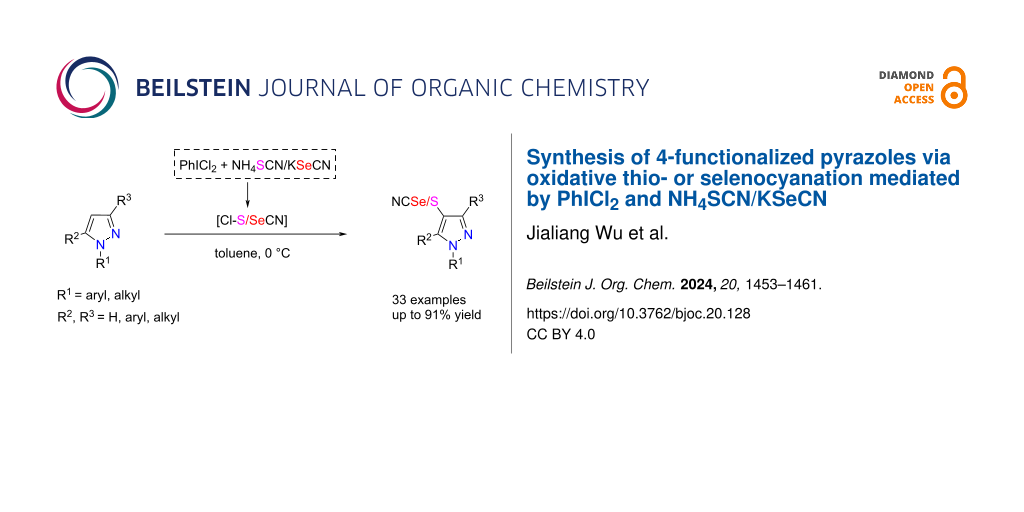
Abstract
A series of 4-thio/seleno-cyanated pyrazoles was conveniently synthesized from 4-unsubstituted pyrazoles using NH4SCN/KSeCN as thio/selenocyanogen sources and PhICl2 as the hypervalent iodine oxidant. This metal-free approach was postulated to involve the in situ generation of reactive thio/selenocyanogen chloride (Cl–SCN/SeCN) from the reaction of PhICl2 and NH4SCN/KSeCN, followed by an electrophilic thio/selenocyanation of the pyrazole skeleton.
Graphical Abstract
Introduction
Pyrazoles and their derivatives are an important class of five-membered heterocyclic compounds [1-5] that have drawn increasing attention from organic chemists, due to their potential biological and pharmaceutical properties including anti-inflammatory [6], antiviral [7], antibacterial [8], antifungal [9], cytotoxic [10], antioxidant [11], and analgesic [12] activities. For instance, celecoxib (I, Figure 1) (for treating rheumatoid arthritis and osteoarthritis), tepoxalin (II, Figure 1) (a veterinary painkiller used to relieve pain from muscle and bone diseases), dimetilan (III, Figure 1) (demonstrating excellent insecticidal effects) [13-15] all possess a pyrazole framework in their respective chemical structure. Considering the pharmaceutical significance of pyrazole compounds, there has been growing interest in the development of efficient strategies for accessing functionalized pyrazole derivatives.
Figure 1: Representative pyrazoles with pharmacological activities and S/Se-containing pharmaceutical molecules.
Figure 1: Representative pyrazoles with pharmacological activities and S/Se-containing pharmaceutical molecul...
Thio/selenocyano groups are widely existing in the core structural motifs of various natural products and pharmaceutical agents [16-20]. Many S/SeCN-containing bioactive small molecules have been proved to possess wide-ranging biological activities. Specifically, representative examples include fasicularin (IV, Figure 1), which possesses cytotoxic properties [21] and psammaplin B (V, Figure 1), which shows antimicrobial and mild tyrosine kinase inhibition activities [22]. In addition, Se-aspirin (VI, Figure 1) has been used as an effective anti-inflammatory pharmaceutical [23]. On the other hand, organic thiocyanates usually serve as useful synthetic intermediates that can be conveniently converted to sulfur-containing derivatives including sulfides [24], disulfides [25], thiocarbamates [26], and trifluoromethyl thioethers [27]. Likewise, selenocyanates can be used as versatile precursors for the synthesis of a variety of selenium-containing compounds [28-32].
As the S/SeCN-containing organic compounds play an important role in organic and medicinal chemistry, organic chemists have devoted a great deal of efforts to developing efficient thio/selenocyanation approaches [33-41]. Specifically, a plethora of synthetic strategies have been reported for the thiocyanation of heteroaromatic compounds including arenes, indoles, carbazoles, pyrroles, and imidazopyridines [42-45]. However, the electrophilic thiocyanation of biologically important pyrazoles has been less explored [46-48]. Among them, the majority of the reported methods proceed through a radical pathway, with the SCN radical generated by the reaction of the thiocyanate source with a corresponding oxidant (Scheme 1a–c) [49]. For example, Xu reported that a series of 4-thiocyanated 5-hydroxy-1H-pyrazoles was synthesized by a K2S2O8-promoted direct thiocyanation of pyrazolin-5-ones at room temperature, using NH4SCN as thiocyanogen source (Scheme 1a) [20]. Similarly, utilizing NH4SCN and K2S2O8, Yotphan and colleagues realized a direct thiocyanation of N-substituted pyrazolones under metal-free conditions [49]. Besides, Choudhury and co-workers developed an additive and metal-free methodology for the C–H thiocyanation of aminopyrazoles, using H2O2 as a benign oxidizing agent (Scheme 1b) [41]. Pan presented a method for the C–H thiocyanation of pyrazoles by using a sustainable catalyst of graphite-phase carbon nitride (g-C3N4) under visible light irradiation (Scheme 1c) [2]. Furthermore, Yao harnessed an electrochemical approach to form the electrophilic SCN+ intermediate, which reacted with pyrazoles to give the corresponding thiocyanated pyrazoles (Scheme 1d) [50]. However, to our knowledge, there are only few reports on the electrophilic selenocyanation of heterocycles [51-53] including the biologically important pyrazoles. In this regard, it should be highly desirable to develop an efficient method for a smooth selenocyanation of pyrazole compounds.
Scheme 1: Approaches for thio/selenocyanation of the pyrazole skeleton.
Scheme 1: Approaches for thio/selenocyanation of the pyrazole skeleton.
Results and Discussion
In our previous work we reported that a regioselective C-5 thiocyanation of the 2-pyridone skeleton could be realized via a PhICl2-mediated electrophilic thiocyanation approach [54]. Inspired by this previous work, we were interested at investigating whether a direct C-4 selenocyanation as well as a thiocyanation of the pyrazole skeleton could be realized using the same protocol. At the outset of the study, 3,5-dimethyl-1-phenyl-1H-pyrazole (1a, 1 equiv) was chosen as the model substrate to react with NH4SCN (1 equiv) and PhICl2 (1 equiv) in THF at 0 °C under N2 atmosphere. To our delight, the desired thiocyanated product 2a was obtained in 68% yield (Table 1, entry 1). Encouraged by this result, we proceeded to investigate the other parameters that would possibly affect the efficiency of the reaction. First, upon a comparison of different reaction temperatures, we found that the reaction operated at 0 °C gave the best result (Table 1, entries 1–3). Then, other SCN-containing inorganic salts including KSCN, AgSCN, and CuSCN were screened, and the results showed that none of them gave better results than NH4SCN (Table 1, entries 4–6). Next, other oxidants including phenyliodine(III) diacetate (PIDA), phenyliodine(III) bis(trifluoroacetate) (PIFA), iodosobenzene (PhIO), and NCS were applied, and the results indicated that PhICl2 was the most effective oxidant (Table 1, entries 7–10). Later on, when the dosage of PhICl2 and NH4SCN was increased to 2.0 equivalents, the yield of product 2a significantly increased to 82% (Table 1, entry 11). However, when the loading of PhICl2 and NH4SCN were further increased to 3.0 equivalents, the reaction did not afford a better outcome (Table 1, entry 12). Furthermore, solvent screening showed that toluene was the most appropriate solvent, while the reaction led to a much lower yield when DMF, MeOH, MeCN, or DCM were used as solvents (Table 1, entries 13–17). On the basis of the above experimental results, the optimized conditions for the thiocyanation of the model substrate were concluded to be: 2.0 equivalents of PhICl2 and NH4SCN in toluene at 0 °C, under N2 atmosphere (Table 1, entry 17).
Table 1: Optimization of oxidative thiocyanation of pyrazole.a
|
|
|||||
| Entry | Oxidant (equiv) | [SCN] (equiv) | Solvent | T (°C) | Yield (%)b |
| 1 | PhICl2 (1.0) | NH4SCN (1.0) | THF | 0 | 68 |
| 2 | PhICl2 (1.0) | NH4SCN (1.0) | THF | 25 | 43 |
| 3 | PhICl2 (1.0) | NH4SCN (1.0) | THF | 40 | 40 |
| 4 | PhICl2 (1.0) | KSCN (1.0) | THF | 0 | 10 |
| 5 | PhICl2 (1.0) | AgSCN (1.0) | THF | 0 | 15 |
| 6 | PhICl2 (1.0) | CuSCN (1.0) | THF | 0 | 12 |
| 7 | PIDA (1.0) | NH4SCN (1.0) | THF | 0 | NRc |
| 8 | PIFA (1.0) | NH4SCN (1.0) | THF | 0 | NR |
| 9 | PhIO (1.0) | NH4SCN (1.0) | THF | 0 | NR |
| 10 | NCS (1.0) | NH4SCN (1.0) | THF | 0 | NDd |
| 11 | PhICl2 (2.0) | NH4SCN (2.0) | THF | 0 | 82 |
| 12 | PhICl2 (3.0) | NH4SCN (3.0) | THF | 0 | 80 |
| 13 | PhICl2 (2.0) | NH4SCN (2.0) | DMF | 0 | NR |
| 14 | PhICl2 (2.0) | NH4SCN (2.0) | MeOH | 0 | 10 |
| 15 | PhICl2 (2.0) | NH4SCN (2.0) | MeCN | 0 | 58 |
| 16 | PhICl2 (2.0) | NH4SCN (2.0) | DCM | 0 | 55 |
| 17 | PhICl2 (2.0) | NH4SCN (2.0) | toluene | 0 | 91 |
aReaction conditions: under N2 atmosphere, a mixture of oxidant and [SCN] in solvent (2 mL) was stirred at 0 °C for 0.5 h, then 1a (0.20 mmol) was added, and stirring continued at 0 °C for 8 h. bYield of the isolated product. cNR = no reaction. dND = no desired product.
With the optimized reaction conditions in hand, the substrate scope of this thiocyanation approach was next investigated (Scheme 2). The results showed that the newly established PhICl2/NH4SCN protocol was suitable for a wide range of substrates. Specifically, when N-aryl substrates containing electron-donating groups (-Me, -OMe) were subjected to the standard reaction conditions, the corresponding products 2b–e were obtained in good yields (80–91%). It was found that there was no significant influence on the outcome of the reactions of various N-aryl-substituted pyrazoles with a methyl group at the ortho-, meta- or para- positions of the phenyl group. Next, N-arylated substrates bearing electron-withdrawing groups (-F, -Cl, -Br, -I, -CF3, -NO2) were tested, and the desired products 2f–k were conveniently obtained in moderate to good yields. Notably, the reaction of the substrate bearing a -CF3 group afforded the corresponding product 2j in 93% yield. However, the substrate possessing a -NO2 substituent gave an inferior yield of the product 2k. Then, we proceeded to investigate the effects of different substituents R2 and R3. When the methyl substituent (R2) was replaced with an aryl group, the corresponding thiocyanated products 2l–o could be obtained in acceptable to moderate yields. On the other hand, the method was equally applicable to the substrate bearing two aryl substituents (R2 and R3), albeit the reaction afforded product 2n in a much lower yield, possibly caused by steric congestion. In addition, when the aryl substituent of R1 was replaced with a tert-butyl group, this method also worked well to give product 2o in moderate yield. Notably, when the C3 and C5-unsubstituted substrate 1p was subjected to the standard conditions, the 4-thiocyanated product 2p was obtained regioselectively in 87% yield. Strikingly, the thiocyanation of the pharmaceutically active compound edaravone could also be realized under the optimized conditions, affording the corresponding product 2q in good yield.
Scheme 2: PhICl2/NH4SCN-mediated thiocyanation of pyrazoles. Reaction conditions: under N2 atmosphere, a mixture of PhICl2 (2.00 mmol) and NH4SCN (2.00 mmol) in toluene (5 mL) was stirred at 0 °C for 0.5 h, then 1a (1.00 mmol) was added and stirring continued at 0 °C for 8 h. Isolated yields are given.
Scheme 2: PhICl2/NH4SCN-mediated thiocyanation of pyrazoles. Reaction conditions: under N2 atmosphere, a mixt...
Furthermore, we turned our attention to the applicability of this protocol for the selenocyanation of the pyrazole skeleton (Scheme 3). Gratifyingly, the method was equally applicable to selenocyanation of pyrazoles bearing various substituents, with the corresponding selenocyanated products 3a–o achieved in acceptable to good yields. Similarly, the selenocyanation of C3- and C5-unsubstituted substrate 1p regioselectively furnished the 4-selenocyanated pyrazole 3p in good yield.
Scheme 3: PhICl2/KSeCN-mediated selenocyanation of pyrazoles. Reaction conditions: under N2 atmosphere, a mixture of PhICl2 (2.00 mmol) and KSeCN (2.00 mmol) in toluene (5 mL) was stirred at 0 °C for 0.5 h, then 1a (1.00 mmol) was added and stirring continued at 0 °C for 8 h. Isolated yields are given.
Scheme 3: PhICl2/KSeCN-mediated selenocyanation of pyrazoles. Reaction conditions: under N2 atmosphere, a mix...
The utility of this approach was further demonstrated by a scale-up experiment. When 10.0 mmol of compound 1a were treated with 20.0 mmol of NH4SCN/KSeCN and PhICl2 under the standard reaction conditions, the desired products 2a and 3a were obtained in 88% and 80% yield, respectively (Scheme 4).
Scheme 4: Gram-scale synthesis of compounds 2a and 3a and their derivatization.
Scheme 4: Gram-scale synthesis of compounds 2a and 3a and their derivatization.
The obtained 4-thio/selenocyanated pyrazoles could be further derivatized by known approaches. Specifically, products 2a and 3a could react with TMSCF3 in the presence of Cs2CO3 [55] to give the corresponding SCF3- and SeCF3-containing compounds 2r and 3q in moderate yields. Moreover, products 2a and 3a could be conveniently transformed into thiomethyl and selenomethyl-substituted pyrazole derivatives 2s and 3r by treatment with CH3MgBr in THF [56] (Scheme 4).
Based on the previous reports [54,57-59], a possible mechanism of this selenocyanation reaction was proposed (Scheme 5). First, the reaction of PhICl2 with KSeCN produces selenocyanogen chloride (Cl–SeCN), which further reacts with selenocyanate to give (SeCN)2 [60]. Then, one selenium atom of (SeCN)2 nucleophilically attacks the iodine center in PhICl2 to generate intermediate A, which was further transformed into intermediate B by release of one molecule of iodobenzene. Next, the nucleophilic attack of chloride anion to the bivalent selenium center of intermediate B resulted in the formation of two molecules of Cl–SeCN. Subsequently, Cl–SeCN undergoes an electrophilic addition reaction with pyrazole 1 to give intermediate C, which, after deprotonative rearomatization affords the 4-selenocyanated pyrazole 3.
Conclusion
In conclusion, we have accomplished the synthesis of a series of C-4 thio/selenocyanated pyrazoles via a hypervalent iodine-mediated electrophilic thio/selenocyanation approach under mild reaction conditions. Furthermore, the obtained S/SeCN-containing pyrazoles can be converted to S/SeCF3- and S/SeMe-containing pyrazole derivatives. Further investigations of the synthetic utility of this approach are currently ongoing in our lab.
Supporting Information
| Supporting Information File 1: Synthetic details and compound characterization data. | ||
| Format: PDF | Size: 5.5 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Sangani, C. B.; Mungra, D. C.; Patel, M. P.; Patel, R. G. Chin. Chem. Lett. 2012, 23, 57–60. doi:10.1016/j.cclet.2011.09.012
Return to citation in text: [1] -
Pan, J.; Liu, C.; Wang, J.; Dai, Y.; Wang, S.; Guo, C. Tetrahedron Lett. 2021, 77, 153253. doi:10.1016/j.tetlet.2021.153253
Return to citation in text: [1] [2] -
Mamaghani, M.; Hossein Nia, R.; Shirini, F.; Tabatabaeian, K.; Rassa, M. Med. Chem. Res. 2015, 24, 1916–1926. doi:10.1007/s00044-014-1271-y
Return to citation in text: [1] -
El-Sabbagh, O. I.; Baraka, M. M.; Ibrahim, S. M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Rashad, A. A. Eur. J. Med. Chem. 2009, 44, 3746–3753. doi:10.1016/j.ejmech.2009.03.038
Return to citation in text: [1] -
Li, G.; Cheng, Y.; Han, C.; Song, C.; Huang, N.; Du, Y. RSC Med. Chem. 2022, 13, 1300–1321. doi:10.1039/d2md00206j
Return to citation in text: [1] -
Gawad, N. M. A.-E.; Georgey, H. H.; Ibrahim, N. A.; Amin, N. H.; Abdelsalam, R. M. Arch. Pharmacal Res. 2012, 35, 807–821. doi:10.1007/s12272-012-0507-y
Return to citation in text: [1] -
Ouyang, G.; Cai, X.-J.; Chen, Z.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 10160–10167. doi:10.1021/jf802489e
Return to citation in text: [1] -
Lupsor, S.; Aonofriesei, F.; Iovu, M. Med. Chem. Res. 2012, 21, 3035–3042. doi:10.1007/s00044-011-9839-2
Return to citation in text: [1] -
Mert, S.; Kasımoğulları, R.; İça, T.; Çolak, F.; Altun, A.; Ok, S. Eur. J. Med. Chem. 2014, 78, 86–96. doi:10.1016/j.ejmech.2014.03.033
Return to citation in text: [1] -
Hassan, G. S.; Kadry, H. H.; Abou-Seri, S. M.; Ali, M. M.; Mahmoud, A. E. E.-D. Bioorg. Med. Chem. 2011, 19, 6808–6817. doi:10.1016/j.bmc.2011.09.036
Return to citation in text: [1] -
Rangaswamy, J.; Vijay Kumar, H.; Harini, S. T.; Naik, N. Bioorg. Med. Chem. Lett. 2012, 22, 4773–4777. doi:10.1016/j.bmcl.2012.05.061
Return to citation in text: [1] -
Palaska, E.; Aytemir, M.; Uzbay, İ. T.; Erol, D. Eur. J. Med. Chem. 2001, 36, 539–543. doi:10.1016/s0223-5234(01)01243-0
Return to citation in text: [1] -
Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Chem. Rev. 2013, 113, 2958–3043. doi:10.1021/cr300176g
Return to citation in text: [1] -
Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. New J. Chem. 2017, 41, 16–41. doi:10.1039/c6nj03181a
Return to citation in text: [1] -
Kang, E.; Kim, H. T.; Joo, J. M. Org. Biomol. Chem. 2020, 18, 6192–6210. doi:10.1039/d0ob01265c
Return to citation in text: [1] -
Yasman; Edrada, R. A.; Wray, V.; Proksch, P. J. Nat. Prod. 2003, 66, 1512–1514. doi:10.1021/np030237j
Return to citation in text: [1] -
Brown, S. P.; Smith, A. B., III. J. Am. Chem. Soc. 2015, 137, 4034–4037. doi:10.1021/ja512880g
Return to citation in text: [1] -
Lawson, A. P.; Long, M. J. C.; Coffey, R. T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Cancer Res. 2015, 75, 5130–5142. doi:10.1158/0008-5472.can-15-1544
Return to citation in text: [1] -
Yang, H.; Duan, X.-H.; Zhao, J.-F.; Guo, L.-N. Org. Lett. 2015, 17, 1998–2001. doi:10.1021/acs.orglett.5b00754
Return to citation in text: [1] -
Mao, X.; Ni, J.; Xu, B.; Ding, C. Org. Chem. Front. 2020, 7, 350–354. doi:10.1039/c9qo01174a
Return to citation in text: [1] [2] -
Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K. S. J. Am. Chem. Soc. 2005, 127, 15004–15005. doi:10.1021/ja053735i
Return to citation in text: [1] -
Jiménez, C.; Crews, P. Tetrahedron 1991, 47, 2097–2102. doi:10.1016/s0040-4020(01)96120-4
Return to citation in text: [1] -
Plano, D.; Karelia, D. N.; Pandey, M. K.; Spallholz, J. E.; Amin, S.; Sharma, A. K. J. Med. Chem. 2016, 59, 1946–1959. doi:10.1021/acs.jmedchem.5b01503
Return to citation in text: [1] -
Nguyen, T.; Rubinstein, M.; Wakselman, C. J. Org. Chem. 1981, 46, 1938–1940. doi:10.1021/jo00322a047
Return to citation in text: [1] -
Prabhu, K. R.; Ramesha, A. R.; Chandrasekaran, S. J. Org. Chem. 1995, 60, 7142–7143. doi:10.1021/jo00127a017
Return to citation in text: [1] -
Riemschneider, R.; Wojahn, F.; Orlick, G. J. Am. Chem. Soc. 1951, 73, 5905–5907. doi:10.1021/ja01156a552
Return to citation in text: [1] -
Goossen, L.; Matheis, C.; Wang, M.; Krause, T. Synlett 2015, 26, 1628–1632. doi:10.1055/s-0034-1378702
Return to citation in text: [1] -
Higuchi, H.; Otsubo, T.; Ogura, F.; Yamaguchi, H.; Sakata, Y.; Misumi, S. Bull. Chem. Soc. Jpn. 1982, 55, 182–187. doi:10.1246/bcsj.55.182
Return to citation in text: [1] -
Mullen, G. P.; Luthra, N. P.; Dunlap, R. B.; Odom, J. D. J. Org. Chem. 1985, 50, 811–816. doi:10.1021/jo00206a017
Return to citation in text: [1] -
Krief, A.; Dumont, W.; Delmotte, C. Angew. Chem., Int. Ed. 2000, 39, 1669–1672. doi:10.1002/(sici)1521-3773(20000502)39:9<1669::aid-anie1669>3.0.co;2-6
Return to citation in text: [1] -
Yu, F.; Li, C.; Wang, C.; Zhang, H.; Cao, Z.-Y. Org. Lett. 2021, 23, 7156–7160. doi:10.1021/acs.orglett.1c02564
Return to citation in text: [1] -
Tao, S.; Jiang, L.; Du, Y. Asian J. Org. Chem. 2022, 11, e202200595. doi:10.1002/ajoc.202200595
Return to citation in text: [1] -
Barbero, M.; Degani, I.; Diulgheroff, N.; Dughera, S.; Fochi, R. Synthesis 2001, 585–590. doi:10.1055/s-2001-12362
Return to citation in text: [1] -
Sun, N.; Che, L.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. Org. Biomol. Chem. 2015, 13, 691–696. doi:10.1039/c4ob02208d
Return to citation in text: [1] -
Fujiki, K.; Yoshida, E. Synth. Commun. 1999, 29, 3289–3294. doi:10.1080/00397919908085956
Return to citation in text: [1] -
Takagi, K.; Takachi, H.; Sasaki, K. J. Org. Chem. 1995, 60, 6552–6556. doi:10.1021/jo00125a047
Return to citation in text: [1] -
Teng, F.; Yu, J.-T.; Yang, H.; Jiang, Y.; Cheng, J. Chem. Commun. 2014, 50, 12139–12141. doi:10.1039/c4cc04578e
Return to citation in text: [1] -
Yang, X.; She, Y.; Chong, Y.; Zhai, H.; Zhu, H.; Chen, B.; Huang, G.; Yan, R. Adv. Synth. Catal. 2016, 358, 3130–3134. doi:10.1002/adsc.201600304
Return to citation in text: [1] -
Zhang, X.-Z.; Ge, D.-L.; Chen, S.-Y.; Yu, X.-Q. RSC Adv. 2016, 6, 66320–66323. doi:10.1039/c6ra13303g
Return to citation in text: [1] -
Jiang, G.; Zhu, C.; Li, J.; Wu, W.; Jiang, H. Adv. Synth. Catal. 2017, 359, 1208–1212. doi:10.1002/adsc.201601142
Return to citation in text: [1] -
Ali, D.; Panday, A. K.; Choudhury, L. H. J. Org. Chem. 2020, 85, 13610–13620. doi:10.1021/acs.joc.0c01738
Return to citation in text: [1] [2] -
Khalili, D. New J. Chem. 2016, 40, 2547–2553. doi:10.1039/c5nj02314a
Return to citation in text: [1] -
Fotouhi, L.; Nikoofar, K. Tetrahedron Lett. 2013, 54, 2903–2905. doi:10.1016/j.tetlet.2013.02.106
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Shubashree, S.; Sadashiv, K. Tetrahedron Lett. 2004, 45, 2951–2954. doi:10.1016/j.tetlet.2004.02.073
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Krishna, A. D.; Reddy, C. S.; Narsaiah, A. V. Synthesis 2005, 961–964. doi:10.1055/s-2005-861852
Return to citation in text: [1] -
Thiruvikraman, S. V.; Seshadri, S. Bull. Chem. Soc. Jpn. 1985, 58, 785–786. doi:10.1246/bcsj.58.785
Return to citation in text: [1] -
Kokorekin, V. A.; Sigacheva, V. L.; Petrosyan, V. A. Tetrahedron Lett. 2014, 55, 4306–4309. doi:10.1016/j.tetlet.2014.06.028
Return to citation in text: [1] -
Finar, I. L.; Godfrey, K. E. J. Chem. Soc. 1954, 2293–2298. doi:10.1039/jr9540002293
Return to citation in text: [1] -
Kittikool, T.; Yotphan, S. Eur. J. Org. Chem. 2020, 961–970. doi:10.1002/ejoc.201901770
Return to citation in text: [1] [2] -
Zhang, Y.; Xu, S.; Zhu, Y.; Xu, Q.; Gao, H.; Liang, Z.; Yao, X. Eur. J. Org. Chem. 2023, 26, e202201278. doi:10.1002/ejoc.202201278
Return to citation in text: [1] -
Dey, A.; Hajra, A. Adv. Synth. Catal. 2019, 361, 842–849. doi:10.1002/adsc.201801232
Return to citation in text: [1] -
Chen, X.-Y.; Kuang, X.; Wu, Y.; Zhou, J.; Wang, P. Chin. J. Chem. 2023, 41, 1979–1986. doi:10.1002/cjoc.202300188
Return to citation in text: [1] -
Zhang, X.; Wang, C.; Jiang, H.; Sun, L. RSC Adv. 2018, 8, 22042–22045. doi:10.1039/c8ra04407d
Return to citation in text: [1] -
Tao, S.; Xiao, J.; Li, Y.; Sun, F.; Du, Y. Chin. J. Chem. 2021, 39, 2536–2546. doi:10.1002/cjoc.202100278
Return to citation in text: [1] [2] -
Jouvin, K.; Matheis, C.; Goossen, L. J. Chem. – Eur. J. 2015, 21, 14324–14327. doi:10.1002/chem.201502914
Return to citation in text: [1] -
Adams, R.; Bramlet, H. B.; Tendick, F. H. J. Am. Chem. Soc. 1920, 42, 2369–2374. doi:10.1021/ja01456a033
Return to citation in text: [1] -
Ito, Y.; Touyama, A.; Uku, M.; Egami, H.; Hamashima, Y. Chem. Pharm. Bull. 2019, 67, 1015–1018. doi:10.1248/cpb.c19-00352
Return to citation in text: [1] -
Tao, S.; Huo, A.; Gao, Y.; Zhang, X.; Yang, J.; Du, Y. Front. Chem. (Lausanne, Switz.) 2022, 10, 859995. doi:10.3389/fchem.2022.859995
Return to citation in text: [1] -
Tao, S.; Xu, L.; Yang, K.; Zhang, J.; Du, Y. Org. Lett. 2022, 24, 4187–4191. doi:10.1021/acs.orglett.2c01468
Return to citation in text: [1] -
For our previous clarification on identifying the formation of (SeCN)2 and Cl–SeCN intermediates, see references [54,58,59].
Return to citation in text: [1]
| 51. | Dey, A.; Hajra, A. Adv. Synth. Catal. 2019, 361, 842–849. doi:10.1002/adsc.201801232 |
| 52. | Chen, X.-Y.; Kuang, X.; Wu, Y.; Zhou, J.; Wang, P. Chin. J. Chem. 2023, 41, 1979–1986. doi:10.1002/cjoc.202300188 |
| 53. | Zhang, X.; Wang, C.; Jiang, H.; Sun, L. RSC Adv. 2018, 8, 22042–22045. doi:10.1039/c8ra04407d |
| 54. | Tao, S.; Xiao, J.; Li, Y.; Sun, F.; Du, Y. Chin. J. Chem. 2021, 39, 2536–2546. doi:10.1002/cjoc.202100278 |
| 55. | Jouvin, K.; Matheis, C.; Goossen, L. J. Chem. – Eur. J. 2015, 21, 14324–14327. doi:10.1002/chem.201502914 |
| 1. | Sangani, C. B.; Mungra, D. C.; Patel, M. P.; Patel, R. G. Chin. Chem. Lett. 2012, 23, 57–60. doi:10.1016/j.cclet.2011.09.012 |
| 2. | Pan, J.; Liu, C.; Wang, J.; Dai, Y.; Wang, S.; Guo, C. Tetrahedron Lett. 2021, 77, 153253. doi:10.1016/j.tetlet.2021.153253 |
| 3. | Mamaghani, M.; Hossein Nia, R.; Shirini, F.; Tabatabaeian, K.; Rassa, M. Med. Chem. Res. 2015, 24, 1916–1926. doi:10.1007/s00044-014-1271-y |
| 4. | El-Sabbagh, O. I.; Baraka, M. M.; Ibrahim, S. M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Rashad, A. A. Eur. J. Med. Chem. 2009, 44, 3746–3753. doi:10.1016/j.ejmech.2009.03.038 |
| 5. | Li, G.; Cheng, Y.; Han, C.; Song, C.; Huang, N.; Du, Y. RSC Med. Chem. 2022, 13, 1300–1321. doi:10.1039/d2md00206j |
| 9. | Mert, S.; Kasımoğulları, R.; İça, T.; Çolak, F.; Altun, A.; Ok, S. Eur. J. Med. Chem. 2014, 78, 86–96. doi:10.1016/j.ejmech.2014.03.033 |
| 25. | Prabhu, K. R.; Ramesha, A. R.; Chandrasekaran, S. J. Org. Chem. 1995, 60, 7142–7143. doi:10.1021/jo00127a017 |
| 8. | Lupsor, S.; Aonofriesei, F.; Iovu, M. Med. Chem. Res. 2012, 21, 3035–3042. doi:10.1007/s00044-011-9839-2 |
| 26. | Riemschneider, R.; Wojahn, F.; Orlick, G. J. Am. Chem. Soc. 1951, 73, 5905–5907. doi:10.1021/ja01156a552 |
| 7. | Ouyang, G.; Cai, X.-J.; Chen, Z.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 10160–10167. doi:10.1021/jf802489e |
| 23. | Plano, D.; Karelia, D. N.; Pandey, M. K.; Spallholz, J. E.; Amin, S.; Sharma, A. K. J. Med. Chem. 2016, 59, 1946–1959. doi:10.1021/acs.jmedchem.5b01503 |
| 6. | Gawad, N. M. A.-E.; Georgey, H. H.; Ibrahim, N. A.; Amin, N. H.; Abdelsalam, R. M. Arch. Pharmacal Res. 2012, 35, 807–821. doi:10.1007/s12272-012-0507-y |
| 24. | Nguyen, T.; Rubinstein, M.; Wakselman, C. J. Org. Chem. 1981, 46, 1938–1940. doi:10.1021/jo00322a047 |
| 13. | Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Chem. Rev. 2013, 113, 2958–3043. doi:10.1021/cr300176g |
| 14. | Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. New J. Chem. 2017, 41, 16–41. doi:10.1039/c6nj03181a |
| 15. | Kang, E.; Kim, H. T.; Joo, J. M. Org. Biomol. Chem. 2020, 18, 6192–6210. doi:10.1039/d0ob01265c |
| 21. | Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K. S. J. Am. Chem. Soc. 2005, 127, 15004–15005. doi:10.1021/ja053735i |
| 60. | For our previous clarification on identifying the formation of (SeCN)2 and Cl–SeCN intermediates, see references [54,58,59]. |
| 12. | Palaska, E.; Aytemir, M.; Uzbay, İ. T.; Erol, D. Eur. J. Med. Chem. 2001, 36, 539–543. doi:10.1016/s0223-5234(01)01243-0 |
| 22. | Jiménez, C.; Crews, P. Tetrahedron 1991, 47, 2097–2102. doi:10.1016/s0040-4020(01)96120-4 |
| 54. | Tao, S.; Xiao, J.; Li, Y.; Sun, F.; Du, Y. Chin. J. Chem. 2021, 39, 2536–2546. doi:10.1002/cjoc.202100278 |
| 58. | Tao, S.; Huo, A.; Gao, Y.; Zhang, X.; Yang, J.; Du, Y. Front. Chem. (Lausanne, Switz.) 2022, 10, 859995. doi:10.3389/fchem.2022.859995 |
| 59. | Tao, S.; Xu, L.; Yang, K.; Zhang, J.; Du, Y. Org. Lett. 2022, 24, 4187–4191. doi:10.1021/acs.orglett.2c01468 |
| 11. | Rangaswamy, J.; Vijay Kumar, H.; Harini, S. T.; Naik, N. Bioorg. Med. Chem. Lett. 2012, 22, 4773–4777. doi:10.1016/j.bmcl.2012.05.061 |
| 56. | Adams, R.; Bramlet, H. B.; Tendick, F. H. J. Am. Chem. Soc. 1920, 42, 2369–2374. doi:10.1021/ja01456a033 |
| 10. | Hassan, G. S.; Kadry, H. H.; Abou-Seri, S. M.; Ali, M. M.; Mahmoud, A. E. E.-D. Bioorg. Med. Chem. 2011, 19, 6808–6817. doi:10.1016/j.bmc.2011.09.036 |
| 16. | Yasman; Edrada, R. A.; Wray, V.; Proksch, P. J. Nat. Prod. 2003, 66, 1512–1514. doi:10.1021/np030237j |
| 17. | Brown, S. P.; Smith, A. B., III. J. Am. Chem. Soc. 2015, 137, 4034–4037. doi:10.1021/ja512880g |
| 18. | Lawson, A. P.; Long, M. J. C.; Coffey, R. T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Cancer Res. 2015, 75, 5130–5142. doi:10.1158/0008-5472.can-15-1544 |
| 19. | Yang, H.; Duan, X.-H.; Zhao, J.-F.; Guo, L.-N. Org. Lett. 2015, 17, 1998–2001. doi:10.1021/acs.orglett.5b00754 |
| 20. | Mao, X.; Ni, J.; Xu, B.; Ding, C. Org. Chem. Front. 2020, 7, 350–354. doi:10.1039/c9qo01174a |
| 54. | Tao, S.; Xiao, J.; Li, Y.; Sun, F.; Du, Y. Chin. J. Chem. 2021, 39, 2536–2546. doi:10.1002/cjoc.202100278 |
| 57. | Ito, Y.; Touyama, A.; Uku, M.; Egami, H.; Hamashima, Y. Chem. Pharm. Bull. 2019, 67, 1015–1018. doi:10.1248/cpb.c19-00352 |
| 58. | Tao, S.; Huo, A.; Gao, Y.; Zhang, X.; Yang, J.; Du, Y. Front. Chem. (Lausanne, Switz.) 2022, 10, 859995. doi:10.3389/fchem.2022.859995 |
| 59. | Tao, S.; Xu, L.; Yang, K.; Zhang, J.; Du, Y. Org. Lett. 2022, 24, 4187–4191. doi:10.1021/acs.orglett.2c01468 |
| 33. | Barbero, M.; Degani, I.; Diulgheroff, N.; Dughera, S.; Fochi, R. Synthesis 2001, 585–590. doi:10.1055/s-2001-12362 |
| 34. | Sun, N.; Che, L.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. Org. Biomol. Chem. 2015, 13, 691–696. doi:10.1039/c4ob02208d |
| 35. | Fujiki, K.; Yoshida, E. Synth. Commun. 1999, 29, 3289–3294. doi:10.1080/00397919908085956 |
| 36. | Takagi, K.; Takachi, H.; Sasaki, K. J. Org. Chem. 1995, 60, 6552–6556. doi:10.1021/jo00125a047 |
| 37. | Teng, F.; Yu, J.-T.; Yang, H.; Jiang, Y.; Cheng, J. Chem. Commun. 2014, 50, 12139–12141. doi:10.1039/c4cc04578e |
| 38. | Yang, X.; She, Y.; Chong, Y.; Zhai, H.; Zhu, H.; Chen, B.; Huang, G.; Yan, R. Adv. Synth. Catal. 2016, 358, 3130–3134. doi:10.1002/adsc.201600304 |
| 39. | Zhang, X.-Z.; Ge, D.-L.; Chen, S.-Y.; Yu, X.-Q. RSC Adv. 2016, 6, 66320–66323. doi:10.1039/c6ra13303g |
| 40. | Jiang, G.; Zhu, C.; Li, J.; Wu, W.; Jiang, H. Adv. Synth. Catal. 2017, 359, 1208–1212. doi:10.1002/adsc.201601142 |
| 41. | Ali, D.; Panday, A. K.; Choudhury, L. H. J. Org. Chem. 2020, 85, 13610–13620. doi:10.1021/acs.joc.0c01738 |
| 27. | Goossen, L.; Matheis, C.; Wang, M.; Krause, T. Synlett 2015, 26, 1628–1632. doi:10.1055/s-0034-1378702 |
| 28. | Higuchi, H.; Otsubo, T.; Ogura, F.; Yamaguchi, H.; Sakata, Y.; Misumi, S. Bull. Chem. Soc. Jpn. 1982, 55, 182–187. doi:10.1246/bcsj.55.182 |
| 29. | Mullen, G. P.; Luthra, N. P.; Dunlap, R. B.; Odom, J. D. J. Org. Chem. 1985, 50, 811–816. doi:10.1021/jo00206a017 |
| 30. | Krief, A.; Dumont, W.; Delmotte, C. Angew. Chem., Int. Ed. 2000, 39, 1669–1672. doi:10.1002/(sici)1521-3773(20000502)39:9<1669::aid-anie1669>3.0.co;2-6 |
| 31. | Yu, F.; Li, C.; Wang, C.; Zhang, H.; Cao, Z.-Y. Org. Lett. 2021, 23, 7156–7160. doi:10.1021/acs.orglett.1c02564 |
| 32. | Tao, S.; Jiang, L.; Du, Y. Asian J. Org. Chem. 2022, 11, e202200595. doi:10.1002/ajoc.202200595 |
| 2. | Pan, J.; Liu, C.; Wang, J.; Dai, Y.; Wang, S.; Guo, C. Tetrahedron Lett. 2021, 77, 153253. doi:10.1016/j.tetlet.2021.153253 |
| 50. | Zhang, Y.; Xu, S.; Zhu, Y.; Xu, Q.; Gao, H.; Liang, Z.; Yao, X. Eur. J. Org. Chem. 2023, 26, e202201278. doi:10.1002/ejoc.202201278 |
| 49. | Kittikool, T.; Yotphan, S. Eur. J. Org. Chem. 2020, 961–970. doi:10.1002/ejoc.201901770 |
| 41. | Ali, D.; Panday, A. K.; Choudhury, L. H. J. Org. Chem. 2020, 85, 13610–13620. doi:10.1021/acs.joc.0c01738 |
| 49. | Kittikool, T.; Yotphan, S. Eur. J. Org. Chem. 2020, 961–970. doi:10.1002/ejoc.201901770 |
| 20. | Mao, X.; Ni, J.; Xu, B.; Ding, C. Org. Chem. Front. 2020, 7, 350–354. doi:10.1039/c9qo01174a |
| 42. | Khalili, D. New J. Chem. 2016, 40, 2547–2553. doi:10.1039/c5nj02314a |
| 43. | Fotouhi, L.; Nikoofar, K. Tetrahedron Lett. 2013, 54, 2903–2905. doi:10.1016/j.tetlet.2013.02.106 |
| 44. | Yadav, J. S.; Reddy, B. V. S.; Shubashree, S.; Sadashiv, K. Tetrahedron Lett. 2004, 45, 2951–2954. doi:10.1016/j.tetlet.2004.02.073 |
| 45. | Yadav, J. S.; Reddy, B. V. S.; Krishna, A. D.; Reddy, C. S.; Narsaiah, A. V. Synthesis 2005, 961–964. doi:10.1055/s-2005-861852 |
| 46. | Thiruvikraman, S. V.; Seshadri, S. Bull. Chem. Soc. Jpn. 1985, 58, 785–786. doi:10.1246/bcsj.58.785 |
| 47. | Kokorekin, V. A.; Sigacheva, V. L.; Petrosyan, V. A. Tetrahedron Lett. 2014, 55, 4306–4309. doi:10.1016/j.tetlet.2014.06.028 |
| 48. | Finar, I. L.; Godfrey, K. E. J. Chem. Soc. 1954, 2293–2298. doi:10.1039/jr9540002293 |
© 2024 Wu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.