illustrated in Figure 3b. This indicates that based on any kind of cyclic hypervalent halogen reagents, we can obtain a rough estimation of the BDEs for others with different halogen centers.
![[1860-5397-20-127-3]](/bjoc/content/figures/1860-5397-20-127-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: a) Linear dependence between the homolytic BDEs of cyclic hypervalent halogen reagents; b) linear dependence between the heterolytic BDEs of cyclic hypervalent halogen reagents.
Figure 3: a) Linear dependence between the homolytic BDEs of cyclic hypervalent halogen reagents; b) linear d...
With these homolytic and heterolytic BDEs in hand, we next attempted to develop a predictive model for BDEs of hypervalent halogen compounds using machine learning algorithms. Graph attention network (GAT) [85] embeds local chemical environment information into the graph network by taking atomic information as node inputs, thus achieving higher predictive capabilities [86]. Building upon the computational studies, we constructed two compound datasets separately, consisting of 296 homolytic BDE data points and 209 heterolytic BDE data points. Taking homolytic BDE datasets as an example (Figure 4a), the distribution of this dataset is illustrated with key bond energy values normalized using min–max scaling. This approach ensures both data consistency and improves training efficiency.
![[1860-5397-20-127-4]](/bjoc/content/figures/1860-5397-20-127-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: a) Composition and distribution of homolytic BDE dataset; b) graph attention network (GAT) model architecture and workflow; c) comparison of prediction performance on the training set using different descriptors (see more details in Supporting Information File 1); d) prediction performance for homolytic BDEs on the test set; e) prediction performance for heterolytic BDEs on the test set.
Figure 4: a) Composition and distribution of homolytic BDE dataset; b) graph attention network (GAT) model ar...
We used the GAT model as the core framework, incorporating ten selected atomic descriptors as local information within the graph structure. Effective molecular transformations into molecular graphs (Figure 4b) were achieved using the RDKit and Deep Graph Library [87]. The dataset was randomly divided into training and testing sets in a 9:1 ratio. Notably, our analysis of descriptor testing revealed that individual inputs, such as neighboring atomic information, atomic charge, and atomic species, did not yield satisfactory results. However, combining all three inputs resulted in highly effective predictions (Figure 4c, see Supporting Information File 1 for detail). The R2, MAE, and RMSE metrics exhibited outstanding performance. The final predictive results yielded excellent performance with an R2 value of 0.955 for homolytic BDEs (Figure 4d) and an R2 value of 0.974 for heterolytic BDEs (Figure 4e). Furthermore, we achieved superior predictive results by not distinguishing between halogen categories in the dataset. This approach is reliable and efficient in assisting chemists in estimating the bond energy ranges of novel cyclic hypervalent halogen reagents.
We conducted additional tests with cyclic hypervalent halogen reagents beyond the training set, employing linear dependence equations and the GAT model for predictions (Table 3). The comparison of the two methods reveals that the GAT model is more reliable, as indicated by the lower root mean square error (RMSE). Moreover, the linear dependence method requires the BDEs of known cyclic hypervalent iodine(III) reagents to deduce the BDEs of the cyclic hypervalent bromine(III) and chlorine(III) reagents. In contrast, the GAT model is more straightforward, relying solely on structural information. Therefore, the GAT model is a superior method to predict the BDEs of cyclic hypervalent halogen reagents.
Table 3: Predictional BDEs of cyclic hypervalent halogen reagents.
|
|
|||||||||||||||
| homolytic BDEs | heterolytic BDEs | ||||||||||||||
| methods | 6-X-F | 1-X-CF2SO2Ph | RMSE | methods | 6-X-F | 1-X-CF2SO2Ph | RMSE | ||||||||
| X = I | X = Br | X = Cl | X = I | X = Br | X = Cl | X = I | X = Br | X = Cl | X = I | X = Br | X = Cl | ||||
| DFTa | 68.3 | 44.1 | 26.2 | 32.6 | 10 | −6.2 | – | DFTb | 367.4 | 342.7 | 328.8 | 79.9 | 57.6 | 41.7 | – |
| LEc | – | 47 | 26.1 | – | 15.2 | −1.2 | 3.9 | LEd | – | 332.2 | 308.6 | – | 56.2 | 44.1 | 11.4 |
| MLe | 72.7 | 45.2 | 27.2 | 38.1 | 13.3 | −4.5 | 3.3 | MLe | 375.8 | 336.8 | 329.1 | 86.3 | 56.6 | 48.9 | 5.7 |
aDFT calculations: M06-2X/def2-TZVPP in gas phase; bDFT calculations: M06-2X/def2-TZVPP in SMD (acetonitrile); clinear dependence equations: these predicted BDEs for hypervalent bromine and hypervalent chlorine are obtained by inserting the calculated hypervalent iodine BDEs into the linear dependence equations: y = 0.89x−13.85 and y = 0.76x−26.02; dlinear dependence equations: y = 0.96x−20.50 and y = 0.92x−29.39; emachine learning.
Conclusion
We have undertaken an extensive computational investigation into the BDEs of cyclic hypervalent halogen reagents. Leveraging this dataset, we have developed a predictive model for both homolytic and heterolytic BDEs of hypervalent halogen compounds employing a graph attention network. We anticipate that the findings from our research will aid the design and development of new hypervalent bromine(III) and chlorine(III) reagents, an area that remains largely underexplored.
Supporting Information
| Supporting Information File 1: Machine learning details and calculation data. | ||
| Format: PDF | Size: 1.3 MB | Download |
Acknowledgements
Thanks to Prof. Dr. Jun Zhang (Shenzhen Bay Laboratory) and Ms. Qiufen Chen (Shenzhen Bay Laboratory & Southern University of Science and Technology) for their help in machine learning as well as Prof. Dr. Xin Li (Nankai University) and Haihe Laboratory of Sustainable Chemical Transformations for computational resources.
Funding
This work was supported by the Ministry of Science and Technology of China (2021YFF0701700), the National Natural Science Foundation of China (Nos. 22122104, 22193012, and 21933004), the CAS Project for Young Scientists in Basic Research (grant no. YSBR-052 and YSBR-095), and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB0590000).
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. doi:10.1021/cr940424+
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+
Return to citation in text: [1] -
Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] -
Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] -
Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154
Return to citation in text: [1] -
Parra, A. Chem. Rev. 2019, 119, 12033–12088. doi:10.1021/acs.chemrev.9b00338
Return to citation in text: [1] -
Flores, A.; Cots, E.; Bergès, J.; Muñiz, K. Adv. Synth. Catal. 2019, 361, 2–25. doi:10.1002/adsc.201800521
Return to citation in text: [1] -
Zhdankin, V. V. ARKIVOC 2020, No. iv, 1–11. doi:10.24820/ark.5550190.p011.145
Return to citation in text: [1] -
Singh, F. V.; Shetgaonkar, S. E.; Krishnan, M.; Wirth, T. Chem. Soc. Rev. 2022, 51, 8102–8139. doi:10.1039/d2cs00206j
Return to citation in text: [1] -
Koser, G. F.; Sun, G.; Porter, C. W.; Youngs, W. J. J. Org. Chem. 1993, 58, 7310–7312. doi:10.1021/jo00077a071
Return to citation in text: [1] -
Zhdankin, V. V.; Kuehl, C. J.; Krasutsky, A. P.; Bolz, J. T.; Mismash, B.; Woodward, J. K.; Simonsen, A. J. Tetrahedron Lett. 1995, 36, 7975–7978. doi:10.1016/0040-4039(95)01720-3
Return to citation in text: [1] [2] -
Eisenberger, P.; Gischig, S.; Togni, A. Chem. – Eur. J. 2006, 12, 2579–2586. doi:10.1002/chem.200501052
Return to citation in text: [1] [2] -
Fernández González, D.; Brand, J. P.; Waser, J. Chem. – Eur. J. 2010, 16, 9457–9461. doi:10.1002/chem.201001539
Return to citation in text: [1] -
Legault, C. Y.; Prévost, J. Acta Crystallogr., Sect. E: Struct. Rep. Online 2012, 68, o1238. doi:10.1107/s1600536812012822
Return to citation in text: [1] -
Ren, J.; Du, F.-H.; Jia, M.-C.; Hu, Z.-N.; Chen, Z.; Zhang, C. Angew. Chem., Int. Ed. 2021, 60, 24171–24178. doi:10.1002/anie.202108589
Return to citation in text: [1] -
Li, Y.; Hari, D. P.; Vita, M. V.; Waser, J. Angew. Chem., Int. Ed. 2016, 55, 4436–4454. doi:10.1002/anie.201509073
Return to citation in text: [1] -
Yoshimura, A.; Saito, A.; Zhdankin, V. V. Adv. Synth. Catal. 2023, 365, 2653–2675. doi:10.1002/adsc.202300275
Return to citation in text: [1] -
Wang, Y.; Hu, X.; Morales-Rivera, C. A.; Li, G.-X.; Huang, X.; He, G.; Liu, P.; Chen, G. J. Am. Chem. Soc. 2018, 140, 9678–9684. doi:10.1021/jacs.8b05753
Return to citation in text: [1] -
Zhang, Y.; Lu, J.; Lan, T.; Cheng, S.; Liu, W.; Chen, C. Eur. J. Org. Chem. 2021, 436–442. doi:10.1002/ejoc.202001373
Return to citation in text: [1] -
Poeira, D. L.; Negrão, A. C. R.; Faustino, H.; Coelho, J. A. S.; Gomes, C. S. B.; Gois, P. M. P.; Marques, M. M. B. Org. Lett. 2022, 24, 776–781. doi:10.1021/acs.orglett.1c04312
Return to citation in text: [1] -
Ilchenko, N. O.; Tasch, B. O. A.; Szabó, K. J. Angew. Chem., Int. Ed. 2014, 53, 12897–12901. doi:10.1002/anie.201408812
Return to citation in text: [1] -
Zheng, L.; Wang, Z.; Li, C.; Wu, Y.; Liu, Z.; Ning, Y. Chem. Commun. 2021, 57, 9874–9877. doi:10.1039/d1cc04268h
Return to citation in text: [1] -
Yuan, W.; Szabó, K. J. Angew. Chem., Int. Ed. 2015, 54, 8533–8537. doi:10.1002/anie.201503373
Return to citation in text: [1] -
Ulmer, A.; Brunner, C.; Arnold, A. M.; Pöthig, A.; Gulder, T. Chem. – Eur. J. 2016, 22, 3660–3664. doi:10.1002/chem.201504749
Return to citation in text: [1] -
Farooq, U.; Shah, A.-u.-H. A.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 1018–1020. doi:10.1002/anie.200805027
Return to citation in text: [1] [2] -
Ochiai, M. Synlett 2009, 159–173. doi:10.1055/s-0028-1087355
Return to citation in text: [1] [2] -
Miyamoto, K.; Uchiyama, M. Chem. Lett. 2021, 50, 832–838. doi:10.1246/cl.200849
Return to citation in text: [1] [2] -
Winterson, B.; Patra, T.; Wirth, T. Synthesis 2022, 54, 1261–1271. doi:10.1055/a-1675-8404
Return to citation in text: [1] [2] -
Ochiai, M.; Miyamoto, K.; Kaneaki, T.; Hayashi, S.; Nakanishi, W. Science 2011, 332, 448–451. doi:10.1126/science.1201686
Return to citation in text: [1] -
Miyamoto, K.; Saito, M.; Tsuji, S.; Takagi, T.; Shiro, M.; Uchiyama, M.; Ochiai, M. J. Am. Chem. Soc. 2021, 143, 9327–9331. doi:10.1021/jacs.1c04536
Return to citation in text: [1] [2] -
Sokolovs, I.; Suna, E. Org. Lett. 2023, 25, 2047–2052. doi:10.1021/acs.orglett.3c00405
Return to citation in text: [1] [2] -
Yoshida, Y.; Ishikawa, S.; Mino, T.; Sakamoto, M. Chem. Commun. 2021, 57, 2519–2522. doi:10.1039/d0cc07733j
Return to citation in text: [1] -
Yoshida, Y.; Ao, T.; Mino, T.; Sakamoto, M. Molecules 2023, 28, 384. doi:10.3390/molecules28010384
Return to citation in text: [1] -
Lanzi, M.; Dherbassy, Q.; Wencel‐Delord, J. Angew. Chem., Int. Ed. 2021, 60, 14852–14857. doi:10.1002/anie.202103625
Return to citation in text: [1] -
Lanzi, M.; Ali Abdine, R. A.; De Abreu, M.; Wencel-Delord, J. Org. Lett. 2021, 23, 9047–9052. doi:10.1021/acs.orglett.1c03278
Return to citation in text: [1] -
Lanzi, M.; Wencel-Delord, J. Chem. Sci. 2024, 15, 1557–1569. doi:10.1039/d3sc05382b
Return to citation in text: [1] -
De Abreu, M.; Rogge, T.; Lanzi, M.; Saiegh, T. J.; Houk, K. N.; Wencel-Delord, J. Angew. Chem., Int. Ed. 2024, 63, e202319960. doi:10.1002/anie.202319960
Return to citation in text: [1] -
Yoshida, Y.; Mino, T.; Sakamoto, M. ACS Catal. 2021, 11, 13028–13033. doi:10.1021/acscatal.1c04070
Return to citation in text: [1] -
Lanzi, M.; Rogge, T.; Truong, T. S.; Houk, K. N.; Wencel-Delord, J. J. Am. Chem. Soc. 2023, 145, 345–358. doi:10.1021/jacs.2c10090
Return to citation in text: [1] -
Huss, C. D.; Yoshimura, A.; Rohde, G. T.; Mironova, I. A.; Postnikov, P. S.; Yusubov, M. S.; Saito, A.; Zhdankin, V. V. ACS Omega 2024, 9, 2664–2673. doi:10.1021/acsomega.3c07512
Return to citation in text: [1] -
Chen, W. W.; Artigues, M.; Font-Bardia, M.; Cuenca, A. B.; Shafir, A. J. Am. Chem. Soc. 2023, 145, 13796–13804. doi:10.1021/jacs.3c02406
Return to citation in text: [1] -
Yang, J.-D.; Li, M.; Xue, X.-S. Chin. J. Chem. 2019, 37, 359–363. doi:10.1002/cjoc.201800549
Return to citation in text: [1] [2] -
Internet Bond-energy Databank (pKa and BDE)-iBonD: http://ibond.chem.tsinghua.edu.cn or http://ibond.nankai.edu.cn.
Return to citation in text: [1] -
Lohithakshamenon, R.; Prasanthkumar, K. P.; Femina, C.; Sajith, P. K. J. Phys. Chem. A 2024, 128, 727–737. doi:10.1021/acs.jpca.3c06378
Return to citation in text: [1] [2] -
Jiang, H.; Sun, T.-Y.; Chen, Y.; Zhang, X.; Wu, Y.-D.; Xie, Y.; Schaefer, H. F., III. Chem. Commun. 2019, 55, 5667–5670. doi:10.1039/c9cc01320b
Return to citation in text: [1] [2] -
Schütt, K. T.; Arbabzadah, F.; Chmiela, S.; Müller, K. R.; Tkatchenko, A. Nat. Commun. 2017, 8, 13890. doi:10.1038/ncomms13890
Return to citation in text: [1] -
Schütt, K. T.; Sauceda, H. E.; Kindermans, P.-J.; Tkatchenko, A.; Müller, K.-R. J. Chem. Phys. 2018, 148, 241722. doi:10.1063/1.5019779
Return to citation in text: [1] -
Yang, Q.; Li, Y.; Yang, J.-D.; Liu, Y.; Zhang, L.; Luo, S.; Cheng, J.-P. Angew. Chem., Int. Ed. 2020, 59, 19282–19291. doi:10.1002/anie.202008528
Return to citation in text: [1] -
St. John, P. C.; Guan, Y.; Kim, Y.; Kim, S.; Paton, R. S. Nat. Commun. 2020, 11, 2328. doi:10.1038/s41467-020-16201-z
Return to citation in text: [1] -
Jeong, W.; Stoneburner, S. J.; King, D.; Li, R.; Walker, A.; Lindh, R.; Gagliardi, L. J. Chem. Theory Comput. 2020, 16, 2389–2399. doi:10.1021/acs.jctc.9b01297
Return to citation in text: [1] -
Wen, M.; Blau, S. M.; Spotte-Smith, E. W. C.; Dwaraknath, S.; Persson, K. A. Chem. Sci. 2021, 12, 1858–1868. doi:10.1039/d0sc05251e
Return to citation in text: [1] -
Yu, H.; Wang, Y.; Wang, X.; Zhang, J.; Ye, S.; Huang, Y.; Luo, Y.; Sharman, E.; Chen, S.; Jiang, J. J. Phys. Chem. A 2020, 124, 3844–3850. doi:10.1021/acs.jpca.0c01280
Return to citation in text: [1] -
Nakajima, M.; Nemoto, T. Sci. Rep. 2021, 11, 20207. doi:10.1038/s41598-021-99369-8
Return to citation in text: [1] [2] -
S. V., S. S.; Kim, Y.; Kim, S.; St. John, P. C.; Paton, R. S. Digital Discovery 2023, 2, 1900–1910. doi:10.1039/d3dd00169e
Return to citation in text: [1] -
Liu, Y.; Yang, Q.; Cheng, J.; Zhang, L.; Luo, S.; Cheng, J.-P. ChemPhysChem 2023, 24, e202300162. doi:10.1002/cphc.202300162
Return to citation in text: [1] [2] -
Saini, V.; Kataria, R.; Rajput, S. Artif. Intell. Chem. 2024, 2, 100032. doi:10.1016/j.aichem.2023.100032
Return to citation in text: [1] -
Li, Y.; Huang, W.-S.; Zhang, L.; Su, D.; Xu, H.; Xue, X.-S. Artif. Intell. Chem. 2024, 2, 100043. doi:10.1016/j.aichem.2024.100043
Return to citation in text: [1] -
Gelžinytė, E.; Öeren, M.; Segall, M. D.; Csányi, G. J. Chem. Theory Comput. 2024, 20, 164–177. doi:10.1021/acs.jctc.3c00710
Return to citation in text: [1] -
Yan, T.; Zhou, B.; Xue, X.-S.; Cheng, J.-P. J. Org. Chem. 2016, 81, 9006–9011. doi:10.1021/acs.joc.6b01642
Return to citation in text: [1] [2] -
Zhou, B.; Yan, T.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 6128–6131. doi:10.1021/acs.orglett.6b03134
Return to citation in text: [1] [2] -
Zhou, B.; Xue, X.-s.; Cheng, J.-p. Tetrahedron Lett. 2017, 58, 1287–1291. doi:10.1016/j.tetlet.2017.02.040
Return to citation in text: [1] [2] -
Zhou, B.; Haj, M. K.; Jacobsen, E. N.; Houk, K. N.; Xue, X.-S. J. Am. Chem. Soc. 2018, 140, 15206–15218. doi:10.1021/jacs.8b05935
Return to citation in text: [1] [2] -
Zheng, H.; Sang, Y.; Houk, K. N.; Xue, X.-S.; Cheng, J.-P. J. Am. Chem. Soc. 2019, 141, 16046–16056. doi:10.1021/jacs.9b08243
Return to citation in text: [1] [2] -
Zheng, H.; Xue, X.-S. Curr. Org. Chem. 2020, 24, 2106–2117. doi:10.2174/1385272824999200620223218
Return to citation in text: [1] [2] -
Zhang, D.; Shao, Y.; Zheng, H.; Zhou, B.; Xue, X.-S. Acta Chim. Sin. (Chin. Ed.) 2021, 79, 1394–1400. doi:10.6023/a21080358
Return to citation in text: [1] [2] -
Chen, Y.; Gu, Y.; Meng, H.; Shao, Q.; Xu, Z.; Bao, W.; Gu, Y.; Xue, X.-S.; Zhao, Y. Angew. Chem., Int. Ed. 2022, 61, e202201240. doi:10.1002/anie.202201240
Return to citation in text: [1] [2] -
Zheng, H.; Cai, L.; Pan, M.; Uyanik, M.; Ishihara, K.; Xue, X.-S. J. Am. Chem. Soc. 2023, 145, 7301–7312. doi:10.1021/jacs.2c13295
Return to citation in text: [1] [2] -
Ge, Y.; Shao, Y.; Wu, S.; Liu, P.; Li, J.; Qin, H.; Zhang, Y.; Xue, X.-s.; Chen, Y. ACS Catal. 2023, 13, 3749–3756. doi:10.1021/acscatal.3c00230
Return to citation in text: [1] [2] -
Shao, Y.; Ren, Z.; Zheng, C.; Xue, X.-S. Adv. Synth. Catal. 2023, 365, 2737–2743. doi:10.1002/adsc.202300375
Return to citation in text: [1] [2] -
Gao, B.; Cai, L.; Zhang, Y.; Huang, H.; Li, Y.; Xue, X.-S. CCS Chem. 2024, in press. doi:10.31635/ccschem.024.202303774
Return to citation in text: [1] [2] -
Jiang, H.; Sun, T.-Y.; Wang, X.; Xie, Y.; Zhang, X.; Wu, Y.-D.; Schaefer, H. F., III. Org. Lett. 2017, 19, 6502–6505. doi:10.1021/acs.orglett.7b03167
Return to citation in text: [1] -
Hyun, S.-M.; Yuan, M.; Maity, A.; Gutierrez, O.; Powers, D. C. Chem 2019, 5, 2388–2404. doi:10.1016/j.chempr.2019.06.006
Return to citation in text: [1] -
Matsumoto, K.; Nakajima, M.; Nemoto, T. J. Phys. Org. Chem. 2019, 32, e3961. doi:10.1002/poc.3961
Return to citation in text: [1] -
Sun, T.-Y.; Chen, K.; Zhou, H.; You, T.; Yin, P.; Wang, X. J. Comput. Chem. 2021, 42, 470–474. doi:10.1002/jcc.26469
Return to citation in text: [1] -
St. John, P. C.; Guan, Y.; Kim, Y.; Etz, B. D.; Kim, S.; Paton, R. S. Sci. Data 2020, 7, 244. doi:10.1038/s41597-020-00588-x
Return to citation in text: [1] -
Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157–167. doi:10.1021/ar700111a
Return to citation in text: [1] -
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a
Return to citation in text: [1] -
Zhao, Y.; Truhlar, D. G. Chem. Phys. Lett. 2011, 502, 1–13. doi:10.1016/j.cplett.2010.11.060
Return to citation in text: [1] -
Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, 2016.
Return to citation in text: [1] -
Ochiai, M.; Sueda, T.; Miyamoto, K.; Kiprof, P.; Zhdankin, V. V. Angew. Chem., Int. Ed. 2006, 45, 8203–8206. doi:10.1002/anie.200603055
Return to citation in text: [1] -
Sajith, P. K.; Suresh, C. H. Inorg. Chem. 2012, 51, 967–977. doi:10.1021/ic202047g
Return to citation in text: [1] -
Sajith, P. K.; Suresh, C. H. Inorg. Chem. 2013, 52, 6046–6054. doi:10.1021/ic400399v
Return to citation in text: [1] -
Veličković, P.; Cucurull, G.; Casanova, A.; Romero, A.; Liò, P.; Bengio, Y. arXiv 2017, 1710.10903. doi:10.48550/arxiv.1710.10903
Return to citation in text: [1] -
Chen, Q.; Zhang, Y.; Gao, P.; Zhang, J. Artif. Intell. Chem. 2023, 1, 100010. doi:10.1016/j.aichem.2023.100010
Return to citation in text: [1] -
Wang, M.; Zheng, D.; Ye, Z.; Gan, Q.; Li, M.; Song, X.; Zhou, J.; Ma, C.; Yu, L.; Gai, Y.; Xiao, T.; He, T.; Karypis, G.; Li, J.; Zhang, Z. arXiv 2019, 1909.01315. doi:10.48550/arxiv.1909.01315
Return to citation in text: [1]
| 46. | Lohithakshamenon, R.; Prasanthkumar, K. P.; Femina, C.; Sajith, P. K. J. Phys. Chem. A 2024, 128, 727–737. doi:10.1021/acs.jpca.3c06378 |
| 47. | Jiang, H.; Sun, T.-Y.; Chen, Y.; Zhang, X.; Wu, Y.-D.; Xie, Y.; Schaefer, H. F., III. Chem. Commun. 2019, 55, 5667–5670. doi:10.1039/c9cc01320b |
| 85. | Veličković, P.; Cucurull, G.; Casanova, A.; Romero, A.; Liò, P.; Bengio, Y. arXiv 2017, 1710.10903. doi:10.48550/arxiv.1710.10903 |
| 86. | Chen, Q.; Zhang, Y.; Gao, P.; Zhang, J. Artif. Intell. Chem. 2023, 1, 100010. doi:10.1016/j.aichem.2023.100010 |
| 1. | Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. doi:10.1021/cr940424+ |
| 2. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 3. | Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 4. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 5. | Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e |
| 6. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 7. | Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154 |
| 8. | Parra, A. Chem. Rev. 2019, 119, 12033–12088. doi:10.1021/acs.chemrev.9b00338 |
| 9. | Flores, A.; Cots, E.; Bergès, J.; Muñiz, K. Adv. Synth. Catal. 2019, 361, 2–25. doi:10.1002/adsc.201800521 |
| 10. | Zhdankin, V. V. ARKIVOC 2020, No. iv, 1–11. doi:10.24820/ark.5550190.p011.145 |
| 11. | Singh, F. V.; Shetgaonkar, S. E.; Krishnan, M.; Wirth, T. Chem. Soc. Rev. 2022, 51, 8102–8139. doi:10.1039/d2cs00206j |
| 18. | Li, Y.; Hari, D. P.; Vita, M. V.; Waser, J. Angew. Chem., Int. Ed. 2016, 55, 4436–4454. doi:10.1002/anie.201509073 |
| 19. | Yoshimura, A.; Saito, A.; Zhdankin, V. V. Adv. Synth. Catal. 2023, 365, 2653–2675. doi:10.1002/adsc.202300275 |
| 40. | Yoshida, Y.; Mino, T.; Sakamoto, M. ACS Catal. 2021, 11, 13028–13033. doi:10.1021/acscatal.1c04070 |
| 41. | Lanzi, M.; Rogge, T.; Truong, T. S.; Houk, K. N.; Wencel-Delord, J. J. Am. Chem. Soc. 2023, 145, 345–358. doi:10.1021/jacs.2c10090 |
| 42. | Huss, C. D.; Yoshimura, A.; Rohde, G. T.; Mironova, I. A.; Postnikov, P. S.; Yusubov, M. S.; Saito, A.; Zhdankin, V. V. ACS Omega 2024, 9, 2664–2673. doi:10.1021/acsomega.3c07512 |
| 14. | Eisenberger, P.; Gischig, S.; Togni, A. Chem. – Eur. J. 2006, 12, 2579–2586. doi:10.1002/chem.200501052 |
| 43. | Chen, W. W.; Artigues, M.; Font-Bardia, M.; Cuenca, A. B.; Shafir, A. J. Am. Chem. Soc. 2023, 145, 13796–13804. doi:10.1021/jacs.3c02406 |
| 13. | Zhdankin, V. V.; Kuehl, C. J.; Krasutsky, A. P.; Bolz, J. T.; Mismash, B.; Woodward, J. K.; Simonsen, A. J. Tetrahedron Lett. 1995, 36, 7975–7978. doi:10.1016/0040-4039(95)01720-3 |
| 33. | Sokolovs, I.; Suna, E. Org. Lett. 2023, 25, 2047–2052. doi:10.1021/acs.orglett.3c00405 |
| 12. | Koser, G. F.; Sun, G.; Porter, C. W.; Youngs, W. J. J. Org. Chem. 1993, 58, 7310–7312. doi:10.1021/jo00077a071 |
| 13. | Zhdankin, V. V.; Kuehl, C. J.; Krasutsky, A. P.; Bolz, J. T.; Mismash, B.; Woodward, J. K.; Simonsen, A. J. Tetrahedron Lett. 1995, 36, 7975–7978. doi:10.1016/0040-4039(95)01720-3 |
| 14. | Eisenberger, P.; Gischig, S.; Togni, A. Chem. – Eur. J. 2006, 12, 2579–2586. doi:10.1002/chem.200501052 |
| 15. | Fernández González, D.; Brand, J. P.; Waser, J. Chem. – Eur. J. 2010, 16, 9457–9461. doi:10.1002/chem.201001539 |
| 16. | Legault, C. Y.; Prévost, J. Acta Crystallogr., Sect. E: Struct. Rep. Online 2012, 68, o1238. doi:10.1107/s1600536812012822 |
| 17. | Ren, J.; Du, F.-H.; Jia, M.-C.; Hu, Z.-N.; Chen, Z.; Zhang, C. Angew. Chem., Int. Ed. 2021, 60, 24171–24178. doi:10.1002/anie.202108589 |
| 34. | Yoshida, Y.; Ishikawa, S.; Mino, T.; Sakamoto, M. Chem. Commun. 2021, 57, 2519–2522. doi:10.1039/d0cc07733j |
| 35. | Yoshida, Y.; Ao, T.; Mino, T.; Sakamoto, M. Molecules 2023, 28, 384. doi:10.3390/molecules28010384 |
| 36. | Lanzi, M.; Dherbassy, Q.; Wencel‐Delord, J. Angew. Chem., Int. Ed. 2021, 60, 14852–14857. doi:10.1002/anie.202103625 |
| 37. | Lanzi, M.; Ali Abdine, R. A.; De Abreu, M.; Wencel-Delord, J. Org. Lett. 2021, 23, 9047–9052. doi:10.1021/acs.orglett.1c03278 |
| 38. | Lanzi, M.; Wencel-Delord, J. Chem. Sci. 2024, 15, 1557–1569. doi:10.1039/d3sc05382b |
| 39. | De Abreu, M.; Rogge, T.; Lanzi, M.; Saiegh, T. J.; Houk, K. N.; Wencel-Delord, J. Angew. Chem., Int. Ed. 2024, 63, e202319960. doi:10.1002/anie.202319960 |
| 27. | Farooq, U.; Shah, A.-u.-H. A.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 1018–1020. doi:10.1002/anie.200805027 |
| 28. | Ochiai, M. Synlett 2009, 159–173. doi:10.1055/s-0028-1087355 |
| 29. | Miyamoto, K.; Uchiyama, M. Chem. Lett. 2021, 50, 832–838. doi:10.1246/cl.200849 |
| 30. | Winterson, B.; Patra, T.; Wirth, T. Synthesis 2022, 54, 1261–1271. doi:10.1055/a-1675-8404 |
| 27. | Farooq, U.; Shah, A.-u.-H. A.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 1018–1020. doi:10.1002/anie.200805027 |
| 28. | Ochiai, M. Synlett 2009, 159–173. doi:10.1055/s-0028-1087355 |
| 29. | Miyamoto, K.; Uchiyama, M. Chem. Lett. 2021, 50, 832–838. doi:10.1246/cl.200849 |
| 30. | Winterson, B.; Patra, T.; Wirth, T. Synthesis 2022, 54, 1261–1271. doi:10.1055/a-1675-8404 |
| 25. | Yuan, W.; Szabó, K. J. Angew. Chem., Int. Ed. 2015, 54, 8533–8537. doi:10.1002/anie.201503373 |
| 26. | Ulmer, A.; Brunner, C.; Arnold, A. M.; Pöthig, A.; Gulder, T. Chem. – Eur. J. 2016, 22, 3660–3664. doi:10.1002/chem.201504749 |
| 32. | Miyamoto, K.; Saito, M.; Tsuji, S.; Takagi, T.; Shiro, M.; Uchiyama, M.; Ochiai, M. J. Am. Chem. Soc. 2021, 143, 9327–9331. doi:10.1021/jacs.1c04536 |
| 23. | Ilchenko, N. O.; Tasch, B. O. A.; Szabó, K. J. Angew. Chem., Int. Ed. 2014, 53, 12897–12901. doi:10.1002/anie.201408812 |
| 24. | Zheng, L.; Wang, Z.; Li, C.; Wu, Y.; Liu, Z.; Ning, Y. Chem. Commun. 2021, 57, 9874–9877. doi:10.1039/d1cc04268h |
| 87. | Wang, M.; Zheng, D.; Ye, Z.; Gan, Q.; Li, M.; Song, X.; Zhou, J.; Ma, C.; Yu, L.; Gai, Y.; Xiao, T.; He, T.; Karypis, G.; Li, J.; Zhang, Z. arXiv 2019, 1909.01315. doi:10.48550/arxiv.1909.01315 |
| 20. | Wang, Y.; Hu, X.; Morales-Rivera, C. A.; Li, G.-X.; Huang, X.; He, G.; Liu, P.; Chen, G. J. Am. Chem. Soc. 2018, 140, 9678–9684. doi:10.1021/jacs.8b05753 |
| 21. | Zhang, Y.; Lu, J.; Lan, T.; Cheng, S.; Liu, W.; Chen, C. Eur. J. Org. Chem. 2021, 436–442. doi:10.1002/ejoc.202001373 |
| 22. | Poeira, D. L.; Negrão, A. C. R.; Faustino, H.; Coelho, J. A. S.; Gomes, C. S. B.; Gois, P. M. P.; Marques, M. M. B. Org. Lett. 2022, 24, 776–781. doi:10.1021/acs.orglett.1c04312 |
| 31. | Ochiai, M.; Miyamoto, K.; Kaneaki, T.; Hayashi, S.; Nakanishi, W. Science 2011, 332, 448–451. doi:10.1126/science.1201686 |
| 32. | Miyamoto, K.; Saito, M.; Tsuji, S.; Takagi, T.; Shiro, M.; Uchiyama, M.; Ochiai, M. J. Am. Chem. Soc. 2021, 143, 9327–9331. doi:10.1021/jacs.1c04536 |
| 33. | Sokolovs, I.; Suna, E. Org. Lett. 2023, 25, 2047–2052. doi:10.1021/acs.orglett.3c00405 |
| 55. | Nakajima, M.; Nemoto, T. Sci. Rep. 2021, 11, 20207. doi:10.1038/s41598-021-99369-8 |
| 44. | Yang, J.-D.; Li, M.; Xue, X.-S. Chin. J. Chem. 2019, 37, 359–363. doi:10.1002/cjoc.201800549 |
| 45. | Internet Bond-energy Databank (pKa and BDE)-iBonD: http://ibond.chem.tsinghua.edu.cn or http://ibond.nankai.edu.cn. |
| 46. | Lohithakshamenon, R.; Prasanthkumar, K. P.; Femina, C.; Sajith, P. K. J. Phys. Chem. A 2024, 128, 727–737. doi:10.1021/acs.jpca.3c06378 |
| 47. | Jiang, H.; Sun, T.-Y.; Chen, Y.; Zhang, X.; Wu, Y.-D.; Xie, Y.; Schaefer, H. F., III. Chem. Commun. 2019, 55, 5667–5670. doi:10.1039/c9cc01320b |
| 48. | Schütt, K. T.; Arbabzadah, F.; Chmiela, S.; Müller, K. R.; Tkatchenko, A. Nat. Commun. 2017, 8, 13890. doi:10.1038/ncomms13890 |
| 49. | Schütt, K. T.; Sauceda, H. E.; Kindermans, P.-J.; Tkatchenko, A.; Müller, K.-R. J. Chem. Phys. 2018, 148, 241722. doi:10.1063/1.5019779 |
| 50. | Yang, Q.; Li, Y.; Yang, J.-D.; Liu, Y.; Zhang, L.; Luo, S.; Cheng, J.-P. Angew. Chem., Int. Ed. 2020, 59, 19282–19291. doi:10.1002/anie.202008528 |
| 51. | St. John, P. C.; Guan, Y.; Kim, Y.; Kim, S.; Paton, R. S. Nat. Commun. 2020, 11, 2328. doi:10.1038/s41467-020-16201-z |
| 52. | Jeong, W.; Stoneburner, S. J.; King, D.; Li, R.; Walker, A.; Lindh, R.; Gagliardi, L. J. Chem. Theory Comput. 2020, 16, 2389–2399. doi:10.1021/acs.jctc.9b01297 |
| 53. | Wen, M.; Blau, S. M.; Spotte-Smith, E. W. C.; Dwaraknath, S.; Persson, K. A. Chem. Sci. 2021, 12, 1858–1868. doi:10.1039/d0sc05251e |
| 54. | Yu, H.; Wang, Y.; Wang, X.; Zhang, J.; Ye, S.; Huang, Y.; Luo, Y.; Sharman, E.; Chen, S.; Jiang, J. J. Phys. Chem. A 2020, 124, 3844–3850. doi:10.1021/acs.jpca.0c01280 |
| 55. | Nakajima, M.; Nemoto, T. Sci. Rep. 2021, 11, 20207. doi:10.1038/s41598-021-99369-8 |
| 56. | S. V., S. S.; Kim, Y.; Kim, S.; St. John, P. C.; Paton, R. S. Digital Discovery 2023, 2, 1900–1910. doi:10.1039/d3dd00169e |
| 57. | Liu, Y.; Yang, Q.; Cheng, J.; Zhang, L.; Luo, S.; Cheng, J.-P. ChemPhysChem 2023, 24, e202300162. doi:10.1002/cphc.202300162 |
| 58. | Saini, V.; Kataria, R.; Rajput, S. Artif. Intell. Chem. 2024, 2, 100032. doi:10.1016/j.aichem.2023.100032 |
| 59. | Li, Y.; Huang, W.-S.; Zhang, L.; Su, D.; Xu, H.; Xue, X.-S. Artif. Intell. Chem. 2024, 2, 100043. doi:10.1016/j.aichem.2024.100043 |
| 60. | Gelžinytė, E.; Öeren, M.; Segall, M. D.; Csányi, G. J. Chem. Theory Comput. 2024, 20, 164–177. doi:10.1021/acs.jctc.3c00710 |
| 82. | Ochiai, M.; Sueda, T.; Miyamoto, K.; Kiprof, P.; Zhdankin, V. V. Angew. Chem., Int. Ed. 2006, 45, 8203–8206. doi:10.1002/anie.200603055 |
| 83. | Sajith, P. K.; Suresh, C. H. Inorg. Chem. 2012, 51, 967–977. doi:10.1021/ic202047g |
| 84. | Sajith, P. K.; Suresh, C. H. Inorg. Chem. 2013, 52, 6046–6054. doi:10.1021/ic400399v |
| 44. | Yang, J.-D.; Li, M.; Xue, X.-S. Chin. J. Chem. 2019, 37, 359–363. doi:10.1002/cjoc.201800549 |
| 78. | Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157–167. doi:10.1021/ar700111a |
| 79. | Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a |
| 80. | Zhao, Y.; Truhlar, D. G. Chem. Phys. Lett. 2011, 502, 1–13. doi:10.1016/j.cplett.2010.11.060 |
| 61. | Yan, T.; Zhou, B.; Xue, X.-S.; Cheng, J.-P. J. Org. Chem. 2016, 81, 9006–9011. doi:10.1021/acs.joc.6b01642 |
| 62. | Zhou, B.; Yan, T.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 6128–6131. doi:10.1021/acs.orglett.6b03134 |
| 63. | Zhou, B.; Xue, X.-s.; Cheng, J.-p. Tetrahedron Lett. 2017, 58, 1287–1291. doi:10.1016/j.tetlet.2017.02.040 |
| 64. | Zhou, B.; Haj, M. K.; Jacobsen, E. N.; Houk, K. N.; Xue, X.-S. J. Am. Chem. Soc. 2018, 140, 15206–15218. doi:10.1021/jacs.8b05935 |
| 65. | Zheng, H.; Sang, Y.; Houk, K. N.; Xue, X.-S.; Cheng, J.-P. J. Am. Chem. Soc. 2019, 141, 16046–16056. doi:10.1021/jacs.9b08243 |
| 66. | Zheng, H.; Xue, X.-S. Curr. Org. Chem. 2020, 24, 2106–2117. doi:10.2174/1385272824999200620223218 |
| 67. | Zhang, D.; Shao, Y.; Zheng, H.; Zhou, B.; Xue, X.-S. Acta Chim. Sin. (Chin. Ed.) 2021, 79, 1394–1400. doi:10.6023/a21080358 |
| 68. | Chen, Y.; Gu, Y.; Meng, H.; Shao, Q.; Xu, Z.; Bao, W.; Gu, Y.; Xue, X.-S.; Zhao, Y. Angew. Chem., Int. Ed. 2022, 61, e202201240. doi:10.1002/anie.202201240 |
| 69. | Zheng, H.; Cai, L.; Pan, M.; Uyanik, M.; Ishihara, K.; Xue, X.-S. J. Am. Chem. Soc. 2023, 145, 7301–7312. doi:10.1021/jacs.2c13295 |
| 70. | Ge, Y.; Shao, Y.; Wu, S.; Liu, P.; Li, J.; Qin, H.; Zhang, Y.; Xue, X.-s.; Chen, Y. ACS Catal. 2023, 13, 3749–3756. doi:10.1021/acscatal.3c00230 |
| 71. | Shao, Y.; Ren, Z.; Zheng, C.; Xue, X.-S. Adv. Synth. Catal. 2023, 365, 2737–2743. doi:10.1002/adsc.202300375 |
| 72. | Gao, B.; Cai, L.; Zhang, Y.; Huang, H.; Li, Y.; Xue, X.-S. CCS Chem. 2024, in press. doi:10.31635/ccschem.024.202303774 |
| 73. | Jiang, H.; Sun, T.-Y.; Wang, X.; Xie, Y.; Zhang, X.; Wu, Y.-D.; Schaefer, H. F., III. Org. Lett. 2017, 19, 6502–6505. doi:10.1021/acs.orglett.7b03167 |
| 74. | Hyun, S.-M.; Yuan, M.; Maity, A.; Gutierrez, O.; Powers, D. C. Chem 2019, 5, 2388–2404. doi:10.1016/j.chempr.2019.06.006 |
| 75. | Matsumoto, K.; Nakajima, M.; Nemoto, T. J. Phys. Org. Chem. 2019, 32, e3961. doi:10.1002/poc.3961 |
| 76. | Sun, T.-Y.; Chen, K.; Zhou, H.; You, T.; Yin, P.; Wang, X. J. Comput. Chem. 2021, 42, 470–474. doi:10.1002/jcc.26469 |
| 77. | St. John, P. C.; Guan, Y.; Kim, Y.; Etz, B. D.; Kim, S.; Paton, R. S. Sci. Data 2020, 7, 244. doi:10.1038/s41597-020-00588-x |
| 57. | Liu, Y.; Yang, Q.; Cheng, J.; Zhang, L.; Luo, S.; Cheng, J.-P. ChemPhysChem 2023, 24, e202300162. doi:10.1002/cphc.202300162 |
| 61. | Yan, T.; Zhou, B.; Xue, X.-S.; Cheng, J.-P. J. Org. Chem. 2016, 81, 9006–9011. doi:10.1021/acs.joc.6b01642 |
| 62. | Zhou, B.; Yan, T.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 6128–6131. doi:10.1021/acs.orglett.6b03134 |
| 63. | Zhou, B.; Xue, X.-s.; Cheng, J.-p. Tetrahedron Lett. 2017, 58, 1287–1291. doi:10.1016/j.tetlet.2017.02.040 |
| 64. | Zhou, B.; Haj, M. K.; Jacobsen, E. N.; Houk, K. N.; Xue, X.-S. J. Am. Chem. Soc. 2018, 140, 15206–15218. doi:10.1021/jacs.8b05935 |
| 65. | Zheng, H.; Sang, Y.; Houk, K. N.; Xue, X.-S.; Cheng, J.-P. J. Am. Chem. Soc. 2019, 141, 16046–16056. doi:10.1021/jacs.9b08243 |
| 66. | Zheng, H.; Xue, X.-S. Curr. Org. Chem. 2020, 24, 2106–2117. doi:10.2174/1385272824999200620223218 |
| 67. | Zhang, D.; Shao, Y.; Zheng, H.; Zhou, B.; Xue, X.-S. Acta Chim. Sin. (Chin. Ed.) 2021, 79, 1394–1400. doi:10.6023/a21080358 |
| 68. | Chen, Y.; Gu, Y.; Meng, H.; Shao, Q.; Xu, Z.; Bao, W.; Gu, Y.; Xue, X.-S.; Zhao, Y. Angew. Chem., Int. Ed. 2022, 61, e202201240. doi:10.1002/anie.202201240 |
| 69. | Zheng, H.; Cai, L.; Pan, M.; Uyanik, M.; Ishihara, K.; Xue, X.-S. J. Am. Chem. Soc. 2023, 145, 7301–7312. doi:10.1021/jacs.2c13295 |
| 70. | Ge, Y.; Shao, Y.; Wu, S.; Liu, P.; Li, J.; Qin, H.; Zhang, Y.; Xue, X.-s.; Chen, Y. ACS Catal. 2023, 13, 3749–3756. doi:10.1021/acscatal.3c00230 |
| 71. | Shao, Y.; Ren, Z.; Zheng, C.; Xue, X.-S. Adv. Synth. Catal. 2023, 365, 2737–2743. doi:10.1002/adsc.202300375 |
| 72. | Gao, B.; Cai, L.; Zhang, Y.; Huang, H.; Li, Y.; Xue, X.-S. CCS Chem. 2024, in press. doi:10.31635/ccschem.024.202303774 |
© 2024 Shao et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.