Abstract
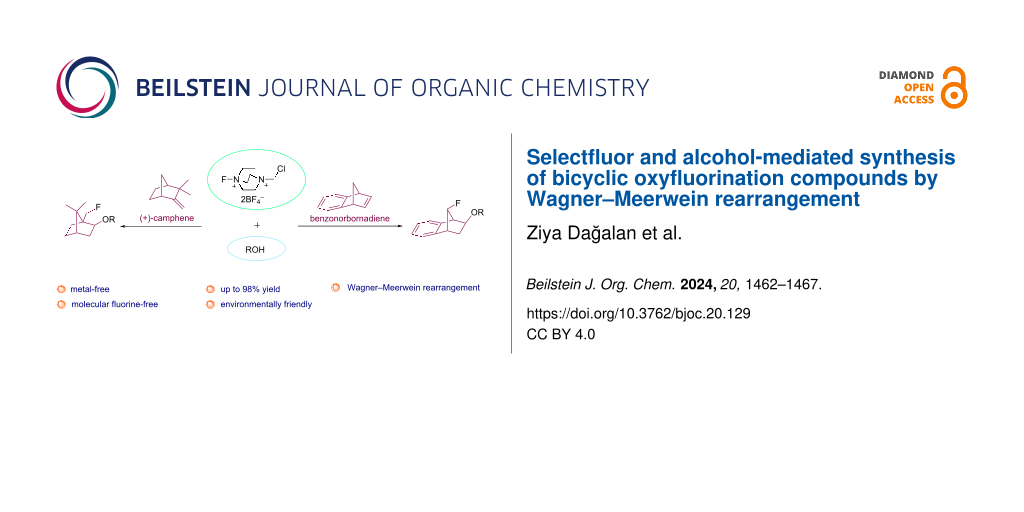
Herein, we report the first environmentally friendly systematic fluoroalkoxylation reactions in bicyclic systems. New oxyfluorination products were obtained with excellent yields (up to 98%) via Wagner–Meerwein rearrangement using benzonorbornadiene and the chiral natural compound (+)-camphene as bicyclic alkenes, selectfluor as an electrophilic fluorine source, and water and various alcohols as nucleophile sources. The structure of bicyclic oxy- and alkoxyfluorine compounds was determined by NMR and QTOF-MS analyses.
Graphical Abstract
Introduction
Organofluorines are of great importance in the pharmaceutical and agrochemical industries, as the presence of fluorine has a serious effect on the biological activities of organic compounds by changing their metabolic stability, hydrogen bonding ability, lipophilicity, solubility, bioavailability, conformation and general structure [1-4]. About 20% of commercially available drugs contain fluorine, and this ratio is estimated to increase further [5,6]. Among organofluorines, oxyfluorines are an important subclass used as an active ingredient in many different drugs such as fludrocortisone (the first fluorine-containing commercial drug) [7,8], sofosbuvir (antihepatitis C) [9], dexamethasone (to treat ashma, severe allergies) [10], difluprednate (ocular anti-inflammatory) [11,12] and many more (Figure 1). On the other hand, with unusual geometry and high reactivity norbornadiene and benzonorbornadiene derivative bicyclic compounds attract great attention by researchers with their use as building blocks in different application areas such as polymers, solar energy storage materials, supramolecular and bioactive compounds [13-17]. To the best of our knowledge, although the oxyfluorination of various olefins with water and alcohols is known in the literature [18-26], there is no systematic study on the oxyfluorination of bicyclic alkenes. We previously developed a dihomohalogenation method using selectfluor as an oxidant [27]. Herein, we synthesized bicyclic oxy- and alkoxyfluorine compounds using selectflour as an electrophilic fluorination reagent, water and various alcohols as an nucleophile.
Results and Discussion
In this study, benzonorbornadiene (1a) and the chiral natural product (+)-camphene (1b) were used as bicyclic alkenes. Safe, easily soluble, easy to use, stable solid, reactive and commercial available selectfluor [18,27,28] was selected for electrophilic fluorination source. Water and various alcohols were used as nucleophiles.
First, optimization experiments were carried out for fluoroalkoxy reactions with benzonorbornadiene (1a, Table 1). As a result of experiments conducted in six different solvents at room temperature with 1.0 equivalent of selectflor and 1.0 equivalent of methanol, it was observed that there was a 12% conversion with CH3CN and a 10% conversion with nitromethane, while no conversion occurred with the other solvents including, CH2Cl2, EtOAc, 1,4-dioxane and DMF (Table 1, entries 1–6). To see the effect of reactant ratios on yields, when reactants were gradually increased at room temperature, the best result was obtained with 1.2 equivalents of selectfluor and 2.4 equivalents of methanol with 21% conversion (Table 1, entry 7). There was no significant change at higher equivalents. At 50 °C, 30% conversion was achieved with 1.2 equivalents of selectfluor and 2.4 equivalents of methanol for one hour, while at 90 °C, a 67% conversion was obtained (Table 1, entries 8 and 9). Finally, when the reaction time was increased to two hours at 90 °C, the product was obtained with a 98% conversion (Table 1, entry 10).
Table 1: Optimizing the conditions for the oxyfluorination of bicyclic alkenesa.
|
|
||||||
| Entry | Solvent |
Selectfluor
(equiv) |
CH3OH
(equiv) |
Temperature (°C) | Time | Conversion (%) |
| 1 | CH3CN | 1 | 1 | rt | 1 h | 12 |
| 2 | CH2Cl2 | 1 | 1 | rt | 1 h | – |
| 3 | EtOAc | 1 | 1 | rt | 1 h | – |
| 4 | 1,4-dioxane | 1 | 1 | rt | 1 h | – |
| 5 | DMF | 1 | 1 | rt | 1 h | – |
| 6 | CH3NO2 | 1 | 1 | rt | 1 h | 10 |
| 7 | CH3CN | 1.2 | 2.4 | rt | 1 h | 21 |
| 8 | CH3CN | 1.2 | 2.4 | 50 °C | 1 h | 30 |
| 9 | CH3CN | 1.2 | 2.4 | 90 °C | 1 h | 67 |
| 10 | CH3CN | 1.2 | 2.4 | 90 °C | 2 h | 98 |
aReaction conditions: Benzonorbornadiene (1a, 0.5 mmol), selectfluor (215 mg, 0.61 mmol) and MeOH (1.2 mmol), 2 mL of CH3CN, 2 h and 90 °C. Conversions were calculated by 1H NMR with 1,3-dinitrobenzene as an internal standard.
After obtaining the optimum fluoroalkoxylation conditions from benzonorbornadiene (1a), the reactions of benzonorbornadiene (1a) with selectfluor and 10 different alcohol derivatives were examined (Scheme 1). Under optimum conditions, fluoroalkoxy compounds 3a–j were obtained in excellent yields (91–98%) by the reaction of benzonorbornadiene (1a) with selectfluor and alcohols (Scheme 1). The configurations of fluoroalkoxy compounds 3a–j were confirmed by the COSY 2D-NMR spectrum of compound 3a (Supporting Information File 1). Additionally, (+)-camphene (1b), a chiral natural product, was used as another alkene for fluoroalkoxy reactions. From (+)-camphene (1b), fluoroalkoxy compounds 4a–j were also obtained in very good yields (60–98%, Scheme 2). Since the reaction mechanism proceeding with a Wagner–Meerwein rearrangement does not cause racemization or a diastereomeric mixture and preserves the initial enantiomeric excess in the camphene's fluoroalkoxy derivatives (Scheme 4, below), optical rotations of the fluoroalkoxy derivatives of camphene 4a–j were also determined (Supporting Information File 1).
Scheme 1: Oxyfluorination of benzonorbornadien (1a) with Selectfluor and alcohols. All reactions were carried out using 0.5 mmol of benzonorbornadiene (1a), 0.6 mmol of selectfluor, and 1.2 mmol alcohol in 2 mL of CH3CN at 90 °C for 2 h. Isolated yields.
Scheme 1: Oxyfluorination of benzonorbornadien (1a) with Selectfluor and alcohols. All reactions were carried...
Scheme 2: Oxyfluorination of (+)-camphene (1b) with selectfluor and alcohols. All reactions were carried out using 0.5 mmol of (+)-camphene, 0.6 mmol of selectfluor, and 1.2 mmol alcohol in 2 mL of CH3CN at 90 °C for 2 h. Isolated yields.
Scheme 2: Oxyfluorination of (+)-camphene (1b) with selectfluor and alcohols. All reactions were carried out ...
In order to demonstrate the gram-scale applicability of fluoroalkoxylation reactions in bicyclic systems using optimized reaction conditions with (+)-camphene (1b, 1.0 g, 7.34 mmol), scale-up experiments were conducted. The isolated yield of 4b (1.26 g, 90% yield) is quite satisfactory, as can be seen from Scheme 3.
Scheme 3: Scale-up experiments. Reaction conditions: (+)-Camphene (1b) (1.0 g, 7.34 mmol), selectfluor (3.12 g, 8.81 mmol), MeOH (17.62 mmol), CH3CN (20 mL), 90 °C, 2 h.
Scheme 3: Scale-up experiments. Reaction conditions: (+)-Camphene (1b) (1.0 g, 7.34 mmol), selectfluor (3.12 ...
For the fluoroalkoxylation, we propose the mechanism given in Scheme 4. In this mechanism, first the double bond in (+)-camphene attacks the fluorine in the selectfluor and a carbocation is formed by bonding with fluorine. Subsequently, fluoroalkoxy compound 4 is formed by Wagner–Meerwein rearrangement followed by alcohol addition and deprotonation.
Scheme 4: Proposed mechanism for fluoroalkoxylation of (+)-camphene by Wagner–Meerwein rearrangement.
Scheme 4: Proposed mechanism for fluoroalkoxylation of (+)-camphene by Wagner–Meerwein rearrangement.
Conclusion
New bicyclic fluoroalkoxy compounds were synthesized by a molecular fluorine and metal-free methodology. An environmentally friendly approach was pursued by using safe, easily soluble, easy to use, stable, solid and reactive selectfluor as an electrophilic fluorination reagent, and water and various alcohols as a nucleophile source. Besides being novel, the presented oxyfluorination protocol provides distinct advantageous such as (i) the methodology does not require the presence of any metal moities, (ii) enables the synthesis of corresponding oxyfluorinated analogues with high yields and selectivity, (iii) allows derivatization of natural chiral molecules, (iv) uses a safe solvent in mild reaction parameters. We hope that these potentially biologically active bicyclic fluoroalkoxy compounds will find a place in various application areas in biological systems.
Supporting Information
| Supporting Information File 1: Experimental procedures, copies of 1H NMR, 13C NMR, and HRMS(Q-TOF) spectra. | ||
| Format: PDF | Size: 5.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Zhang, Z.; Wang, F.; Mu, X.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2013, 52, 7549–7553. doi:10.1002/anie.201301891
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a
Return to citation in text: [1] -
Zhang, J.; Wang, H.; Ren, S.; Zhang, W.; Liu, Y. Org. Lett. 2015, 17, 2920–2923. doi:10.1021/acs.orglett.5b01110
Return to citation in text: [1] -
Cresswell, A. J.; Davies, S. G.; Lee, J. A.; Morris, M. J.; Roberts, P. M.; Thomson, J. E. J. Org. Chem. 2012, 77, 7262–7281. doi:10.1021/jo301056r
Return to citation in text: [1] -
Kumar, A.; Singh, T. V.; Venugopalan, P. J. Fluorine Chem. 2013, 150, 72–77. doi:10.1016/j.jfluchem.2013.02.014
Return to citation in text: [1] -
Sedgwick, D. M.; López, I.; Román, R.; Kobayashi, N.; Okoromoba, O. E.; Xu, B.; Hammond, G. B.; Barrio, P.; Fustero, S. Org. Lett. 2018, 20, 2338–2341. doi:10.1021/acs.orglett.8b00681
Return to citation in text: [1] -
Zhu, Q.; Ji, D.; Liang, T.; Wang, X.; Xu, Y. Org. Lett. 2015, 17, 3798–3801. doi:10.1021/acs.orglett.5b01774
Return to citation in text: [1] -
Yancey, A.; Armbruster, A.; Tackett, S. J. Pharm. Technol. 2015, 31, 29–37. doi:10.1177/8755122514548897
Return to citation in text: [1] -
Maria Faisca Phillips, A.; Pombeiro, A. J. L. Eur. J. Org. Chem. 2021, 3938–3969. doi:10.1002/ejoc.202100364
Return to citation in text: [1] -
Mulki, L.; Foster, C. S. Drugs Today 2011, 47, 327–333. doi:10.1358/dot.2011.47.5.1590791
Return to citation in text: [1] -
Jamal, K. N.; Callanan, D. G. Clin. Ophthalmol. 2009, 381–390. doi:10.2147/opth.s4460
Return to citation in text: [1] -
Nişancı, B.; Dalkılıç, E.; Güney, M.; Daştan, A. Beilstein J. Org. Chem. 2009, 5, 39. doi:10.3762/bjoc.5.39
Return to citation in text: [1] -
Hermann, K.; Nakhla, M.; Gallucci, J.; Dalkilic, E.; Dastan, A.; Badjić, J. D. Angew. Chem. 2013, 125, 11523–11526. doi:10.1002/ange.201305761
Return to citation in text: [1] -
Tsubata, A.; Uchiyama, T.; Kameyama, A.; Nishikubo, T. Macromolecules 1997, 30, 5649–5654. doi:10.1021/ma970431a
Return to citation in text: [1] -
Nishimura, I.; Kameyama, A.; Nishikubo, T. Macromolecules 1998, 31, 2789–2796. doi:10.1021/ma9718098
Return to citation in text: [1] -
Kocak, R.; Akın, E. T.; Kalın, P.; Talaz, O.; Saracoglu, N.; Dastan, A.; Gülçin, İ.; Durdagi, S. J. Heterocycl. Chem. 2016, 53, 2049–2056. doi:10.1002/jhet.2558
Return to citation in text: [1] -
Nyffeler, P. T.; Durón, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
Return to citation in text: [1] [2] -
Lozano, O.; Blessley, G.; Martinez del Campo, T.; Thompson, A. L.; Giuffredi, G. T.; Bettati, M.; Walker, M.; Borman, R.; Gouverneur, V. Angew. Chem., Int. Ed. 2011, 50, 8105–8109. doi:10.1002/anie.201103151
Return to citation in text: [1] -
Greedy, B.; Gouverneur, V. Chem. Commun. 2001, 233–234. doi:10.1039/b009179k
Return to citation in text: [1] -
Wilkinson, S. C.; Lozano, O.; Schuler, M.; Pacheco, M. C.; Salmon, R.; Gouverneur, V. Angew. Chem., Int. Ed. 2009, 48, 7083–7086. doi:10.1002/anie.200901795
Return to citation in text: [1] -
Vincent, S. P.; Burkart, M. D.; Tsai, C.-Y.; Zhang, Z.; Wong, C.-H. J. Org. Chem. 1999, 64, 5264–5279. doi:10.1021/jo990686h
Return to citation in text: [1] -
Chang, M.-Y.; Lee, N.-C.; Lee, M.-F.; Huang, Y.-P.; Lin, C.-H. Tetrahedron Lett. 2010, 51, 5900–5903. doi:10.1016/j.tetlet.2010.08.090
Return to citation in text: [1] -
Parmar, D.; Rueping, M. Chem. Commun. 2014, 50, 13928–13931. doi:10.1039/c4cc05027d
Return to citation in text: [1] -
Yuan, Z.; Peng, H.; Liu, G. Chin. J. Chem. 2013, 31, 908–914. doi:10.1002/cjoc.201300437
Return to citation in text: [1] -
Li, Y.; Jiang, X.; Zhao, C.; Fu, X.; Xu, X.; Tang, P. ACS Catal. 2017, 7, 1606–1609. doi:10.1021/acscatal.6b03529
Return to citation in text: [1] -
Dağalan, Z.; Koçak, R.; Daştan, A.; Nişancı, B. Org. Lett. 2022, 24, 8261–8264. doi:10.1021/acs.orglett.2c02627
Return to citation in text: [1] [2] -
Yang, K.; Song, M.; Ali, A. I. M.; Mudassir, S. M.; Ge, H. Chem. – Asian J. 2020, 15, 729–741. doi:10.1002/asia.202000011
Return to citation in text: [1]
| 1. | Zhang, Z.; Wang, F.; Mu, X.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2013, 52, 7549–7553. doi:10.1002/anie.201301891 |
| 2. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 3. | Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a |
| 4. | Zhang, J.; Wang, H.; Ren, S.; Zhang, W.; Liu, Y. Org. Lett. 2015, 17, 2920–2923. doi:10.1021/acs.orglett.5b01110 |
| 10. | Maria Faisca Phillips, A.; Pombeiro, A. J. L. Eur. J. Org. Chem. 2021, 3938–3969. doi:10.1002/ejoc.202100364 |
| 9. | Yancey, A.; Armbruster, A.; Tackett, S. J. Pharm. Technol. 2015, 31, 29–37. doi:10.1177/8755122514548897 |
| 7. | Sedgwick, D. M.; López, I.; Román, R.; Kobayashi, N.; Okoromoba, O. E.; Xu, B.; Hammond, G. B.; Barrio, P.; Fustero, S. Org. Lett. 2018, 20, 2338–2341. doi:10.1021/acs.orglett.8b00681 |
| 8. | Zhu, Q.; Ji, D.; Liang, T.; Wang, X.; Xu, Y. Org. Lett. 2015, 17, 3798–3801. doi:10.1021/acs.orglett.5b01774 |
| 5. | Cresswell, A. J.; Davies, S. G.; Lee, J. A.; Morris, M. J.; Roberts, P. M.; Thomson, J. E. J. Org. Chem. 2012, 77, 7262–7281. doi:10.1021/jo301056r |
| 6. | Kumar, A.; Singh, T. V.; Venugopalan, P. J. Fluorine Chem. 2013, 150, 72–77. doi:10.1016/j.jfluchem.2013.02.014 |
| 27. | Dağalan, Z.; Koçak, R.; Daştan, A.; Nişancı, B. Org. Lett. 2022, 24, 8261–8264. doi:10.1021/acs.orglett.2c02627 |
| 18. | Nyffeler, P. T.; Durón, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648 |
| 19. | Lozano, O.; Blessley, G.; Martinez del Campo, T.; Thompson, A. L.; Giuffredi, G. T.; Bettati, M.; Walker, M.; Borman, R.; Gouverneur, V. Angew. Chem., Int. Ed. 2011, 50, 8105–8109. doi:10.1002/anie.201103151 |
| 20. | Greedy, B.; Gouverneur, V. Chem. Commun. 2001, 233–234. doi:10.1039/b009179k |
| 21. | Wilkinson, S. C.; Lozano, O.; Schuler, M.; Pacheco, M. C.; Salmon, R.; Gouverneur, V. Angew. Chem., Int. Ed. 2009, 48, 7083–7086. doi:10.1002/anie.200901795 |
| 22. | Vincent, S. P.; Burkart, M. D.; Tsai, C.-Y.; Zhang, Z.; Wong, C.-H. J. Org. Chem. 1999, 64, 5264–5279. doi:10.1021/jo990686h |
| 23. | Chang, M.-Y.; Lee, N.-C.; Lee, M.-F.; Huang, Y.-P.; Lin, C.-H. Tetrahedron Lett. 2010, 51, 5900–5903. doi:10.1016/j.tetlet.2010.08.090 |
| 24. | Parmar, D.; Rueping, M. Chem. Commun. 2014, 50, 13928–13931. doi:10.1039/c4cc05027d |
| 25. | Yuan, Z.; Peng, H.; Liu, G. Chin. J. Chem. 2013, 31, 908–914. doi:10.1002/cjoc.201300437 |
| 26. | Li, Y.; Jiang, X.; Zhao, C.; Fu, X.; Xu, X.; Tang, P. ACS Catal. 2017, 7, 1606–1609. doi:10.1021/acscatal.6b03529 |
| 13. | Nişancı, B.; Dalkılıç, E.; Güney, M.; Daştan, A. Beilstein J. Org. Chem. 2009, 5, 39. doi:10.3762/bjoc.5.39 |
| 14. | Hermann, K.; Nakhla, M.; Gallucci, J.; Dalkilic, E.; Dastan, A.; Badjić, J. D. Angew. Chem. 2013, 125, 11523–11526. doi:10.1002/ange.201305761 |
| 15. | Tsubata, A.; Uchiyama, T.; Kameyama, A.; Nishikubo, T. Macromolecules 1997, 30, 5649–5654. doi:10.1021/ma970431a |
| 16. | Nishimura, I.; Kameyama, A.; Nishikubo, T. Macromolecules 1998, 31, 2789–2796. doi:10.1021/ma9718098 |
| 17. | Kocak, R.; Akın, E. T.; Kalın, P.; Talaz, O.; Saracoglu, N.; Dastan, A.; Gülçin, İ.; Durdagi, S. J. Heterocycl. Chem. 2016, 53, 2049–2056. doi:10.1002/jhet.2558 |
| 11. | Mulki, L.; Foster, C. S. Drugs Today 2011, 47, 327–333. doi:10.1358/dot.2011.47.5.1590791 |
| 12. | Jamal, K. N.; Callanan, D. G. Clin. Ophthalmol. 2009, 381–390. doi:10.2147/opth.s4460 |
| 18. | Nyffeler, P. T.; Durón, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648 |
| 27. | Dağalan, Z.; Koçak, R.; Daştan, A.; Nişancı, B. Org. Lett. 2022, 24, 8261–8264. doi:10.1021/acs.orglett.2c02627 |
| 28. | Yang, K.; Song, M.; Ali, A. I. M.; Mudassir, S. M.; Ge, H. Chem. – Asian J. 2020, 15, 729–741. doi:10.1002/asia.202000011 |
© 2024 Dağalan et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.