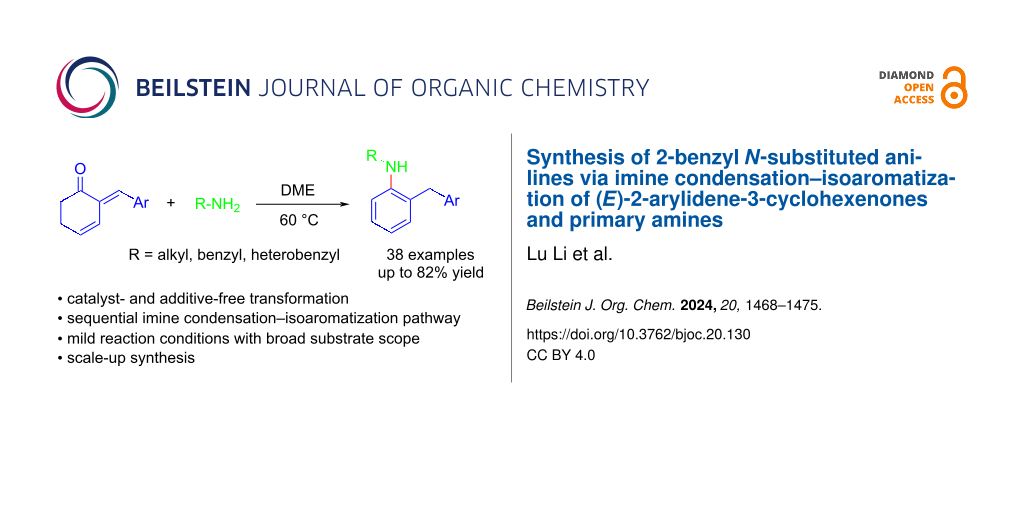
Abstract
A catalyst- and additive-free synthesis of 2-benzyl N-substituted anilines from (E)-2-arylidene-3-cyclohexenones and primary amines has been reported. The reaction proceeds smoothly through a sequential imine condensation–isoaromatization pathway, affording a series of synthetically useful aniline derivatives in acceptable to high yields. Mild reaction conditions, no requirement of metal catalysts, operational simplicity and the potential for scale-up production are some of the highlighted advantages of this transformation.
Graphical Abstract
Introduction
Aniline derivatives possessing arylmethyl substituents at the ortho position are an important class of amines. They have a wide variety of practical applications, ranging from anti-depression [1], being δ receptor stimulants in analgesic pharmaceuticals [2], to antioxidant additives in the petrochemical industry [3]. Besides, 2-benzylanilines also serve as valuable building blocks in synthetic chemistry [4]. The classical route to this kind of aniline derivatives usually starts from parent anilines, which undergo Friedel–Crafts reaction with acyl halides followed by carbonyl reduction. This electrophilic aromatic substitution usually needs harsh reaction conditions, tedious synthetic procedures and sometimes encounters the trouble of separating positional isomers caused by orientation or steric effects of the pre-existed amino group on the aryl moiety. Nevertheless, anilines are not always readily accessible. Typically, the preparation methods involve SNAr reactions with N-centered nucleophiles [5], nitroarene reduction [6] and transition metal (e.g., Pd, Cu)-catalyzed C–N cross coupling of aryl halides, aryl sulfonates or arylboronic acid reagents with ammonia or NH substrates [7,8]. Pre-functionalized arenes are essential precusors in all of these general approaches.
2-Cyclohexenones are fundamental and versatile organic synthetic materials [9,10]. They have been applied as an ideal arylation platform to construct functionalized anilines via an amination–dehydrogenative aromatization strategy with amines as nucleophiles [11,12]. For instance, the groups of Deng and Li reported the Pd catalyzed oxidative coupling of 2-cyclohexenones with amines [13]. Later, the same group demonstrated the direct amination of phenols by reductive coupling of in situ generated 2-cyclohexenones with nucleophilic nitrogen sources like ammonia, amines and hydrazine [14]. The reactions were regarded as via simple nucleophilic addition along with Pd-catalyzed dehydrogenative aromatization in these elegant works (Scheme 1, (1)). The Semmler–Wolff reaction is often implemented in the synthesis of anilines through Brønsted acid or transition-metal-promoted conversion of 2-cyclohexanone oximes [15-18] (Scheme 1, (2)). Moreover, Strauss and co-workers described a green, multicomponent reaction of aromatic aldehydes, 2-cyclohexenone and amines to afford 2-arylmethyl N-substituted anilines [19] (Scheme 1, (3)). To date, although plentiful amination–aromatization approaches for the preparation of anilines have been well-established, to develop novel and efficient synthetic methods still remains highly desirable. In continuation of our recent studies on synthetic applications of Morita–Baylis–Hillman (MBH) adducts [20,21], we were interested in further utilizing (E)-2-arylidene-3-cyclohexenones that can be facilely synthesized from MBH alcohols to build functionalized molecules. Herein, we wish to report our preliminary study on a catalyst- and additive-free synthesis of 2-benzyl-N-substituted anilines. In this work, (E)-2-arylidene-3-cyclohexenones firstly react with primary amines to form cyclohexenylimine intermediates I. Afterward, isoaromatization resulting from imine–enamine tautomerization and exocyclic double bond shift occurs to give rise to stable aniline products. Interestingly, when the 2-arylidene-3-cyclohexenones bearing a strong electron-withdrawing group take part in the reaction, a base-promoted phenol formation via self-tautomerization of cyclohexenones emerges as a competing reaction pathway (Scheme 1, this work).
Scheme 1: Synthesis of aniline derivatives from 2-cyclohexenones or derivatives thereof.
Scheme 1: Synthesis of aniline derivatives from 2-cyclohexenones or derivatives thereof.
Results and Discussion
The present study began with the preparation of the (E)-2-arylidene-3-cyclohexenones 2 via DMAP-catalyzed elimination reaction of 2-cyclohexenone-MBH alcohols 1 and di-tert-butyl dicarbonate [22] as depicted in Scheme 2. Starting materials 2 were prepared in moderate to high yields.
Scheme 2: Synthesis of (E)-2-arylidene-3-cyclohexenones 2.
Scheme 2: Synthesis of (E)-2-arylidene-3-cyclohexenones 2.
Next, we chose (E)-2-benzylidenecyclohex-3-en-1-one (2a) and 4-methoxybenzylamine (3a) as starting materials to investigate the feasibility and efficiency of the reaction (Table 1). Initially, the reaction was conducted in the presence of 2.0 equiv of 3a in toluene at 60 °C without any addition of catalysts or additives. To our delight, 2a could be fully converted and the 2-benzylaniline 4aa was obtained in 52% yield after 46 h (Table 1, entry 1). Some commonly used acid catalysts were tested, however, neither Brønsted acids such as AcOH and TsOH, nor Lewis acids such as FeCl3 and BF3·Et2O, showed a promoting effect on the aimed transformation (Table 1, entries 2–5). Thiourea, which is regarded as a classic H-bond donor in carbonyl activation, also could not boost the yield (Table 1, entry 6). Fortunately, it was found that the yield of 4aa was gradually increased to 64% upon adding 3a from 2.0 to 10.0 equiv (Table 1, entries 7 and 8). However, carrying out the reaction under neat conditions rendered to dramatically dropped yield (Table 1, entry 9). It indicates that the involvement of conventional organic solvents into the reaction system seems to be critical for target transformation. Though the exact reason is not apparent now, we speculate that the solvation effect might be beneficial for stabilizing the condensation intermediate I and avoiding further unwanted conversions, e.g., nucleophilic attack of excessive benzylamine to the intermediate I. The following examination on solvents demonstrated that using ether solvents as the reaction media led to higher yields. More precisely, dimethoxyethane (DME) gave a superior result to THF, affording 4aa in 82% yield (Table 1, entries 10–16). Subsequently, modification of the reaction temperature or concentration turned out to be unsatisfactory (Table 1, entries 17–19). We also added 4 Å molecular sieves as water scavengers, but it showed no positive influence on the reaction efficiency (Table 1, entry 20). It should be noted that no competing aza-Michael adduct was monitored in all of the evaluated reaction conditions.
Table 1: Optimization studies.a
|
|
|||||
| Entry | 3a (equiv) | Solvent | Additives (amount) | Time (h) | Yield (%)b |
| 1 | 2.0 | toluene | – | 46 | 52 |
| 2 | 2.0 | toluene | FeCl3 (0.3 equiv) | 48 | 30 |
| 3 | 2.0 | toluene | BF3·Et2O (0.3 equiv) | 30 | 34 |
| 4 | 2.0 | toluene | AcOH (0.3 equiv) | 38 | 21 |
| 5 | 2.0 | toluene | TsOH (0.3 equiv) | 35 | 20 |
| 6 | 2.0 | toluene | thiourea (0.3 equiv) | 42 | 45 |
| 7 | 5.0 | toluene | – | 33 | 58 |
| 8 | 10.0 | toluene | – | 14 | 64 |
| 9 | 10.0 | – | – | 12 | 27 |
| 10 | 10.0 | PhCF3 | – | 14 | 65 |
| 11 | 10.0 | CH3CN | – | 21 | 57 |
| 12 | 10.0 | CHCl3 | – | 18 | 35 |
| 13 | 10.0 | THF | – | 25 | 73 |
| 14 | 10.0 | DME | – | 11 | 82 |
| 15 | 10.0 | EtOH | – | 18 | 42 |
| 16 | 10.0 | DMF | – | 18 | 20 |
| 17c | 10.0 | DME | – | 16 | 66 |
| 18d | 10.0 | DME | – | 11 | 71 |
| 19e | 10.0 | DME | – | 13 | 81 |
| 20 | 10.0 | DME | 4 Å MS (50 mg) | 12 | 74 |
aUnless otherwise noted, the reactions were performed with 2a (0.20 mmol) and 3a (2.0 mmol) in solvent (2 mL) at 60 °C. bYields of isolated products. cAt 50 °C. d1.0 mL of solvent. e4.0 mL of solvent.
According to the above screening results, the generality of the reaction was examined under the optimal reaction conditions as outlined in Table 1, entry 14. Firstly, the substrate range of (E)-2-arylidene-3-cyclohexenones 2 were investigated while keeping 4-methoxybenzylamine (3a) as the nucleophile (Scheme 3). Generally speaking, the method exhibited good tolerance to various aryl moieties except for those containing strong electron-withdrawing substituent (i.e., -CN, -NO2). 3-Cyclohexenones 2 bearing a halogen group (i.e., -Cl, -Br) as well as an electron-donating group (i.e., -Me, -OMe, -Ph) worked well under the optimized reaction conditions, delivering the expected products 4ba–ea and 4ga–ka in 28–78% yields. 3-Cyclohexenones possessing a bulky 2-naphthyl group or heteroaryl group (i.e., 2-furyl, 2-thienyl) also smoothly took part in the reaction to afford 4la–na in 42–76% yields. In the case of 3-cyclohexenone possessing a 3-NO2 group, the main reaction pathway appeared to proceed via self-tautomerization, since 2-benzylphenol 5f was separated in 43% yield along with only 23% yield of normal product 4fa. When 4-CN substituted 3-cyclohexanone was investigated, phenol 5o was isolated exclusively. This was probably due to the significantly enhanced acidity of 3-cyclohexanones caused by the strong electron-withdrawing effect, which made 3a as a base rather than a nucleophile under such conditions. The location of substituents was found to affect the product yield greatly, considering that the reactions worked better with 3-cyclohexenones bearing a para-substituent as compared to their ortho- or meta-substituted counterparts. It might be explained by the less obvious steric resistance of the former in the process of Schiff base formation. In addition, an alkylidenyl-equipped 3-cyclohexanone was found to be incompatible with the current reaction system, only generating a mixture of unidentifiable byproducts.
Scheme 3: Substrate scope of (E)-2-arylidene-3-cyclohexenones 2. Conditions: reactions were conducted with 2a–o (0.2 mmol) and 3a (2.0 mmol) were stirred in DME (2 mL) at 60 °C; Isolated yields; a2-Benzylaniline 4fa and 2-benzylphenol 5f were delivered synchronously.
Scheme 3: Substrate scope of (E)-2-arylidene-3-cyclohexenones 2. Conditions: reactions were conducted with 2a...
Next, we explored the scope of various primary amines under the optimal conditions (Scheme 4). With 3-cyclohexenones 2a and 2p used, a variety of primary amines 3 successfully participated in the reaction to produce N-substituted anilines 4 in moderate to good yields. The electronic properties of the substituents, irrespective of their positions on benzylamines, displayed no substantial disparity on the reaction outcomes, leading to the formation of 4ab–ap in 50–80% yields. Both the heteroarylmethylamines and sterically hindered α-methylbenzylamine reacted nicely to afford 4aq–as in 60–74% yields. This method was equally valid for β-phenylethylamine to provide 4at in 53% yield. Notably, not only linear but also cyclic primary amines were applicable for the established transformation, and targeted products 4au–ax and 4py were synthesized in 44–72% yields. Finally, when we switched our attention to the generality of primary aromatic amines such as aniline or secondary amines such as dibenzylamine, it was found that only the starting materials were recovered after work-up of the reaction mixture.
Scheme 4: Substrate scope of primary amines 3. Conditions: reactions conducted with 2 (0.2 mmol) and 3b–y (2.0 mmol) were stirred in DME (2 mL) at 60 °C; isolated yields.
Scheme 4: Substrate scope of primary amines 3. Conditions: reactions conducted with 2 (0.2 mmol) and 3b–y (2....
The structure of 2-benzyl N-substituted anilines 4 were determined by detailed analysis of their NMR spectral data. In particular, 1H NMR spectrum of the representative compound 4aa shows two characteristic signals at δ = 4.16 (singlet) and 3.89 (singlet) that correspond to the two groups of benzylic protons, respectively. Two peaks at δ = 3.77 (singlet) and 3.81 (broad singlet) are attributed to the -OMe group and active hydrogen of -NH group, respectively. The aromatic protons, shown as multi- or doublet signals at δ = 7.21, 7.07, 6.71 and 6.63 indicate the newly formed aromatic protons derived from the isoaromatization of the fragment of 3-cyclohexenone. This is further supported by the 13C NMR spectrum, which contains two peaks at δ = 38.4 and 47.7 indicating the two types of benzylic carbons. The NMR data of known compound 4ab were also in good correlation with previously reported data [19].
The synthetic practicability of the protocol was further demonstrated. As depicted in Scheme 5, we first attempted scale-up synthesis of product 4aa. Pleasingly, when starting from 2a on a 6.0 mmol scale, the product 4aa was afforded in 74% yield. We also conducted the successive synthesis of 4aa in a manner of one-pot procedure. On the condition of full conversion of 2a under the standard reactions, 1.0 equiv of each substrate was added synchronously. After running 5 times, 65% yield was obtained within a total reaction time of 60 h.
Scheme 5: Gram-scale reaction and successive one-pot synthesis.
Scheme 5: Gram-scale reaction and successive one-pot synthesis.
Finally, we explored the synthetic versatility of the products in this methodology. Debenzylation of product 4aa could be easily carried out by catalytic hydrogenation to produce 6 (Scheme 6a). On the other hand, 4ax could smoothly undergo N-methylation with MeI to give product 7 in quantitative yield (Scheme 6b).
Conclusion
In conclusion, we have developed an efficient method to rapidly synthesize 2-benzyl-N-substituted anilines from (E)-2-arylidene-3-cyclohexenones and primary aliphatic amines. The reaction proceeds through an imine condensation–isoaromatization approach under catalyst- and additive-free conditions, allowing the generation of synthetically useful aniline derivatives in 23–82% yields. This method provides an alternative to the construction of anilines via an amination–aromatization strategy. Furthermore, it is also characterized by simple operation, mild reaction conditions, broad substrate scope and efficiency on a gram-scale preparation, thus allowing a new and convenient process to assemble synthetically valuable industrial or fine chemicals. Further exploration into more synthetic application of (E)-2-arylidene-3-cyclohexenones is in progress.
Experimental
General procedure for the preparation of 2-benzyl-N-substituted anilines 4 and 2-benzylphenols 5f/5o
In a vial containing a magnetic stirrer was placed (E)-2-arylidene-3-cyclohexenone 2 (0.2 mmol), primary aliphatic amine 3 (2.0 mmol) and DME (2 mL). The reaction mixture was stirred at 60 °C and the reaction process was monitored by TLC analysis. After completion, the solvent was concentrated under reduced pressure and the residue was purified by column chromatography on silica gel to give product 4. In the case of reacting with 3-NO2 bearing 2f, 2-benzylphenol 5f was partially obtained together with normal product 4fa. 4-CN substituted 2o generated 2-benzylphenol 5o exclusively.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, and copies of NMR spectra of all new compounds. | ||
| Format: PDF | Size: 6.3 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Curtis, A. L.; Valentino, R. J. Psychopharmacology (Heidelberg, Ger.) 1991, 103, 330–338. doi:10.1007/bf02244286
Return to citation in text: [1] -
Field, G. F.; Zally, W. J. 2-[[2-Methyl-1-[2-benzoyl(or benzyl)phenyl]-1H-imidazol-5-yl]methyl]-1H-isoindole-1,3(2H)-diones. U.S. Patent US4194049, March 18, 1980.
Return to citation in text: [1] -
Roberts, J. T. N,N',N''-trisubstituted-bis-(p-aminobenzyl) anilines as antioxidants. U.S. Patent US4411805, Oct 25, 1983.
Return to citation in text: [1] -
Lawrence, S. A. Amines: synthesis, properties and applications; Cambridge University Press: Cambridge, UK, 2004.
Return to citation in text: [1] -
Neri, J. M.; Cavalcanti, L. N.; Araújo, R. M.; Menezes, F. G. Arabian J. Chem. 2020, 13, 721–739. doi:10.1016/j.arabjc.2017.07.012
Return to citation in text: [1] -
Formenti, D.; Ferretti, F.; Scharnagl, F. K.; Beller, M. Chem. Rev. 2019, 119, 2611–2680. doi:10.1021/acs.chemrev.8b00547
Return to citation in text: [1] -
Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512
Return to citation in text: [1] -
West, M. J.; Fyfe, J. W. B.; Vantourout, J. C.; Watson, A. J. B. Chem. Rev. 2019, 119, 12491–12523. doi:10.1021/acs.chemrev.9b00491
Return to citation in text: [1] -
Yang, X.; Wang, J.; Li, P. Org. Biomol. Chem. 2014, 12, 2499–2513. doi:10.1039/c3ob42293c
Return to citation in text: [1] -
Bamfield, P.; Gordon, P. F. Chem. Soc. Rev. 1984, 13, 441–488. doi:10.1039/cs9841300441
Return to citation in text: [1] -
Girard, S. A.; Huang, H.; Zhou, F.; Deng, G.-J.; Li, C.-J. Org. Chem. Front. 2015, 2, 279–287. doi:10.1039/c4qo00358f
Return to citation in text: [1] -
Qiu, Z.; Zeng, H.; Li, C.-J. Acc. Chem. Res. 2020, 53, 2395–2413. doi:10.1021/acs.accounts.0c00479
Return to citation in text: [1] -
Girard, S. A.; Hu, X.; Knauber, T.; Zhou, F.; Simon, M.-O.; Deng, G.-J.; Li, C.-J. Org. Lett. 2012, 14, 5606–5609. doi:10.1021/ol3027279
Return to citation in text: [1] -
Qiu, Z.; Lv, L.; Li, J.; Li, C.-C.; Li, C.-J. Chem. Sci. 2019, 10, 4775–4781. doi:10.1039/c9sc00595a
Return to citation in text: [1] -
Tabolin, A. A.; Ioffe, S. L. Chem. Rev. 2014, 114, 5426–5476. doi:10.1021/cr400196x
Return to citation in text: [1] -
Matsumoto, M.; Tomizuka, J.; Suzuki, M. Synth. Commun. 1994, 24, 1441–1446. doi:10.1080/00397919408011748
Return to citation in text: [1] -
Wang, S.-K.; You, X.; Zhao, D.-Y.; Mou, N.-J.; Luo, Q.-L. Chem. – Eur. J. 2017, 23, 11757–11760. doi:10.1002/chem.201701712
Return to citation in text: [1] -
Hong, W. P.; Iosub, A. V.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 13664–13667. doi:10.1021/ja4073172
Return to citation in text: [1] -
Rosamilia, A. E.; Scott, J. L.; Strauss, C. R. Org. Lett. 2005, 7, 1525–1528. doi:10.1021/ol0501828
Return to citation in text: [1] [2] -
Jiang, L.; Peng, P.; Yu, L.; Jiang, D.; Wang, Y.; Li, H.; Yuan, M.; Yuan, M. Tetrahedron 2021, 98, 132430. doi:10.1016/j.tet.2021.132430
Return to citation in text: [1] -
Jiang, L.; Li, L.; Li, M.; Yuan, M.-W.; Yuan, M.-L. Synth. Commun. 2023, 53, 1520–1528. doi:10.1080/00397911.2023.2235625
Return to citation in text: [1] -
Ren, H.-X.; Song, X.-J.; Wu, L.; Huang, Z.-C.; Zou, Y.; Li, X.; Chen, X.-W.; Tian, F.; Wang, L.-X. Eur. J. Org. Chem. 2019, 715–719. doi:10.1002/ejoc.201801301
Return to citation in text: [1]
| 1. | Curtis, A. L.; Valentino, R. J. Psychopharmacology (Heidelberg, Ger.) 1991, 103, 330–338. doi:10.1007/bf02244286 |
| 5. | Neri, J. M.; Cavalcanti, L. N.; Araújo, R. M.; Menezes, F. G. Arabian J. Chem. 2020, 13, 721–739. doi:10.1016/j.arabjc.2017.07.012 |
| 22. | Ren, H.-X.; Song, X.-J.; Wu, L.; Huang, Z.-C.; Zou, Y.; Li, X.; Chen, X.-W.; Tian, F.; Wang, L.-X. Eur. J. Org. Chem. 2019, 715–719. doi:10.1002/ejoc.201801301 |
| 4. | Lawrence, S. A. Amines: synthesis, properties and applications; Cambridge University Press: Cambridge, UK, 2004. |
| 19. | Rosamilia, A. E.; Scott, J. L.; Strauss, C. R. Org. Lett. 2005, 7, 1525–1528. doi:10.1021/ol0501828 |
| 3. | Roberts, J. T. N,N',N''-trisubstituted-bis-(p-aminobenzyl) anilines as antioxidants. U.S. Patent US4411805, Oct 25, 1983. |
| 19. | Rosamilia, A. E.; Scott, J. L.; Strauss, C. R. Org. Lett. 2005, 7, 1525–1528. doi:10.1021/ol0501828 |
| 2. | Field, G. F.; Zally, W. J. 2-[[2-Methyl-1-[2-benzoyl(or benzyl)phenyl]-1H-imidazol-5-yl]methyl]-1H-isoindole-1,3(2H)-diones. U.S. Patent US4194049, March 18, 1980. |
| 20. | Jiang, L.; Peng, P.; Yu, L.; Jiang, D.; Wang, Y.; Li, H.; Yuan, M.; Yuan, M. Tetrahedron 2021, 98, 132430. doi:10.1016/j.tet.2021.132430 |
| 21. | Jiang, L.; Li, L.; Li, M.; Yuan, M.-W.; Yuan, M.-L. Synth. Commun. 2023, 53, 1520–1528. doi:10.1080/00397911.2023.2235625 |
| 11. | Girard, S. A.; Huang, H.; Zhou, F.; Deng, G.-J.; Li, C.-J. Org. Chem. Front. 2015, 2, 279–287. doi:10.1039/c4qo00358f |
| 12. | Qiu, Z.; Zeng, H.; Li, C.-J. Acc. Chem. Res. 2020, 53, 2395–2413. doi:10.1021/acs.accounts.0c00479 |
| 14. | Qiu, Z.; Lv, L.; Li, J.; Li, C.-C.; Li, C.-J. Chem. Sci. 2019, 10, 4775–4781. doi:10.1039/c9sc00595a |
| 9. | Yang, X.; Wang, J.; Li, P. Org. Biomol. Chem. 2014, 12, 2499–2513. doi:10.1039/c3ob42293c |
| 10. | Bamfield, P.; Gordon, P. F. Chem. Soc. Rev. 1984, 13, 441–488. doi:10.1039/cs9841300441 |
| 15. | Tabolin, A. A.; Ioffe, S. L. Chem. Rev. 2014, 114, 5426–5476. doi:10.1021/cr400196x |
| 16. | Matsumoto, M.; Tomizuka, J.; Suzuki, M. Synth. Commun. 1994, 24, 1441–1446. doi:10.1080/00397919408011748 |
| 17. | Wang, S.-K.; You, X.; Zhao, D.-Y.; Mou, N.-J.; Luo, Q.-L. Chem. – Eur. J. 2017, 23, 11757–11760. doi:10.1002/chem.201701712 |
| 18. | Hong, W. P.; Iosub, A. V.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 13664–13667. doi:10.1021/ja4073172 |
| 7. | Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512 |
| 8. | West, M. J.; Fyfe, J. W. B.; Vantourout, J. C.; Watson, A. J. B. Chem. Rev. 2019, 119, 12491–12523. doi:10.1021/acs.chemrev.9b00491 |
| 6. | Formenti, D.; Ferretti, F.; Scharnagl, F. K.; Beller, M. Chem. Rev. 2019, 119, 2611–2680. doi:10.1021/acs.chemrev.8b00547 |
| 13. | Girard, S. A.; Hu, X.; Knauber, T.; Zhou, F.; Simon, M.-O.; Deng, G.-J.; Li, C.-J. Org. Lett. 2012, 14, 5606–5609. doi:10.1021/ol3027279 |
© 2024 Li et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.