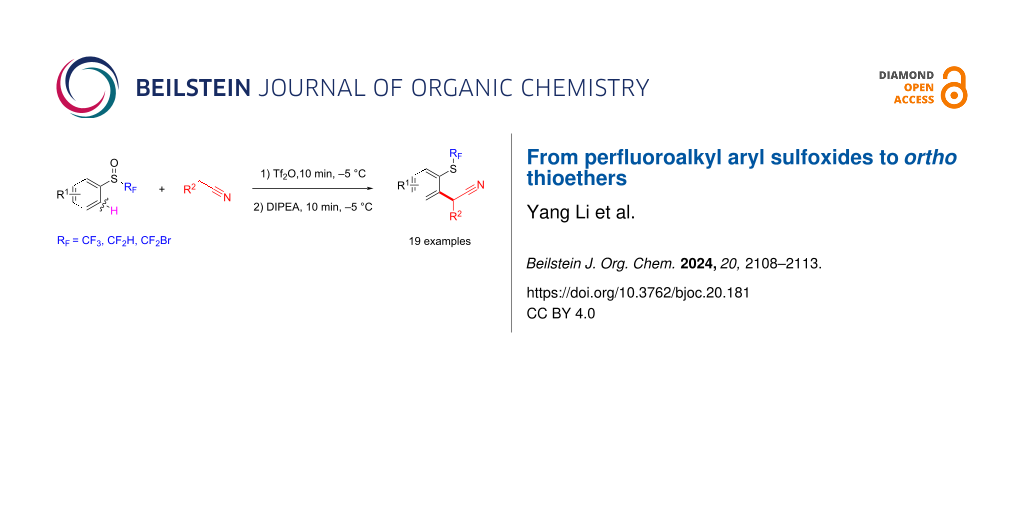
Abstract
Access to original ortho thioether derivatives was achieved through a [3,3]-rearrangement in a one-pot two-step protocol. Several aryl-SCF3 compounds are reported by variation of the nitrile or of the trifluoroalkyl sulfoxide starting material. The variation of the perfluoroalkyl chain was also possible.
Graphical Abstract
Introduction
Since decades, sigmatropic rearrangements have established themselves as robust and versatile tools for many transformations in organic synthesis [1-3]. They were widely employed with a wide range of substrates. With a peculiar type of scaffold, S-perfluoroalkyl aryl sulfoxides, in 2009, we were the first to demonstrate their ability to be engaged in such a rearrangement [4,5]. Upon activation with trifluoromethanesulfonic anhydride and under heating, we showed their transformation to ortho thioethers with a fairly acceptable selectivity towards the pathway of sulfilimine synthesis (Scheme 1b). Following our seminal study, many research groups described a strategy for ortho-C–H functionalization of aryl sulfoxides with various nucleophiles via a cascade reaction of interrupted Pummerer reaction/sigmatropic rearrangement (Scheme 1a) [6-11]. A large range of nucleophiles, such as phenols [12-16], anilines [17], carbonyls [18-21], propargyl/allylsilanes [22-34], ynamides [35-37], and alkyl nitriles [38-40], were found to be suitable for this process. Whereas the addition of fluorine atoms to molecules is a well-established strategy to improve or modulate their physicochemical and biological properties [41-45], only few publications have reported a [3,3]-rearrangement with fluorinated molecules (Scheme 1c). In 2020, Wang and co-workers have developed a one-pot [3,3]-sigmatropic rearrangement/Haller–Bauer reaction of aryl sulfoxides with difluoroenoxysilanes as nucleophile under mild reaction conditions [46]. This provided access to organosulfur compounds ortho-functionalized by CF2H. At the same time Peng and co-workers described the dearomatization of aryl sulfoxides using the same difluoroenol silyl ether with trifluoromethanesulfonic anhydride, allowing the incorporation of two difluoroalkyl groups [47]. By blocking the rearomatization after the [3,3]-rearrangement, external nucleophiles could be trapped to give mono-difluoroalkylated cycles. More recently, in 2019, Peng’s group reported also the ortho-cyanoalkylation of benzoyl or ester group-substituted fluoroalkyl aryl sulfoxides with various alkyl nitriles in two steps [48]. The addition of a base in the second step easily enabled the [3,3]-rearrangement, allowing for the addition of two functional groups – the cyano group and difluoromethylthio group – to arenes in good yield.
Scheme 1: [3,3]-Rearrangement of aryl sulfoxides.
Scheme 1: [3,3]-Rearrangement of aryl sulfoxides.
Inspired and stimulated by this abundant literature, and as part of our research program focused on creating novel perfluoroalkylsulfur derivatives, we became interested in a reappraisal of our previous study with the aim of increasing its scope as well as the yield and selectivity (Scheme 1d). It is important to mention that during the preparation of this paper, a similar study appeared. Peng and co-workers demonstrated the efficient use of acetonitrile as nucleophile with various aryl difluoroalkyl sulfoxides but only one example of an SCF3 compound was reported [49].
Results and Discussion
We started our optimization with the reaction between acetonitrile and phenyl trifluoromethyl sulfoxide (1a, Table 1). We firstly chose the same stoichiometry as described in our previous study and tried to reduce the reaction time by the help of microwave heating (Table 1, entry 1). Under these conditions, a significant amount of degradation products was observed and the yield was rather low. The same result was obtained when the reagent was first added at 0 °C and then heated for one hour under microwave irradiation (Table 1, entry 2). To avoid degradation, the temperature was reduced while the reaction time was increased with twice the number of equivalents of acetonitrile (−15 °C to rt, for 12 hours, entry 3 in Table 1) without any significant improvement in the yield. As previously reported, the use of an organic base can improve the yield of this reaction [26,38,40,48]. Therefore, we decided to use 2 equivalents of DIPEA at low temperature. After ten minutes at −15 °C to allow for the reaction between phenyl trifluoromethyl sulfoxide (1a) and acetonitrile, the base was added and the reaction was stirred for the same amount of time. To our delight, a good NMR yield of 74% was received under these conditions (Table 1, entry 4). The importance of the temperature was then evaluated (Table 1, entries 5–7). A too low value was deleterious to the yield, whereas −5 °C appeared as the conditions of choice. Finally, by adjusting to 5 equivalents of nitrile and base, resulted in the optimal conditions (Table 1, entry 9). Other organic nitrogenous bases were tested (Table 1, entries 10–12). Et3N gave nearly the same result, while DBU seemed less efficient. The use of the inorganic base K2CO3 resulted in poor outcomes.
Table 1: Optimization of the reaction conditions.
|
|
||||||
| Entry | T (°C) | t | x | base | y | NMR yield (%)a,b |
| 1 | 50 °C (MW) | 1 h | 1.5 | – | 21 | |
| 2 | 0 to 50 °C (MW) | 1 h | 1.5 | – | 21 | |
| 3 | −15 to 20 °C | 12 h | 3 | – | 38 | |
| 4 | −15 °C | 10 min | 3 | DIPEA | 2 | 74 |
| 5 | −30 °C | 10 min | 3 | DIPEA | 2 | 41 |
| 6 | −5 °C | 10 min | 3 | DIPEA | 2 | 77 |
| 7 | 0 °C | 10 min | 3 | DIPEA | 2 | 75 |
| 8 | −5 °C | 10 min | 5 | DIPEA | 2.5 | 80 |
| 9 | −5 °C | 10 min | 5 | DIPEA | 5 | 95 (79) |
| 10 | −5 °C | 10 min | 5 | Et3N | 5 | 85 |
| 11 | −5 °C | 10 min | 5 | DBU | 5 | 48 |
| 12 | −5 °C | 10 min | 5 | K2CO3 | 5 | 2 |
aExperimental conditions: 1a (0.5 mmol), Tf2O (1.5 equiv), T (°C), t (min or h), then addition of base (y equiv) at the same temperature and time as the first step (T, t). b19F NMR spectroscopic yields, isolated yields in parentheses.
With the optimized conditions in hand, a scale-up was successfully performed, resulting in the production of 1.88 g of product 2a corresponding to 84% yield (Scheme 2). The reaction with other aryl sulfoxides was then investigated. We observed that the rearrangement product was isolated in good yield (2b–d) when the sulfoxide is para-substituted whereas the meta or difunctionalization led to lower yields (2e,f). The product of rearrangement 2a was oxidized into the sulfoxide and re-engaged under the optimized conditions to afford the compound of bis-rearrangement 2g in a good yield of 49%. This compound is then the result of an iterative rearrangement. Difluorinated sulfoxides 1h–j proved also efficient for this rearrangement giving rise to the corresponding thioethers 2h–j in good NMR yields and lower isolated yield in the case of the more volatile adduct 2i. Finally, trifluoromethyl selenoxide 1k was tested as a substrate, and the rearranged product was obtained in a low yield of 15%. The main product obtained was phenyl(trifluoromethyl)selane, a reduction product of the selenoxide. Despite a low yield, this result is encouraging because it is the first example of rearrangement with aryl trifluoromethyl selenoxide.
Scheme 2: The scope of aryl perfluoromethyl sulfoxides and a selenoxide.
Scheme 2: The scope of aryl perfluoromethyl sulfoxides and a selenoxide.
We further investigated the generality of the reaction using a series of nitriles with the sulfoxide 1a (Scheme 3). We noticed that the length of the alkyl chain has no impact on the yield (3a,b). However, the use of benzyl cyanide is completely deleterious for the reaction as no product was observed (3c). The presence of a chlorine atom at the alpha-position of the nitrile is also detrimental to the reaction, resulting in less than 30% yield of the desired product 3d. Nevertheless, the reaction is compatible with halogens elsewhere in longer nitrile alkyl chains (3e,g). Finally, it was possible to obtain the terminal alkene 3f with a yield of 58% using hex-5-enenitrile.
Conclusion
In summary, fine-tuning of the experimental conditions gave us access to original ortho-cyanoalkylated aryl perfluoroalkylsulfur derivatives. We have also shown that structural diversity is possible by varying the substituents on the aromatic ring, the perfluoroalkyl chain, and the alkyl chain linking the cyano functional groups. The [3,3]-sigmatropic rearrangement of perfluoroalkyl selenoxides needs to be optimized to improve the yield and decrease the amount of reduction product. The complete evaluation of the potential of these new compounds will be provided in the future.
Experimental
General procedure for the rearrangement process
Sulfoxide (0.5 mmol, 1 equiv), nitrile (5 equiv) and Tf2O (1.5 equiv) were added in the described order to a 5 mL flask under an argon atmosphere, maintained at −5 °C. The reaction mixture was stirred for 10 min, then DIPEA (5 equiv) was slowly added to the flask with a syringe and the reaction was stirred for another 10 min. At the end of the reaction, 1 mL of chloroform and a known amount of trifluoromethoxybenzene were added to the flask in order to determine the 19F NMR yield. To purify the product, the reaction mixture was mixed with a sufficient volume of a saturated NH4Cl solution, then extracted 3 times with diethyl ether. The combined organic layers were dried over MgSO4, filtered, concentrated under reduced pressure, and purified by preparative TLC or flash chromatography.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data of all isolated products as well as copies of NMR spectra for novel compounds. | ||
| Format: PDF | Size: 6.6 MB | Download |
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Hiersemann, M.; Nubbemeyer, U., Eds. The Claisen Rearrangement: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2007. doi:10.1002/9783527610549
Return to citation in text: [1] -
Martín Castro, A. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u
Return to citation in text: [1] -
Ito, H.; Taguchi, T. Chem. Soc. Rev. 1999, 28, 43–50. doi:10.1039/a706415b
Return to citation in text: [1] -
Macé, Y.; Urban, C.; Pradet, C.; Blazejewski, J.-C.; Magnier, E. Eur. J. Org. Chem. 2009, 5313–5316. doi:10.1002/ejoc.200900873
Return to citation in text: [1] -
Pégot, B.; Urban, C.; Diter, P.; Magnier, E. Eur. J. Org. Chem. 2013, 7800–7808. doi:10.1002/ejoc.201301017
Return to citation in text: [1] -
Zhang, L.; Hu, M.; Peng, B. Synlett 2019, 30, 2203–2208. doi:10.1055/s-0039-1690212
Return to citation in text: [1] -
Yorimitsu, H. Chem. Rec. 2017, 17, 1156–1167. doi:10.1002/tcr.201700017
Return to citation in text: [1] -
Yanagi, T.; Nogi, K.; Yorimitsu, H. Tetrahedron Lett. 2018, 59, 2951–2959. doi:10.1016/j.tetlet.2018.06.055
Return to citation in text: [1] -
Pulis, A. P.; Procter, D. J. Angew. Chem., Int. Ed. 2016, 55, 9842–9860. doi:10.1002/anie.201601540
Return to citation in text: [1] -
Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111
Return to citation in text: [1] -
Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J. Angew. Chem., Int. Ed. 2010, 49, 5832–5844. doi:10.1002/anie.201000517
Return to citation in text: [1] -
Yanagi, T.; Otsuka, S.; Kasuga, Y.; Fujimoto, K.; Murakami, K.; Nogi, K.; Yorimitsu, H.; Osuka, A. J. Am. Chem. Soc. 2016, 138, 14582–14585. doi:10.1021/jacs.6b10278
Return to citation in text: [1] -
Murakami, K.; Yorimitsu, H.; Osuka, A. Angew. Chem., Int. Ed. 2014, 53, 7510–7513. doi:10.1002/anie.201403288
Return to citation in text: [1] -
Shrives, H. J.; Fernández-Salas, J. A.; Hedtke, C.; Pulis, A. P.; Procter, D. J. Nat. Commun. 2017, 8, 14801. doi:10.1038/ncomms14801
Return to citation in text: [1] -
Okamoto, K.; Hori, M.; Yanagi, T.; Murakami, K.; Nogi, K.; Yorimitsu, H. Angew. Chem., Int. Ed. 2018, 57, 14230–14234. doi:10.1002/anie.201809035
Return to citation in text: [1] -
Kobatake, T.; Fujino, D.; Yoshida, S.; Yorimitsu, H.; Oshima, K. J. Am. Chem. Soc. 2010, 132, 11838–11840. doi:10.1021/ja1030134
Return to citation in text: [1] -
Yanagi, T.; Nogi, K.; Yorimitsu, H. Chem. – Eur. J. 2020, 26, 783–787. doi:10.1002/chem.201903570
Return to citation in text: [1] -
Peng, B.; Geerdink, D.; Farès, C.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 5462–5466. doi:10.1002/anie.201402229
Return to citation in text: [1] -
Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510–8513. doi:10.1021/ja2031882
Return to citation in text: [1] -
Huang, X.; Patil, M.; Farès, C.; Thiel, W.; Maulide, N. J. Am. Chem. Soc. 2013, 135, 7312–7323. doi:10.1021/ja4017683
Return to citation in text: [1] -
Meng, X.; Chen, D.; Cao, X.; Luo, J.; Wang, F.; Huang, S. Chem. Commun. 2019, 55, 12495–12498. doi:10.1039/c9cc06505a
Return to citation in text: [1] -
Kaiser, D.; Veiros, L. F.; Maulide, N. Chem. – Eur. J. 2016, 22, 4727–4732. doi:10.1002/chem.201600432
Return to citation in text: [1] -
Šiaučiulis, M.; Sapmaz, S.; Pulis, A. P.; Procter, D. J. Chem. Sci. 2018, 9, 754–759. doi:10.1039/c7sc04723a
Return to citation in text: [1] -
Akai, S.; Kawashita, N.; Satoh, H.; Wada, Y.; Kakiguchi, K.; Kuriwaki, I.; Kita, Y. Org. Lett. 2004, 6, 3793–3796. doi:10.1021/ol0484310
Return to citation in text: [1] -
Hu, L.; Gui, Q.; Chen, X.; Tan, Z.; Zhu, G. J. Org. Chem. 2016, 81, 4861–4868. doi:10.1021/acs.joc.6b00535
Return to citation in text: [1] -
Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J. J. Am. Chem. Soc. 2016, 138, 790–793. doi:10.1021/jacs.5b12579
Return to citation in text: [1] [2] -
Eberhart, A. J.; Procter, D. J. Angew. Chem., Int. Ed. 2013, 52, 4008–4011. doi:10.1002/anie.201300223
Return to citation in text: [1] -
Eberhart, A. J.; Imbriglio, J. E.; Procter, D. J. Org. Lett. 2011, 13, 5882–5885. doi:10.1021/ol2025197
Return to citation in text: [1] -
Eberhart, A. J.; Cicoira, C.; Procter, D. J. Org. Lett. 2013, 15, 3994–3997. doi:10.1021/ol401786d
Return to citation in text: [1] -
Baldassari, L. L.; Mantovani, A. C.; Senoner, S.; Maryasin, B.; Maulide, N.; Lüdtke, D. S. Org. Lett. 2018, 20, 5881–5885. doi:10.1021/acs.orglett.8b02544
Return to citation in text: [1] -
Kaiser, D.; Veiros, L. F.; Maulide, N. Adv. Synth. Catal. 2017, 359, 64–77. doi:10.1002/adsc.201600860
Return to citation in text: [1] -
Akai, S.; Kawashita, N.; Wada, Y.; Satoh, H.; Alinejad, A. H.; Kakiguchi, K.; Kuriwaki, I.; Kita, Y. Tetrahedron Lett. 2006, 47, 1881–1884. doi:10.1016/j.tetlet.2006.01.090
Return to citation in text: [1] -
Eberhart, A. J.; Shrives, H. J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D. J. Chem. – Eur. J. 2015, 21, 7428–7434. doi:10.1002/chem.201406424
Return to citation in text: [1] -
Pons, A.; Michalland, J.; Zawodny, W.; Chen, Y.; Tona, V.; Maulide, N. Angew. Chem., Int. Ed. 2019, 58, 17303–17306. doi:10.1002/anie.201909381
Return to citation in text: [1] -
Peng, B.; Huang, X.; Xie, L.-G.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 8718–8721. doi:10.1002/anie.201310865
Return to citation in text: [1] -
Kaldre, D.; Maryasin, B.; Kaiser, D.; Gajsek, O.; González, L.; Maulide, N. Angew. Chem., Int. Ed. 2017, 56, 2212–2215. doi:10.1002/anie.201610105
Return to citation in text: [1] -
Maryasin, B.; Kaldre, D.; Galaverna, R.; Klose, I.; Ruider, S.; Drescher, M.; Kählig, H.; González, L.; Eberlin, M. N.; Jurberg, I. D.; Maulide, N. Chem. Sci. 2018, 9, 4124–4131. doi:10.1039/c7sc04736c
Return to citation in text: [1] -
Shang, L.; Chang, Y.; Luo, F.; He, J.-N.; Huang, X.; Zhang, L.; Kong, L.; Li, K.; Peng, B. J. Am. Chem. Soc. 2017, 139, 4211–4217. doi:10.1021/jacs.7b00969
Return to citation in text: [1] [2] -
Luo, F.; Lu, Y.; Hu, M.; Tian, J.; Zhang, L.; Bao, W.; Yan, C.; Huang, X.; Wang, Z.-X.; Peng, B. Org. Chem. Front. 2018, 5, 1756–1762. doi:10.1039/c8qo00268a
Return to citation in text: [1] -
Zhang, L.; He, J.-N.; Liang, Y.; Hu, M.; Shang, L.; Huang, X.; Kong, L.; Wang, Z.-X.; Peng, B. Angew. Chem., Int. Ed. 2019, 58, 5316–5320. doi:10.1002/anie.201900434
Return to citation in text: [1] [2] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Nair, A. S.; Singh, A. K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V. P.; Pappachen, L. K.; Rangarajan, T. M.; Kim, H.; Mathew, B. Processes 2022, 10, 2054. doi:10.3390/pr10102054
Return to citation in text: [1] -
Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315–6386. doi:10.1021/acs.jmedchem.9b01877
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. doi:10.1002/9783527651351
Return to citation in text: [1] -
Li, J.; Chen, Y.; Zhong, R.; Zhang, Y.; Yang, J.; Ding, H.; Wang, Z. Org. Lett. 2020, 22, 1164–1168. doi:10.1021/acs.orglett.0c00018
Return to citation in text: [1] -
Huang, X.; Zhang, Y.; Liang, W.; Zhang, Q.; Zhan, Y.; Kong, L.; Peng, B. Chem. Sci. 2020, 11, 3048–3053. doi:10.1039/d0sc00244e
Return to citation in text: [1] -
Hu, M.; He, J.-N.; Liu, Y.; Dong, T.; Chen, M.; Yan, C.; Ye, Y.; Peng, B. Eur. J. Org. Chem. 2020, 193–197. doi:10.1002/ejoc.201901577
Return to citation in text: [1] [2] -
Ye, S.; Wang, H.; Liang, G.; Hu, Z.; Wan, K.; Zhang, L.; Peng, B. Org. Biomol. Chem. 2024, 22, 1495–1499. doi:10.1039/d3ob02102e
Return to citation in text: [1]
| 1. | Hiersemann, M.; Nubbemeyer, U., Eds. The Claisen Rearrangement: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2007. doi:10.1002/9783527610549 |
| 2. | Martín Castro, A. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u |
| 3. | Ito, H.; Taguchi, T. Chem. Soc. Rev. 1999, 28, 43–50. doi:10.1039/a706415b |
| 17. | Yanagi, T.; Nogi, K.; Yorimitsu, H. Chem. – Eur. J. 2020, 26, 783–787. doi:10.1002/chem.201903570 |
| 26. | Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J. J. Am. Chem. Soc. 2016, 138, 790–793. doi:10.1021/jacs.5b12579 |
| 38. | Shang, L.; Chang, Y.; Luo, F.; He, J.-N.; Huang, X.; Zhang, L.; Kong, L.; Li, K.; Peng, B. J. Am. Chem. Soc. 2017, 139, 4211–4217. doi:10.1021/jacs.7b00969 |
| 40. | Zhang, L.; He, J.-N.; Liang, Y.; Hu, M.; Shang, L.; Huang, X.; Kong, L.; Wang, Z.-X.; Peng, B. Angew. Chem., Int. Ed. 2019, 58, 5316–5320. doi:10.1002/anie.201900434 |
| 48. | Hu, M.; He, J.-N.; Liu, Y.; Dong, T.; Chen, M.; Yan, C.; Ye, Y.; Peng, B. Eur. J. Org. Chem. 2020, 193–197. doi:10.1002/ejoc.201901577 |
| 12. | Yanagi, T.; Otsuka, S.; Kasuga, Y.; Fujimoto, K.; Murakami, K.; Nogi, K.; Yorimitsu, H.; Osuka, A. J. Am. Chem. Soc. 2016, 138, 14582–14585. doi:10.1021/jacs.6b10278 |
| 13. | Murakami, K.; Yorimitsu, H.; Osuka, A. Angew. Chem., Int. Ed. 2014, 53, 7510–7513. doi:10.1002/anie.201403288 |
| 14. | Shrives, H. J.; Fernández-Salas, J. A.; Hedtke, C.; Pulis, A. P.; Procter, D. J. Nat. Commun. 2017, 8, 14801. doi:10.1038/ncomms14801 |
| 15. | Okamoto, K.; Hori, M.; Yanagi, T.; Murakami, K.; Nogi, K.; Yorimitsu, H. Angew. Chem., Int. Ed. 2018, 57, 14230–14234. doi:10.1002/anie.201809035 |
| 16. | Kobatake, T.; Fujino, D.; Yoshida, S.; Yorimitsu, H.; Oshima, K. J. Am. Chem. Soc. 2010, 132, 11838–11840. doi:10.1021/ja1030134 |
| 6. | Zhang, L.; Hu, M.; Peng, B. Synlett 2019, 30, 2203–2208. doi:10.1055/s-0039-1690212 |
| 7. | Yorimitsu, H. Chem. Rec. 2017, 17, 1156–1167. doi:10.1002/tcr.201700017 |
| 8. | Yanagi, T.; Nogi, K.; Yorimitsu, H. Tetrahedron Lett. 2018, 59, 2951–2959. doi:10.1016/j.tetlet.2018.06.055 |
| 9. | Pulis, A. P.; Procter, D. J. Angew. Chem., Int. Ed. 2016, 55, 9842–9860. doi:10.1002/anie.201601540 |
| 10. | Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111 |
| 11. | Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J. Angew. Chem., Int. Ed. 2010, 49, 5832–5844. doi:10.1002/anie.201000517 |
| 48. | Hu, M.; He, J.-N.; Liu, Y.; Dong, T.; Chen, M.; Yan, C.; Ye, Y.; Peng, B. Eur. J. Org. Chem. 2020, 193–197. doi:10.1002/ejoc.201901577 |
| 4. | Macé, Y.; Urban, C.; Pradet, C.; Blazejewski, J.-C.; Magnier, E. Eur. J. Org. Chem. 2009, 5313–5316. doi:10.1002/ejoc.200900873 |
| 5. | Pégot, B.; Urban, C.; Diter, P.; Magnier, E. Eur. J. Org. Chem. 2013, 7800–7808. doi:10.1002/ejoc.201301017 |
| 49. | Ye, S.; Wang, H.; Liang, G.; Hu, Z.; Wan, K.; Zhang, L.; Peng, B. Org. Biomol. Chem. 2024, 22, 1495–1499. doi:10.1039/d3ob02102e |
| 38. | Shang, L.; Chang, Y.; Luo, F.; He, J.-N.; Huang, X.; Zhang, L.; Kong, L.; Li, K.; Peng, B. J. Am. Chem. Soc. 2017, 139, 4211–4217. doi:10.1021/jacs.7b00969 |
| 39. | Luo, F.; Lu, Y.; Hu, M.; Tian, J.; Zhang, L.; Bao, W.; Yan, C.; Huang, X.; Wang, Z.-X.; Peng, B. Org. Chem. Front. 2018, 5, 1756–1762. doi:10.1039/c8qo00268a |
| 40. | Zhang, L.; He, J.-N.; Liang, Y.; Hu, M.; Shang, L.; Huang, X.; Kong, L.; Wang, Z.-X.; Peng, B. Angew. Chem., Int. Ed. 2019, 58, 5316–5320. doi:10.1002/anie.201900434 |
| 46. | Li, J.; Chen, Y.; Zhong, R.; Zhang, Y.; Yang, J.; Ding, H.; Wang, Z. Org. Lett. 2020, 22, 1164–1168. doi:10.1021/acs.orglett.0c00018 |
| 35. | Peng, B.; Huang, X.; Xie, L.-G.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 8718–8721. doi:10.1002/anie.201310865 |
| 36. | Kaldre, D.; Maryasin, B.; Kaiser, D.; Gajsek, O.; González, L.; Maulide, N. Angew. Chem., Int. Ed. 2017, 56, 2212–2215. doi:10.1002/anie.201610105 |
| 37. | Maryasin, B.; Kaldre, D.; Galaverna, R.; Klose, I.; Ruider, S.; Drescher, M.; Kählig, H.; González, L.; Eberlin, M. N.; Jurberg, I. D.; Maulide, N. Chem. Sci. 2018, 9, 4124–4131. doi:10.1039/c7sc04736c |
| 47. | Huang, X.; Zhang, Y.; Liang, W.; Zhang, Q.; Zhan, Y.; Kong, L.; Peng, B. Chem. Sci. 2020, 11, 3048–3053. doi:10.1039/d0sc00244e |
| 22. | Kaiser, D.; Veiros, L. F.; Maulide, N. Chem. – Eur. J. 2016, 22, 4727–4732. doi:10.1002/chem.201600432 |
| 23. | Šiaučiulis, M.; Sapmaz, S.; Pulis, A. P.; Procter, D. J. Chem. Sci. 2018, 9, 754–759. doi:10.1039/c7sc04723a |
| 24. | Akai, S.; Kawashita, N.; Satoh, H.; Wada, Y.; Kakiguchi, K.; Kuriwaki, I.; Kita, Y. Org. Lett. 2004, 6, 3793–3796. doi:10.1021/ol0484310 |
| 25. | Hu, L.; Gui, Q.; Chen, X.; Tan, Z.; Zhu, G. J. Org. Chem. 2016, 81, 4861–4868. doi:10.1021/acs.joc.6b00535 |
| 26. | Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J. J. Am. Chem. Soc. 2016, 138, 790–793. doi:10.1021/jacs.5b12579 |
| 27. | Eberhart, A. J.; Procter, D. J. Angew. Chem., Int. Ed. 2013, 52, 4008–4011. doi:10.1002/anie.201300223 |
| 28. | Eberhart, A. J.; Imbriglio, J. E.; Procter, D. J. Org. Lett. 2011, 13, 5882–5885. doi:10.1021/ol2025197 |
| 29. | Eberhart, A. J.; Cicoira, C.; Procter, D. J. Org. Lett. 2013, 15, 3994–3997. doi:10.1021/ol401786d |
| 30. | Baldassari, L. L.; Mantovani, A. C.; Senoner, S.; Maryasin, B.; Maulide, N.; Lüdtke, D. S. Org. Lett. 2018, 20, 5881–5885. doi:10.1021/acs.orglett.8b02544 |
| 31. | Kaiser, D.; Veiros, L. F.; Maulide, N. Adv. Synth. Catal. 2017, 359, 64–77. doi:10.1002/adsc.201600860 |
| 32. | Akai, S.; Kawashita, N.; Wada, Y.; Satoh, H.; Alinejad, A. H.; Kakiguchi, K.; Kuriwaki, I.; Kita, Y. Tetrahedron Lett. 2006, 47, 1881–1884. doi:10.1016/j.tetlet.2006.01.090 |
| 33. | Eberhart, A. J.; Shrives, H. J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D. J. Chem. – Eur. J. 2015, 21, 7428–7434. doi:10.1002/chem.201406424 |
| 34. | Pons, A.; Michalland, J.; Zawodny, W.; Chen, Y.; Tona, V.; Maulide, N. Angew. Chem., Int. Ed. 2019, 58, 17303–17306. doi:10.1002/anie.201909381 |
| 18. | Peng, B.; Geerdink, D.; Farès, C.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 5462–5466. doi:10.1002/anie.201402229 |
| 19. | Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510–8513. doi:10.1021/ja2031882 |
| 20. | Huang, X.; Patil, M.; Farès, C.; Thiel, W.; Maulide, N. J. Am. Chem. Soc. 2013, 135, 7312–7323. doi:10.1021/ja4017683 |
| 21. | Meng, X.; Chen, D.; Cao, X.; Luo, J.; Wang, F.; Huang, S. Chem. Commun. 2019, 55, 12495–12498. doi:10.1039/c9cc06505a |
| 41. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 42. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 43. | Nair, A. S.; Singh, A. K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V. P.; Pappachen, L. K.; Rangarajan, T. M.; Kim, H.; Mathew, B. Processes 2022, 10, 2054. doi:10.3390/pr10102054 |
| 44. | Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315–6386. doi:10.1021/acs.jmedchem.9b01877 |
| 45. | Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. doi:10.1002/9783527651351 |
© 2024 Li et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.