Abstract
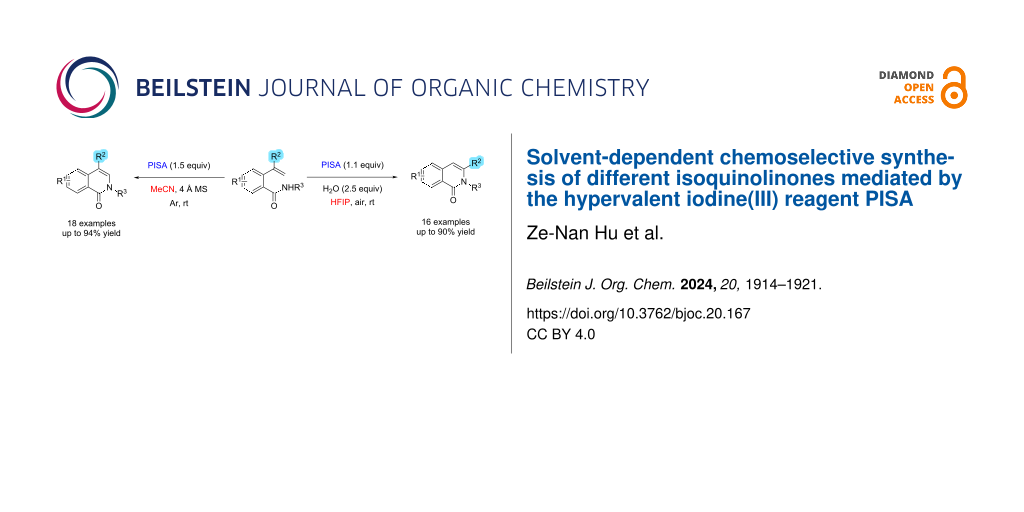
Isoquinolinone is an important heterocyclic framework in natural products and biologically active molecules, and the efficient synthesis of this structural motif has received much attention in recent years. Herein, we report a (phenyliodonio)sulfamate (PISA)-mediated, solvent-dependent synthesis of different isoquinolinone derivatives. The method provides highly chemoselective access to 3- or 4-substituted isoquinolinone derivatives by reacting o-alkenylbenzamide derivatives with PISA in either acetonitrile or wet hexafluoro-2-isopropanol.
Graphical Abstract
Introduction
Isoquinolinone is an important heterocyclic structure found in many natural products and biologically active compounds, including pharmaceuticals [1]. For instance, lycoricidine, found in the medicinal plant Lycoris radiata, may inhibit the MCPyV LT protein activity and thus block cancer formation [2]. Alangiumkaloids A, an isoquinolinone alkaloid isolated from Alangium salviiforlium, was reported to exhibit cytotoxic activity against cancer cells [3]. In 2018, duvelisib, a dual inhibitor of phosphoinositide-3 kinases, was firstly approved by the FDA for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukaemia or small lymphocytic lymphoma [4]. Palonosetron is a key component of Akynzeo®, used for the prevention of acute and delayed nausea and vomiting of cancer patients who are receiving chemotherapy [5]. As an active compound, PF-06821497 showed potent tumor growth inhibition in mouse xenograft models [6]. CRA-680 was efficacious in both a house dust mouse model of allergic lung inflammation and a guinea pig allergen challenge model of lung inflammation [7]. In addition, isoquinolinone compounds not only prevent and control plant diseases but also have some herbicidal activity. Compound I showed good inhibitory activity against Sclerotinia sclerotiorum on detached oilseed rape leaves [8], and compound II has excellent herbicidal activity against dicot weeds, such as Zinnia elegans Jacq. and Abutilon theophrasti Medicus (Figure 1) [9]. Therefore, in recent years, isoquinolinone derivatives have attracted considerable attention, and successful synthetic methods involving the isoquinolinone framework have been reported.
Figure 1: Selected natural products, pharmaceuticals, and biologically active compounds having an isoquinolinone scaffold.
Figure 1: Selected natural products, pharmaceuticals, and biologically active compounds having an isoquinolin...
A number of appealing methods for the synthesis of isoquinolinone scaffolds using transition metal reagents, including cobalt [10], copper [11], rhodium [12-14], palladium [15-17], silver [18], and gold [19] catalysts, have been reported. However, compared to the widespread use of metal catalysts, the synthesis of isoquinolinone scaffolds mediated by environmentally friendly nonmetallic reagents as an attractive alternative is less developed. In 2014, Antonchick and Manna firstly reported the synthesis of a series of 3,4-diaryl-substituted isoquinolinone derivatives through oxidative annulation between alkynes and benzamide derivatives using iodobenzene as a catalyst and peracetic acid as a terminal oxidant [20]. Recently, Kočovský et al. disclosed a method employing 2-methylbenzamide and benzonitrile to yield 3-aryl-substituted isoquinolinone derivatives in the presence of n-butyllithium [21]. On the other hand, the intramolecular oxidative cyclization is also a viable option for the preparation of isoquinolinone derivatives. In 2020, two reports have been published on the conversion of alkyne-tethered N-alkoxybenzamides to isoquinolinones by intramolecular oxidative annulation, either electrochemically or using the hypervalent iodine reagent phenyliodine(III) diacetate (PIDA) [22,23]. And more recently, Du and our group have developed a method for the chemoselective cycloisomerization of o-alkenylbenzamides to 3-arylisoquinolinones, using PhIO as oxidant in combination with a catalytic amount of trimethylsilyl trifluoromethanesulfonate [24]. Although considerable progress has been made in the synthesis of isoquinolinone derivatives, there is still the need to develop chemoselective strategies based on easily adjustable factors, such as solvent selection to obtain 3- or 4-substituted isoquinolinone derivatives.
In 2018, our group has reported the zwitterionic water-soluble hypervalent iodine reagent (phenyliodonio)sulfamate (PISA). In water, PISA is strongly acidic, and the pH value can reach 2.05 in a saturated aqueous solution. With PISA, various indoles have been synthesized via C–H amination of 2-alkenylanilines involving an aryl migration–intramolecular cyclization cascade with excellent chemoselectivity in aqueous CH3CN [25]. Herein, as part of our continuing studies of heterocyclic scaffold synthesis mediated by hypervalent iodine reagents, we present the solvent-dependent chemoselective synthesis of a series of isoquinolinones mediated by PISA using 2-alkenylbenzamide derivatives as substrates (Scheme 1).
Scheme 1: Chemoselective and PISA-mediated, solvent-controlled synthesis of different isoquinolinone derivatives 2 and 3.
Scheme 1: Chemoselective and PISA-mediated, solvent-controlled synthesis of different isoquinolinone derivati...
Results and Discussion
We began by exploring the reaction of N-methoxy-2-(prop-1-en-2-yl)benzamide (1a) with PISA (1.5 equiv) in anhydrous acetonitrile at room temperature under argon atmosphere. 4-Methylisoquinolinone 2a was the sole product in the reaction, with a yield of 86% in 20 minutes (Table 1, entry 1). Encouraged by this result, we added additives to the reaction with the aim of further increasing the chemical yield of 2a. When 1.5 equivalents of water were added to the reaction, the yield of 2a dropped to 79% (Table 1, entry 2). The reduced yield of 2a indicated that this reaction could benefit from a dry solvent. Therefore, 4 Å molecular sieves or anhydrous sodium sulfate were added to the reaction mixture. When 4 Å molecular sieves were added, the yield of 2a slightly increased to 88%, which was superior to using Na2SO4 (Table 1, entries 3 and 4). Next, different commercially available iodanes were employed as oxidants, such as PIDA, phenyliodine(III) bis(trifluoroacetate) (PIFA), N-tosyliminobenzyliodinane (PhINTs), iodosylbenzene (PhIO), and Koser’s reagent (HTIB) (Table 1, entries 5–9). Of the reagents tested, PISA gave the best result. Furthermore, screening of different solvents showed that acetonitrile was superior for this reaction (Table S1, Supporting Information File 1). Based on the screening results, the optimized reaction conditions for the conversion of 1a to the 4-subsitituted isoquinolinone 2a were as follows: 1.5 equivalents of PISA and 4 Å MS in anhydrous CH3CN (0.1 M of 2a) under argon atmosphere at room temperature for 20 min.
Table 1: Optimization of the reaction conditions for the synthesis of 4-substituted isoquinolinone 2aa.
|
|
|||
| entry | iodane | additive | yield of 2a, %b |
| 1 | PISA | — | 86 |
| 2 | PISA | H2O (1.5 equiv) | 79 |
| 3 | PISA | 4 Å MS | 88 |
| 4 | PISA | Na2SO4 | 81 |
| 5 | PIDA | 4 Å MS | 52 |
| 6 | PIFA | 4 Å MS | 77 |
| 7 | PhINTs | 4 Å MS | 61 |
| 8 | PhIO | 4 Å MS | 0 |
| 9 | HTIB | 4 Å MS | 52 |
aReactions were carried out using 1a (0.2 mmol), hypervalent iodine reagent (1.5 equiv), and 4 Å MS (7.6 mg) in MeCN (2.0 mL) at room temperature under argon atmosphere. bIsolated yield.
With the optimal reaction conditions in hand, we explored the scope of the method by testing various 2-alkenylbenzamide derivatives 1 (Scheme 2). When R1 was ethyl, isopropyl, cyclopropyl, phenyl, or hydrogen, respectively, the intramolecular amination smoothly gave the corresponding 4-substituted isoquinolinone products 2b,c,d–f in 51–94% yield. Notably, when 1c was used as the substrate, the cycloisomerization product 2c' was observed in 31% yield besides 2c in 51% yield. Additional experiments were then carried out using N-methoxy-2-(prop-1-en-2-yl)benzamide with different substituents R2. Both electron-donating (methyl, alkoxy, dimethylamino) and electron-withdrawing substituents (fluoro, chloro, trifluoromethyl) were well tolerated on the phenyl ring and gave the desired products 2g–n in 68–95% yield. Furthermore, a substrate containing a naphthalene moiety was also compatible with the reaction conditions, giving the corresponding ring-fused product 2o in 40% yield. It is worth noting that when the N-substituent was phenyl, benzyloxy, or phenylamino, the reaction still proceeded well, and the corresponding products 2p,q,r were obtained in 69%, 41%, and 40% yield, respectively.
Scheme 2: Substrate scope for the synthesis of 4-substituted isoquinolinones 2. Reaction conditions: 1 (0.3 mmol), PISA (1.5 equiv), and 4 Å MS (10 mg) in MeCN (3.0 mL).
Scheme 2: Substrate scope for the synthesis of 4-substituted isoquinolinones 2. Reaction conditions: 1 (0.3 m...
Interestingly, when screening solvents for the synthesis of 4-methylisoquinolinones, we were surprised to discover that when hexafluoro-2-propanol (HFIP) was used as the solvent, 3-methylisoquinolinone 3a, an isomer of 2a, was formed in 51% yield. Apparently, the change of solvent resulted in a different chemoselectivity of the reaction. With this in mind, we investigated the reaction conditions (see Supporting Information File 1 for details) and obtained the optimal conditions for the synthesis of 3-methylisoquinolinone as follows: reacting 1.1 equivalents of PISA in HFIP (0.1 M of 1a) containing 2.5 equivalents of H2O at room temperature for 20 minutes (Scheme 3).
Scheme 3: Optimal reaction conditions for the synthesis of 3-substituted isoquinolinone 3a.
Scheme 3: Optimal reaction conditions for the synthesis of 3-substituted isoquinolinone 3a.
The general applicability of PISA in wet HFIP solvent was studied. When R1 was ethyl, isopropyl, cyclopropyl, or hydrogen, respectively, the substrates could be successfully converted to the products 3b–d and 2f in 52–87% yield with this method. In addition, a good or high yield of 3-methylisoquinolinones 3e–k, with different substituents on the phenyl ring, was also obtained. It is worth noting that when an electron-withdrawing group (trifluoromethyl, fluoro, chloro) was located on the phenyl ring, various amounts of 4-substituted isoquinolinone derivatives 2k,m,n were observed along with the formation of 3h,j,k, respectively. Furthermore, a substrate containing a naphthalene unit was also compatible with the reaction conditions, leading to 3l. In particular, the presence of diverse nitrogen protecting groups, such as benzyloxy, phenyl, and alkyl, did not affect the smooth reaction, affording 3m–o in a moderate yield of 32–64% (Scheme 4). Just by changing the solvent of the reaction, we were able to obtain the isomeric 3- or 4-substituted isoquinolinone derivatives with excellent chemoselectivity. These interesting findings led us to investigate the reaction mechanism.
Scheme 4: Substrate scope for the synthesis of 3-substituted isoquinolinones 3. Reaction conditions: 1 (0.3 mmol), PISA (1.1 equiv), and H2O (2.5 equiv) in HFIP (3.0 mL) at room temperature. Isolated yield is stated. aThe yield of 2k was 56%. bThe yield of 2m was 24%. cThe yield of 2n was 11%.
Scheme 4: Substrate scope for the synthesis of 3-substituted isoquinolinones 3. Reaction conditions: 1 (0.3 m...
To gain insight into the mechanism and chemoselectivity of the reactions above, we performed a control experiment. With acetonitrile as the solvent, a radical clock experiment was carried out with 1d under the optimal reaction conditions, resulting in the formation of 2d in 54% yield, and no cyclopropyl ring opening products were observed. This result suggested that no radical intermediates were generated during the reaction (Scheme 5).
Scheme 5: Control experiment to test for radical intermediates.
Scheme 5: Control experiment to test for radical intermediates.
According to the aforementioned control experiment and literature precedents, we proposed a mechanism for the formation of 4-substituted isoquinolinone derivatives, including 2a. The reaction begins by tautomerization of 1a, and PISA undergoes an electrophilic reaction with 1a' to form the iodane intermediate A. The iodane A then undergoes a proton shift to provide intermediate B. Intermediate B collapses via reductive elimination to give nitrenium ion C, along with the release of iodobenzene and sulfamate. Finally, nucleophilic attack of the olefin moiety of C on the electrophilic nitrogen atom, followed by the deprotonation with sulfamate, gives the 4-substituted isoquinolinone derivative 2a (Scheme 6).
Scheme 6: Proposed mechanism for the reaction between 1a and PISA in anhydrous acetonitrile.
Scheme 6: Proposed mechanism for the reaction between 1a and PISA in anhydrous acetonitrile.
Looking into the formation of 2c' from 1c (Scheme 2), two other resonance structures for the initially formed intermediate 1CC, namely 1CC' and 1CC'', are shown in Scheme 7. The oxygen atom in the amide motif of the substrate 1c may act as an electrophilic center, forming a C–O bond with the alkenyl group to give the isochromen-1-one oxime product 2c'.
Scheme 7: Two other resonance structures of the intermediate 1CC.
Scheme 7: Two other resonance structures of the intermediate 1CC.
When wet HFIP was used as the solvent, the reaction followed a different pathway. HFIP, a strong hydrogen bonding donor [26-28], interacts with the amide moiety of the substrate, and thus preventing the possible interaction between the amide moiety and PISA, as opposed to CH3CN. The olefin moiety of the complex then interacts with the exposed central iodine(III) atom in PISA [25], forming the intermediate D. Similar cyclic iodonium intermediates were also postulated for the synthesis of benzofuran derivatives from styrene derivatives by iodane reagents [29,30]. Subsequently, intermediate D is attacked by H2O at the benzylic carbon atom to afford intermediate E. Intramolecular proton shift occurs, generating the intermediate F, which undergoes phenyl migration and reductive elimination, along with the release of iodobenzene and sulfamic acid. Cyclization of protonated G takes place to afford the intermediate H. Finally, release of water and β-proton elimination produces the rearranged product 3a (Scheme 8).
Scheme 8: Proposed mechanism for the reaction between 1a and PISA in wet HFIP.
Scheme 8: Proposed mechanism for the reaction between 1a and PISA in wet HFIP.
Conclusion
In summary, we reported the efficient synthesis of isoquinolinone derivatives using a PISA-mediated methodology that chemoselectively yielded 3- or 4-substituted isoquinolinone derivatives by simply adjusting the solvent. When acetonitrile was used, the 4-substituted isoquinolinone derivatives were the reaction products, whereas hexafluoro-2-propanol led to 3-substituted isoquinolinones. The solvent-dependent chemoselective synthesis of isoquinolinone derivatives is interesting and unprecedented. Further research on synthetic utility of PISA, a unique zwitterionic hypervalent iodine(III) reagent, is underway in our laboratory.
Supporting Information
| Supporting Information File 1: Experimental details, optimization studies, compound characterization data, and spectra. | ||
| Format: PDF | Size: 6.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Rao, L. B.; Sreenivasulu, C.; Kishore, D. R.; Satyanarayana, G. Tetrahedron 2022, 127, 133093. doi:10.1016/j.tet.2022.133093
Return to citation in text: [1] -
Asseri, A. H.; Alam, M. J.; Alzahrani, F.; Khames, A.; Pathan, M. T.; Abourehab, M. A. S.; Hosawi, S.; Ahmed, R.; Sultana, S. A.; Alam, N. F.; Alam, N.-U.; Alam, R.; Samad, A.; Pokhrel, S.; Kim, J. K.; Ahammad, F.; Kim, B.; Tan, S. C. Pharmaceuticals 2022, 15, 501. doi:10.3390/ph15050501
Return to citation in text: [1] -
Nishiyama, T.; Hironaka, M.; Taketomi, M.; Taguchi, E.; Kotouge, R.; Shigemori, Y.; Hatae, N.; Ishikura, M.; Choshi, T. Eur. J. Org. Chem. 2018, 673–678. doi:10.1002/ejoc.201701557
Return to citation in text: [1] -
Blair, H. A. Drugs 2018, 78, 1847–1853. doi:10.1007/s40265-018-1013-4
Return to citation in text: [1] -
Gao, A.; Guan, S.; Sun, Y.; Wang, L.; Meng, F.; Liu, X.; Gu, L.; Li, G.; Zhong, D.; Zhang, L. BMC Cancer 2023, 23, 609. doi:10.1186/s12885-023-11070-3
Return to citation in text: [1] -
Kung, P.-P.; Bingham, P.; Brooun, A.; Collins, M.; Deng, Y.-L.; Dinh, D.; Fan, C.; Gajiwala, K. S.; Grantner, R.; Gukasyan, H. J.; Hu, W.; Huang, B.; Kania, R.; Kephart, S. E.; Krivacic, C.; Kumpf, R. A.; Khamphavong, P.; Kraus, M.; Liu, W.; Maegley, K. A.; Nguyen, L.; Ren, S.; Richter, D.; Rollins, R. A.; Sach, N.; Sharma, S.; Sherrill, J.; Spangler, J.; Stewart, A. E.; Sutton, S.; Uryu, S.; Verhelle, D.; Wang, H.; Wang, S.; Wythes, M.; Xin, S.; Yamazaki, S.; Zhu, H.; Zhu, J.; Zehnder, L.; Edwards, M. J. Med. Chem. 2018, 61, 650–665. doi:10.1021/acs.jmedchem.7b01375
Return to citation in text: [1] -
Kaila, N.; Follows, B.; Leung, L.; Thomason, J.; Huang, A.; Moretto, A.; Janz, K.; Lowe, M.; Mansour, T. S.; Hubeau, C.; Page, K.; Morgan, P.; Fish, S.; Xu, X.; Williams, C.; Saiah, E. J. Med. Chem. 2014, 57, 1299–1322. doi:10.1021/jm401509e
Return to citation in text: [1] -
Li, M.; Yuan, C.; Fang, Y.; Zhang, Z.; Wang, D. Chin. J. Pestic. Sci. 2023, 25, 62–72. doi:10.16801/j.issn.1008-7303.2022.0102
Return to citation in text: [1] -
Li, B.; Wu, H.; Cui, D.; Yu, H.; Xu, J.; Yang, H. Isoquinolinone compounds and their applications. Chin. Patent CN1687061, Oct 26, 2005.
Return to citation in text: [1] -
Li, X.; Huang, T.; Song, Y.; Qi, Y.; Li, L.; Li, Y.; Xiao, Q.; Zhang, Y. Org. Lett. 2020, 22, 5925–5930. doi:10.1021/acs.orglett.0c02016
Return to citation in text: [1] -
Zhao, S.; Gong, X.; Gan, Z.; Yan, Q.; Liu, X.; Yang, D. Chin. J. Org. Chem. 2021, 41, 258–266. doi:10.6023/cjoc202008045
Return to citation in text: [1] -
Bian, M.; Mawjuda, H.; Gao, H.; Xu, H.; Zhou, Z.; Yi, W. Org. Lett. 2020, 22, 9677–9682. doi:10.1021/acs.orglett.0c03734
Return to citation in text: [1] -
Mochida, S.; Umeda, N.; Hirano, K.; Satoh, T.; Miura, M. Chem. Lett. 2010, 39, 744–746. doi:10.1246/cl.2010.744
Return to citation in text: [1] -
Huang, J.-R.; Bolm, C. Angew. Chem., Int. Ed. 2017, 56, 15921–15925. doi:10.1002/anie.201710776
Return to citation in text: [1] -
Zhong, R.; Xu, Y.; Sun, M.; Wang, Y. J. Org. Chem. 2021, 86, 5255–5264. doi:10.1021/acs.joc.1c00150
Return to citation in text: [1] -
Zhong, H.; Yang, D.; Wang, S.; Huang, J. Chem. Commun. 2012, 48, 3236–3238. doi:10.1039/c2cc17859a
Return to citation in text: [1] -
Zheng, Z.; Alper, H. Org. Lett. 2008, 10, 4903–4906. doi:10.1021/ol801991m
Return to citation in text: [1] -
Dell’Acqua, M.; Castano, B.; Cecchini, C.; Pedrazzini, T.; Pirovano, V.; Rossi, E.; Caselli, A.; Abbiati, G. J. Org. Chem. 2014, 79, 3494–3505. doi:10.1021/jo5002559
Return to citation in text: [1] -
Wang, A.; Xie, X.; Zhang, C.; Liu, Y. Chem. Commun. 2020, 56, 15581–15584. doi:10.1039/d0cc06875f
Return to citation in text: [1] -
Manna, S.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 7324–7327. doi:10.1002/anie.201404222
Return to citation in text: [1] -
Matouš, P.; Májek, M.; Kysilka, O.; Kuneš, J.; Maříková, J.; Růžička, A.; Pour, M.; Kočovský, P. J. Org. Chem. 2021, 86, 8078–8088. doi:10.1021/acs.joc.1c00561
Return to citation in text: [1] -
Zhang, L.-B.; Geng, R.-S.; Wang, Z.-C.; Ren, G.-Y.; Wen, L.-R.; Li, M. Green Chem. 2020, 22, 16–21. doi:10.1039/c9gc03290h
Return to citation in text: [1] -
Wen, L.-R.; Ren, G.-Y.; Geng, R.-S.; Zhang, L.-B.; Li, M. Org. Biomol. Chem. 2020, 18, 225–229. doi:10.1039/c9ob02430a
Return to citation in text: [1] -
He, J.; Du, F.-H.; Zhang, C.; Du, Y. Commun. Chem. 2023, 6, 126. doi:10.1038/s42004-023-00930-5
Return to citation in text: [1] -
Xia, H.-D.; Zhang, Y.-D.; Wang, Y.-H.; Zhang, C. Org. Lett. 2018, 20, 4052–4056. doi:10.1021/acs.orglett.8b01615
Return to citation in text: [1] [2] -
An, X.-D.; Xiao, J. Chem. Rec. 2020, 20, 142–161. doi:10.1002/tcr.201900020
Return to citation in text: [1] -
Tian, F.-X.; Qu, J. J. Org. Chem. 2022, 87, 1814–1829. doi:10.1021/acs.joc.1c02361
Return to citation in text: [1] -
Cheng, Y.-X.; Yang, X.-G.; Du, F.-H.; Zhang, C. Green Chem. 2024, 26, 5914–5920. doi:10.1039/d4gc00622d
Return to citation in text: [1] -
Mangaonkar, S. R.; Shetgaonkar, S. E.; Vernekar, A. A.; Singh, F. V. ChemistrySelect 2020, 5, 10754–10758. doi:10.1002/slct.202002860
Return to citation in text: [1] -
Singh, F. V.; Mangaonkar, S. R. Synthesis 2018, 50, 4940–4948. doi:10.1055/s-0037-1610650
Return to citation in text: [1]
| 24. | He, J.; Du, F.-H.; Zhang, C.; Du, Y. Commun. Chem. 2023, 6, 126. doi:10.1038/s42004-023-00930-5 |
| 21. | Matouš, P.; Májek, M.; Kysilka, O.; Kuneš, J.; Maříková, J.; Růžička, A.; Pour, M.; Kočovský, P. J. Org. Chem. 2021, 86, 8078–8088. doi:10.1021/acs.joc.1c00561 |
| 22. | Zhang, L.-B.; Geng, R.-S.; Wang, Z.-C.; Ren, G.-Y.; Wen, L.-R.; Li, M. Green Chem. 2020, 22, 16–21. doi:10.1039/c9gc03290h |
| 23. | Wen, L.-R.; Ren, G.-Y.; Geng, R.-S.; Zhang, L.-B.; Li, M. Org. Biomol. Chem. 2020, 18, 225–229. doi:10.1039/c9ob02430a |
| 1. | Rao, L. B.; Sreenivasulu, C.; Kishore, D. R.; Satyanarayana, G. Tetrahedron 2022, 127, 133093. doi:10.1016/j.tet.2022.133093 |
| 5. | Gao, A.; Guan, S.; Sun, Y.; Wang, L.; Meng, F.; Liu, X.; Gu, L.; Li, G.; Zhong, D.; Zhang, L. BMC Cancer 2023, 23, 609. doi:10.1186/s12885-023-11070-3 |
| 19. | Wang, A.; Xie, X.; Zhang, C.; Liu, Y. Chem. Commun. 2020, 56, 15581–15584. doi:10.1039/d0cc06875f |
| 20. | Manna, S.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 7324–7327. doi:10.1002/anie.201404222 |
| 3. | Nishiyama, T.; Hironaka, M.; Taketomi, M.; Taguchi, E.; Kotouge, R.; Shigemori, Y.; Hatae, N.; Ishikura, M.; Choshi, T. Eur. J. Org. Chem. 2018, 673–678. doi:10.1002/ejoc.201701557 |
| 15. | Zhong, R.; Xu, Y.; Sun, M.; Wang, Y. J. Org. Chem. 2021, 86, 5255–5264. doi:10.1021/acs.joc.1c00150 |
| 16. | Zhong, H.; Yang, D.; Wang, S.; Huang, J. Chem. Commun. 2012, 48, 3236–3238. doi:10.1039/c2cc17859a |
| 17. | Zheng, Z.; Alper, H. Org. Lett. 2008, 10, 4903–4906. doi:10.1021/ol801991m |
| 2. | Asseri, A. H.; Alam, M. J.; Alzahrani, F.; Khames, A.; Pathan, M. T.; Abourehab, M. A. S.; Hosawi, S.; Ahmed, R.; Sultana, S. A.; Alam, N. F.; Alam, N.-U.; Alam, R.; Samad, A.; Pokhrel, S.; Kim, J. K.; Ahammad, F.; Kim, B.; Tan, S. C. Pharmaceuticals 2022, 15, 501. doi:10.3390/ph15050501 |
| 18. | Dell’Acqua, M.; Castano, B.; Cecchini, C.; Pedrazzini, T.; Pirovano, V.; Rossi, E.; Caselli, A.; Abbiati, G. J. Org. Chem. 2014, 79, 3494–3505. doi:10.1021/jo5002559 |
| 9. | Li, B.; Wu, H.; Cui, D.; Yu, H.; Xu, J.; Yang, H. Isoquinolinone compounds and their applications. Chin. Patent CN1687061, Oct 26, 2005. |
| 11. | Zhao, S.; Gong, X.; Gan, Z.; Yan, Q.; Liu, X.; Yang, D. Chin. J. Org. Chem. 2021, 41, 258–266. doi:10.6023/cjoc202008045 |
| 25. | Xia, H.-D.; Zhang, Y.-D.; Wang, Y.-H.; Zhang, C. Org. Lett. 2018, 20, 4052–4056. doi:10.1021/acs.orglett.8b01615 |
| 8. | Li, M.; Yuan, C.; Fang, Y.; Zhang, Z.; Wang, D. Chin. J. Pestic. Sci. 2023, 25, 62–72. doi:10.16801/j.issn.1008-7303.2022.0102 |
| 12. | Bian, M.; Mawjuda, H.; Gao, H.; Xu, H.; Zhou, Z.; Yi, W. Org. Lett. 2020, 22, 9677–9682. doi:10.1021/acs.orglett.0c03734 |
| 13. | Mochida, S.; Umeda, N.; Hirano, K.; Satoh, T.; Miura, M. Chem. Lett. 2010, 39, 744–746. doi:10.1246/cl.2010.744 |
| 14. | Huang, J.-R.; Bolm, C. Angew. Chem., Int. Ed. 2017, 56, 15921–15925. doi:10.1002/anie.201710776 |
| 29. | Mangaonkar, S. R.; Shetgaonkar, S. E.; Vernekar, A. A.; Singh, F. V. ChemistrySelect 2020, 5, 10754–10758. doi:10.1002/slct.202002860 |
| 30. | Singh, F. V.; Mangaonkar, S. R. Synthesis 2018, 50, 4940–4948. doi:10.1055/s-0037-1610650 |
| 7. | Kaila, N.; Follows, B.; Leung, L.; Thomason, J.; Huang, A.; Moretto, A.; Janz, K.; Lowe, M.; Mansour, T. S.; Hubeau, C.; Page, K.; Morgan, P.; Fish, S.; Xu, X.; Williams, C.; Saiah, E. J. Med. Chem. 2014, 57, 1299–1322. doi:10.1021/jm401509e |
| 25. | Xia, H.-D.; Zhang, Y.-D.; Wang, Y.-H.; Zhang, C. Org. Lett. 2018, 20, 4052–4056. doi:10.1021/acs.orglett.8b01615 |
| 6. | Kung, P.-P.; Bingham, P.; Brooun, A.; Collins, M.; Deng, Y.-L.; Dinh, D.; Fan, C.; Gajiwala, K. S.; Grantner, R.; Gukasyan, H. J.; Hu, W.; Huang, B.; Kania, R.; Kephart, S. E.; Krivacic, C.; Kumpf, R. A.; Khamphavong, P.; Kraus, M.; Liu, W.; Maegley, K. A.; Nguyen, L.; Ren, S.; Richter, D.; Rollins, R. A.; Sach, N.; Sharma, S.; Sherrill, J.; Spangler, J.; Stewart, A. E.; Sutton, S.; Uryu, S.; Verhelle, D.; Wang, H.; Wang, S.; Wythes, M.; Xin, S.; Yamazaki, S.; Zhu, H.; Zhu, J.; Zehnder, L.; Edwards, M. J. Med. Chem. 2018, 61, 650–665. doi:10.1021/acs.jmedchem.7b01375 |
| 10. | Li, X.; Huang, T.; Song, Y.; Qi, Y.; Li, L.; Li, Y.; Xiao, Q.; Zhang, Y. Org. Lett. 2020, 22, 5925–5930. doi:10.1021/acs.orglett.0c02016 |
| 26. | An, X.-D.; Xiao, J. Chem. Rec. 2020, 20, 142–161. doi:10.1002/tcr.201900020 |
| 27. | Tian, F.-X.; Qu, J. J. Org. Chem. 2022, 87, 1814–1829. doi:10.1021/acs.joc.1c02361 |
| 28. | Cheng, Y.-X.; Yang, X.-G.; Du, F.-H.; Zhang, C. Green Chem. 2024, 26, 5914–5920. doi:10.1039/d4gc00622d |
© 2024 Hu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.