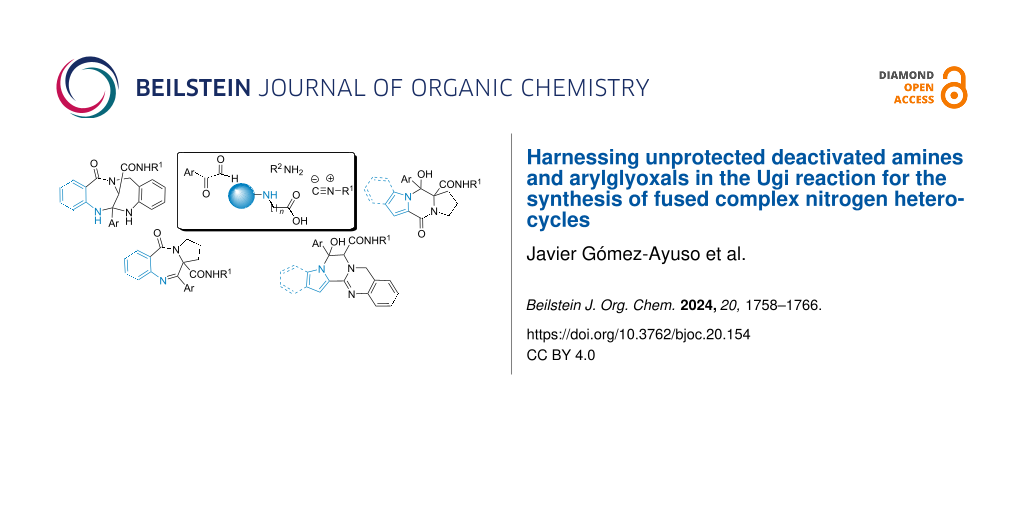
Abstract
Piperazines and diazepines are examples of nitrogen heterocycles present in many marketed drugs highlighting their importance in the discovery of novel bioactive compounds. However, their synthesis often faces challenges, including complex functionalization and lengthy reaction sequences. Multicomponent reactions, notably the Ugi reaction, have emerged as powerful tools to address these hurdles. Here, we have demonstrated the possibility of using the combination of arylglyoxals and carboxylic acids tethered to nonprotected deactivated amines as a powerful strategy for the synthesis of complex fused heterocycles. The limited nucleophilic character of the amino group of the anthranilic acid, indole-2-carboxylic acid, pyrrole-2-carboxylic acid or N-phenylglycine has allowed the use of these compounds in the Ugi reaction without triggering competitive reactions. The additional functional group present in the resulting Ugi adduct can be leveraged in different post-condensation strategies to easily generate multiple fused nitrogen heterocycles including benzodiazepinone and piperazinone cores.
Graphical Abstract
Introduction
Nitrogen heterocycles such as piperazines or diazepines represent important systems in the search of new bioactive compounds, as evidenced by the fact that they are present in many marketed drugs [1,2]. These substructures are found fused with other heterocycles in many cases, as illustrated by the antineoplastic dibromophakellstatin [3-6], the CDK inhibitor trilaciclib [7] or the kinase inhibitor tinengotinib [8] (Figure 1). Despite the interest of these structures, several drawbacks are typically found during their syntheses, for instance the difficulty in obtaining certain functionalizations or the need of long, elaborated reaction sequences.
Figure 1: Fused heterocycles containing the piperazine and diazepine core.
Figure 1: Fused heterocycles containing the piperazine and diazepine core.
In recent years, multicomponent reactions have become a useful tool for the synthesis of complex structures which overcome many of these problems, with the Ugi reaction leading these strategies. The scope of the Ugi reaction further increases when coupled with post-condensation reactions, which usually require the use of starting reagents that bear two functional groups. In order to avoid competitive reactions, these groups need to be compatible with the components of the Ugi reaction or the intermediates generated during the reaction. Thus, an amine can rarely be directly incorporated as an additional functional group, because of different competitive reactions triggered by it, such as the interrupted Ugi reaction resulting from the competitive addition over the nitrilium intermediate [9,10] or the split-Ugi reaction arising from a competitive O,N-acyl transference on the imidate intermediate, through a remote Mumm rearrangement [11].
Different strategies have been developed to avoid these competitive reactions, the most common ones being the use of protecting groups (Ugi/deprotection/cyclization strategy) [12-14] or of surrogates of amines [15]. However, direct incorporation of the second amine without derivatization is the most desirable strategy from the point-of view of sustainability. This has been achieved following two different approaches: the blockage of the amine group throughout the reaction by the incorporation of an additional group on the carbonyl component [16-18] or the use of amine groups with a limited reactivity, modulated by their substitution [19].
Results and Discussion
Synthesis of 3H-benzo[e][1,4]diazepin-5-ones
As part of our continued interest in developing new and efficient strategies for synthesizing complex fused nitrogen heterocycles, we decided to evaluate the use of amine groups with a reduced reactivity in the synthesis of 3H-benzo[e][1,4]diazepin-5-ones, heterocycles previously synthesized by our research group through Ugi/Staudinger/aza-Wittig and Ugi/reduction/cyclization sequences, using 2-azidobenzoic [20] and 2-nitrobenzoic [21] acids, respectively. The first methodology has several drawbacks, as the need to synthesise the 2-azidobenzoic acid from anthranilic acid and sodium azide, the requirement of anhydrous conditions to perform the Staudinger/aza-Wittig sequence or the generation of large quantities of triphenylphosphine oxide which needs to be removed by chromatography column, while the more eco-friendly second strategy needs an additional stage for the reduction of the nitro group on the Ugi adduct.
In order to find a more efficient synthesis, we thought that the second nitrogen in the diazepine nucleus could be incorporated without the need of surrogates or protecting groups, as the reduced nucleophilic character of the amino group of the anthranilic acid would prevent its participation in the Ugi reaction. In this way, the Ugi reaction was performed using arylglyoxals 1, anthranilic acid derivatives 2, amines 3 and isocyanides 4 following the most common procedure. Thus, initially the amine 3 (1 equiv) was added to a solution of the arylglyoxal 1 (1 equiv) in methanol and the mixture was stirred for 15 min. Then, the acid 2 (1 equiv) and the isocyanide 4 (1 equiv) were added and the reaction was stirred at room temperature for 24 h. Fortunately, after the workup the only product observed was the corresponding benzodiazepinone 5, resulting from a spontaneous cyclization of the Ugi adduct, in a six-center four-component Ugi reaction (U-6C-4CR), which prevents the need of additional steps (Scheme 1, Table 1).
Scheme 1: Synthesis of benzodiazepinones 5 from anthranilic acid derivatives.
Scheme 1: Synthesis of benzodiazepinones 5 from anthranilic acid derivatives.
Table 1: Results obtained in the synthesis of benzodiazepinones 5 from anthranilic acid derivatives.
| Entry | 1 (Ar) | 2 (R1, R2) | 3 (R3) | 4 (R4) | 5 (%)a |
| 1 | 1a (C6H5) | 2a (H, H) | 3a (C6H5CH2) | 4a (cC6H11) | 5a (66)b |
| 2 | 1a (C6H5) | 2a (H, H) | 3b (2-NO2C6H4CH2) | 4a (cC6H11) | 5b (80) |
| 3 | 1b (4-CH3C6H4) | 2a (H, H) | 3c (CH2CH2CH2Br) | 4a (cC6H11) | 5c (58)c |
| 4 | 1a (C6H5) | 2a (H, H) | 3a (C6H5CH2) | 4b (C6H5CH2) | 5d (63) |
| 5 | 1a (C6H5) | 2b (H, NO2) | 3a (C6H5CH2) | 4a (cC6H11) | 5e (68) |
| 6 | 1a (C6H5) | 2c (NO2, H) | 3a (C6H5CH2) | 4a (cC6H11) | 5f (63) |
| 7 | 1a (C6H5) | 2d (I, H) | 3a (C6H5CH2) | 4a (cC6H11) | 5g (67) |
| 8 | 1a (C6H5) | 2d (I, H) | 3b (2-NO2C6H4CH2) | 4a (cC6H11) | 5h (61) |
aYield after purification; bglobal yield for the synthesis of 5a from Ugi/Staudinger/aza-Wittig sequence 42% [20], and from Ugi/reduction/cyclization sequence 55% [21]; cthe formation of pyrrolobenzodiazepine 8 (35%) was observed (see Scheme 4).
Due to the interest of these results and considering the diastereoselectivity observed when enantiopure (S)-α-methylbenzylamine was used as chiral component in the two-step syntheses from amine surrogates [22], we assayed our new strategy to achieve a one-pot diastereoselective synthesis of benzodiazepinones 6 (Scheme 2, Table 2). Interestingly, the reaction took place with a α,3-like relative configuration on the major diastereomer, similar to that obtained for the Ugi/Staudinger/aza-Wittig sequence and complementary to that observed for the Ugi/reduction/cyclization sequence (see Supporting Information File 1, Figure S2).
Scheme 2: Diastereoselective one-pot synthesis of benzodiazepinones 6.
Scheme 2: Diastereoselective one-pot synthesis of benzodiazepinones 6.
Table 2: Results obtained in the diastereoselective one-pot synthesis of benzodiazepinones 6.
| Entry | 1 (Ar) | 2 (R1, R2) | 4 (R4) | 6 (%)a | drb (l:u)c |
| 1 | 1a (C6H5) | 2a (H, H) | 4a (cC6H11) | 6a (66) | 79:21 |
| 2 | 1a (C6H5) | 2a (H, H) | 4b (C6H5CH2) | 6b (80) | 76:24 |
| 3 | 1c (4-FC6H4) | 2a (H, H) | 4a (cC6H11) | 6c (58) | 74:26 |
| 4 | 1a (C6H5) | 2b (H, NO2) | 4a (cC6H11) | 6d (63) | 72:28 |
| 5 | 1a (C6H5) | 2c (NO2, H) | 4a (cC6H11) | 6e (63) | 68:32 |
| 6 | 1a (C6H5) | 2d (I, H) | 4a (cC6H11) | 6f (67) | 76:24 |
aYield referred to the major diastereomer after purification; bdetermined by 1H NMR of the reaction mixture; cconfiguration l(αS,3S):u(αS,3R) [22].
With the aim of building more complex systems, we explored post-condensation reactions on the 2-nitrobenzylamine and 3-bromopropylamine derivatives. Thus, we carried out the reduction of the nitro group on derivatives 5b and 5h employing tin(II) chloride and chlorhydric acid in boiling n-butanol (120 °C), conditions previously assayed in our group. Under these conditions, the bis-1,4-benzodiazepines 7 resulting from the reaction of the amino group with the imine on the benzodiazepine were obtained as a single diastereomer. On the basis of the preferred conformation for the 1,4-benzodiazepin-5-ones 5 and 6 [20,22], where the amide substituent in C3 is pseudoaxially oriented in an all-trans conformation, only the unlike attack would be allowed (Scheme 3).
Scheme 3: Synthesis of bis-1,4-benzodiazepines 7.
Scheme 3: Synthesis of bis-1,4-benzodiazepines 7.
On the other hand, when 3-bromopropanamine was used, the formation of the pyrrolobenzodiazepinone system 8 was observed albeit along with benzodiazepinone 5c (Scheme 4). In order to improve the synthesis of this fused heterocycle, different strategies were tried, e.g., performing the Ugi reaction in different solvents (dichloromethane, ethyl acetate, methanol) to prevent the precipitation of benzodiazepine 5c, conducting the Ugi reaction in boiling methanol or treating the benzodiazepine with different bases (caesium carbonate, sodium hydroxide). However, the complete cyclisation to the pyrrolobenzodiazepinone was not achieved, so the described three-step strategy starting from 2-nitrobenzoic acid (Ugi reaction/cyclization to pyrrolidine/reduction sequence) remains as a better choice [23].
Scheme 4: Synthesis of benzodiazepinone 5c and pyrrolobenzodiazepinone 8 from anthranilic acid and 3-bromopropanamine.
Scheme 4: Synthesis of benzodiazepinone 5c and pyrrolobenzodiazepinone 8 from anthranilic acid and 3-bromopro...
Synthesis of piperazinones
On the basis of these results, we planned to synthesise piperazinone derivatives following a similar strategy. Thus, we chose different deactivated 2-amino-substituted carboxylic acid derivatives such as the indole-2-carboxylic acid, recently used in a similar strategy with aromatic amines [24], pyrrole-2-carboxylic acid and N-phenylglycine, and carried out their reactions with arylglyoxals, alkylamines and isocyanides (Scheme 5). As we expected, the cyclization product 9 was observed, although the complete cyclization of the Ugi adduct, found as the enol tautomer, needed the addition of hydrochloric acid (1 equiv) in the case of the tert-butylamine derivatives. However, the nature of the cyclization product depended on the carboxylic acid employed in the Ugi reaction. Thus, the indole and pyrrole derivatives yielded the corresponding hemiaminal 9 as a single diastereomer (Scheme 5, Table 3), while N-phenylglycine afforded the enamine derivative 10 (Scheme 6, Table 4). Both structures were confirmed by single-crystal X-ray diffraction of compounds 9a and 10a (Figure 2). The diastereoselectivity observed in the hemiaminals and their stability can be explained by the intramolecular hydrogen bond formed between the hydroxy group and the carbamide substituent, which explains the high deshielding observed in the 1H NMR spectra for the signal of the OH group (around 8.5 ppm).
Scheme 5: Synthesis of pyrrolopiperazinones 9 from pyrrole and indole carboxylic acids.
Scheme 5: Synthesis of pyrrolopiperazinones 9 from pyrrole and indole carboxylic acids.
Table 3: Results obtained in the synthesis of pyrrolopiperazinones 9 from pyrrole and indole carboxylic acids.
| Entry | 1 (Ar) | 2 | 3 (R3) | 4 (R4) | 9 (%)a,b |
| 1 | 1a (C6H5) | 2e | 3a (C6H5CH2) | 4c (C(CH3)3) | 9a (61)b |
| 2 | 1a (C6H5) | 2e | 3d (C(CH3)3) | 4a (cC6H11) | 9b (52)c |
| 3 | 1a (C6H5) | 2e | 3c (CH2CH2CH2Br) | 4a (cC6H11) | 9c (16)d |
| 4 | 1a (C6H5) | 2e | 3c (CH2CH2CH2Br) | 4a (C(CH3)3) | 9d (24)d |
| 5 | 1c (4-FC6H4) | 2e | 3c (CH2CH2CH2Br) | 4c (C(CH3)3) | 9e (22)d |
| 6 | 1a (C6H5) | 2e | 3b (2-NO2C6H4CH2) | 4a (cC6H11) | 9f (77) |
| 7 | 1d (4-CF3C6H4) | 2e | 3b (2-NO2C6H4CH2) | 4c (C(CH3)3) | 9g (69) |
| 8 | 1a (C6H5) | 2f | 3a (C6H5CH2) | 4a (cC6H11) | 9h (49) |
| 9 | 1c (4-FC6H4) | 2f | 3a (C6H5CH2) | 4a (cC6H11) | 9i (58) |
| 10 | 1a (C6H5) | 2f | 3d (C(CH3)3) | 4c (C(CH3)3) | 9j (65)c |
| 11 | 1a (C6H5) | 2f | 3c (CH2CH2CH2Br) | 4c (C(CH3)3) | 9k (22)d |
| 12 | 1a (C6H5) | 2f | 3b (2-NO2C6H4CH2) | 4a (cC6H11) | 9l (72) |
| 13 | 1c (4-FC6H4) | 2f | 3b (2-NO2C6H4CH2) | 4a (cC6H11) | 9m (75) |
| 14 | 1e (4-CH3OC6H4) | 2f | 3b (2-NO2C6H4CH2) | 4c (C(CH3)3) | 9n (76) |
| 15 | 1d (4-CF3C6H4) | 2f | 3b (2-NO2C6H4CH2) | 4c (C(CH3)3) | 9o (71) |
aYield after purification; bthe only diastereomer observed was the (3R*,4R*); cyield obtained after treatment of the reaction mixture with HCl (1 equiv) for 24 h; dthe corresponding dipyrrolopiperazinone 12 resulting from a second cyclization was isolated from the reaction mixture (12a (32%), 12b (7%), 12c (13%), 12d (15%)) (see Scheme 8).
Scheme 6: Synthesis of pyrrolopiperazinones 10 from N-phenylglicine.
Scheme 6: Synthesis of pyrrolopiperazinones 10 from N-phenylglicine.
Table 4: Results obtained in the synthesis of pyrrolopiperazinones 10 from N-phenylglycine.
| Entry | 1 (Ar) | 3 (R3) | 10 (%)a |
| 1 | 1a (C6H5) | 3a (C6H5CH2) | 10a (54) |
| 2 | 1b (4-FC6H4) | 3a (C6H5CH2) | 10b (49) |
| 3 | 1a (C6H5) | 3b (2-NO2C6H4CH2) | 10c (−)b,c |
aYield after purification; bunstable; cused in the following step without purification.
![[1860-5397-20-154-2]](/bjoc/content/figures/1860-5397-20-154-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray diffraction structures of pyrrolopiperazinones 9a (left) and 10a (right). The thermal ellipsoid plot (Olex2) is at the 40% probability level.
Figure 2: X-ray diffraction structures of pyrrolopiperazinones 9a (left) and 10a (right). The thermal ellipso...
As before, we also explored the stereochemical outcome in the synthesis of pyrrolopiperazinones when enantiopure (S)-α-methylbenzylamine was used as chiral component. In this case, although the relative configuration on C3,C4 remained unchanged ((3R*,4R*)), an equimolar mixture of diastereomers (2(1S),3R,4R) and (2(1S),3S,4S) was obtained (Scheme 7).
Scheme 7: Synthesis of pyrrolopiperazinone 11 using (S)-α-methylbenzylamine.
Scheme 7: Synthesis of pyrrolopiperazinone 11 using (S)-α-methylbenzylamine.
These results indicate that these reactions seem to take place through conjugated additions on the enol tautomer of the Ugi adduct. Indeed, the enolate intermediate would explain the stereochemical results, controlled by the configuration in the hemiaminal intermediate and not by the chiral information on the amine, unlike in the case of benzodiazepinones, where the protonation would be exclusively controlled by the chiral amine in the synthesis of 6. Moreover, this proposed mechanism also explains the spontaneous cyclization in the absence of a base when 3-bromopropanamine was used (Scheme 8).
Scheme 8: Proposed mechanism in the spontaneous cyclization of Ugi adducts obtained from arylglyoxals and deactivated amines.
Scheme 8: Proposed mechanism in the spontaneous cyclization of Ugi adducts obtained from arylglyoxals and dea...
Moreover, we envisaged the possibility of obtaining piperazinoquinazoline derivatives, a core found in fungal metabolites [25,26], systems with promising antitumour activity [27], as well as dipyrrolopiperazinone derivatives, substructure found in some alkaloids such as dibromophakellin or the palau’amine [28], which possess immunosuppressive and cytotoxic properties [29], through post-condensation reactions.
Following the methodology previously described in our group [30], the reduction of the nitro group on indole and pyrrole derivatives 9f,g,l–o (Scheme 9, Table 5) employing tin(II) chloride under acidic conditions in boiling n-butanol (120 °C) afforded the corresponding pyrrolopiperazinoquinazolines 13 with high yields. Interestingly, despite the acidic medium and high temperatures, the hemiaminal group was conserved, showing the high stability of these systems. On the other hand, the reduction of the N-phenylglycine derivative 10c, used without purification after the Ugi/cyclization sequence, yielded the corresponding piperazinoquinazoline 14 with a high global yield (Scheme 10).
Scheme 9: Synthesis of pyrrolopiperazinoquinazolines 13.
Scheme 9: Synthesis of pyrrolopiperazinoquinazolines 13.
Table 5: Results obtained in the synthesis of pyrrolopiperazinoquinazolines 13.
| Entry | 9 (R1, R2, R4) | 13 (%)a,b |
| 1 | 9f (pyrrole, H, cC6H11) | 13a (70) |
| 2 | 9g (pyrrole, 4-CF3, C(CH3)3) | 13b (70) |
| 3 | 9l (indole, H, cC6H11) | 13c (72) |
| 4 | 9m (indole, 4-F, cC6H11) | 13d (68) |
| 5 | 9n (indole, 4-CH3O, C(CH3)3) | 13e (69) |
| 6 | 9o (indole, 4-CF3, C(CH3)3) | 13f (68) |
aYield after purification; bthe only diastereomer observed was the (3R*,4R*).
Scheme 10: Synthesis of piperazinoquinazoline 14.
Scheme 10: Synthesis of piperazinoquinazoline 14.
Moreover, the treatment of piperazinones 9c–e,k derived from 3-bromopropanamine with caesium carbonate in boiling acetonitrile for 1 h afforded the corresponding dipyrrolopiperazinone 12 quantitatively. In view of the interest of these structures and the poor global yield obtained because of the purification in the first step, we tried the synthesis of these polycyclic systems in a one-pot sequence. In this way, after performing the Ugi reaction for 24 h, the reaction mixture, without purification, was treated with caesium carbonate (1 equiv) and heated to reflux for an hour. Gratefully, the dipyrrolopiperazinone derivatives 12 were obtained with high yields and complete diastereoselectivity in an efficient and simple way (Scheme 11, Table 6). The structure of these systems was further confirmed by single-crystal X-ray diffraction of compound 12c (Figure 3).
Scheme 11: Synthesis of dipyrrolopiperazinones 12.
Scheme 11: Synthesis of dipyrrolopiperazinones 12.
Table 6: Results obtained in the synthesis of dipyrrolopiperazinones 12.
| Entry | 1 (Ar) | 2 | 4 (R4) | 12 (%)a,b |
| 1 | 1a (C6H5) | 2e | 4a (cC6H11) | 12a (93) |
| 2 | 1a (C6H5) | 2e | 4c (C(CH3)3) | 12b (74) |
| 3 | 1c (4-FC6H4) | 2e | 4c (C(CH3)3) | 12c (85) |
| 4 | 1a (C6H5) | 2f | 4c (C(CH3)3) | 12d (80) |
aYield after purification; bthe only diastereomer observed was the (3R*,4R*).
![[1860-5397-20-154-3]](/bjoc/content/figures/1860-5397-20-154-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: X-ray diffraction structure of dipyrrolopiperazinone 12c. The thermal ellipsoid plot (Olex2) is at the 40 % probability level.
Figure 3: X-ray diffraction structure of dipyrrolopiperazinone 12c. The thermal ellipsoid plot (Olex2) is at ...
Conclusion
In this work, we have demonstrated the straightforward access to a variety of complex nitrogen heterocycles by using unprotected deactivated amines tethered to carboxylic acids combined with arylglyoxals in the Ugi reaction. The reduced nucleophilic character of the amino group of the anthranilic acid, indole-2-carboxylic acid, pyrrole-2-carboxylic acid or N-phenylglycine allowed the use of these compounds in this multicomponent reaction without triggering competitive reactions. The presence of an additional functional group in the resulting Ugi adduct can be exploited in different post-condensation strategies to generate multiple fused nitrogen heterocycles in an easy manner.
Supporting Information
| Supporting Information File 1: General synthetic procedures and characterisation. | ||
| Format: PDF | Size: 7.1 MB | Download |
Funding
Funding from MCIN/AEI/10.13039/501100011033 [grant PID2020-117610RB-I00] is gratefully acknowledged. J. G.-A. and I. C.-B. thank Consejería de Educación de la Junta de Castilla y León, European Social Fund (ESF) and ERDF for their predoctoral (J. G.-A.) and post-doctoral (I. C.-B.) contracts.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b
Return to citation in text: [1] -
Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845–5859. doi:10.1021/jm4017625
Return to citation in text: [1] -
Burkholder, P. R.; Sharma, G. M. Lloydia 1969, 32, 466–483.
Return to citation in text: [1] -
Sharma, G. M.; Burkholder, P. R. J. Chem. Soc. D 1971, 151–152. doi:10.1039/c29710000151
Return to citation in text: [1] -
Poullennec, K. G.; Romo, D. J. Am. Chem. Soc. 2003, 125, 6344–6345. doi:10.1021/ja034575i
Return to citation in text: [1] -
Wang, S.; Romo, D. Angew. Chem., Int. Ed. 2008, 47, 1284–1286. doi:10.1002/anie.200703998
Return to citation in text: [1] -
Dhillon, S. Drugs 2021, 81, 867–874. doi:10.1007/s40265-021-01508-y
Return to citation in text: [1] -
Piha-Paul, S. A.; Xu, B.; Dumbrava, E. E.; Fu, S.; Karp, D. D.; Meric-Bernstam, F.; Hong, D. S.; Rodon, J. A.; Tsimberidou, A. M.; Raghav, K.; Ajani, J. A.; Conley, A. P.; Mott, F.; Fan, Y.; Fan, J.; Peng, P.; Wang, H.; Ni, S.; Sun, C.; Qiang, X.; Levin, W. J.; Ngo, B.; Ru, Q. C.; Wu, F.; Javle, M. M. Oncologist 2024, 29, e514–e525. doi:10.1093/oncolo/oyad338
Return to citation in text: [1] -
Giustiniano, M.; Moni, L.; Sangaletti, L.; Pelliccia, S.; Basso, A.; Novellino, E.; Tron, C. G. Synthesis 2018, 50, 3549–3570. doi:10.1055/s-0037-1610193
Return to citation in text: [1] -
Trudel, V.; Tien, C.-H.; Trofimova, A.; Yudin, A. K. Nat. Rev. Chem. 2021, 5, 604–623. doi:10.1038/s41570-021-00304-2
Return to citation in text: [1] -
Giovenzana, G. B.; Tron, G. C.; Di Paola, S.; Menegotto, I. G.; Pirali, T. Angew. Chem., Int. Ed. 2006, 45, 1099–1102. doi:10.1002/anie.200503095
Return to citation in text: [1] -
Hulme, C.; Peng, J.; Tang, S.-Y.; Burns, C. J.; Morize, I.; Labaudiniere, R. J. Org. Chem. 1998, 63, 8021–8023. doi:10.1021/jo980622r
Return to citation in text: [1] -
Hulme, C.; Ma, L.; Romano, J.; Morrissette, M. Tetrahedron Lett. 1999, 40, 7925–7928. doi:10.1016/s0040-4039(99)01580-4
Return to citation in text: [1] -
Xu, Z.; De Moliner, F.; Cappelli, A. P.; Ayaz, M.; Hulme, C. Synlett 2014, 25, 225–228. doi:10.1055/s-0033-1340219
Return to citation in text: [1] -
Neo, A. G.; Ramiro, J. L.; García-Valverde, M.; Díaz, J.; Marcos, C. F. Mol. Diversity 2024, 28, 335–418. doi:10.1007/s11030-023-10641-7
Return to citation in text: [1] -
Pertejo, P.; González-Saiz, B.; Quesada, R.; García-Valverde, M. J. Org. Chem. 2020, 85, 14240–14245. doi:10.1021/acs.joc.0c02103
Return to citation in text: [1] -
v. Zychlinski, A.; Ugi, I. Heterocycles 1998, 49, 29–32. doi:10.3987/com-97-s2
Return to citation in text: [1] -
Rossen, K.; Sager, J.; DiMichele, L. M. Tetrahedron Lett. 1997, 38, 3183–3186. doi:10.1016/s0040-4039(97)00607-2
Return to citation in text: [1] -
Keating, T. A.; Armstrong, R. W. J. Org. Chem. 1996, 61, 8935–8939. doi:10.1021/jo961517p
Return to citation in text: [1] -
Sañudo, M.; García-Valverde, M.; Marcaccini, S.; Delgado, J. J.; Rojo, J.; Torroba, T. J. Org. Chem. 2009, 74, 2189–2192. doi:10.1021/jo8025862
Return to citation in text: [1] [2] [3] -
Pertejo, P.; García-Valverde, M.; Peña, P.; Cordero, N. A.; Torroba, T.; González-Ortega, A. Org. Biomol. Chem. 2014, 12, 4905–4916. doi:10.1039/c4ob00444b
Return to citation in text: [1] [2] -
Pertejo, P.; Corres, N.; Torroba, T.; García-Valverde, M. Org. Lett. 2015, 17, 612–615. doi:10.1021/ol503628r
Return to citation in text: [1] [2] [3] -
Pertejo, P.; Carreira-Barral, I.; Peña-Calleja, P.; Quesada, R.; García-Valverde, M. J. Org. Chem. 2020, 85, 2291–2302. doi:10.1021/acs.joc.9b02995
Return to citation in text: [1] -
Shourkaei, F. A.; Lish, A. B.; Talebi, M.; Abdoli, S.; Zare, N.; Amanlou, M.; Ranjbar, P. R.; mahdavi, M. J. Mol. Struct. 2024, 1297, 136939. doi:10.1016/j.molstruc.2023.136939
Return to citation in text: [1] -
Resende, D. I. S. P.; Boonpothong, P.; Sousa, E.; Kijjoa, A.; Pinto, M. M. M. Nat. Prod. Rep. 2019, 36, 7–34. doi:10.1039/c8np00043c
Return to citation in text: [1] -
Xin, Z. H.; Fang, Y.; Du, L.; Zhu, T.; Duan, L.; Chen, J.; Gu, Q.-Q.; Zhu, W.-M. J. Nat. Prod. 2007, 70, 853–855. doi:10.1021/np060516h
Return to citation in text: [1] -
Wang, L.; Fang, K.; Cheng, J.; Li, Y.; Huang, Y.; Chen, S.; Dong, G.; Wu, S.; Sheng, C. J. Med. Chem. 2020, 63, 696–713. doi:10.1021/acs.jmedchem.9b01626
Return to citation in text: [1] -
Lindel, T. Chemistry and biology of the pyrrole-imidazole alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press, 2017; Vol. 77, pp 117–219. doi:10.1016/bs.alkal.2016.12.001
Return to citation in text: [1] -
Lansdell, T. A.; Hewlett, N. M.; Skoumbourdis, A. P.; Fodor, M. D.; Seiple, I. B.; Su, S.; Baran, P. S.; Feldman, K. S.; Tepe, J. J. J. Nat. Prod. 2012, 75, 980–985. doi:10.1021/np300231f
Return to citation in text: [1] -
Pertejo, P.; Peña-Calleja, P.; Carreira-Barral, I.; Quesada, R.; Cordero, N. A.; Rodríguez, F. J.; García-Valverde, M. Org. Biomol. Chem. 2017, 15, 7549–7557. doi:10.1039/c7ob01807j
Return to citation in text: [1]
| 24. | Shourkaei, F. A.; Lish, A. B.; Talebi, M.; Abdoli, S.; Zare, N.; Amanlou, M.; Ranjbar, P. R.; mahdavi, M. J. Mol. Struct. 2024, 1297, 136939. doi:10.1016/j.molstruc.2023.136939 |
| 20. | Sañudo, M.; García-Valverde, M.; Marcaccini, S.; Delgado, J. J.; Rojo, J.; Torroba, T. J. Org. Chem. 2009, 74, 2189–2192. doi:10.1021/jo8025862 |
| 22. | Pertejo, P.; Corres, N.; Torroba, T.; García-Valverde, M. Org. Lett. 2015, 17, 612–615. doi:10.1021/ol503628r |
| 23. | Pertejo, P.; Carreira-Barral, I.; Peña-Calleja, P.; Quesada, R.; García-Valverde, M. J. Org. Chem. 2020, 85, 2291–2302. doi:10.1021/acs.joc.9b02995 |
| 1. | Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b |
| 2. | Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845–5859. doi:10.1021/jm4017625 |
| 9. | Giustiniano, M.; Moni, L.; Sangaletti, L.; Pelliccia, S.; Basso, A.; Novellino, E.; Tron, C. G. Synthesis 2018, 50, 3549–3570. doi:10.1055/s-0037-1610193 |
| 10. | Trudel, V.; Tien, C.-H.; Trofimova, A.; Yudin, A. K. Nat. Rev. Chem. 2021, 5, 604–623. doi:10.1038/s41570-021-00304-2 |
| 22. | Pertejo, P.; Corres, N.; Torroba, T.; García-Valverde, M. Org. Lett. 2015, 17, 612–615. doi:10.1021/ol503628r |
| 8. | Piha-Paul, S. A.; Xu, B.; Dumbrava, E. E.; Fu, S.; Karp, D. D.; Meric-Bernstam, F.; Hong, D. S.; Rodon, J. A.; Tsimberidou, A. M.; Raghav, K.; Ajani, J. A.; Conley, A. P.; Mott, F.; Fan, Y.; Fan, J.; Peng, P.; Wang, H.; Ni, S.; Sun, C.; Qiang, X.; Levin, W. J.; Ngo, B.; Ru, Q. C.; Wu, F.; Javle, M. M. Oncologist 2024, 29, e514–e525. doi:10.1093/oncolo/oyad338 |
| 22. | Pertejo, P.; Corres, N.; Torroba, T.; García-Valverde, M. Org. Lett. 2015, 17, 612–615. doi:10.1021/ol503628r |
| 20. | Sañudo, M.; García-Valverde, M.; Marcaccini, S.; Delgado, J. J.; Rojo, J.; Torroba, T. J. Org. Chem. 2009, 74, 2189–2192. doi:10.1021/jo8025862 |
| 30. | Pertejo, P.; Peña-Calleja, P.; Carreira-Barral, I.; Quesada, R.; Cordero, N. A.; Rodríguez, F. J.; García-Valverde, M. Org. Biomol. Chem. 2017, 15, 7549–7557. doi:10.1039/c7ob01807j |
| 3. | Burkholder, P. R.; Sharma, G. M. Lloydia 1969, 32, 466–483. |
| 4. | Sharma, G. M.; Burkholder, P. R. J. Chem. Soc. D 1971, 151–152. doi:10.1039/c29710000151 |
| 5. | Poullennec, K. G.; Romo, D. J. Am. Chem. Soc. 2003, 125, 6344–6345. doi:10.1021/ja034575i |
| 6. | Wang, S.; Romo, D. Angew. Chem., Int. Ed. 2008, 47, 1284–1286. doi:10.1002/anie.200703998 |
| 21. | Pertejo, P.; García-Valverde, M.; Peña, P.; Cordero, N. A.; Torroba, T.; González-Ortega, A. Org. Biomol. Chem. 2014, 12, 4905–4916. doi:10.1039/c4ob00444b |
| 16. | Pertejo, P.; González-Saiz, B.; Quesada, R.; García-Valverde, M. J. Org. Chem. 2020, 85, 14240–14245. doi:10.1021/acs.joc.0c02103 |
| 17. | v. Zychlinski, A.; Ugi, I. Heterocycles 1998, 49, 29–32. doi:10.3987/com-97-s2 |
| 18. | Rossen, K.; Sager, J.; DiMichele, L. M. Tetrahedron Lett. 1997, 38, 3183–3186. doi:10.1016/s0040-4039(97)00607-2 |
| 20. | Sañudo, M.; García-Valverde, M.; Marcaccini, S.; Delgado, J. J.; Rojo, J.; Torroba, T. J. Org. Chem. 2009, 74, 2189–2192. doi:10.1021/jo8025862 |
| 28. | Lindel, T. Chemistry and biology of the pyrrole-imidazole alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press, 2017; Vol. 77, pp 117–219. doi:10.1016/bs.alkal.2016.12.001 |
| 15. | Neo, A. G.; Ramiro, J. L.; García-Valverde, M.; Díaz, J.; Marcos, C. F. Mol. Diversity 2024, 28, 335–418. doi:10.1007/s11030-023-10641-7 |
| 21. | Pertejo, P.; García-Valverde, M.; Peña, P.; Cordero, N. A.; Torroba, T.; González-Ortega, A. Org. Biomol. Chem. 2014, 12, 4905–4916. doi:10.1039/c4ob00444b |
| 29. | Lansdell, T. A.; Hewlett, N. M.; Skoumbourdis, A. P.; Fodor, M. D.; Seiple, I. B.; Su, S.; Baran, P. S.; Feldman, K. S.; Tepe, J. J. J. Nat. Prod. 2012, 75, 980–985. doi:10.1021/np300231f |
| 12. | Hulme, C.; Peng, J.; Tang, S.-Y.; Burns, C. J.; Morize, I.; Labaudiniere, R. J. Org. Chem. 1998, 63, 8021–8023. doi:10.1021/jo980622r |
| 13. | Hulme, C.; Ma, L.; Romano, J.; Morrissette, M. Tetrahedron Lett. 1999, 40, 7925–7928. doi:10.1016/s0040-4039(99)01580-4 |
| 14. | Xu, Z.; De Moliner, F.; Cappelli, A. P.; Ayaz, M.; Hulme, C. Synlett 2014, 25, 225–228. doi:10.1055/s-0033-1340219 |
| 25. | Resende, D. I. S. P.; Boonpothong, P.; Sousa, E.; Kijjoa, A.; Pinto, M. M. M. Nat. Prod. Rep. 2019, 36, 7–34. doi:10.1039/c8np00043c |
| 26. | Xin, Z. H.; Fang, Y.; Du, L.; Zhu, T.; Duan, L.; Chen, J.; Gu, Q.-Q.; Zhu, W.-M. J. Nat. Prod. 2007, 70, 853–855. doi:10.1021/np060516h |
| 11. | Giovenzana, G. B.; Tron, G. C.; Di Paola, S.; Menegotto, I. G.; Pirali, T. Angew. Chem., Int. Ed. 2006, 45, 1099–1102. doi:10.1002/anie.200503095 |
| 19. | Keating, T. A.; Armstrong, R. W. J. Org. Chem. 1996, 61, 8935–8939. doi:10.1021/jo961517p |
| 27. | Wang, L.; Fang, K.; Cheng, J.; Li, Y.; Huang, Y.; Chen, S.; Dong, G.; Wu, S.; Sheng, C. J. Med. Chem. 2020, 63, 696–713. doi:10.1021/acs.jmedchem.9b01626 |
© 2024 Gómez-Ayuso et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.