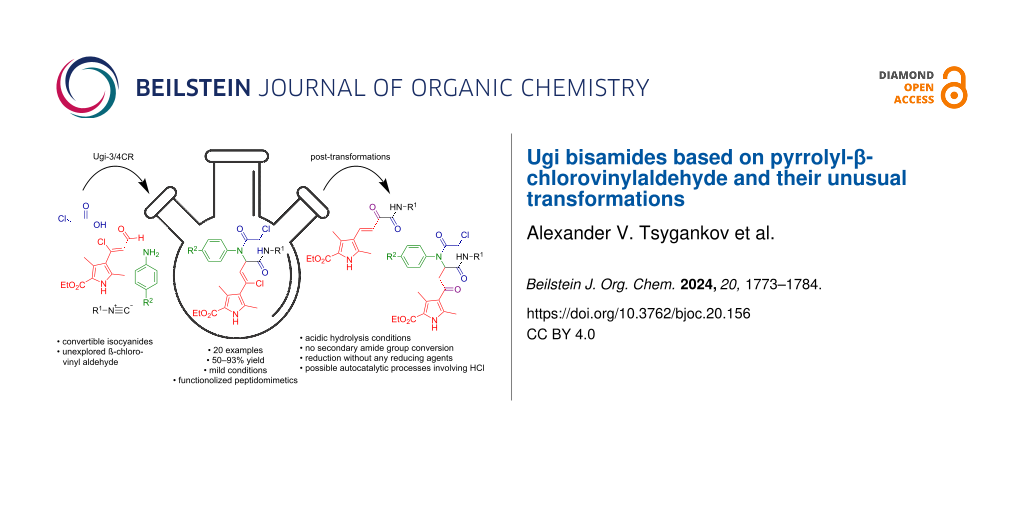
Abstract
By one-pot four- and three-component Ugi reactions involving convertible isocyanides and unexplored pyrrole-containing β-chlorovinylaldehyde, a small library of 20 bisamides with unusual behavior in post-Ugi transformations was prepared and characterized. Surprisingly, a well-documented approach to obtain peptide-containing carboxylic acids through acid hydrolysis of the convertible isocyanide moiety in the Ugi bisamides proceeded in an unexpected manner in our case, leading to the formation of derivatives of amides of heterylidenepyruvic acid. An optimized synthetic protocol for this transformation was elaborated and a plausible sequence involving the elimination of the 2-chloroacetamide moiety and the conversion of the β-chlorovinyl fragment into a vinyl one is provided.
Graphical Abstract
Introduction
One of the keys to the development of mankind is the constant search for new substances and advanced materials. Today, we have powerful tools at our disposal that allow us to create entire libraries of structurally complex organic compounds [1-6] to expand and systematically explore the chemical space within the concepts of molecular diversity chemistry (diversity oriented synthesis) [6] and biologically oriented synthesis [7].
Among the multicomponent processes, the four-component Ugi reaction (Ugi-4CR) [8-13] is characterized by the greatest versatility, through the variability of the starting components leading to a variety of possible products [12-17]. The Ugi-4CR has been used for the synthesis of numerous natural substances, e.g., bicyclomycin, furanomycin, penicillin, etc. [15,18]. Further, the application of reagents with additional functional groups in the Ugi reaction makes it possible to further increase the complexity of the product structures, also due to possible post-transformation reactions. For example, if an unsaturated bond is present in the aldehyde component, after the formation of the expected Ugi bisamide products, subsequent post-transformations allow the synthesis of products of intramolecular cyclization [19-22] and/or products of a tandem combination of several reactions (Scheme 1) [22-26].
Scheme 1: The use of α,β-unsaturated aldehydes in the Ugi reaction.
Scheme 1: The use of α,β-unsaturated aldehydes in the Ugi reaction.
At the same time, the use of so-called convertible isocyanides [27-36] in Ugi-4CR makes it possible to obtain carboxylic acids or esters after hydrolysis of the secondary amide group in the Ugi products (Scheme 2) [27-29,31,32,34-36]. Ugi bisamides modified in this way may be subsequently used as acid components in tandem combinations of various multicomponent processes such as Ugi and Ugi, azido-Ugi and Ugi, Ugi and Passerini, Groebke–Blackburn–Bienaymé and Ugi, etc. [15,17,37,38].
Scheme 2: Comparison of isocyanide conversion conditions.
Scheme 2: Comparison of isocyanide conversion conditions.
The creation of hybrid molecules by using primary and post-modified Ugi products in combination with other isocyanide MCRs is effective and one promising direction to increase the diversity of new peptidomimetics is the use of, for example, α,β-unsaturated aldehydes including those containing a halogen atom in the β-position, in the Ugi-4CR reaction [1,39].
As our previous studies have shown, azomethines based on aromatic amines and substituted pyrrolecarbaldehyde [40] or pyrrolyl-β-chlorovinylaldehyde [39], contain several frequently encountered motifs in drugs and drug candidates – a pyrrole heterocycle and an azomethine C=N fragment (Figure 1) – and exhibit some biological activity. Thus, Ugi bisamides based on the same aldehydes and amines may also demonstrate biological activity.
Figure 1: Azomethines based on ethyl 4-acetyl-3,5-dimethyl-1H-pyrrole-2-carboxylate and 4-[(E)-1-chloro-3-oxoprop-1-enyl]-3,5-dimethyl-1H-pyrrole-2-carboxylate.
Figure 1: Azomethines based on ethyl 4-acetyl-3,5-dimethyl-1H-pyrrole-2-carboxylate and 4-[(E)-1-chloro-3-oxo...
In view of these facts, we decided to develop a new approach for the synthesis of hybrid molecules containing substituted heterocyclic and peptidomimetic moieties. The first stage of this approach was the preparation of Ugi bisamides based on pyrrole-containing β-chlorovinylaldehyde and convertible isocyanides. The subsequent post-transformation of the products by acidic hydrolysis conditions should then lead to an acidic component. However, due to the cascade nature of the multicomponent processes and the presence of several alternative reaction centers in the structure of our substances, we sometimes encountered unexpected and intriguing results.
Results and Discussion
Synthesis of Ugi bisamides
Four-component and three-component Ugi reactions
The combination of pyrrole-containing α,β-unsaturated aldehyde 1, which contains a chlorine atom in the β-position, with convertible isocyanides 4a–d, para-substituted anilines 2a–e and monochloroacetic acid (3) as the smallest building blocks in a four-component reaction leads to the formation of the Ugi bisamides 5–8 (Table 1). Their structures offer several possibilities for subsequent post-transformation reactions.
Table 1: Library of Ugi bisamides 5–8 containing a β-chlorovinyl fragment.
|
|
||||
| Bisamide | R1 | R2 | Yield 4CRa | Yield 3CRa |
| 5a | 2-NO2-Bn | OMe | 55 | 47 |
| 5b | Me | 64 | 47 | |
| 5c | Br | 63 | – | |
| 5d | CF3 | 54 | – | |
| 5e | Cl | 50 | – | |
| 6a | Bn | OMe | 54b | 26b |
| 6b | Me | 69 | – | |
| 6c | Br | 72 | – | |
| 6d | CF3 | 49b | – | |
| 6e | Cl | 93 | – | |
| 7a | Cy | OMe | 59 | – |
| 7b | Me | 87 | – | |
| 7c | Br | 64 | – | |
| 7d | CF3 | 79 | – | |
| 7c | Cl | 72 | – | |
| 8a | t-Bu | OMe | 65 | 27 |
| 8b | Me | 69 | 66 | |
| 8c | Br | 79 | 53 | |
| 8d | CF3 | 64 | – | |
| 8e | Cl | 58 | – | |
aIsolated, EtOH as a solvent; bthe first precipitated compound was amide 10b.
The synthesis of the target Ugi bisamides 5–8 was carried out at room temperature in ethanol with stirring for 24–48 hours (depending on the type of starting material) with a yield of 54–93% (Table 1).
It is worth noting that the Ugi-4CR reaction also led to the formation of bisamides 5–8 when other solvents were used, e.g., methanol or acetonitrile. However, the yields of the targeted reaction products in methanol were generally lower than in ethanol, while the procedure in acetonitrile was not suitable for all reagents.
It is known that the Ugi-4CR proceeds through the formation of an azomethine (Schiff base) in the first stage [39,40]. Therefore, considering the results of our previous work [39] on the nature and properties of azomethines based on β-chlorovinylaldehyde 1 (Figure 1), we decided to study the possibility of using the three-component Ugi reaction (Ugi-3CR) with preliminary synthesis of azomethines 9a–c (Table 1). In this case, the Ugi bisamides 5, 6, 8 were formed, however, it was found that the application of the Ugi-3CR approach had no significant effect on the yields of the target products. Considering the additional reaction step for the synthesis and purification of the starting azomethines 9, we cannot propose the Ugi-3CR approach as more suitable compared to the Ugi-4CR approach.
The structure of the Ugi bisamides 5–8 were proved by X-ray diffraction study on the example of substance 8c (Figure 2), according to which the Z-configuration of the chlorovinyl fragment was detected.
![[1860-5397-20-156-2]](/bjoc/content/figures/1860-5397-20-156-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of ethyl (Z)-4-(3-(N-(4-bromophenyl)-2-chloroacetamido)-4-(tert-butylamino)-1-chloro-4-oxobut-1-en-1-yl)-3,5-dimethyl-1H-pyrrole-2-carboxylate (8c) according to the X-ray diffraction study. Non-hydrogen atoms are presented as thermal ellipsoids with 50% probability.
Figure 2: Molecular structure of ethyl (Z)-4-(3-(N-(4-bromophenyl)-2-chloroacetamido)-4-(tert-butylamino)-1-c...
Post-Ugi transformations
As previously mentioned [32], the introduction of the convertible 2‑bromo-6-isocyanopyridine into the Ugi bisamide structure allows the conversion of the newly formed amide into a carboxylic acid fragment after acid hydrolysis. Similar results were obtained by Dömling and co-workers [27], who used 2-nitrobenzyl isocyanide as a universal convertible isocyanide, and the amide group was also converted into a carboxylic acid under the conditions of acid hydrolysis (Scheme 2). Therefore, taking into account the experience of the authors [27,32], we tried to apply the described hydrolysis conditions (5 equiv HCl in MeOH) to our products (compounds 5). As a model reaction, we heated a mixture of Ugi bisamide 5d and an aqueous solution of HCl in methanol (glycerol bath, 80 °C) in a hermetically sealed vial with stirring for three hours (Scheme 3, conditions A).
Scheme 3: Hydrolysis of Ugi bisamide 5d in the presence of HCl. Conditions: (A) 5 equiv HCl, MeOH, 80 °C, 3 h; (B) 5 equiv HCl, EtOH, MW 120 °C, 15 min; (C) 5 equiv HCl, MeCN, MW 100 °C, 20 min.
Scheme 3: Hydrolysis of Ugi bisamide 5d in the presence of HCl. Conditions: (A) 5 equiv HCl, MeOH, 80 °C, 3 h...
However, the results of this attempted post-Ugi transformation were quite unexpected: Instead of acid 11, we isolated the amide of the unsaturated derivative of pyruvic acid 10a according to the 1H and 13C NMR spectra and mass spectrometry data. In order to drive the process towards the desired hydrolysis of the secondary amide group, we performed the post-Ugi transformation under MW activation in ethanol or acetonitrile (Scheme 3, conditions B or C). However, the application of MW irradiation did not change the course of the reaction, and as under conventional thermal heating, amide 10a was isolated, albeit in a lower yield and accompanied with tar formation.
In addition to the 1H, 13C NMR spectra and mass spectrometry data, the structure of compound 10d was established by X-ray diffraction analysis (Figure 3). It was also found that the substituents at the double bond are trans configured.
![[1860-5397-20-156-3]](/bjoc/content/figures/1860-5397-20-156-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of ethyl (E)-4-(4-(tert-butylamino)-3,4-dioxobut-1-en-1-yl)-3,5-dimethyl-1H-pyrrole-2-carboxylate (10d) according to X-ray diffraction data. Non-hydrogen atoms are presented as thermal ellipsoids with 50% probability.
Figure 3: Molecular structure of ethyl (E)-4-(4-(tert-butylamino)-3,4-dioxobut-1-en-1-yl)-3,5-dimethyl-1H-pyr...
To find out the patterns of the new reaction, we applied different conditions to a wider range of starting bisamides 5–8 (Table 2). As in the model reaction (Scheme 3), the main products of the transformation were the corresponding amides 10a–d (Table 2).
Table 2: Post-Ugi transformations of bisamides 5–8 under different conditions.
|
|
|||||||||
| Entry | Bisamide | Conditions | Yielda | ||||||
| R1 | R2 | No. | solvent | cat | temperatureb, °C | time, h | amide 10 | ketobisamide 12 | |
| 1 | 2-NO2-Bn | Br | 5c | MeOH | HCl 5 equivc | 70 | 3 | 10a, 13 | – |
| 2 | CF3 | 5d | MeOH | HCl 5 equivc | 80 | 3 | 10a, 42 | – | |
| 3 | CF3 | 5d | EtOH | – | 80 | 3.5 | 10a, 44 | 12a, 19 | |
| 4 | CF3 | 5d | EtOH | HCl 1 equivc | 80 | 3.5 | 10a, 47 | 12a, 20 | |
| 5 | CF3 | 5d | MeOH | HCl 5 equivc | 85, MW | 0.5 | 10a, 40 | – | |
| 6 | CF3 | 5d | EtOH | HCl 5 equivc | 100, MW | 0.5 | 10a, 39 | – | |
| 7 | CF3 | 5d | EtOH | HCl 5 equivc | 120, MW | 0.25 | 10a, 35 | – | |
| 8 | CH3 | 5b | MeCN | HCl 5 equivc | 50 | 6 | 10a, 35 | – | |
| 9 | CF3 | 5d | MeCN | HCl 5 equivc | 100, MW | 0.5 | 10a, 26 | – | |
| 10 | Bn | Br | 6c | EtOH | – | 80 | 3 | 10b,42 | – |
| 11 | Me | 6b | EtOH | – | 80 | 3 | 10b, 35 | – | |
| 12 | Cl | 6e | EtOH | HCl 1 equivc | 50 | 168 | 10b, 20 | – | |
| 13 | Cl | 6e | EtOH | HCl 1 equivc | 80 | 3 | 10b, 40 | – | |
| 14 | Br | 6c | EtOH | HCl 1 equivc | 80 | 2.5 | 10b, 25 | – | |
| 15 | OMe | 6a | EtOH | HCl 1 equivc | 80 | 3 | 10b, 39 | 12b, traces | |
| 16 | Me | 6b | EtOH | HCl 1 equivc | 80 | 3 | 10b, 38 | – | |
| 17 | Br | 6c | MeCN | HCl 1 equivc | 25 | 216 | 10b, 20 | 12c, 9 | |
| 18 | Cy | Me | 7b | EtOH | – | 25 | 850 | 10c, 18 | 12d, 71 |
| 19 | Me | 7b | EtOH | HCl 5 equivc | 80 | 3 | 10c, 34 | – | |
| 20 | Br | 7c | EtOH | HCl 5 equivc | 80 | 4.5 | 10c, 40 | – | |
| 21 | OMe | 7a | EtOH | HCl 5 equivc | 80 | 3 | 10c, 38 | – | |
| 23 | t-Bu | Br | 8c | EtOH | – | 25 | 840 | 10d, 6 | 12e, 6 |
| 24 | Br | 8c | EtOH | – | 80 | 3 | 10d, 56 | 12e, 15 | |
| 25 | Br | 8c | EtOH | HCl 1 equivc | 25 | 850 | 10d, 14 | 12e, 5 | |
| 26 | Br | 8c | EtOH | DIPEA 2 equiv | 80 | 3 | – | – | |
| 27 | Br | 8c | EtOH | Et3N 2 equiv | 80 | 3 | – | – | |
| 28 | Br | 8c | EtOH | MCA 1 equiv | 80 | 2 | 10d, 52 | 12e, 13 | |
| 29 | Br | 8c | EtOH | HCl 0.5 equivc | 80 | 3 | 10d, 64 | 12e, 12 | |
| 30 | Br | 8c | EtOH | HCl 1 equivc | 80 | 3 | 10d, 46 | 12e, 12 | |
| 31 | Br | 8c | EtOH | HCl 2 equivc | 80 | 3 | 10d, 47 | 12e, 12 | |
| 32 | Br | 8c | EtOH | HCl 5 equivc | 80 | 3 | 10d, 51 | 12e, 12 | |
| 33 | OMe | 8a | EtOH | HCl 5 equivc | 80 | 3 | 10d, 62 | 12f, 21 | |
| 34 | Br | 8c | MeCN | HCl 1 equivc | 25 | 72 | 10d, 34 | 12e, 46 | |
| 35 | Br | 8c | MeCN | – | 80 | 3 | 10d, 10 | 12e, 18 | |
aIsolated yield; bthe temperature in bath; cwater 36% solution.
In the case of bisamides 5d, 6a, 6c, 7b, 8a, and 8c (Table 2), additional transformation products were also isolated from the reaction mixture. According to 1H and 13C NMR, MS, and X-ray diffraction studies these were the corresponding ketobisamides 12, which are products of a nucleophilic substitution of the chlorine atom in the chlorovinyl fragment to the hydroxy group, probably under the influence of water (Figure 4).
![[1860-5397-20-156-4]](/bjoc/content/figures/1860-5397-20-156-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Molecular structure of ethyl 4-(3-(N-(4-bromophenyl)-2-chloroacetamido)-4-(tert-butylamino)-4-oxobutanoyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate (12e) according to the X-ray diffraction data. Non-hydrogen atoms are presented as thermal ellipsoids with 50% probability.
Figure 4: Molecular structure of ethyl 4-(3-(N-(4-bromophenyl)-2-chloroacetamido)-4-(tert-butylamino)-4-oxobu...
It is worth noting that the synthesis of Ugi bisamides 5–8 (Table 1) yielded compounds 10 and ketobisamide 12 in some cases. For example, in the Ugi reaction involving benzyl isocyanide (4b) and p-anisidine (2a), the product that precipitated first from the reaction mixture was 2-oxo-4-(1H-pyrrol-3-yl)but-3-enoic acid amide 10b (Scheme 4; Table 1, footnote b). Pure Ugi bisamide 6a could only be obtained when the reaction was carried out in MeCN or EtOH. The same was observed in the case of p-CF3-substituted aniline when synthesizing bisamide 6d. The yield of the amide 10c was about 8% and it was also the first to precipitate.
Scheme 4: The Ugi-4CR with the participation of p-anisidine and benzyl isocyanide.
Scheme 4: The Ugi-4CR with the participation of p-anisidine and benzyl isocyanide.
In addition, it is worth mentioning that the corresponding amides 10 were observed in 1H NMR spectra and LC–MS analysis in many mother liquors after filtration of the bisamides 6–8. Moreover, traces of the ketobisamides 12 were also observed when the synthesis was carried out in 96% EtOH. The one-pot synthesis allows to combine two consecutive steps, the Ugi-4CR and the post-Ugi reaction, by keeping the mixture of starting materials 1, 2c, 3 and 4d at 25 °C for two days and then heating the reaction mixture in a closed vessel at 80 °C for 3 hours (Scheme 5). This procedure led to the formation and isolation of the products 10d and 12e with yields which are similar to the tandem reaction.
Scheme 5: Successful attempt at tandem one-pot coupling of the Ugi-4CR reaction and post-transformation of the corresponding bisamide 8c.
Scheme 5: Successful attempt at tandem one-pot coupling of the Ugi-4CR reaction and post-transformation of th...
Using the example of bisamide 8c, it was found that changing the amount of HCl or replacing it with chloroacetic acid under otherwise identical conditions had no significant effect on the yields of products 10 and 12 (Table 2, entries 28–32). At the same time, decreasing the temperature of the post-Ugi transformation of bisamide 8c in the presence of HCl to 25 °C significantly slowed down the reaction (Table 2, entry 25) and after 36 days a large amount of the starting bisamide remained unchanged in the reaction mixture, while the target amide 10d and ketobisamide 12e were observed in low yield. However, when the same reaction was carried out in MeCN, the bisamide 8c completely disappeared within 3 days (Table 2, entry 34), and the amide 10d and the ketobisamide 12e were isolated from the reaction mixture in sufficiently higher yields than when the reaction was carried out in ethanol.
Moreover, the post-Ugi transformation of bisamides 6b, 6c, 7b, and 8c without addition of acid also led to the formation of amides 10b–d as main products and the corresponding ketobisamides 12d–e as minor products (Table 2, entries 10, 11, 18, 23, and 24). This can be explained by the formation of HCl in the reaction mixture due to the substitution of chlorine in the vinyl chloride moiety under the influence of water. The appearance of HCl in these cases was identified by the specific odor and detected by pH measurements. It is likely that this catalytic amount of HCl is enough for the conversion and formation of the amides 10.
To confirm the influence of HCl and its necessity to initiate the formation of amide 10, the post-Ugi transformation of bisamide 8c was carried out in the presence of Et3N or DIPEA (Table 2, entries 26 and 27). The expected corresponding quaternary ammonium salts were isolated, and no trace of amide 10d was observed.
It should also be noted that in the 1H NMR spectra of the reaction mixtures and the various mother liquors, the signals of the 2-chloroacetamides 13a–d (Scheme 6) [41] were clearly recognizable in many cases and were sometimes isolated individually. In addition, the hydrochlorides of the corresponding para-substituted anilines were identified. The amount of salt formed increased with increasing HCl excess, indicating that they were formed by the acidic hydrolysis of the corresponding 2-chloroacetamides 13a–e. The amount of either 2-chloroacetamide or 2-chloroacetamide and ammonium salt together correlated well with the amount of the corresponding amide 10.
Scheme 6: Plausible transformation sequence of the formation of amides 10 and ketobisamides 12.
Scheme 6: Plausible transformation sequence of the formation of amides 10 and ketobisamides 12.
The expected fact that the amides 10 could not be formed from the ketobisamides 12 (Table 3) was also confirmed; stirring the latter in MeCN or EtOH in the presence of HCl at room temperature or under heating did not lead to formation, not even to the appearance of traces of the amide 10d.
Based on the above facts, we have proposed a plausible transformation sequence for the formation of amides 10 and ketobisamides 12 (Scheme 6).
Conclusion
Thus, in this work, the multicomponent reaction of pyrrole-containing β-chlorovinylaldehyde, para-substituted anilines, monochloroacetic acid, and different convertible isocyanides gives rise to products of the classic Ugi reaction, ethyl (E)-4-(4-(R1-amino)-1-chloro-3-(2-chloro-N-(4-(R2)phenyl)acetamido)-4-oxobut-1-en-1-yl)-3,5-dimethyl-1H-pyrrole-2-carboxylates, which, however, exhibit unusual behavior in post-Ugi transformations. The attempt to apply a well-documented approach for the subsequent synthesis of peptide-containing carboxylic acids by acid hydrolysis of the convertible isocyanide moiety in the Ugi bisamides proceeded in an unexpected manner: their treatment with acids led to elimination of the 2-chloroacetamide moiety and conversion of the β-chlorovinyl fragment into a vinyl fragment giving rise to ethyl (E)-4-(4-(R1-amino)-3,4-dioxobut-1-en-1-yl)-3,5-dimethyl-1H-pyrrole-2-carboxylates. Another direction of the post-transformation was the replacement of the chlorine atom in the β-chlorovinyl group of the Ugi bisamides with a hydroxy group and the formation of a different type of peptidomimetic, namely ethyl 4-(3-(N-(4-R2-phenyl)-2-chloroacetamido)-4-(R1-amino))-4-oxobutanoyl)-3,5-dimethyl-1H-pyrrole-2-carboxylates. It was also found that these two unusual products of acidic transformation were observed as byproducts of the Ugi reaction. Optimized synthetic protocols were developed for all reactions and a plausible sequence of the post-Ugi transformation was provided.
Supporting Information
| Supporting Information File 1: Experimental section, NMR and LC–MS spectra as well as X-ray data. | ||
| Format: PDF | Size: 11.5 MB | Download |
Acknowledgements
The authors thank all brave defenders of Ukraine who allow us to continue our scientific work and prepare this publication. We also express our gratitude to the scientists who were not included in the main list of authors and took part in the initial stages of the work: Professor Olena I. Mikhedkina, Dr. Ihor I. Melnik, Dr. Anna I. Larina. Parts of the work were presented at the following conferences: International scientific and practical conferences MicroCAD 2022 and 2023, Kharkiv, Ukraine; XIX Scientific Conference Lviv Chemical Readings 2023, Lviv, Ukraine; Conferences XXIII and XXIV ”Advances in the Chemistry of Heteroorganic Compounds” 2023 and 2024, Lodz, Poland; VII International (XVII Ukrainian) Scientific Conference for Students and Young Scientists 2024, Vinnitsa, Ukraine; XVI All-Ukrainian Scientific conference of students and graduate students 2024 (HCC'24), Kharkiv, Ukraine.
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Koopmanschap, G.; Ruijter, E.; Orru, R. V. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50
Return to citation in text: [1] [2] -
Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566–583. doi:10.1007/s10593-012-1030-2
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent-Switched Reactions in Synthesis of Heterocycles. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Van der Eycken, E. V.; Sharma, U. K., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8
Return to citation in text: [1] -
Murlykina, M. V.; Morozova, A. D.; Zviagin, I. M.; Sakhno, Y. I.; Desenko, S. M.; Chebanov, V. A. Front. Chem. (Lausanne, Switz.) 2018, 6, 527. doi:10.3389/fchem.2018.00527
Return to citation in text: [1] -
Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244
Return to citation in text: [1] -
Schreiber, S. L. Science 2000, 287, 1964–1969. doi:10.1126/science.287.5460.1964
Return to citation in text: [1] [2] -
Nören-Müller, A.; Reis-Corrêa, I., Jr.; Prinz, H.; Rosenbaum, C.; Saxena, K.; Schwalbe, H. J.; Vestweber, D.; Cagna, G.; Schunk, S.; Schwarz, O.; Schiewe, H.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10606–10611. doi:10.1073/pnas.0601490103
Return to citation in text: [1] -
Ugi, I.; Werner, B.; Dömling, A. Molecules 2003, 8, 53–66. doi:10.3390/80100053
Return to citation in text: [1] -
Van der Eycken, E.; Sharma, U. K., Eds. Multicomponent Reactions towards Heterocycles: Concepts and Applications; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527832439
Return to citation in text: [1] -
Hulme, C.; Gore, V. Curr. Med. Chem. 2003, 10, 51–80. doi:10.2174/0929867033368600
Return to citation in text: [1] -
Váradi, A.; Palmer, T. C.; Dardashti, R. N.; Majumdar, S. Molecules 2016, 21, 19. doi:10.3390/molecules21010019
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u
Return to citation in text: [1] [2] -
Zhu, J.; Bienayme, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118
Return to citation in text: [1] [2] -
Bode, M. L.; Gravestock, D.; Rousseau, A. L. Org. Prep. Proced. Int. 2016, 48, 89–221. doi:10.1080/00304948.2016.1138072
Return to citation in text: [1] -
Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a
Return to citation in text: [1] [2] [3] -
Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126
Return to citation in text: [1] -
Kolomiiets, O. V.; Tsygankov, A. V.; Kornet, M. N.; Brazhko, A. A.; Musatov, V. I.; Chebanov, V. A. Beilstein J. Org. Chem. 2023, 19, 727–735. doi:10.3762/bjoc.19.53
Return to citation in text: [1] [2] -
Ugi, I. Angew. Chem., Int. Ed. Engl. 1982, 21, 810–819. doi:10.1002/anie.198208101
Return to citation in text: [1] -
Bossio, R.; Marcos, C. F.; Marcaccini, S.; Pepino, R. Tetrahedron Lett. 1997, 38, 2519–2520. doi:10.1016/s0040-4039(97)00389-4
Return to citation in text: [1] -
Bossio, R.; Marcos, C. F.; Marcaccini, S.; Pepino, R. Heterocycles 1997, 45, 1589–1592. doi:10.3987/com-97-7855
Return to citation in text: [1] -
Marcos, C. F.; Bossio, R.; Marcaccini, S.; Pepino, R. J. Chem. Educ. 2000, 77, 382–384. doi:10.1021/ed077p382
Return to citation in text: [1] -
Huang, J.; Du, X.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Eur. J. Org. Chem. 2017, 4379–4388. doi:10.1002/ejoc.201700747
Return to citation in text: [1] [2] -
Thessing, A.; Ayres, R.; Jones, J.; Bauer, H.; Zangi, M.; Luesse, S. B. Results Chem. 2022, 4, 100416. doi:10.1016/j.rechem.2022.100416
Return to citation in text: [1] -
Kalinski, C.; Umkehrer, M.; Schmidt, J.; Ross, G.; Kolb, J.; Burdack, C.; Hiller, W.; Hoffmann, S. D. Tetrahedron Lett. 2006, 47, 4683–4686. doi:10.1016/j.tetlet.2006.04.127
Return to citation in text: [1] -
Lu, K.; Luo, T.; Xiang, Z.; You, Z.; Fathi, R.; Chen, J.; Yang, Z. J. Comb. Chem. 2005, 7, 958–967. doi:10.1021/cc050099b
Return to citation in text: [1] -
Pokhodylo, N. T.; Тupychak, M. A.; Goreshnik, E. A.; Obushak, M. D. Synthesis 2023, 55, 977–988. doi:10.1055/s-0042-1751382
Return to citation in text: [1] -
Chandgude, A. L.; Li, J.; Dömling, A. Asian J. Org. Chem. 2017, 6, 798–801. doi:10.1002/ajoc.201700177
Return to citation in text: [1] [2] [3] [4] -
Strocker, A. M.; Keating, T. A.; Tempest, P. A.; Armstrong, R. W. Tetrahedron Lett. 1996, 37, 1149–1152. doi:10.1016/0040-4039(96)00012-3
Return to citation in text: [1] [2] -
Keating, T. A.; Armstrong, R. W. J. Am. Chem. Soc. 1995, 117, 7842–7843. doi:10.1021/ja00134a044
Return to citation in text: [1] [2] -
Gilley, C. B.; Buller, M. J.; Kobayashi, Y. Org. Lett. 2007, 9, 3631–3634. doi:10.1021/ol701446y
Return to citation in text: [1] -
Neves Filho, R. A. W.; Stark, S.; Morejon, M. C.; Westermann, B.; Wessjohann, L. A. Tetrahedron Lett. 2012, 53, 5360–5363. doi:10.1016/j.tetlet.2012.07.064
Return to citation in text: [1] [2] -
van der Heijden, G.; Jong, J. A. W.; Ruijter, E.; Orru, R. V. A. Org. Lett. 2016, 18, 984–987. doi:10.1021/acs.orglett.6b00091
Return to citation in text: [1] [2] [3] [4] -
Hulme, C.; Ma, L.; Cherrier, M.-P.; Romano, J. J.; Morton, G.; Duquenne, C.; Salvino, J.; Labaudiniere, R. Tetrahedron Lett. 2000, 41, 1883–1887. doi:10.1016/s0040-4039(00)00052-6
Return to citation in text: [1] -
Hollanders, C.; Elsocht, M.; Van der Poorten, O.; Jida, M.; Renders, E.; Maes, B. U. W.; Ballet, S. Chem. Commun. 2021, 57, 6863–6866. doi:10.1039/d1cc01701b
Return to citation in text: [1] [2] -
Balalaie, S.; Motaghedi, H.; Tahmassebi, D.; Bararjanian, M.; Bijanzadeh, H. R. Tetrahedron Lett. 2012, 53, 6177–6181. doi:10.1016/j.tetlet.2012.08.096
Return to citation in text: [1] [2] -
Borase, B. B.; Godbole, H. M.; Singh, G. P.; Upadhyay, P. R.; Trivedi, A.; Bhat, V.; Shenoy, G. G. J. Chem. Sci. 2021, 133, 35. doi:10.1007/s12039-021-01892-8
Return to citation in text: [1] [2] -
Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e
Return to citation in text: [1] -
Barreto, A. d. F. S.; Santos, V. A. d.; Andrade, C. K. Z. Beilstein J. Org. Chem. 2017, 13, 2596–2602. doi:10.3762/bjoc.13.256
Return to citation in text: [1] -
Mikhedkina, E. I.; Ananieva, V. V.; Tsygankov, A. V.; Osolodchenko, T. P.; Ponomarenko, S. V.; Chebanov, V. A. Funct. Mater. 2021, 28, 587–596. doi:10.15407/fm28.03.587
Return to citation in text: [1] [2] [3] [4] -
Mikhedkina, O. I.; Ananieva, V. V.; Sakhno, Y. I.; Melnyk, I. I.; Vereshchak, V. O.; Osolodchenko, T. P.; Shishkina, S. V.; Tsygankov, A. V.; Chebanov, V. A. Chem. Heterocycl. Compd. 2023, 59, 449–455. doi:10.1007/s10593-023-03215-w
Return to citation in text: [1] [2] -
Peixoto, I. N.; Souza, H. D. S.; Lira, B. F.; Silva, D. F.; Lima, E. O.; José, B.-F.; Athayde-Filho, P. F. J. Braz. Chem. Soc. 2016, 27, 1807–1813. doi:10.5935/0103-5053.20160063
Return to citation in text: [1]
| 27. | Chandgude, A. L.; Li, J.; Dömling, A. Asian J. Org. Chem. 2017, 6, 798–801. doi:10.1002/ajoc.201700177 |
| 32. | van der Heijden, G.; Jong, J. A. W.; Ruijter, E.; Orru, R. V. A. Org. Lett. 2016, 18, 984–987. doi:10.1021/acs.orglett.6b00091 |
| 32. | van der Heijden, G.; Jong, J. A. W.; Ruijter, E.; Orru, R. V. A. Org. Lett. 2016, 18, 984–987. doi:10.1021/acs.orglett.6b00091 |
| 27. | Chandgude, A. L.; Li, J.; Dömling, A. Asian J. Org. Chem. 2017, 6, 798–801. doi:10.1002/ajoc.201700177 |
| 1. | Koopmanschap, G.; Ruijter, E.; Orru, R. V. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50 |
| 2. | Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566–583. doi:10.1007/s10593-012-1030-2 |
| 3. | Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent-Switched Reactions in Synthesis of Heterocycles. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Van der Eycken, E. V.; Sharma, U. K., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8 |
| 4. | Murlykina, M. V.; Morozova, A. D.; Zviagin, I. M.; Sakhno, Y. I.; Desenko, S. M.; Chebanov, V. A. Front. Chem. (Lausanne, Switz.) 2018, 6, 527. doi:10.3389/fchem.2018.00527 |
| 5. | Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244 |
| 6. | Schreiber, S. L. Science 2000, 287, 1964–1969. doi:10.1126/science.287.5460.1964 |
| 12. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u |
| 13. | Zhu, J.; Bienayme, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118 |
| 14. | Bode, M. L.; Gravestock, D.; Rousseau, A. L. Org. Prep. Proced. Int. 2016, 48, 89–221. doi:10.1080/00304948.2016.1138072 |
| 15. | Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a |
| 16. | Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126 |
| 17. | Kolomiiets, O. V.; Tsygankov, A. V.; Kornet, M. N.; Brazhko, A. A.; Musatov, V. I.; Chebanov, V. A. Beilstein J. Org. Chem. 2023, 19, 727–735. doi:10.3762/bjoc.19.53 |
| 39. | Mikhedkina, E. I.; Ananieva, V. V.; Tsygankov, A. V.; Osolodchenko, T. P.; Ponomarenko, S. V.; Chebanov, V. A. Funct. Mater. 2021, 28, 587–596. doi:10.15407/fm28.03.587 |
| 40. | Mikhedkina, O. I.; Ananieva, V. V.; Sakhno, Y. I.; Melnyk, I. I.; Vereshchak, V. O.; Osolodchenko, T. P.; Shishkina, S. V.; Tsygankov, A. V.; Chebanov, V. A. Chem. Heterocycl. Compd. 2023, 59, 449–455. doi:10.1007/s10593-023-03215-w |
| 8. | Ugi, I.; Werner, B.; Dömling, A. Molecules 2003, 8, 53–66. doi:10.3390/80100053 |
| 9. | Van der Eycken, E.; Sharma, U. K., Eds. Multicomponent Reactions towards Heterocycles: Concepts and Applications; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527832439 |
| 10. | Hulme, C.; Gore, V. Curr. Med. Chem. 2003, 10, 51–80. doi:10.2174/0929867033368600 |
| 11. | Váradi, A.; Palmer, T. C.; Dardashti, R. N.; Majumdar, S. Molecules 2016, 21, 19. doi:10.3390/molecules21010019 |
| 12. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u |
| 13. | Zhu, J.; Bienayme, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118 |
| 39. | Mikhedkina, E. I.; Ananieva, V. V.; Tsygankov, A. V.; Osolodchenko, T. P.; Ponomarenko, S. V.; Chebanov, V. A. Funct. Mater. 2021, 28, 587–596. doi:10.15407/fm28.03.587 |
| 7. | Nören-Müller, A.; Reis-Corrêa, I., Jr.; Prinz, H.; Rosenbaum, C.; Saxena, K.; Schwalbe, H. J.; Vestweber, D.; Cagna, G.; Schunk, S.; Schwarz, O.; Schiewe, H.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10606–10611. doi:10.1073/pnas.0601490103 |
| 40. | Mikhedkina, O. I.; Ananieva, V. V.; Sakhno, Y. I.; Melnyk, I. I.; Vereshchak, V. O.; Osolodchenko, T. P.; Shishkina, S. V.; Tsygankov, A. V.; Chebanov, V. A. Chem. Heterocycl. Compd. 2023, 59, 449–455. doi:10.1007/s10593-023-03215-w |
| 6. | Schreiber, S. L. Science 2000, 287, 1964–1969. doi:10.1126/science.287.5460.1964 |
| 39. | Mikhedkina, E. I.; Ananieva, V. V.; Tsygankov, A. V.; Osolodchenko, T. P.; Ponomarenko, S. V.; Chebanov, V. A. Funct. Mater. 2021, 28, 587–596. doi:10.15407/fm28.03.587 |
| 27. | Chandgude, A. L.; Li, J.; Dömling, A. Asian J. Org. Chem. 2017, 6, 798–801. doi:10.1002/ajoc.201700177 |
| 28. | Strocker, A. M.; Keating, T. A.; Tempest, P. A.; Armstrong, R. W. Tetrahedron Lett. 1996, 37, 1149–1152. doi:10.1016/0040-4039(96)00012-3 |
| 29. | Keating, T. A.; Armstrong, R. W. J. Am. Chem. Soc. 1995, 117, 7842–7843. doi:10.1021/ja00134a044 |
| 30. | Gilley, C. B.; Buller, M. J.; Kobayashi, Y. Org. Lett. 2007, 9, 3631–3634. doi:10.1021/ol701446y |
| 31. | Neves Filho, R. A. W.; Stark, S.; Morejon, M. C.; Westermann, B.; Wessjohann, L. A. Tetrahedron Lett. 2012, 53, 5360–5363. doi:10.1016/j.tetlet.2012.07.064 |
| 32. | van der Heijden, G.; Jong, J. A. W.; Ruijter, E.; Orru, R. V. A. Org. Lett. 2016, 18, 984–987. doi:10.1021/acs.orglett.6b00091 |
| 33. | Hulme, C.; Ma, L.; Cherrier, M.-P.; Romano, J. J.; Morton, G.; Duquenne, C.; Salvino, J.; Labaudiniere, R. Tetrahedron Lett. 2000, 41, 1883–1887. doi:10.1016/s0040-4039(00)00052-6 |
| 34. | Hollanders, C.; Elsocht, M.; Van der Poorten, O.; Jida, M.; Renders, E.; Maes, B. U. W.; Ballet, S. Chem. Commun. 2021, 57, 6863–6866. doi:10.1039/d1cc01701b |
| 35. | Balalaie, S.; Motaghedi, H.; Tahmassebi, D.; Bararjanian, M.; Bijanzadeh, H. R. Tetrahedron Lett. 2012, 53, 6177–6181. doi:10.1016/j.tetlet.2012.08.096 |
| 36. | Borase, B. B.; Godbole, H. M.; Singh, G. P.; Upadhyay, P. R.; Trivedi, A.; Bhat, V.; Shenoy, G. G. J. Chem. Sci. 2021, 133, 35. doi:10.1007/s12039-021-01892-8 |
| 15. | Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a |
| 17. | Kolomiiets, O. V.; Tsygankov, A. V.; Kornet, M. N.; Brazhko, A. A.; Musatov, V. I.; Chebanov, V. A. Beilstein J. Org. Chem. 2023, 19, 727–735. doi:10.3762/bjoc.19.53 |
| 37. | Zhi, S.; Ma, X.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632–7650. doi:10.1039/c9ob00772e |
| 38. | Barreto, A. d. F. S.; Santos, V. A. d.; Andrade, C. K. Z. Beilstein J. Org. Chem. 2017, 13, 2596–2602. doi:10.3762/bjoc.13.256 |
| 22. | Huang, J.; Du, X.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Eur. J. Org. Chem. 2017, 4379–4388. doi:10.1002/ejoc.201700747 |
| 23. | Thessing, A.; Ayres, R.; Jones, J.; Bauer, H.; Zangi, M.; Luesse, S. B. Results Chem. 2022, 4, 100416. doi:10.1016/j.rechem.2022.100416 |
| 24. | Kalinski, C.; Umkehrer, M.; Schmidt, J.; Ross, G.; Kolb, J.; Burdack, C.; Hiller, W.; Hoffmann, S. D. Tetrahedron Lett. 2006, 47, 4683–4686. doi:10.1016/j.tetlet.2006.04.127 |
| 25. | Lu, K.; Luo, T.; Xiang, Z.; You, Z.; Fathi, R.; Chen, J.; Yang, Z. J. Comb. Chem. 2005, 7, 958–967. doi:10.1021/cc050099b |
| 26. | Pokhodylo, N. T.; Тupychak, M. A.; Goreshnik, E. A.; Obushak, M. D. Synthesis 2023, 55, 977–988. doi:10.1055/s-0042-1751382 |
| 1. | Koopmanschap, G.; Ruijter, E.; Orru, R. V. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50 |
| 39. | Mikhedkina, E. I.; Ananieva, V. V.; Tsygankov, A. V.; Osolodchenko, T. P.; Ponomarenko, S. V.; Chebanov, V. A. Funct. Mater. 2021, 28, 587–596. doi:10.15407/fm28.03.587 |
| 19. | Bossio, R.; Marcos, C. F.; Marcaccini, S.; Pepino, R. Tetrahedron Lett. 1997, 38, 2519–2520. doi:10.1016/s0040-4039(97)00389-4 |
| 20. | Bossio, R.; Marcos, C. F.; Marcaccini, S.; Pepino, R. Heterocycles 1997, 45, 1589–1592. doi:10.3987/com-97-7855 |
| 21. | Marcos, C. F.; Bossio, R.; Marcaccini, S.; Pepino, R. J. Chem. Educ. 2000, 77, 382–384. doi:10.1021/ed077p382 |
| 22. | Huang, J.; Du, X.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Eur. J. Org. Chem. 2017, 4379–4388. doi:10.1002/ejoc.201700747 |
| 41. | Peixoto, I. N.; Souza, H. D. S.; Lira, B. F.; Silva, D. F.; Lima, E. O.; José, B.-F.; Athayde-Filho, P. F. J. Braz. Chem. Soc. 2016, 27, 1807–1813. doi:10.5935/0103-5053.20160063 |
| 15. | Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a |
| 18. | Ugi, I. Angew. Chem., Int. Ed. Engl. 1982, 21, 810–819. doi:10.1002/anie.198208101 |
| 27. | Chandgude, A. L.; Li, J.; Dömling, A. Asian J. Org. Chem. 2017, 6, 798–801. doi:10.1002/ajoc.201700177 |
| 28. | Strocker, A. M.; Keating, T. A.; Tempest, P. A.; Armstrong, R. W. Tetrahedron Lett. 1996, 37, 1149–1152. doi:10.1016/0040-4039(96)00012-3 |
| 29. | Keating, T. A.; Armstrong, R. W. J. Am. Chem. Soc. 1995, 117, 7842–7843. doi:10.1021/ja00134a044 |
| 31. | Neves Filho, R. A. W.; Stark, S.; Morejon, M. C.; Westermann, B.; Wessjohann, L. A. Tetrahedron Lett. 2012, 53, 5360–5363. doi:10.1016/j.tetlet.2012.07.064 |
| 32. | van der Heijden, G.; Jong, J. A. W.; Ruijter, E.; Orru, R. V. A. Org. Lett. 2016, 18, 984–987. doi:10.1021/acs.orglett.6b00091 |
| 34. | Hollanders, C.; Elsocht, M.; Van der Poorten, O.; Jida, M.; Renders, E.; Maes, B. U. W.; Ballet, S. Chem. Commun. 2021, 57, 6863–6866. doi:10.1039/d1cc01701b |
| 35. | Balalaie, S.; Motaghedi, H.; Tahmassebi, D.; Bararjanian, M.; Bijanzadeh, H. R. Tetrahedron Lett. 2012, 53, 6177–6181. doi:10.1016/j.tetlet.2012.08.096 |
| 36. | Borase, B. B.; Godbole, H. M.; Singh, G. P.; Upadhyay, P. R.; Trivedi, A.; Bhat, V.; Shenoy, G. G. J. Chem. Sci. 2021, 133, 35. doi:10.1007/s12039-021-01892-8 |
© 2024 Tsygankov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.