Abstract
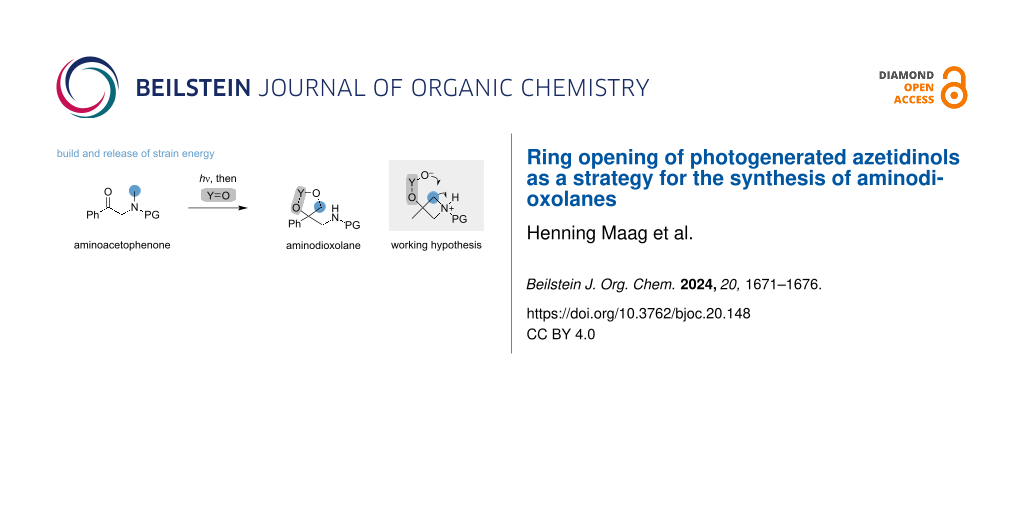
α-Aminoacetophenones are identified as promising building blocks for the synthesis of highly substituted dioxolanes. The presented strategy is founded on the build and release of molecular strain and achieves a formal transposition of a methyl group. During light irradiation, 3-phenylazetidinols are forged as reaction intermediates, which readily undergo ring opening upon the addition of electron-deficient ketones or boronic acids. Key to the successful development of this two-step process is the identification of a benzhydryl-protecting group, which orchestrates the photochemical Norrish–Yang cyclization and facilitates the subsequent ring opening.
Graphical Abstract
Introduction
Identifying efficient methods for the preparation of densely functionalized molecules is one of the central goals of modern organic chemistry. In this context, application of strained molecules has garnered increasing attention due to their intrinsic reactivity [1,2]. However, accessing strained molecules continues to pose a synthetic challenge because many reported methods require harsh conditions for the preparation. An attractive alternative to traditional synthesis can be found in photochemical methods, bypassing energetic constraints by the utilization of photon energy. Thus, endergonic transformations can be realized [3]. A promising strategy for the synthesis of complex products lies in the combination of photochemical cyclization and strain-release reaction (Scheme 1a) [4]. In this ‘build and release approach’, a simple precursor is cyclized upon irradiation. Subsequently, a functionalization step such as a ring-opening event is implemented, facilitated by the pre-installed strain energy of the four-membered ring [5-7].
Scheme 1: Build and release approach for the functionalization of simple precursors. a) General overview. b) Embedding azetidines into the general synthetic strategy.
Scheme 1: Build and release approach for the functionalization of simple precursors. a) General overview. b) ...
The implementation of a build and release strategy, as depicted in Scheme 1a, necessitates the full compatibility of both individual reaction steps, thus placing stringent demands on the selection and assessment of substrates. In our quest to identify a suitable system, we were attracted by the use of azetidines as potential reaction intermediates (Scheme 1b) [8-10]. Azetidines were chosen because of existing literature precedent for their photochemical assembly by Norrish–Yang cyclization, which employs easily accessible α-aminoacetophenones as starting materials [11-15]. Furthermore, ring-opening reactions of azetidines have been recently achieved using sulfur and oxygen nucleophiles [16-25]. However, methods that target a combination of photochemical ring closure and subsequent functionalization are still underdeveloped [4-7]. Within this work, we describe our endeavours in identifying a suitable substrate to meet the photochemical requirements of the Norrish–Yang cyclization and allow for subsequent ring-opening reactions. In this regard, we uncovered a novel entry to dioxolanes by intramolecular ring opening of azetidines using ketones and boronic acids.
Results and Discussion
Photocyclization
We initiated our study by a systematic investigating of the Norrish–Yang cyclization for the synthesis of azetidinols. Mechanistically, the Norrish–Yang cyclization involves a 1,5-hydrogen abstraction (HAT) step followed by ring closure to forge the azetidine scaffold (Scheme 2a, 1 → 3, via 1,4-biradical 2) [26]. The respective α-aminoacetophenones 1 were synthesized using a modular approach starting from α-bromoacetophenones 4 and disubstituted amines 5 (Scheme 2b). Three different criteria were chosen to evaluate the photochemical cyclization (Scheme 2c). We hypothesized that changes in the π-system of the aromatic ring would have an impact on the efficiency of triplet generation (1 → 31). The corresponding protecting group (PG) was thought to control the conformation of the 1,4-biradical 2, which is known to be important for efficient Norrish–Yang cyclizations [27,28]. Furthermore, the PG was deemed crucial for the development of further functionalizations of the azetidinols (vide infra). Thirdly, we wanted to assess substitution α to the nitrogen (R), as this was thought to impact the stability of the intermediary 1,4-biradical 2 [29,30]. To obtain comparable results, all reactions were performed in a LuzChem photoreactor under identical conditions. Deuterated acetonitrile was chosen as a preferred solvent because it is known to facilitate Norrish–Yang-cyclizations and allows simple analysis by nuclear magnetic resonance (NMR) spectroscopy [10].
Scheme 2: Modularity of the Norrish–Yang cyclization for the synthesis of azetidines.
Scheme 2: Modularity of the Norrish–Yang cyclization for the synthesis of azetidines.
Our results are summarized in Table 1. When p-toluenesulfonyl (Ts) was used as a PG at nitrogen, azetidine 3a was obtained in 81% yield along with 14% acetophenone (6) as the major side product (Table 1, entry 1). Acetophenone (6) is thought to be generated by a Norrish type II cleavage of biradical 2a. Changes at the aromatic core, as demonstrated by acetonaphthone 1b, resulted in low conversions (Table 1, entry 2). When extending the reaction time to reach full conversion, significant amounts of Norrish type II cleavage were obtained and product 3b was isolated in only 16% yield. This substrate was therefore abandoned. Interestingly, ethyl (1c) and benzyl (1d) substitution was detrimental to the cyclization, indicated by the 54% and 63% yield, respectively (Table 1, entries 3 and 4). We observed an increase in Norrish II fragmentation in these cases. The observed diastereoselectivity was poor, supporting the radical character of the ring-closing event. A similar trend was observed when studying the methanesulfonyl (Ms) protecting group (entries 5–7 in Table 1). When changing to phenyl substitution at the nitrogen, azetidinol 3h was formed in 12% yield with a substantial amount of unselective side reactions (Table 1, entry 8). By introducing an ethyl group (1i), only 27% conversion were achieved, with 2% product formation (Table 1, entry 9). The notable influence of steric and electronic parameters of the PG prompted us to investigate a range of sterically diverse alkyl substituents. Interestingly, while only fragmentation products were obtained for methyl and benzyl groups (Table 1, entries 10 and 11), the sterically demanding benzhydryl (Bzh) group showed progress towards azetidinol 3l with a yield of 10% (Table 1, entry 12).
Table 1: Comparison of the effect of structural variations on the photoreaction.
|
|
||||||
| Entry | Substrates 1 | Conversion (%) | Yield 3 (%) | Yield 6 (%) | ||
| 1 | Ar = Ph | PG = Ts | R = H (1a) | >99 | 81 | 14 |
| 2 | Ar = Nph | PG = Ts | R = H (1b) | 23 | 11 | 4 |
| 3 | Ar = Ph | PG = Ts | R = CH3 (1c) | >99 | 54 (dr 54:46) | 26 |
| 4 | Ar = Ph | PG = Ts | R = Ph (1d) | >99 | 63 (dr 60:40) | 20 |
| 5 | Ar = Ph | PG = Ms | R = H (1e) | >99 | 67 | 14 |
| 6 | Ar = Ph | PG = Ms | R = CH3 (1f) | >99 | 44 (dr 51:49) | 42 |
| 7 | Ar = Ph | PG = Ms | R = Ph (1g) | >99 | 37 (dr 52:48) | 22 |
| 8 | Ar = Ph | PG = Ph | R = H (1h) | 90 | 12 | 3 |
| 9 | Ar = Ph | PG = Ph | R = CH3 (1i) | 27 | 2 (dr 52:48) | 6 |
| 10 | Ar = Ph | PG = Me | R = H (1j) | 87 | <1 | 53 |
| 11 | Ar = Ph | PG = Bn | R = H (1k) | >99 | <1 | 65 |
| 12 | Ar = Ph | PG = Bzh | R = H (1l) | 94 | 10 | 74 |
aConversions and yields were determined by 1H NMR spectroscopy using mesitylene as an internal standard. bDiastereomeric ratios were determined by 1H NMR spectroscopy. Reactions were run on 25 µM scale. Ts = p-toluenesulfonyl. Nph = 2-naphthyl. Ms = methanesulfonyl. Bzh = benzhydryl = CH(Ph)2.
As it can be extracted from our study, sulfonyl-based protecting groups hold a unique role in the Norrish–Yang cyclization of α-aminoacetophenones, which was underpinned by the good performances of substrates 1a and 1e. On the other hand, increasing steric demand at the α-aminocarbon has a detrimental effect on the cyclization, as indicated by the poor performances of ethyl and benzyl substitution (1c and 1d). In contrast, sterically demanding groups at the nitrogen can have a positive effect on product formation, potentially by changing the substrate’s conformation.
Ring opening
Based on its outstanding performance in the photoreaction, we selected Ts-protected azetidinol 3a as our preferred substrate to test ring-opening reactions. However, initial attempts were met with limited success. Being conscious about the challenges associated with intermolecular ring openings at four-membered heterocycles, we considered an in situ tethering approach, which has proved an attractive alternative in our recent study on oxetane-ring openings [31]. Therefore, electron-deficient ketone 7 was added to 3a (Scheme 3a). Formation of hemiketal 8 was expected to occur, which would facilitate ring opening by a 5-exo-tet cyclization. While we did observe the formation of hemiketal 8 by NMR spectroscopy, we were unable to detect any ring-opened products 9, even when adding Lewis acids or Brønsted acids to the reaction mixture (see Supporting Information File 1 for further details). Finally, we decided to evaluate the electronic effects of the PG on the ring opening, which is why we opted to try the benzhydryl-protected substrate 3l in our envisioned transformation. Several recent publications have indicated the unique role of the benzhydryl group for azetidinol reactivity [32-34]. To our delight, 3l cleanly underwent ring opening via hemiketal 10 to deliver dioxolane 11 as a single diastereomer in 93% yield (Scheme 3b). With this result at hand, we tested several other carbonyls known to form stable hemiacetals [35-40]. While we were unable to isolate ring-opened products from trifluoroacetophenone (12), chloral (13), and indane-1,2,3-trione (14), ethyl trifluoropyruvate (15) furnished the desired dioxolane 16, which was isolated as a mixture of diastereomers in 73% yield. Mechanistically, we believe that the hemiketal protonates the azetidine before a nucleophilic attack can occur. Therefore, the ring opening is critical to the acidity of the transient hemiacetals and to the basicity of the respective azetidines, explaining the unsuccessful attempts with 3a.
Scheme 3: Ring-opening reactions using electron-deficient ketones and boronic acids.
Scheme 3: Ring-opening reactions using electron-deficient ketones and boronic acids.
Dioxolanes 11 and 16 are inherently stable and we were unable to cleave the ketal after reaction. Thus, we investigated the feasibility to use boronic acids in the ring-opening reaction (Scheme 3c). Phenylboronic acid 17 is known to exhibit a similar acidity as the corresponding hemiketals providing a promising starting point for pursuing this ring-opening attempt [41]. We hypothesized that this approach would allow the synthesis of 3-amino-1,2-diols, a motif commonly found in pharmaceuticals such as, e.g., β-blockers [42]. Our envisioned one-pot protocol features protonation of the azetidine from boronic monoester (18 → 19) followed by ring opening, in accordance to the hemiketal reactions. We decided to directly cleave dioxaborolane 20 after the reaction has stalled to facilitate separation. After a brief optimization of the reaction conditions (details are provided in Supporting Information File 1), we successfully conducted the desired sequence by raising the temperature to 100 °C to initiate ring opening, and employing a mild transesterification method for diol release [43-45]. Thus, we were able to isolate 3-amino-1,2-diol 21 in 49% yield.
Conclusion
Within this work, we demonstrated that the build and release of strain energy can be combined in a simple reaction sequence when appropriately tuning the reaction parameters. Our system relies on a photochemical Norrish–Yang cyclization of α-aminoacetophenones, which provides a sustainable entry to highly strained azetidinols. It was shown that subsequent ring opening can be triggered by the addition of electron-deficient ketones or boronic acids, which resembles a novel strategy for azetidinol desymmetrization. The nature of the protecting group was found to be critical for the two-step process to be effective, which resulted in the synthesis of highly functionalized dioxolanes and 3-amino-1,2-diols. We believe that the concept of building and releasing strain energy, particularly when paired with photochemical means, is of general interest and its structural simplicity should allow for an expansion to other substrates.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data and copies of NMR spectra. | ||
| Format: PDF | Size: 5.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Turkowska, J.; Durka, J.; Gryko, D. Chem. Commun. 2020, 56, 5718–5734. doi:10.1039/d0cc01771j
Return to citation in text: [1] -
Bellotti, P.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 20716–20732. doi:10.1021/jacs.3c08206
Return to citation in text: [1] -
Wang, H.; Tian, Y.-M.; König, B. Nat. Rev. Chem. 2022, 6, 745–755. doi:10.1038/s41570-022-00421-6
Return to citation in text: [1] -
Luque, A.; Paternoga, J.; Opatz, T. Chem. – Eur. J. 2021, 27, 4500–4516. doi:10.1002/chem.202004178
Return to citation in text: [1] [2] -
Ishida, N.; Sawano, S.; Murakami, M. Nat. Commun. 2014, 5, 3111. doi:10.1038/ncomms4111
See for a selected example using this concept.
Return to citation in text: [1] [2] -
Ishida, N.; Nečas, D.; Masuda, Y.; Murakami, M. Angew. Chem., Int. Ed. 2015, 54, 7418–7421. doi:10.1002/anie.201502584
See for a selected example using this concept.
Return to citation in text: [1] [2] -
Sandvoß, A.; Wahl, J. M. Org. Lett. 2023, 25, 5795–5799. doi:10.1021/acs.orglett.3c02048
See for a selected example using this concept.
Return to citation in text: [1] [2] -
Ishida, N.; Shimamoto, Y.; Murakami, M. Angew. Chem., Int. Ed. 2012, 51, 11750–11752. doi:10.1002/anie.201206166
See for an example using azetidines as intermediates.
Return to citation in text: [1] -
Ishida, N.; Necas, D.; Shimamoto, Y.; Murakami, M. Chem. Lett. 2013, 42, 1076–1078. doi:10.1246/cl.130463
See for an example using azetidines as intermediates.
Return to citation in text: [1] -
Ruggeri, M.; Dombrowski, A. W.; Djuric, S. W.; Baxendale, I. R. J. Org. Chem. 2020, 85, 7276–7286. doi:10.1021/acs.joc.0c00656
See for an example using azetidines as intermediates.
Return to citation in text: [1] [2] -
Yang, N. C.; Yang, D.-D. H. J. Am. Chem. Soc. 1958, 80, 2913–2914. doi:10.1021/ja01544a092
See for a historic background on the Norrish-Yang cyclization.
Return to citation in text: [1] -
Chen, C. Org. Biomol. Chem. 2016, 14, 8641–8647. doi:10.1039/c6ob01214k
See for a historic background on the Norrish-Yang cyclization.
Return to citation in text: [1] -
Gold, E. H. J. Am. Chem. Soc. 1971, 93, 2793–2795. doi:10.1021/ja00740a041
See for a selected example for photochemical azetidine synthesis.
Return to citation in text: [1] -
Aoyama, H.; Sakamoto, M.; Omote, Y. J. Chem. Soc., Chem. Commun. 1982, 119–120. doi:10.1039/c39820000119
See for a selected example for photochemical azetidine synthesis.
Return to citation in text: [1] -
Ruggeri, M.; Dombrowski, A. W.; Djuric, S. W.; Baxendale, I. R. ChemPhotoChem 2019, 3, 1212–1218. doi:10.1002/cptc.201900188
See for a selected example for photochemical azetidine synthesis.
Return to citation in text: [1] -
Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988–4035. doi:10.1021/cr800325e
See for an overview of azetidines.
Return to citation in text: [1] -
Couty, F.; Drouillat, B.; Evano, G.; David, O. Eur. J. Org. Chem. 2013, 2045–2056. doi:10.1002/ejoc.201201685
See for an overview of azetidines.
Return to citation in text: [1] -
Masson, G.; Gomez Pardo, D.; Cossy, J. Chirality 2021, 33, 5–21. doi:10.1002/chir.23280
See for an overview of azetidines.
Return to citation in text: [1] -
Kenis, S.; D’hooghe, M.; Verniest, G.; Dang Thi, T. A.; Pham The, C.; Van Nguyen, T.; De Kimpe, N. J. Org. Chem. 2012, 77, 5982–5992. doi:10.1021/jo300694y
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles.
Return to citation in text: [1] -
Wang, Z.; Sheong, F. K.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Sun, J. J. Am. Chem. Soc. 2015, 137, 5895–5898. doi:10.1021/jacs.5b03083
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles.
Return to citation in text: [1] -
Qian, D.; Chen, M.; Bissember, A. C.; Sun, J. Angew. Chem., Int. Ed. 2018, 57, 3763–3766. doi:10.1002/anie.201712395
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles.
Return to citation in text: [1] -
Jeziorna, A.; Krawiecka, B. Tetrahedron: Asymmetry 2005, 16, 1577–1581. doi:10.1016/j.tetasy.2005.03.022
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles.
Return to citation in text: [1] -
Dwivedi, S. K.; Gandhi, S.; Rastogi, N.; Singh, V. K. Tetrahedron Lett. 2007, 48, 5375–5377. doi:10.1016/j.tetlet.2007.06.033
See for oxygen nucleophiles.
Return to citation in text: [1] -
Boddy, A. J.; Cordier, C. J.; Goldberg, K.; Madin, A.; Spivey, A. C.; Bull, J. A. Org. Lett. 2019, 21, 1818–1822. doi:10.1021/acs.orglett.9b00407
See for oxygen nucleophiles.
Return to citation in text: [1] -
Bertolini, F.; Crotti, S.; Di Bussolo, V.; Macchia, F.; Pineschi, M. J. Org. Chem. 2008, 73, 8998–9007. doi:10.1021/jo801568a
See for oxygen nucleophiles.
Return to citation in text: [1] -
Wagner, P. J. Top. Curr. Chem. 1976, 1–52. doi:10.1007/bfb0047763
See for an overview of the reactivity of excited ketones.
Return to citation in text: [1] -
Ihmels, H.; Scheffer, J. R. Tetrahedron 1999, 55, 885–907. doi:10.1016/s0040-4020(98)01019-9
See for examples highlighting the importance of conformation.
Return to citation in text: [1] -
Griesbeck, A. G.; Heckroth, H. J. Am. Chem. Soc. 2002, 124, 396–403. doi:10.1021/ja0111941
See for examples highlighting the importance of conformation.
Return to citation in text: [1] -
Hill, J.; Zakaria, M. M.; Mumford, D. J. Chem. Soc., Perkin Trans. 1 1983, 2455–2458. doi:10.1039/p19830002455
Return to citation in text: [1] -
Allworth, K. L.; El-Hamamy, A. A.; Hesabi, M. M.; Hill, J. J. Chem. Soc., Perkin Trans. 1 1980, 1671. doi:10.1039/p19800001671
Return to citation in text: [1] -
Sandvoß, A.; Maag, H.; Daniliuc, C. G.; Schollmeyer, D.; Wahl, J. M. Chem. Sci. 2022, 13, 6297–6302. doi:10.1039/d2sc01547a
Return to citation in text: [1] -
Krishna Reddy, V.; Ramamohan, H.; Ganesh, A.; Srinivas, K.; Mukkanti, K.; Madhusudhan, G. Asian J. Chem. 2012, 24, 3468–3472.
Return to citation in text: [1] -
Roagna, G.; Ascough, D. M. H.; Ibba, F.; Vicini, A. C.; Fontana, A.; Christensen, K. E.; Peschiulli, A.; Oehlrich, D.; Misale, A.; Trabanco, A. A.; Paton, R. S.; Pupo, G.; Gouverneur, V. J. Am. Chem. Soc. 2020, 142, 14045–14051. doi:10.1021/jacs.0c05131
Return to citation in text: [1] -
Li, Z.; Wang, Y.; Liu, D.; Ning, L.; Pu, M.; Lin, L.; Feng, X. Org. Lett. 2023, 25, 7612–7616. doi:10.1021/acs.orglett.3c02728
Return to citation in text: [1] -
Sumerford, W. T.; Cronic, F. M. J. Am. Chem. Soc. 1948, 70, 448. doi:10.1021/ja01182a003
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Shostakovskii, M. F.; Atavin, A. S.; Al'pert, M. L.; Lenskikh, G. V. Russ. Chem. Bull. 1965, 14, 1081–1082. doi:10.1007/bf00845771
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Maeda, S.; Sudo, A.; Endo, T. Tetrahedron Lett. 2016, 57, 1061–1065. doi:10.1016/j.tetlet.2015.12.112
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Huang, S.; Miller, A. K.; Wu, W. Tetrahedron Lett. 2009, 50, 6584–6585. doi:10.1016/j.tetlet.2009.09.054
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Guthrie, J. P. Can. J. Chem. 1975, 53, 898–906. doi:10.1139/v75-125
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Tobin, J. B.; Frey, P. A. J. Am. Chem. Soc. 1996, 118, 12253–12260. doi:10.1021/ja950573p
See for the formation of hemiacetals using electron-deficient carbonyls.
Return to citation in text: [1] -
Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. D.; King, E. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2017, 139, 13156–13165. doi:10.1021/jacs.7b07444
Return to citation in text: [1] -
Ogrodowczyk, M.; Dettlaff, K.; Jelinska, A. Mini-Rev. Med. Chem. 2016, 16, 40–54. doi:10.2174/1389557515666151016125948
Return to citation in text: [1] -
Hall, D. G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications. In Boronic Acids; Hall, D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp 1–99. doi:10.1002/3527606548.ch1
Return to citation in text: [1] -
Duggan, P. J.; Tyndall, E. M. J. Chem. Soc., Perkin Trans. 1 2002, 1325–1339. doi:10.1039/b006767i
Return to citation in text: [1] -
Coutts, S. J.; Adams, J.; Krolikowski, D.; Snow, R. J. Tetrahedron Lett. 1994, 35, 5109–5112. doi:10.1016/s0040-4039(00)77040-7
Return to citation in text: [1]
| 42. | Ogrodowczyk, M.; Dettlaff, K.; Jelinska, A. Mini-Rev. Med. Chem. 2016, 16, 40–54. doi:10.2174/1389557515666151016125948 |
| 43. | Hall, D. G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications. In Boronic Acids; Hall, D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp 1–99. doi:10.1002/3527606548.ch1 |
| 44. | Duggan, P. J.; Tyndall, E. M. J. Chem. Soc., Perkin Trans. 1 2002, 1325–1339. doi:10.1039/b006767i |
| 45. | Coutts, S. J.; Adams, J.; Krolikowski, D.; Snow, R. J. Tetrahedron Lett. 1994, 35, 5109–5112. doi:10.1016/s0040-4039(00)77040-7 |
| 1. | Turkowska, J.; Durka, J.; Gryko, D. Chem. Commun. 2020, 56, 5718–5734. doi:10.1039/d0cc01771j |
| 2. | Bellotti, P.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 20716–20732. doi:10.1021/jacs.3c08206 |
| 8. |
Ishida, N.; Shimamoto, Y.; Murakami, M. Angew. Chem., Int. Ed. 2012, 51, 11750–11752. doi:10.1002/anie.201206166
See for an example using azetidines as intermediates. |
| 9. |
Ishida, N.; Necas, D.; Shimamoto, Y.; Murakami, M. Chem. Lett. 2013, 42, 1076–1078. doi:10.1246/cl.130463
See for an example using azetidines as intermediates. |
| 10. |
Ruggeri, M.; Dombrowski, A. W.; Djuric, S. W.; Baxendale, I. R. J. Org. Chem. 2020, 85, 7276–7286. doi:10.1021/acs.joc.0c00656
See for an example using azetidines as intermediates. |
| 35. |
Sumerford, W. T.; Cronic, F. M. J. Am. Chem. Soc. 1948, 70, 448. doi:10.1021/ja01182a003
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 36. |
Shostakovskii, M. F.; Atavin, A. S.; Al'pert, M. L.; Lenskikh, G. V. Russ. Chem. Bull. 1965, 14, 1081–1082. doi:10.1007/bf00845771
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 37. |
Maeda, S.; Sudo, A.; Endo, T. Tetrahedron Lett. 2016, 57, 1061–1065. doi:10.1016/j.tetlet.2015.12.112
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 38. |
Huang, S.; Miller, A. K.; Wu, W. Tetrahedron Lett. 2009, 50, 6584–6585. doi:10.1016/j.tetlet.2009.09.054
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 39. |
Guthrie, J. P. Can. J. Chem. 1975, 53, 898–906. doi:10.1139/v75-125
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 40. |
Tobin, J. B.; Frey, P. A. J. Am. Chem. Soc. 1996, 118, 12253–12260. doi:10.1021/ja950573p
See for the formation of hemiacetals using electron-deficient carbonyls. |
| 5. |
Ishida, N.; Sawano, S.; Murakami, M. Nat. Commun. 2014, 5, 3111. doi:10.1038/ncomms4111
See for a selected example using this concept. |
| 6. |
Ishida, N.; Nečas, D.; Masuda, Y.; Murakami, M. Angew. Chem., Int. Ed. 2015, 54, 7418–7421. doi:10.1002/anie.201502584
See for a selected example using this concept. |
| 7. |
Sandvoß, A.; Wahl, J. M. Org. Lett. 2023, 25, 5795–5799. doi:10.1021/acs.orglett.3c02048
See for a selected example using this concept. |
| 41. | Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. D.; King, E. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2017, 139, 13156–13165. doi:10.1021/jacs.7b07444 |
| 4. | Luque, A.; Paternoga, J.; Opatz, T. Chem. – Eur. J. 2021, 27, 4500–4516. doi:10.1002/chem.202004178 |
| 31. | Sandvoß, A.; Maag, H.; Daniliuc, C. G.; Schollmeyer, D.; Wahl, J. M. Chem. Sci. 2022, 13, 6297–6302. doi:10.1039/d2sc01547a |
| 3. | Wang, H.; Tian, Y.-M.; König, B. Nat. Rev. Chem. 2022, 6, 745–755. doi:10.1038/s41570-022-00421-6 |
| 32. | Krishna Reddy, V.; Ramamohan, H.; Ganesh, A.; Srinivas, K.; Mukkanti, K.; Madhusudhan, G. Asian J. Chem. 2012, 24, 3468–3472. |
| 33. | Roagna, G.; Ascough, D. M. H.; Ibba, F.; Vicini, A. C.; Fontana, A.; Christensen, K. E.; Peschiulli, A.; Oehlrich, D.; Misale, A.; Trabanco, A. A.; Paton, R. S.; Pupo, G.; Gouverneur, V. J. Am. Chem. Soc. 2020, 142, 14045–14051. doi:10.1021/jacs.0c05131 |
| 34. | Li, Z.; Wang, Y.; Liu, D.; Ning, L.; Pu, M.; Lin, L.; Feng, X. Org. Lett. 2023, 25, 7612–7616. doi:10.1021/acs.orglett.3c02728 |
| 26. |
Wagner, P. J. Top. Curr. Chem. 1976, 1–52. doi:10.1007/bfb0047763
See for an overview of the reactivity of excited ketones. |
| 29. | Hill, J.; Zakaria, M. M.; Mumford, D. J. Chem. Soc., Perkin Trans. 1 1983, 2455–2458. doi:10.1039/p19830002455 |
| 30. | Allworth, K. L.; El-Hamamy, A. A.; Hesabi, M. M.; Hill, J. J. Chem. Soc., Perkin Trans. 1 1980, 1671. doi:10.1039/p19800001671 |
| 4. | Luque, A.; Paternoga, J.; Opatz, T. Chem. – Eur. J. 2021, 27, 4500–4516. doi:10.1002/chem.202004178 |
| 5. |
Ishida, N.; Sawano, S.; Murakami, M. Nat. Commun. 2014, 5, 3111. doi:10.1038/ncomms4111
See for a selected example using this concept. |
| 6. |
Ishida, N.; Nečas, D.; Masuda, Y.; Murakami, M. Angew. Chem., Int. Ed. 2015, 54, 7418–7421. doi:10.1002/anie.201502584
See for a selected example using this concept. |
| 7. |
Sandvoß, A.; Wahl, J. M. Org. Lett. 2023, 25, 5795–5799. doi:10.1021/acs.orglett.3c02048
See for a selected example using this concept. |
| 10. |
Ruggeri, M.; Dombrowski, A. W.; Djuric, S. W.; Baxendale, I. R. J. Org. Chem. 2020, 85, 7276–7286. doi:10.1021/acs.joc.0c00656
See for an example using azetidines as intermediates. |
| 16. |
Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988–4035. doi:10.1021/cr800325e
See for an overview of azetidines. |
| 17. |
Couty, F.; Drouillat, B.; Evano, G.; David, O. Eur. J. Org. Chem. 2013, 2045–2056. doi:10.1002/ejoc.201201685
See for an overview of azetidines. |
| 18. |
Masson, G.; Gomez Pardo, D.; Cossy, J. Chirality 2021, 33, 5–21. doi:10.1002/chir.23280
See for an overview of azetidines. |
| 19. |
Kenis, S.; D’hooghe, M.; Verniest, G.; Dang Thi, T. A.; Pham The, C.; Van Nguyen, T.; De Kimpe, N. J. Org. Chem. 2012, 77, 5982–5992. doi:10.1021/jo300694y
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles. |
| 20. |
Wang, Z.; Sheong, F. K.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Sun, J. J. Am. Chem. Soc. 2015, 137, 5895–5898. doi:10.1021/jacs.5b03083
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles. |
| 21. |
Qian, D.; Chen, M.; Bissember, A. C.; Sun, J. Angew. Chem., Int. Ed. 2018, 57, 3763–3766. doi:10.1002/anie.201712395
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles. |
| 22. |
Jeziorna, A.; Krawiecka, B. Tetrahedron: Asymmetry 2005, 16, 1577–1581. doi:10.1016/j.tetasy.2005.03.022
See for a selection of ring-opening reactions at azetidines using sulfur nucleophiles. |
| 23. |
Dwivedi, S. K.; Gandhi, S.; Rastogi, N.; Singh, V. K. Tetrahedron Lett. 2007, 48, 5375–5377. doi:10.1016/j.tetlet.2007.06.033
See for oxygen nucleophiles. |
| 24. |
Boddy, A. J.; Cordier, C. J.; Goldberg, K.; Madin, A.; Spivey, A. C.; Bull, J. A. Org. Lett. 2019, 21, 1818–1822. doi:10.1021/acs.orglett.9b00407
See for oxygen nucleophiles. |
| 25. |
Bertolini, F.; Crotti, S.; Di Bussolo, V.; Macchia, F.; Pineschi, M. J. Org. Chem. 2008, 73, 8998–9007. doi:10.1021/jo801568a
See for oxygen nucleophiles. |
| 11. |
Yang, N. C.; Yang, D.-D. H. J. Am. Chem. Soc. 1958, 80, 2913–2914. doi:10.1021/ja01544a092
See for a historic background on the Norrish-Yang cyclization. |
| 12. |
Chen, C. Org. Biomol. Chem. 2016, 14, 8641–8647. doi:10.1039/c6ob01214k
See for a historic background on the Norrish-Yang cyclization. |
| 13. |
Gold, E. H. J. Am. Chem. Soc. 1971, 93, 2793–2795. doi:10.1021/ja00740a041
See for a selected example for photochemical azetidine synthesis. |
| 14. |
Aoyama, H.; Sakamoto, M.; Omote, Y. J. Chem. Soc., Chem. Commun. 1982, 119–120. doi:10.1039/c39820000119
See for a selected example for photochemical azetidine synthesis. |
| 15. |
Ruggeri, M.; Dombrowski, A. W.; Djuric, S. W.; Baxendale, I. R. ChemPhotoChem 2019, 3, 1212–1218. doi:10.1002/cptc.201900188
See for a selected example for photochemical azetidine synthesis. |
| 27. |
Ihmels, H.; Scheffer, J. R. Tetrahedron 1999, 55, 885–907. doi:10.1016/s0040-4020(98)01019-9
See for examples highlighting the importance of conformation. |
| 28. |
Griesbeck, A. G.; Heckroth, H. J. Am. Chem. Soc. 2002, 124, 396–403. doi:10.1021/ja0111941
See for examples highlighting the importance of conformation. |
© 2024 Maag et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.