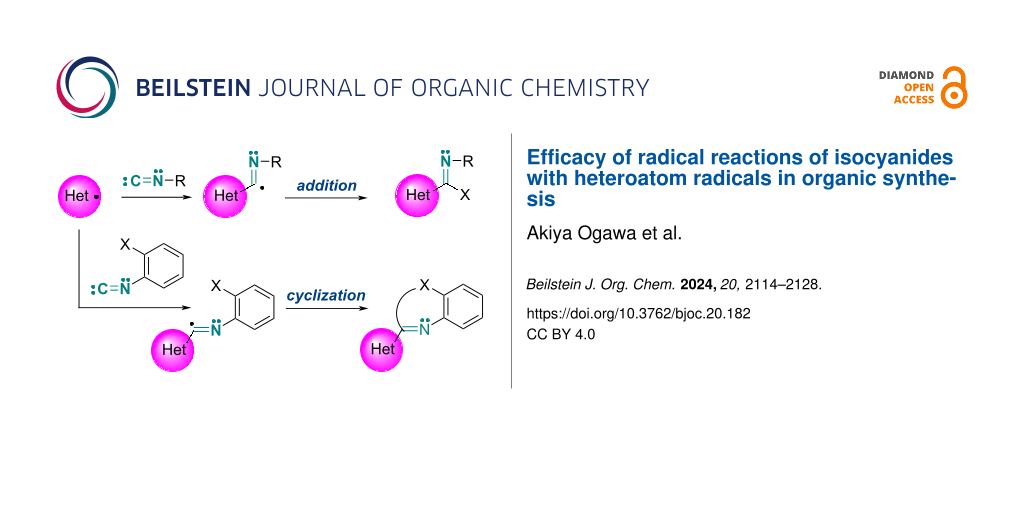
Abstract
Isocyanide is a promising synthetic reagent not only as a one-carbon homologation reagent but also as a nitrogen source for nitrogen-containing molecules. Because of their isoelectronic structure with carbon monoxide, isocyanides also react with nucleophiles, electrophiles, carbon radicals, and transition metal reagents, and are widely used in organic synthesis. On the other hand, the use of isocyanides in reactions with heteroatom radicals is limited. However, the reaction of isocyanides with heteroatom radicals is a promising synthetic tool for the construction of nitrogen-containing organic molecules modified with a variety of heteroatoms. In this Perspective, we review the addition and cyclization reactions of heteroatom radicals with isocyanides and discuss the synthetic prospects of the reaction of isocyanides with heteroatom radicals.
Graphical Abstract
Introduction
Carbon monoxide is a very important C1 resource in both synthetic and industrial chemistry and is not only capable of reacting with a variety of active species such as carbon cations, carbon anions, and carbon radicals (Figure 1), but is also widely used in transition-metal-catalyzed carbonylation reactions [1,2]. However, carbon monoxide is a flammable gas with a wide explosive range, although colorless and odorless, and requires special care in handling due to its high toxicity. In addition, when carbon monoxide is used in a reaction, pressurization in an autoclave or other pressurization device is required to increase the CO concentration.
Figure 1: Resonance structures and reactivity of carbon monoxide.
Figure 1: Resonance structures and reactivity of carbon monoxide.
Isocyanides, on the other hand, have an isoelectronic structure with carbon monoxide and are expected to be not only a promising C1 resource but also an important synthetic reagent for nitrogen-containing compounds [3-7]. Furthermore, by adjusting the substituents on the nitrogen, reactivity can be controlled and solubility in various solvents can be tuned (Figure 2).
Figure 2: Resonance structures and reactivity of isocyanides.
Figure 2: Resonance structures and reactivity of isocyanides.
However, the use of isocyanide as a C1 resource is somewhat limited compared to that of carbon monoxide [8] because isocyanide is susceptible to multiple imidoylation [9-11], whereas carbon monoxide is less susceptible to multiple carbonylation. Therefore, precise control of the reaction is required for selective formation of the monoimidoylation product.
Regarding the radical reaction of isocyanides, the reaction of carbon radicals with isocyanides generates imidoyl radicals as key active species [12], and addition and cyclization reactions using these radical species are useful in synthetic organic chemistry, especially multicomponent synthesis. If various functional groups can be appropriately attached to imidoyl units generated in situ by radical addition to isocyanides, innovative molecules with a variety of functions can be obtained. In other words, if functional groups can be prepared simultaneously with the formation of an imidoyl group, it would be an extremely useful method for the synthesis of nitrogen-containing functional molecules. To achieve this goal, it is expected to be effective to develop a new method to react heteroatom radicals with isocyanides to generate imidoyl radicals to which various heteroatom groups are attached and to use them as synthetic reagents. However, systematic studies of addition reactions of heteroatom radicals to isocyanides are still limited. In this perspective paper, we systematically review the addition reactions of heteroatom radicals to isocyanides and discuss prospects.
Discussion
1,1-Addition of heteroatom radicals to isocyanides
Generation of heteroatom radicals
When reacting a heteroatom radical with an isocyanide, the first thing to consider is the method of generating the heteroatom radicals [13-15]. As mentioned above, isocyanides are readily polymerizable molecules, so to suppress the polymerization of isocyanides, it is necessary to understand the conditions for the generation of heteroatom radicals. In addition, from the perspective of recent green chemistry, the development of environmentally friendly synthetic methods is strongly demanded. In other words, a new synthetic method should have excellent atom economy, produce no waste, be aware of resource recycling, and promote the use of natural energy [16].
The following three methods are generally used to generate heteroatom radicals (E•) (Scheme 1). In method 1, E• is generated by hydrogen abstraction from E–H by cyanoisopropyl radicals generated by thermal decomposition of 2,2'-azobis(isobutyronitrile) (AIBN). Then, E• adds to isocyanide 1 to form imidoyl radical 2, which abstracts hydrogen from E–H. The addition reaction proceeds by a radical chain mechanism, producing the 1,1-addition product 3 with regeneration of E•. In method 2, E• is generated by homolysis of a heteroatom–heteroatom bonded compound (E–E) upon heating or photoirradiation. Similarly, E• adds to 1 to form 2, which undergoes atom (or group)-transfer from E–E to give the 1,1-addition product 4 with regeneration of E• [17,18]. In method 3, the photoinduced redox reaction of a heteroatom compound takes place using metal complex or functional dye as a photocatalyst (PC) [19,20]. Recently, some important reviews summarize and discuss the use of method 3 in synthetic organic chemistry [21,22]; in contrast, there is little detailed and coherent literature on the overall research trends regarding the latest research on molecular transformations by the reactions of heteroatom radicals with isocyanides using methods 1 and 2. Thus, in this perspective, we mainly focused on the use of method 1 and method 2 for the generation of heteroatom radicals and the reactivity of them with isocyanides.
Scheme 1: Possible three pathways of the E• formation for imidoylation.
Scheme 1: Possible three pathways of the E• formation for imidoylation.
Among these three methods, methods 1 and 3 require the addition of a radical initiator and a photocatalyst, respectively. In contrast, method 2 does not require any additive, although, if the heating or photoirradiation is performed by electricity, the combustion of fossil fuels may cause environmental pollution. However, when using sunlight, which is an inexhaustible natural energy, it is expected to be the most environmentally friendly method.
The homolysis of E–E upon visible light irradiation is induced by exciting one electron of the isolated electron pair on E to the anti-bonding orbital of the E–E-bond (σ* orbital). Such an n–σ* transition usually indicates the maximum absorption in the near-UV region, and the absorption cutoff reaches the visible region, especially in the case of highly periodic E–E. Therefore, interelement compounds E–E with groups 15–17 heteroatoms have isolated electron pairs, and E• can be generated by photoirradiation. On the other hand, the photoinduced homolysis of groups 13 and 14 interelement compounds with B–B, Si–Si, Sn–Sn bonds, etc. is generally impossible, because such E–E compounds have no isolated electronic pair. Therefore, the use of a photocatalyst (method 3) or the combination with groups 15–17 interelement compounds would be considered effective for the generation of groups 13 and 14 heteroatom radicals.
Radical addition of group 17 compounds to isocyanides
If isocyanides (RNC) can undergo a radical addition of hydrogen halides (HX) and molecular halogens (X2), 1,1-addition products RN=CH-X, 3 (E = X: F, Cl, Br, I) and RN=CX2, 4 (E = X), respectively, could be formed. In practice, however, very few examples of such radical addition to isocyanides are known, and the 1,1-addition products 3 and 4 have usually been synthesized by ionic reactions. 1,1-Addition of molecular bromine to phenyl isocyanide was reported by E. Kühle et al. to afford the corresponding 1,1-adduct (PhN=CBr2) [23]. Since dichloro compounds (RN=CCl2) [24] are the imino derivatives of highly toxic phosgene (O=CCl2), reactions using them as key intermediates are not safe synthetic methods. For these reasons, it is no exaggeration to say that radical reactions of group 17 interelement compounds with isocyanides have hardly been developed.
Upon exposure to near-UV light, perfluoroalkyl iodides (RFI) undergo homolysis to form perfluoroalkyl radicals (RF•) and iodine radical (I•). The perfluoroalkyl radical, as a carbon radical, rather than iodine radical can add to isocyanides to form imidoyl radicals. Then, the iodine atom of RFI can trap the imidoyl radicals to give the corresponding 1,1-adducts (R–N=C(I)–RF) in good yields [25,26].
Radical addition of group 16 compounds to isocyanides
In a pioneering study, Ito and Saegusa et al. reported the radical addition of thiols to isocyanides (Scheme 2) [27]. Thermal decomposition of AIBN as a radical initiator generates the 2-cyano-2-propyl radical ((NC)(CH3)2C•), which abstracts hydrogen from the thiol (R’SH) to form the thiyl radical (R’S•). The formed R’S• adds to isocyanide (RNC) to generate imidoyl radical intermediate 2 (E = R’S), which abstracts hydrogen from thiol to give the corresponding thioformimidate 3 (E = R’S) with regeneration of R’S•. Thus, the hydrothiolation of isocyanides with thiols proceeds by the radical chain mechanism. In the case of tertiary alkanethiols and arylmethanethiols, the corresponding imidoyl radicals 2 decompose to give tertiary alkyl and benzylic radicals, respectively, to form isothiocyanates 5.
Scheme 2: Radical addition of thiols to isocyanides.
Scheme 2: Radical addition of thiols to isocyanides.
On the other hand, we have investigated the radical addition of diphenyl disulfide to isocyanides under photoirradiation. The photoinduced radical addition of the disulfide to aliphatic isocyanides hardly proceeds, whereas the radical addition to aromatic isocyanides proceeds under high concentration conditions using excess amounts of (PhS)2, selectively yielding 1,1-addition product 4 (R = 2,6-xylyl, E = PhS) [28]. In the case of aromatic isocyanides, the 1,1-addition reaction is probably more likely to proceed because the C–N double bond of the 1,1-addition product 4 (R = Ar, E = PhS) is conjugated to the aromatic ring, which stabilizes it compared to the corresponding adduct with aliphatic isocyanides. Because of this conjugation, the aromatic ring at the N of the 1,1-addition product 4 (R = Ar, E = PhS) geometrically isomerizes faster than the NMR timescale, so that the two thio groups of 4 are observed to be equivalent in NMR spectroscopy.
In the case of diphenyl diselenide as a representative organic diselenide, the addition of PhSe• to alkenes proceeds 10 to 50 times slower than PhS• [29]. For this reason, the addition of (PhSe)2 to isocyanides, whether aliphatic or aromatic, rarely proceeds. The exception is the addition to p-nitrophenyl isocyanides, which does proceed, but this is because the electron-withdrawing group improves the stability of the product 4 (R = p-O2N-C6H4, E = PhSe) and also because phase separation is caused by the product precipitation from the reaction solution. On the other hand, the addition reaction of (PhTe)2 to isocyanides does not proceed at all [30]. This is because the addition product 4 (E = PhTe) is unstable under photoirradiation conditions.
The photoinduced 1,1-dithiolation reaction of isocyanides required an excess of (PhS)2 due to the low carbon radical capturing ability of (PhS)2 (kS = 7.6 × 104 M−1s−1). In contrast, the carbon radical capturing ability of (PhSe)2 (kSe = 1.2 × 107 M−1s−1) is known to be ca. 160 times higher than that of (PhS)2 [31]. Therefore, we investigated the radical addition to isocyanides using a disulfide–diselenide binary system under photoirradiation and found that the thioselenation of aromatic isocyanides proceeded efficiently to afford the corresponding thioselenation products 6 (Scheme 3a).
Scheme 3: Selective thioselenation and catalytic dithiolation of isocyanides.
Scheme 3: Selective thioselenation and catalytic dithiolation of isocyanides.
More interestingly, it was found that the thioselenation of aliphatic isocyanides also proceeds in the initial stage of the reaction, but the formed thioselenation products 6 were gradually converted to the dithiolation products 4 under photoirradiation conditions (Scheme 3b). Thus, it was shown that the photoirradiated dithiolation of aliphatic isocyanides with (PhS)2 proceeded as a catalytic reaction of (PhSe)2 (30 mol %). In the dithiolation products 4 from aliphatic isocyanides, two PhS-groups were observed non-equivalently, suggesting the lack of geometrical isomerization of the C–N double bond of the dithiolation products [32].
The thioselenation product 6’, an imine derivative, can be converted to a β-lactam derivative by [2 + 2] cycloaddition with ketene generated in situ. For example, thioselenation of RNC (R = (EtO)2P(O)–CH2) gave imine 6’ (96%), which underwent [2 + 2] cycloaddition with methoxyketene to afford β-lactam derivative 7 (79%) (Scheme 4). Selective replacement of the PhSe group of 7 with a 3-butanonyl group (34%) and the subsequent intramolecular Horner–Emmons reaction successfully led to carbacephem skeleton 8 (96%).
Scheme 4: Synthesis of carbacephem framework.
Scheme 4: Synthesis of carbacephem framework.
Since it is known that the carbon radical capturing ability of (PhTe)2 is even four times higher than that of (PhSe)2 [31], we investigated the radical addition to isocyanides using a disulfide–ditelluride binary system under photoirradiation. The visible-light-irradiated thiotelluration reaction did not proceed at all for normal isocyanides, but for aromatic isocyanides with electron-withdrawing groups (EWG) such as p-NO2, p-CF3, p-CN, p-Cl, and m-MeO, the desired thiotelluration reaction proceeded under visible light irradiation to successfully afford the corresponding thiotelluration products 4 (R = EWG-C6H4, E = PhS and PhTe) in moderate to high yields [30].
Very few examples of intermolecular cascade reactions with imidoyl radicals as key intermediates have been reported. The intermolecular cascade reaction of diselenide, electron-deficient acetylene, and isocyanide under photoirradiation yields the sequential addition products 9 in moderate to excellent yields (Scheme 5) [33]. The product can be used as a precursor for the carbapenem scaffold, one of the basic scaffolds of antibiotics.
Scheme 5: Sequential addition of (PhSe)2 to ethyl propiolate and isocyanide.
Scheme 5: Sequential addition of (PhSe)2 to ethyl propiolate and isocyanide.
Thermal or photoirradiated decomposition of organotellurium compounds generates carbon radicals that can add to isocyanides to form the imidoyl radicals. In this reaction, telluro radicals (ArTe•) also forms in situ, but the relative reactivity of them toward isocyanides might be very low. In addition, dimerization of ArTe• to (ArTe)2 is very fast, and therefore, the ArTe-substituted imidoyl radical (ArTe-C•(=NR’)) could not be observed. However, the tellurium group of RTeAr can successfully trap the imidoyl radicals to yield the corresponding isocyanide-inserted organotellurium compounds (Scheme 6) [34-36].
Scheme 6: Isocyanide insertion reaction into carbon-tellurium bonds.
Scheme 6: Isocyanide insertion reaction into carbon-tellurium bonds.
Radical addition of group 15 compounds to isocyanides
Tetrakis(trimethylsilyl)hydrazine ((Me3Si)2N–N(SiMe3)2, 10) undergoes ultraviolet light-induced homolysis of the N–N single bond of 10 to form bis(trimethylsilyl)aminyl radical ((Me3Si)2N•, 11). The aminyl radical 11 adds to tert-butyl isocyanide to form the corresponding imidoyl radical 2 (R = t-Bu, E = (Me3Si)2N), as confirmed by ESR measurement [37].
Similar to the thiol addition to isocyanides, disubstituted phosphines (R’2PH) induce radical addition to isocyanides in the presence of AIBN as a radical initiator yielding the corresponding iminoformyl phosphines, R’2P–CH=NR, (12, R = c-C6H11 or n-C6H11, E = Et2P) in good yields. In the case of tert-butyl and benzyl isocyanides, the substituents on the nitrogen of imidoyl radical 2 (R = t-Bu or PhCH2, E = Et2P or Ph2P) were eliminated to give cyanophosphines (R’2P–CN, Scheme 7) [38].
Scheme 7: Radical addition to isocyanides with disubstituted phosphines.
Scheme 7: Radical addition to isocyanides with disubstituted phosphines.
On the other hand, we attempted a photoinduced addition of phosphorus–phosphorus interelement compounds such as Ph2P–PPh2 and Ph2P(S) –PPh2 to phenyl isocyanide, but the addition did not proceed at all. This is most likely due to the bulkiness of the Ph2P and Ph2P(S) groups (Scheme 8) [39].
Scheme 8: Radical addition to phenyl isocyanides with diphosphines.
Scheme 8: Radical addition to phenyl isocyanides with diphosphines.
In sharp contrast, the addition of a germyl phosphine (Et2P–GeEt3) to phenyl isocyanide was reported to give the corresponding 1,1-adduct (Et2P–C(=NPh)–GeEt3, 13) in 46% yield [40]. Similarly, Me2N-SnMe3 was known to add to p-tolyl isocyanide to give Me2N–C(=N–C6H4–p-Me)–SnMe3 (14) in good yield [41]. However, the authors did not specify whether the addition reactions proceeded by a radical or ionic mechanism.
Radical addition of group 14 compounds to isocyanides
Group 14 compounds with an E–H or E–E bond (E = Si, Ge, Sn) have no lone-pair electrons and therefore cannot generate group 14 heteroatom radicals by homolysis via the n–σ* transition. To generate group 14 heteroatom radicals, the hydrogen abstraction reaction from tin hydride or hydrosilane by radical initiators such as AIBN has effectively been used. When tin and silyl radicals generated in this way are reacted with isocyanides, they are more susceptible to steric hindrance than group 16 or 15 heteroatom radicals due to the greater number of substituents on the heteroatom. For this reason, the 1,1-addition is less likely to proceed as with group 16 or 15 heteroatom radicals. In the case of stannyl and silyl radicals, the alkyl group of the isocyanide is eliminated as an alkyl radical from the imidoyl radical intermediate 2 [42]. The formed alkyl radicals abstract hydrogen from the tin hydride or hydrosilane, and the reduction reaction proceeds with the concomitant formation of stannyl or silyl cyanide 15 as byproducts (Scheme 9a) [38,43].
Scheme 9: Radical reaction of tin hydride and hydrosilane toward isocyanide.
Scheme 9: Radical reaction of tin hydride and hydrosilane toward isocyanide.
In the presence of acrylonitrile, the formed alkyl radical can add to acrylonitrile, affording the addition product (c-C6H11CH2CH2CN) after hydrogen abstraction from tris(trimethylsilyl)silane (TTMSS, (Me3Si)3SiH) (Scheme 9b) [44]. Triethylsilane (Et3SiH), one of the most popular hydrosilanes, has a strong Si–H bond (90 kcal/mol), and therefore the radical-chain reaction using Et3SiH is often difficult to perform. In contrast, tris(trimethylsilyl)silane, (Me3Si)3SiH, has a bond dissociation energy similar to that of n-Bu3SnH (74 kcal/mol) and can be used as an efficient reducing agent/mediator.
Radical addition of group 13 compounds to isocyanides
Boron, a group 13 typical element, also lacks a non-covalent electron pair, making it impossible to generate boron radicals by homolysis via the n–σ* transition. In addition, since boron has an empty orbital, it forms ate complexes when Lewis base compounds coexist. As the result, the boryl groups of the ate complexes are bulky and often cause steric hindrance. However, several examples of isocyanide insertion reactions into B–H and B–B bonds are known. For example, isocyanides coordinate to diborane (B2H6) or trialkylboranes (BR'3) to form Lewis acid–base complexes (RNC→BH3 or RNC→BR'3), but these complexes are thermally labile, and hydrogen or alkyl groups on boron are 1,2-shifted to the isocyanide carbon, yielding the compounds (H2B-C(=NR)-H or R'2B-C(=NR)-R') with the isocyanide inserted between the B–H or B–alkyl bond [45,46]. The insertion products easily underwent dimerization to afford 2,5-diboradihydropyrazine derivatives 16 (Scheme 10a). Since this 1,2-shift reaction proceeds under mild conditions and in the absence of a radical initiator, it is thought to proceed by an ionic rather than a radical mechanism.
Scheme 10: Isocyanide insertion into boron compounds.
Scheme 10: Isocyanide insertion into boron compounds.
Alkoxy and cyclopropyl radicals, which are more reactive than the usual alkyl radicals, are capable of abstracting hydrogen from Lewis acid–base complexes (t-BuNC→BH3) to generate the corresponding isocyanide–boryl radicals 17 (t-BuNC→BH2•), which can be observed by ESR (Scheme 10b) [47]. However, the synthetic application of this boryl radical has not been investigated.
Among the cyclic diboron compounds, a series of five-membered cyclic diboron compounds 18 undergo an insertion reaction of isocyanides into the boron–boron single bond of 18 under mild conditions without the addition of any additives (Scheme 11a) [48]. The reaction is thought to proceed by an ionic mechanism.
Scheme 11: Isocyanide insertion into cyclic compounds containing boron units.
Scheme 11: Isocyanide insertion into cyclic compounds containing boron units.
Recently, several insertion-type reactions of isocyanides into diboron compounds have been reported to proceed by an ionic mechanism [49,50]. As an interesting example, a frustrated Lewis ion pair 19 consisting of a boryl and a phosphinyl group undergoes an isocyanide insertion reaction (Scheme 11b) [51,52]. As described above, the isocyanide insertion reaction into B–H or B–B bonds has been reported, but the reactions by a radical mechanism are largely unknown.
Very recently, Turlik and Schuppe reported a novel generation of nucleophilic boryl radicals using hydrogen atom transfer (HAT) and photoredox catalysis. Furthermore, its reaction with isocyanides forms boron-substituted imidoyl radical intermediates and rapid β-scission then causes elimination of the substituents on the nitrogen (Scheme 12) [53].
Scheme 12: Photoinduced hydrodefunctionalization of isocyanides.
Scheme 12: Photoinduced hydrodefunctionalization of isocyanides.
Radical cyclization via formation of imidoyl radical species
In the former chapter, we discussed 1,1-addition reactions of typical element compounds to isocyanides using imidoyl radicals as key intermediates. However, as the number of substituents on typical elements increases, intermolecular 1,1-addition reactions become more difficult due to the increase in steric hindrance. Therefore, it is expected that intramolecular cyclization of imidoyl radicals will be possible by introducing an unsaturated group at an appropriate position in the isocyanide molecule, since intramolecular reactions are generally 103 times faster than intermolecular reactions. This chapter discusses the intramolecular radical cyclization reactions of isocyanides with alkenyl, alkynyl, aryl, and isocyano groups as unsaturated groups.
Intramolecular cyclization of ortho-alkynylaryl- or ortho-alkenylaryl isocyanides
Fukuyama et al. reported that the reaction of an aryl isocyanide with an alkenyl group at the ortho-position with tin hydride in the presence of AIBN generates a stannylated imidoyl radical 20. The subsequent 5-exo cyclization, hydrogen abstraction from n-Bu3SnH, and aromatization successfully afforded the stannylated indole derivative 21 (Scheme 13) [8,54-56]. The stannyl group of 21 could be transferred to aryl or vinyl group by cross-coupling reaction.
Scheme 13: Tin hydride-mediated indole synthesis and cross-coupling.
Scheme 13: Tin hydride-mediated indole synthesis and cross-coupling.
At the same time, Bachi et al. succeeded in synthesizing a 5-membered nitrogen-containing heterocycle based on the 5-exo cyclization of isocyanides with alkenyl or alkynyl groups using thiols as mediators (Scheme 14) [57].
Scheme 14: 2-Thioethanol-mediated radical cyclization of alkenyl isocyanide.
Scheme 14: 2-Thioethanol-mediated radical cyclization of alkenyl isocyanide.
The generated thiyl radical attacks the isocyano group and forms imidoyl radical, which induces 5-exo cyclization. The following hydrogen abstraction, intramolecular ionic cyclization, and hydrolysis during chromatography on silica gel affords the cyclic amide in good yield. They further applied this radical cyclization reaction as a key step in the synthesis of (±)-α-kainic acid [58].
Rainier et al. reported the thiol-mediated 5-exo cyclization of o-alkynylaryl isocyanides, which successfully afforded dithiolated indoles 22 (Scheme 15) [59]. However, depending on the reaction conditions, quinoline derivatives were also produced as byproducts (vide infra).
Scheme 15: Thiol-mediated radical cyclization of o-alkenylaryl isocyanide.
Scheme 15: Thiol-mediated radical cyclization of o-alkenylaryl isocyanide.
The photoinduced reaction of o-ethenylaryl isocyanides with disulfides in the presence of diphenyl ditelluride yields the corresponding dithiolated indole derivatives 23 (Scheme 16) [60]. Initially, the thiotelluration products via 5-exo cyclization are formed in situ. The subsequent aromatization followed by photoinduced displacement of the PhTe group with the PhS group afford 23. Furthermore, the photoinduced reaction of ortho-ethenylaryl isocyanides with bis(2-aminophenyl) disulfides affords tetracyclic compounds 24 in a single step.
Scheme 16: (PhTe)2-assisted dithiolative cyclization of o-alkenylaryl isocyanide.
Scheme 16: (PhTe)2-assisted dithiolative cyclization of o-alkenylaryl isocyanide.
Not only alkynyl and alkenyl groups, but also heteroatom moieties such as azido and sulfide groups can intramolecularly capture imidoyl radicals generated in situ, yielding the corresponding benzoimidazoles and benzothiazoles, respectively (Scheme 17). Upon treatment with Mn(OAc)3·H2O, diphenylphosphine oxide (Ph2P(O)H) and organoboron reagents (ArB(OH)2) generate Ph2P(O)• and Ar•, respectively, which add to isocyanides to form the imidoyl radicals. The capture of the imidoyl radicals with the azido group proceeds with the release of N2, and the amino radical formed abstracts hydrogen from the surroundings (Scheme 17a and 17b) [61]. The imidoyl radical formed by the addition of Ph2P(O)• to isocyanide can also be trapped intramolecularly by the methylthio group (Scheme 17c) [62].
Scheme 17: Trapping imidoyl radicals with heteroatom moieties.
Scheme 17: Trapping imidoyl radicals with heteroatom moieties.
In the case of 1,2-diisocyanoarenes, the quinoxaline synthesis can be achieved via radical cyclization of imidoyl radical species, which proceeds by intramolecular capture with the o-isocyano group (Scheme 18) [63-66].
Scheme 18: Trapping imidoyl radicals with isocyano group.
Scheme 18: Trapping imidoyl radicals with isocyano group.
The iodoperfluoroalkylation with radical cyclization of ortho-diisocyanoarenes proceeded efficiently by using AIBN as initiator or using a hexabutyldistannane under visible light irradiation to afford the quinoxaline derivative 25 in good yields (Scheme 18a) [63]. At the same time, a similar quinoxaline synthesis was reported to proceed by irradiation with visible light in the presence of dibenzylamine ((PhCH2)2NH, MeCN, rt, blue LED) [64]. This reaction involves a visible-light-induced single electron transfer (SET) process. An efficient radical cascade cyclization has also been reported, in which a wide-range of 2-phosphoryl-substituted quinoxalines 26 were prepared in one pot via reaction of ortho-diisocyanoarenes with diarylphosphine oxides in the presence of AgNO3 (Scheme 18b) [65]. Chalcogen compounds such as PhSH and (PhSe)2 can be used as chalcogeno radical sources for the photoinduced radical cyclization of ortho-diisocyanoarenes to afford the corresponding 2-thiolated and 2,3-diselenated quinoxaline derivatives (e.g., 27), respectively (Scheme 18c) [66]. Although diphosphines such as (Ph2P)2 and Ph2P(S)PPh2 did not intermolecularly add to isocyanides under radical reaction conditions (as mentiond above), they worked well for the photoinduced radical cyclization of o-diisocyanoarenes (Scheme 18d) [39]. The obtained quinoxaline-2,3-diphosphines 28 are promising ligands for transition metal catalysts such as palladium catalysts.
Aza-Bergman cyclization of o-alkynylaryl isocyanates
In the case of o-alkenyl isocyanides, it has already been described that the 5-exo cyclization reaction proceeds selectively, yielding nitrogen-containing 5-membered cyclic products. In sharp contrast, when the photoirradiated reaction of ortho-alkynyl isocyanides was carried out, we found that an electrocyclization reaction, the aza-Bergmann cyclization, takes place, selectively yielding nitrogen-containing 6-membered cyclization products (Scheme 19) [67]. The aza-Bergmann cyclization of ortho-alkynyl isocyanides proceeds under milder conditions than the Bergmann cyclization of endiynes, forming quinoline-2,4-biradical intermediates. When diselenide, ditelluride, and diiodide are used as radical mediators, seleno, telluro, and iodo groups are introduced at the 2,4-positions of the quinoline, respectively [68-70].
Scheme 19: Quinoline synthesis via aza-Bergman cyclization.
Scheme 19: Quinoline synthesis via aza-Bergman cyclization.
On the other hand, in the case of germyl hydride, hydrosilane (TTMSS), selenol, and aliphatic thiols, hydrogen abstraction reaction by quinoline-2,4-biradical intermediates occurred to give 2,4-hydrogenated quinolines [69]. In the case of aromatic thiols, the ionic cycloaddition reaction proceeds to give 2-thiolated quinoline derivatives. In addition, the aza-Bergman cyclization can be thermally induced [71].
Radical cyclization of 2-isocyanobiarenes
The cycloaddition reaction with 2-isocyanobiaryls 29 under radical conditions is an excellent synthetic method for nitrogen-containing fused heterocycles such as phenanthridine derivatives 31 (Scheme 20) [72,73]. The reaction proceeds by addition of radical species to the isocyano group of 29 to form the imidoyl radical 30 as a key intermediate, which adds intramolecularly to the ortho-aryl group. The subsequent aromatization with the release of hydrogen (or proton) affords 31 in good yields.
Scheme 20: Phenanthridine synthesis via radical cyclization of 2-isocyanobiaryls.
Scheme 20: Phenanthridine synthesis via radical cyclization of 2-isocyanobiaryls.
Nanni et al. reported the reaction of 2-isocyanobiphenyl with AIBN or dibenzoyl peroxide (DBP) affords 6-cyanoisopropyl- or 6-phenyl-substituted phenanthridine derivatives. When the reaction was performed in the presence of TTMSS and AIBN, 6-tris(trimethylsilyl)-substituted phenanthridine derivatives were mainly obtained (Scheme 21) [74].
Scheme 21: Phenanthridine synthesis by radical reactions with AIBN, DBP and TTMSS.
Scheme 21: Phenanthridine synthesis by radical reactions with AIBN, DBP and TTMSS.
Tobisu and Chatani et al. reported that a carbon radical generated by the reaction of RB(OH)2 with Mn(acac)3, added to isocyano groups, is leading to intramolecular cyclization with an ortho-aryl group. The formed aryl radical is oxidized by Mn(acac)3 to convert into an aryl cation, which can be deprotonated to synthesize tricyclic pyridine derivatives in a single step (Scheme 22) [75].
Scheme 22: Phenanthridine synthesis by oxidative cyclization of 2-isocyanobiaryls.
Scheme 22: Phenanthridine synthesis by oxidative cyclization of 2-isocyanobiaryls.
Zhang and Yu et al. also developed a cyclization of 2-isocyanobiaryls using a photoredox system [76-80] in which carbon radicals were generated by a photoredox reaction of α-bromopropanoates under visible light irradiation (Scheme 23) [81].
Scheme 23: Phenanthridine synthesis using a photoredox system.
Scheme 23: Phenanthridine synthesis using a photoredox system.
After these pioneering reports mentioned above, many examples of cyclization of 2-isocyanobiaryls using a metal-assisted system [82-86], photoredox system [21,87-90], or some other oxidation systems [91-93] were developed as excellent synthetic methods for nitrogen-containing fused heterocycles.
On the other hand, there are not as many examples of reactions in which the addition of a heteroatom radical to 2-isocyanobiaryls generates an imidoyl radical intermediate to yield nitrogen-containing fused ring compounds. A few examples have been reported in which phosphorus-centered radicals generated from diarylphosphine oxides by Mn(OAc)3-assisted oxidation [94] or the photoredox system [95-97] were used in the radical cyclization reaction of 2-isocyanobiaryls (Scheme 24).
Scheme 24: Phenanthridine synthesis induced by phosphorus-centered radicals.
Scheme 24: Phenanthridine synthesis induced by phosphorus-centered radicals.
Yadav and Sigh et al. reported the direct synthesis of 6-sulfonylated phenanthridines via silver-catalyzed radical sulfonylation–cyclization of 2-isocyanobiphenyls (Scheme 25) [98].
Scheme 25: Phenanthridine synthesis induced by sulfur-centered radicals.
Scheme 25: Phenanthridine synthesis induced by sulfur-centered radicals.
Wang et al. reported a radical borylative cyclization of 2-isocyanobiaryls with N-heterocyclic carbene borane (Scheme 26) [99]. The boryl radical generated via hydrogen abstraction in the presence of di-(tert-butylperoxy)-2-methylpropane (DTBP) as the radical initiator attacks isocyanide units, and the subsequent radical cyclization successfully proceeds to form a variety of borylated phenanthridines in moderate to good yields.
Scheme 26: Phenanthridine synthesis induced by boron-centered radicals.
Scheme 26: Phenanthridine synthesis induced by boron-centered radicals.
Although the previous examples described the synthesis of phenanthridines via the radical addition of hetroatom radicals to 2-isocyanobiphenyl, another pathway to form phenanthridine scaffolds is the radical reaction of 2-aminobiaryls with free isocyanides. For example, oxidative generation of amino radicals from 2-aminobiaryls followed by intermolecular addition to isocyanides forms the imidoyl radicals, which undergo intramolecular cyclization and oxidative dehydrogenation to give phenanthridines (Scheme 27) [100].
Scheme 27: Phenanthridine synthesis by oxidative cyclization of 2-aminobiaryls.
Scheme 27: Phenanthridine synthesis by oxidative cyclization of 2-aminobiaryls.
Conclusion
In this Perspective, the addition and cycloaddition reactions of heteroatom radicals with isocyanides have been described in detail and their synthetic application has been discussed. A number of useful synthetic reactions have been developed from the reactions of group 15 and 16 heteroatom radicals with isocyanides. On the other hand, the use of other heteroatoms in radical reactions with isocyanides has been limited due to the limitations of methods for generating these heteroatom radicals. It is highly expected that the radical reaction with isocyanides will be extended to many heteroatom radicals in the future, leading to the development of nitrogen-containing functional molecules modified with a variety of heteroatom functional groups. We hope that this Perspective will help in the development of such new reactions.
Data Availability Statement
Data sharing is not applicable as no new data was generated or analyzed in this study.
References
-
Gabrielu, B. Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry; Wiley-VCH: Weinheim, Germany, 2021. doi:10.1002/9783527829354
Return to citation in text: [1] -
Singh, J.; Sharma, S.; Sharma, A. J. Org. Chem. 2021, 86, 24–48. doi:10.1021/acs.joc.0c02205
Return to citation in text: [1] -
Collet, J. W.; Roose, T. R.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2020, 59, 540–558. doi:10.1002/anie.201905838
Return to citation in text: [1] -
Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103–1123. doi:10.1039/c6cs00384b
Return to citation in text: [1] -
Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c
Return to citation in text: [1] -
Neo, A. G.; Ramiro, J. L.; García-Valverde, M.; Díaz, J.; Marcos, C. F. Mol. Diversity 2024, 28, 335–418. doi:10.1007/s11030-023-10641-7
Return to citation in text: [1] -
Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a
Return to citation in text: [1] -
Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626
Return to citation in text: [1] [2] -
Zhang, S.; Wang, Y.; Huang, H.; Cao, D. Angew. Chem., Int. Ed. 2023, 62, e202308524. doi:10.1002/anie.202308524
Return to citation in text: [1] -
Čamdžić, L.; Stache, E. E. J. Am. Chem. Soc. 2023, 145, 20311–20318. doi:10.1021/jacs.3c04595
Return to citation in text: [1] -
Liu, N.; Zhou, L.; Wu, Z.-Q. Acc. Chem. Res. 2021, 54, 3953–3967. doi:10.1021/acs.accounts.1c00489
Return to citation in text: [1] -
Gomes, G. d. P.; Loginova, Y.; Vatsadze, S. Z.; Alabugin, I. V. J. Am. Chem. Soc. 2018, 140, 14272–14288. doi:10.1021/jacs.8b08513
Return to citation in text: [1] -
Taniguchi, T. Synthesis 2017, 49, 3511–3534. doi:10.1055/s-0036-1588481
Return to citation in text: [1] -
Dénès, F. Heteroatom-Centred Radicals for the Synthesis of Heterocyclic Compounds. In Free-Radical Synthesis and Functionaization of Heterocycles; Landais, Y., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Vol. 54, pp 151–230. doi:10.1007/7081_2018_19
Return to citation in text: [1] -
Linker, T. Chemistry (Basel, Switz.) 2020, 2, 80–92. doi:10.3390/chemistry2010008
Return to citation in text: [1] -
Sheldon, R. A. Green Chem. 2023, 25, 1704–1728. doi:10.1039/d2gc04747k
Return to citation in text: [1] -
Yamamoto, Y.; Ogawa, A. Molecules 2023, 28, 787. doi:10.3390/molecules28020787
Return to citation in text: [1] -
Ogawa, A.; Yamamoto, Y. Molecules 2023, 28, 6356. doi:10.3390/molecules28176356
Return to citation in text: [1] -
Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035–10074. doi:10.1021/acs.chemrev.6b00018
Return to citation in text: [1] -
Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057
Return to citation in text: [1] -
Russo, C.; Brunelli, F.; Tron, G. C.; Giustiniano, M. Chem. – Eur. J. 2023, 29, e202203150. doi:10.1002/chem.202203150
Return to citation in text: [1] [2] -
Russo, C.; Brunelli, F.; Tron, G. C.; Giustiniano, M. Eur. J. Org. Chem. 2023, 26, e202300743. doi:10.1002/ejoc.202300743
Return to citation in text: [1] -
Kühle, E.; Anders, B.; Zumach, G. Angew. Chem. 1967, 79, 663–680. doi:10.1002/ange.19670791504
Return to citation in text: [1] -
Tanaka, S.; Okano, M.; Tanimoto, S. Bull. Chem. Soc. Jpn. 1975, 48, 1862–1864. doi:10.1246/bcsj.48.1862
Return to citation in text: [1] -
Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A. J. Org. Chem. 2004, 69, 6658–6665. doi:10.1021/jo0495889
Return to citation in text: [1] -
Lei, J.; Wu, X.; Zhu, Q. Org. Lett. 2015, 17, 2322–2325. doi:10.1021/acs.orglett.5b00730
Return to citation in text: [1] -
Saegusa, T.; Kobayashi, S.; Ito, Y. J. Org. Chem. 1970, 35, 2118–2121. doi:10.1021/jo00832a003
Return to citation in text: [1] -
Tsuchii, K.; Tsuboi, Y.; Kawaguchi, S.-i.; Takahashi, J.; Sonoda, N.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2007, 72, 415–423. doi:10.1021/jo061704f
Return to citation in text: [1] -
Ito, O. J. Am. Chem. Soc. 1983, 105, 850–853. doi:10.1021/ja00342a034
Return to citation in text: [1] -
Mitamura, T.; Tsuboi, Y.; Iwata, K.; Tsuchii, K.; Nomoto, A.; Sonoda, M.; Ogawa, A. Tetrahedron Lett. 2007, 48, 5953–5957. doi:10.1016/j.tetlet.2007.06.126
Return to citation in text: [1] [2] -
Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069
Return to citation in text: [1] [2] -
Yamamoto, Y.; Chen, Q.; Ogawa, A. Molecules 2023, 28, 2450. doi:10.3390/molecules28062450
Return to citation in text: [1] -
Ogawa, A.; Doi, M.; Tsuchii, K.; Hirao, T. Tetrahedron Lett. 2001, 42, 2317–2319. doi:10.1016/s0040-4039(01)00123-x
Return to citation in text: [1] -
Miyazoe, H.; Yamago, S.; Yoshida, J.-i. Angew. Chem., Int. Ed. 2000, 39, 3669–3671. doi:10.1002/1521-3773(20001016)39:20<3669::aid-anie3669>3.0.co;2-4
Return to citation in text: [1] -
Yamago, S.; Miyazoe, H.; Goto, R.; Hashidume, M.; Sawazaki, T.; Yoshida, J.-i. J. Am. Chem. Soc. 2001, 123, 3697–3705. doi:10.1021/ja003879r
Return to citation in text: [1] -
Yamago, S. Synlett 2004, 1875–1890. doi:10.1055/s-2004-830883
Return to citation in text: [1] -
Roberts, B. P.; Winter, J. N. J. Chem. Soc., Chem. Commun. 1978, 545–546. doi:10.1039/c39780000545
Return to citation in text: [1] -
Saegusa, T.; Ito, Y.; Yasuda, N.; Hotaka, T. J. Org. Chem. 1970, 35, 4238–4240. doi:10.1021/jo00837a625
Return to citation in text: [1] [2] -
Yamamoto, Y.; Ogawa, A. Chem. – Asian J. 2023, 18, e202201269. doi:10.1002/asia.202201269
Return to citation in text: [1] [2] -
Satgé, J.; Couret, C.; Escudié, J. J. Organomet. Chem. 1972, 34, 83–92. doi:10.1016/s0022-328x(00)88673-8
Return to citation in text: [1] -
George, T. A.; Lappert, M. F. J. Organomet. Chem. 1968, 14, 327–337. doi:10.1016/s0022-328x(00)87672-x
Return to citation in text: [1] -
Chatgilialoglu, C.; Ingold, K. U.; Scaiano, J. C. J. Am. Chem. Soc. 1983, 105, 3292–3296. doi:10.1021/ja00348a055
Return to citation in text: [1] -
Saegusa, T.; Kobayashi, S.; Ito, Y.; Yasuda, N. J. Am. Chem. Soc. 1968, 90, 4182. doi:10.1021/ja01017a061
Return to citation in text: [1] -
Ballestri, M.; Chatgilialoglu, C.; Clark, K. B.; Griller, D.; Giese, B.; Kopping, B. J. Org. Chem. 1991, 56, 678–683. doi:10.1021/jo00002a035
Return to citation in text: [1] -
Bresadola, S.; Rossetto, F.; Puosi, G. Tetrahedron Lett. 1965, 6, 4775–4778. doi:10.1016/s0040-4039(01)89033-x
Return to citation in text: [1] -
Bresadola, S.; Carraro, G.; Pecile, C.; Turco, A. Tetrahedron Lett. 1964, 5, 3185–3188. doi:10.1016/0040-4039(64)83131-2
Return to citation in text: [1] -
Green, I. G.; Hudson, R. L.; Roberts, B. P. J. Chem. Soc., Perkin Trans. 2 1987, 1773–1779. doi:10.1039/p29870001773
Return to citation in text: [1] -
Teichmann, J.; Stock, H.; Pritzkow, H.; Siebert, W. Eur. J. Inorg. Chem. 1998, 459–463. doi:10.1002/(sici)1099-0682(199804)1998:4<459::aid-ejic459>3.0.co;2-u
Return to citation in text: [1] -
Katsuma, Y.; Tsukahara, N.; Wu, L.; Lin, Z.; Yamashita, M. Angew. Chem., Int. Ed. 2018, 57, 6109–6114. doi:10.1002/anie.201800878
Return to citation in text: [1] -
Yamashita, M. J. Synth. Org. Chem., Jpn. 2018, 76, 1223–1231. doi:10.5059/yukigoseikyokaishi.76.1223
Return to citation in text: [1] -
Ekkert, O.; Miera, G. G.; Wiegand, T.; Eckert, H.; Schirmer, B.; Petersen, J. L.; Daniliuc, C. G.; Fröhlich, R.; Grimme, S.; Kehr, G.; Erker, G. Chem. Sci. 2013, 4, 2657–2664. doi:10.1039/c3sc00082f
Return to citation in text: [1] -
Cardenas, A. J. P.; Hasegawa, Y.; Kehr, G.; Warren, T. H.; Erker, G. Coord. Chem. Rev. 2016, 306, 468–482. doi:10.1016/j.ccr.2015.01.006
Return to citation in text: [1] -
Jiao, Z.; Jaunich, K. T.; Tao, T.; Gottschall, O.; Hughes, M. M.; Turlik, A.; Schuppe, A. W. Angew. Chem., Int. Ed. 2024, 63, e202405779. doi:10.1002/anie.202405779
Return to citation in text: [1] -
Fukuyama, T.; Chen, X.; Peng, G. J. Am. Chem. Soc. 1994, 116, 3127–3128. doi:10.1021/ja00086a054
Return to citation in text: [1] -
Stork, G.; Sher, P. M. J. Am. Chem. Soc. 1983, 105, 6765–6766. doi:10.1021/ja00360a062
Return to citation in text: [1] -
Curran, D. P.; Liu, H. J. Am. Chem. Soc. 1991, 113, 2127–2132. doi:10.1021/ja00006a033
Return to citation in text: [1] -
Bachi, M. D.; Balanov, A.; Bar-Ner, N. J. Org. Chem. 1994, 59, 7752–7758. doi:10.1021/jo00104a035
Return to citation in text: [1] -
Bachi, M. D.; Bar-Ner, N.; Melman, A. J. Org. Chem. 1996, 61, 7116–7124. doi:10.1021/jo9607875
Return to citation in text: [1] -
Rainier, J. D.; Kennedy, A. R. J. Org. Chem. 2000, 65, 6213–6216. doi:10.1021/jo000831n
Return to citation in text: [1] -
Mitamura, T.; Iwata, K.; Ogawa, A. J. Org. Chem. 2011, 76, 3880–3887. doi:10.1021/jo200299d
Return to citation in text: [1] -
Li, D.; Mao, T.; Huang, J.; Zhu, Q. Org. Lett. 2017, 19, 3223–3226. doi:10.1021/acs.orglett.7b01339
Return to citation in text: [1] -
Yang, W.-C.; Wei, K.; Sun, X.; Zhu, J.; Wu, L. Org. Lett. 2018, 20, 3144–3147. doi:10.1021/acs.orglett.8b01278
Return to citation in text: [1] -
Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2016, 55, 11660–11663. doi:10.1002/anie.201606023
Return to citation in text: [1] [2] -
Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638–4641. doi:10.1021/acs.orglett.6b02271
Return to citation in text: [1] [2] -
Liu, Y.; Chen, X.-L.; Zeng, F.-L.; Sun, K.; Qu, C.; Fan, L.-L.; An, Z.-L.; Li, R.; Jing, C.-F.; Wei, S.-K.; Qu, L.-B.; Yu, B.; Sun, Y.-Q.; Zhao, Y.-F. J. Org. Chem. 2018, 83, 11727–11735. doi:10.1021/acs.joc.8b01657
Return to citation in text: [1] [2] -
Tran, C. C.; Kawaguchi, S.-i.; Sato, F.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2020, 85, 7258–7266. doi:10.1021/acs.joc.0c00647
Return to citation in text: [1] [2] -
Ogawa, A.; Tamai, T.; Mitamura, T.; Nomoto, A. Pure Appl. Chem. 2013, 85, 785–799. doi:10.1351/pac-con-12-07-01
Return to citation in text: [1] -
Mitamura, T.; Iwata, K.; Ogawa, A. Org. Lett. 2009, 11, 3422–3424. doi:10.1021/ol901267h
Return to citation in text: [1] -
Mitamura, T.; Iwata, K.; Nomoto, A.; Ogawa, A. Org. Biomol. Chem. 2011, 9, 3768–3775. doi:10.1039/c0ob01168a
Return to citation in text: [1] [2] -
Mitamura, T.; Ogawa, A. J. Org. Chem. 2011, 76, 1163–1166. doi:10.1021/jo1021772
Return to citation in text: [1] -
Mitamura, T.; Ogawa, A. Bull. Chem. Soc. Jpn. 2011, 84, 791–793. doi:10.1246/bcsj.20110041
Return to citation in text: [1] -
Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a
Return to citation in text: [1] -
Doraghi, F.; Amini, A.; Ghanbarlou, M.; Larijani, B.; Mahdavi, M. Mol. Diversity 2024, 28, 419–435. doi:10.1007/s11030-023-10743-2
Return to citation in text: [1] -
Nanni, D.; Pareschi, P.; Rizzoli, C.; Sgarabotto, P.; Tundo, A. Tetrahedron 1995, 51, 9045–9062. doi:10.1016/0040-4020(95)00348-c
Return to citation in text: [1] -
Tobisu, M.; Koh, K.; Furukawa, T.; Chatani, N. Angew. Chem., Int. Ed. 2012, 51, 11363–11366. doi:10.1002/anie.201206115
Return to citation in text: [1] -
Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77–80. doi:10.1126/science.1161976
Return to citation in text: [1] -
Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. doi:10.1021/ja805387f
Return to citation in text: [1] -
Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756–8757. doi:10.1021/ja9033582
Return to citation in text: [1] -
Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237–4352. doi:10.1021/acs.chemrev.2c00478
Return to citation in text: [1] -
Plesniak, M. P.; Huang, H.-M.; Procter, D. J. Nat. Rev. Chem. 2017, 1, 0077. doi:10.1038/s41570-017-0077
Return to citation in text: [1] -
Jiang, H.; Cheng, Y.; Wang, R.; Zheng, M.; Zhang, Y.; Yu, S. Angew. Chem., Int. Ed. 2013, 52, 13289–13292. doi:10.1002/anie.201308376
Return to citation in text: [1] -
Teng, F.; Hu, W.; Hu, H.; Luo, S.; Zhu, Q. Adv. Synth. Catal. 2019, 361, 1414–1418. doi:10.1002/adsc.201801623
Return to citation in text: [1] -
Liu, S.; Fan, F.; Wang, N.; Yuan, D.; Wang, Y.; Luo, Z.; Liu, Z.-Q. Adv. Synth. Catal. 2019, 361, 3086–3093. doi:10.1002/adsc.201900221
Return to citation in text: [1] -
Luo, Z.; Li, R.; Zhu, T.; Liu, C.-F.; Feng, H.; Aboagye Nartey, K.; Liu, Q.; Xu, X. Asian J. Org. Chem. 2021, 10, 926–930. doi:10.1002/ajoc.202100112
Return to citation in text: [1] -
Yuan, S.; Liu, Y.; Ni, M.; Hao, T.; Peng, Y.; Ding, Q. Chem. Commun. 2022, 58, 10985–10988. doi:10.1039/d2cc04348c
Return to citation in text: [1] -
Xiang, H.; Yu, Z.; Xie, T.; Ye, X.-Y.; Ye, Y. Eur. J. Org. Chem. 2022, e202200937. doi:10.1002/ejoc.202200937
Return to citation in text: [1] -
Wang, S.; Jia, W.-L.; Wang, L.; Liu, Q. Eur. J. Org. Chem. 2015, 6817–6821. doi:10.1002/ejoc.201500988
Return to citation in text: [1] -
Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Adv. Synth. Catal. 2015, 357, 3681–3686. doi:10.1002/adsc.201500674
Return to citation in text: [1] -
Qin, W.-B.; Xiong, W.; Li, X.; Chen, J.-Y.; Lin, L.-T.; Wong, H. N. C.; Liu, G.-K. J. Org. Chem. 2020, 85, 10479–10487. doi:10.1021/acs.joc.0c00816
Return to citation in text: [1] -
Zhang, J.; Xu, W.; Qu, Y.; Liu, Y.; Li, Y.; Song, H.; Wang, Q. Chem. Commun. 2020, 56, 15212–15215. doi:10.1039/d0cc06645a
Return to citation in text: [1] -
Wang, L.; Sha, W.; Dai, Q.; Feng, X.; Wu, W.; Peng, H.; Chen, B.; Cheng, J. Org. Lett. 2014, 16, 2088–2091. doi:10.1021/ol500277u
Return to citation in text: [1] -
He, Z.; Bae, M.; Wu, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 14451–14455. doi:10.1002/anie.201408522
Return to citation in text: [1] -
Wan, W.; Xu, X.; Chen, Y.; Jiang, H.; Wang, Y.; Deng, H.; Hao, J. Eur. J. Org. Chem. 2017, 3145–3151. doi:10.1002/ejoc.201700470
Return to citation in text: [1] -
Li, Y.; Qiu, G.; Ding, Q.; Wu, J. Tetrahedron 2014, 70, 4652–4656. doi:10.1016/j.tet.2014.05.039
Return to citation in text: [1] -
Liu, Y.; Chen, X.-L.; Li, X.-Y.; Zhu, S.-S.; Li, S.-J.; Song, Y.; Qu, L.-B.; Yu, B. J. Am. Chem. Soc. 2021, 143, 964–972. doi:10.1021/jacs.0c11138
Return to citation in text: [1] -
Wu, H.-Y.; Cao, Z.; Li, S.-Q.; Fu, Y.-W.; Li, J.-M.; Li, X.-H.; He, C.-M.; Chen, J.-Y. J. Org. Chem. 2023, 88, 17322–17329. doi:10.1021/acs.joc.3c02152
Return to citation in text: [1] -
Liu, S.; Huang, Y.; Wang, J.; Qing, F.-L.; Xu, X.-H. J. Am. Chem. Soc. 2022, 144, 1962–1970. doi:10.1021/jacs.1c12467
Return to citation in text: [1] -
Singh, M.; Yadav, A. K.; Yadav, L. D. S.; Singh, R. K. P. Tetrahedron Lett. 2018, 59, 3198–3201. doi:10.1016/j.tetlet.2018.07.024
Return to citation in text: [1] -
Liu, Y.; Li, J.-L.; Liu, X.-G.; Wu, J.-Q.; Huang, Z.-S.; Li, Q.; Wang, H. Org. Lett. 2021, 23, 1891–1897. doi:10.1021/acs.orglett.1c00309
Return to citation in text: [1] -
Zhu, T.-H.; Wang, S.-Y.; Tao, Y.-Q.; Wei, T.-Q.; Ji, S.-J. Org. Lett. 2014, 16, 1260–1263. doi:10.1021/ol500286x
Return to citation in text: [1]
| 41. | George, T. A.; Lappert, M. F. J. Organomet. Chem. 1968, 14, 327–337. doi:10.1016/s0022-328x(00)87672-x |
| 42. | Chatgilialoglu, C.; Ingold, K. U.; Scaiano, J. C. J. Am. Chem. Soc. 1983, 105, 3292–3296. doi:10.1021/ja00348a055 |
| 38. | Saegusa, T.; Ito, Y.; Yasuda, N.; Hotaka, T. J. Org. Chem. 1970, 35, 4238–4240. doi:10.1021/jo00837a625 |
| 43. | Saegusa, T.; Kobayashi, S.; Ito, Y.; Yasuda, N. J. Am. Chem. Soc. 1968, 90, 4182. doi:10.1021/ja01017a061 |
| 53. | Jiao, Z.; Jaunich, K. T.; Tao, T.; Gottschall, O.; Hughes, M. M.; Turlik, A.; Schuppe, A. W. Angew. Chem., Int. Ed. 2024, 63, e202405779. doi:10.1002/anie.202405779 |
| 8. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 54. | Fukuyama, T.; Chen, X.; Peng, G. J. Am. Chem. Soc. 1994, 116, 3127–3128. doi:10.1021/ja00086a054 |
| 55. | Stork, G.; Sher, P. M. J. Am. Chem. Soc. 1983, 105, 6765–6766. doi:10.1021/ja00360a062 |
| 56. | Curran, D. P.; Liu, H. J. Am. Chem. Soc. 1991, 113, 2127–2132. doi:10.1021/ja00006a033 |
| 49. | Katsuma, Y.; Tsukahara, N.; Wu, L.; Lin, Z.; Yamashita, M. Angew. Chem., Int. Ed. 2018, 57, 6109–6114. doi:10.1002/anie.201800878 |
| 50. | Yamashita, M. J. Synth. Org. Chem., Jpn. 2018, 76, 1223–1231. doi:10.5059/yukigoseikyokaishi.76.1223 |
| 51. | Ekkert, O.; Miera, G. G.; Wiegand, T.; Eckert, H.; Schirmer, B.; Petersen, J. L.; Daniliuc, C. G.; Fröhlich, R.; Grimme, S.; Kehr, G.; Erker, G. Chem. Sci. 2013, 4, 2657–2664. doi:10.1039/c3sc00082f |
| 52. | Cardenas, A. J. P.; Hasegawa, Y.; Kehr, G.; Warren, T. H.; Erker, G. Coord. Chem. Rev. 2016, 306, 468–482. doi:10.1016/j.ccr.2015.01.006 |
| 47. | Green, I. G.; Hudson, R. L.; Roberts, B. P. J. Chem. Soc., Perkin Trans. 2 1987, 1773–1779. doi:10.1039/p29870001773 |
| 48. | Teichmann, J.; Stock, H.; Pritzkow, H.; Siebert, W. Eur. J. Inorg. Chem. 1998, 459–463. doi:10.1002/(sici)1099-0682(199804)1998:4<459::aid-ejic459>3.0.co;2-u |
| 44. | Ballestri, M.; Chatgilialoglu, C.; Clark, K. B.; Griller, D.; Giese, B.; Kopping, B. J. Org. Chem. 1991, 56, 678–683. doi:10.1021/jo00002a035 |
| 45. | Bresadola, S.; Rossetto, F.; Puosi, G. Tetrahedron Lett. 1965, 6, 4775–4778. doi:10.1016/s0040-4039(01)89033-x |
| 46. | Bresadola, S.; Carraro, G.; Pecile, C.; Turco, A. Tetrahedron Lett. 1964, 5, 3185–3188. doi:10.1016/0040-4039(64)83131-2 |
| 57. | Bachi, M. D.; Balanov, A.; Bar-Ner, N. J. Org. Chem. 1994, 59, 7752–7758. doi:10.1021/jo00104a035 |
| 58. | Bachi, M. D.; Bar-Ner, N.; Melman, A. J. Org. Chem. 1996, 61, 7116–7124. doi:10.1021/jo9607875 |
| 59. | Rainier, J. D.; Kennedy, A. R. J. Org. Chem. 2000, 65, 6213–6216. doi:10.1021/jo000831n |
| 65. | Liu, Y.; Chen, X.-L.; Zeng, F.-L.; Sun, K.; Qu, C.; Fan, L.-L.; An, Z.-L.; Li, R.; Jing, C.-F.; Wei, S.-K.; Qu, L.-B.; Yu, B.; Sun, Y.-Q.; Zhao, Y.-F. J. Org. Chem. 2018, 83, 11727–11735. doi:10.1021/acs.joc.8b01657 |
| 66. | Tran, C. C.; Kawaguchi, S.-i.; Sato, F.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2020, 85, 7258–7266. doi:10.1021/acs.joc.0c00647 |
| 63. | Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2016, 55, 11660–11663. doi:10.1002/anie.201606023 |
| 64. | Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638–4641. doi:10.1021/acs.orglett.6b02271 |
| 62. | Yang, W.-C.; Wei, K.; Sun, X.; Zhu, J.; Wu, L. Org. Lett. 2018, 20, 3144–3147. doi:10.1021/acs.orglett.8b01278 |
| 63. | Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2016, 55, 11660–11663. doi:10.1002/anie.201606023 |
| 64. | Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638–4641. doi:10.1021/acs.orglett.6b02271 |
| 65. | Liu, Y.; Chen, X.-L.; Zeng, F.-L.; Sun, K.; Qu, C.; Fan, L.-L.; An, Z.-L.; Li, R.; Jing, C.-F.; Wei, S.-K.; Qu, L.-B.; Yu, B.; Sun, Y.-Q.; Zhao, Y.-F. J. Org. Chem. 2018, 83, 11727–11735. doi:10.1021/acs.joc.8b01657 |
| 66. | Tran, C. C.; Kawaguchi, S.-i.; Sato, F.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2020, 85, 7258–7266. doi:10.1021/acs.joc.0c00647 |
| 60. | Mitamura, T.; Iwata, K.; Ogawa, A. J. Org. Chem. 2011, 76, 3880–3887. doi:10.1021/jo200299d |
| 61. | Li, D.; Mao, T.; Huang, J.; Zhu, Q. Org. Lett. 2017, 19, 3223–3226. doi:10.1021/acs.orglett.7b01339 |
| 67. | Ogawa, A.; Tamai, T.; Mitamura, T.; Nomoto, A. Pure Appl. Chem. 2013, 85, 785–799. doi:10.1351/pac-con-12-07-01 |
| 68. | Mitamura, T.; Iwata, K.; Ogawa, A. Org. Lett. 2009, 11, 3422–3424. doi:10.1021/ol901267h |
| 69. | Mitamura, T.; Iwata, K.; Nomoto, A.; Ogawa, A. Org. Biomol. Chem. 2011, 9, 3768–3775. doi:10.1039/c0ob01168a |
| 70. | Mitamura, T.; Ogawa, A. J. Org. Chem. 2011, 76, 1163–1166. doi:10.1021/jo1021772 |
| 39. | Yamamoto, Y.; Ogawa, A. Chem. – Asian J. 2023, 18, e202201269. doi:10.1002/asia.202201269 |
| 1. | Gabrielu, B. Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry; Wiley-VCH: Weinheim, Germany, 2021. doi:10.1002/9783527829354 |
| 2. | Singh, J.; Sharma, S.; Sharma, A. J. Org. Chem. 2021, 86, 24–48. doi:10.1021/acs.joc.0c02205 |
| 12. | Gomes, G. d. P.; Loginova, Y.; Vatsadze, S. Z.; Alabugin, I. V. J. Am. Chem. Soc. 2018, 140, 14272–14288. doi:10.1021/jacs.8b08513 |
| 28. | Tsuchii, K.; Tsuboi, Y.; Kawaguchi, S.-i.; Takahashi, J.; Sonoda, N.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2007, 72, 415–423. doi:10.1021/jo061704f |
| 81. | Jiang, H.; Cheng, Y.; Wang, R.; Zheng, M.; Zhang, Y.; Yu, S. Angew. Chem., Int. Ed. 2013, 52, 13289–13292. doi:10.1002/anie.201308376 |
| 9. | Zhang, S.; Wang, Y.; Huang, H.; Cao, D. Angew. Chem., Int. Ed. 2023, 62, e202308524. doi:10.1002/anie.202308524 |
| 10. | Čamdžić, L.; Stache, E. E. J. Am. Chem. Soc. 2023, 145, 20311–20318. doi:10.1021/jacs.3c04595 |
| 11. | Liu, N.; Zhou, L.; Wu, Z.-Q. Acc. Chem. Res. 2021, 54, 3953–3967. doi:10.1021/acs.accounts.1c00489 |
| 8. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 25. | Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A. J. Org. Chem. 2004, 69, 6658–6665. doi:10.1021/jo0495889 |
| 26. | Lei, J.; Wu, X.; Zhu, Q. Org. Lett. 2015, 17, 2322–2325. doi:10.1021/acs.orglett.5b00730 |
| 75. | Tobisu, M.; Koh, K.; Furukawa, T.; Chatani, N. Angew. Chem., Int. Ed. 2012, 51, 11363–11366. doi:10.1002/anie.201206115 |
| 3. | Collet, J. W.; Roose, T. R.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2020, 59, 540–558. doi:10.1002/anie.201905838 |
| 4. | Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103–1123. doi:10.1039/c6cs00384b |
| 5. | Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c |
| 6. | Neo, A. G.; Ramiro, J. L.; García-Valverde, M.; Díaz, J.; Marcos, C. F. Mol. Diversity 2024, 28, 335–418. doi:10.1007/s11030-023-10641-7 |
| 7. | Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a |
| 27. | Saegusa, T.; Kobayashi, S.; Ito, Y. J. Org. Chem. 1970, 35, 2118–2121. doi:10.1021/jo00832a003 |
| 76. | Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77–80. doi:10.1126/science.1161976 |
| 77. | Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. doi:10.1021/ja805387f |
| 78. | Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756–8757. doi:10.1021/ja9033582 |
| 79. | Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237–4352. doi:10.1021/acs.chemrev.2c00478 |
| 80. | Plesniak, M. P.; Huang, H.-M.; Procter, D. J. Nat. Rev. Chem. 2017, 1, 0077. doi:10.1038/s41570-017-0077 |
| 19. | Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035–10074. doi:10.1021/acs.chemrev.6b00018 |
| 20. | Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057 |
| 23. | Kühle, E.; Anders, B.; Zumach, G. Angew. Chem. 1967, 79, 663–680. doi:10.1002/ange.19670791504 |
| 72. | Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a |
| 73. | Doraghi, F.; Amini, A.; Ghanbarlou, M.; Larijani, B.; Mahdavi, M. Mol. Diversity 2024, 28, 419–435. doi:10.1007/s11030-023-10743-2 |
| 17. | Yamamoto, Y.; Ogawa, A. Molecules 2023, 28, 787. doi:10.3390/molecules28020787 |
| 18. | Ogawa, A.; Yamamoto, Y. Molecules 2023, 28, 6356. doi:10.3390/molecules28176356 |
| 24. | Tanaka, S.; Okano, M.; Tanimoto, S. Bull. Chem. Soc. Jpn. 1975, 48, 1862–1864. doi:10.1246/bcsj.48.1862 |
| 74. | Nanni, D.; Pareschi, P.; Rizzoli, C.; Sgarabotto, P.; Tundo, A. Tetrahedron 1995, 51, 9045–9062. doi:10.1016/0040-4020(95)00348-c |
| 69. | Mitamura, T.; Iwata, K.; Nomoto, A.; Ogawa, A. Org. Biomol. Chem. 2011, 9, 3768–3775. doi:10.1039/c0ob01168a |
| 13. | Taniguchi, T. Synthesis 2017, 49, 3511–3534. doi:10.1055/s-0036-1588481 |
| 14. | Dénès, F. Heteroatom-Centred Radicals for the Synthesis of Heterocyclic Compounds. In Free-Radical Synthesis and Functionaization of Heterocycles; Landais, Y., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Vol. 54, pp 151–230. doi:10.1007/7081_2018_19 |
| 15. | Linker, T. Chemistry (Basel, Switz.) 2020, 2, 80–92. doi:10.3390/chemistry2010008 |
| 21. | Russo, C.; Brunelli, F.; Tron, G. C.; Giustiniano, M. Chem. – Eur. J. 2023, 29, e202203150. doi:10.1002/chem.202203150 |
| 22. | Russo, C.; Brunelli, F.; Tron, G. C.; Giustiniano, M. Eur. J. Org. Chem. 2023, 26, e202300743. doi:10.1002/ejoc.202300743 |
| 71. | Mitamura, T.; Ogawa, A. Bull. Chem. Soc. Jpn. 2011, 84, 791–793. doi:10.1246/bcsj.20110041 |
| 32. | Yamamoto, Y.; Chen, Q.; Ogawa, A. Molecules 2023, 28, 2450. doi:10.3390/molecules28062450 |
| 30. | Mitamura, T.; Tsuboi, Y.; Iwata, K.; Tsuchii, K.; Nomoto, A.; Sonoda, M.; Ogawa, A. Tetrahedron Lett. 2007, 48, 5953–5957. doi:10.1016/j.tetlet.2007.06.126 |
| 31. | Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069 |
| 91. | Wang, L.; Sha, W.; Dai, Q.; Feng, X.; Wu, W.; Peng, H.; Chen, B.; Cheng, J. Org. Lett. 2014, 16, 2088–2091. doi:10.1021/ol500277u |
| 92. | He, Z.; Bae, M.; Wu, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 14451–14455. doi:10.1002/anie.201408522 |
| 93. | Wan, W.; Xu, X.; Chen, Y.; Jiang, H.; Wang, Y.; Deng, H.; Hao, J. Eur. J. Org. Chem. 2017, 3145–3151. doi:10.1002/ejoc.201700470 |
| 94. | Li, Y.; Qiu, G.; Ding, Q.; Wu, J. Tetrahedron 2014, 70, 4652–4656. doi:10.1016/j.tet.2014.05.039 |
| 82. | Teng, F.; Hu, W.; Hu, H.; Luo, S.; Zhu, Q. Adv. Synth. Catal. 2019, 361, 1414–1418. doi:10.1002/adsc.201801623 |
| 83. | Liu, S.; Fan, F.; Wang, N.; Yuan, D.; Wang, Y.; Luo, Z.; Liu, Z.-Q. Adv. Synth. Catal. 2019, 361, 3086–3093. doi:10.1002/adsc.201900221 |
| 84. | Luo, Z.; Li, R.; Zhu, T.; Liu, C.-F.; Feng, H.; Aboagye Nartey, K.; Liu, Q.; Xu, X. Asian J. Org. Chem. 2021, 10, 926–930. doi:10.1002/ajoc.202100112 |
| 85. | Yuan, S.; Liu, Y.; Ni, M.; Hao, T.; Peng, Y.; Ding, Q. Chem. Commun. 2022, 58, 10985–10988. doi:10.1039/d2cc04348c |
| 86. | Xiang, H.; Yu, Z.; Xie, T.; Ye, X.-Y.; Ye, Y. Eur. J. Org. Chem. 2022, e202200937. doi:10.1002/ejoc.202200937 |
| 21. | Russo, C.; Brunelli, F.; Tron, G. C.; Giustiniano, M. Chem. – Eur. J. 2023, 29, e202203150. doi:10.1002/chem.202203150 |
| 87. | Wang, S.; Jia, W.-L.; Wang, L.; Liu, Q. Eur. J. Org. Chem. 2015, 6817–6821. doi:10.1002/ejoc.201500988 |
| 88. | Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Adv. Synth. Catal. 2015, 357, 3681–3686. doi:10.1002/adsc.201500674 |
| 89. | Qin, W.-B.; Xiong, W.; Li, X.; Chen, J.-Y.; Lin, L.-T.; Wong, H. N. C.; Liu, G.-K. J. Org. Chem. 2020, 85, 10479–10487. doi:10.1021/acs.joc.0c00816 |
| 90. | Zhang, J.; Xu, W.; Qu, Y.; Liu, Y.; Li, Y.; Song, H.; Wang, Q. Chem. Commun. 2020, 56, 15212–15215. doi:10.1039/d0cc06645a |
| 39. | Yamamoto, Y.; Ogawa, A. Chem. – Asian J. 2023, 18, e202201269. doi:10.1002/asia.202201269 |
| 40. | Satgé, J.; Couret, C.; Escudié, J. J. Organomet. Chem. 1972, 34, 83–92. doi:10.1016/s0022-328x(00)88673-8 |
| 37. | Roberts, B. P.; Winter, J. N. J. Chem. Soc., Chem. Commun. 1978, 545–546. doi:10.1039/c39780000545 |
| 38. | Saegusa, T.; Ito, Y.; Yasuda, N.; Hotaka, T. J. Org. Chem. 1970, 35, 4238–4240. doi:10.1021/jo00837a625 |
| 33. | Ogawa, A.; Doi, M.; Tsuchii, K.; Hirao, T. Tetrahedron Lett. 2001, 42, 2317–2319. doi:10.1016/s0040-4039(01)00123-x |
| 99. | Liu, Y.; Li, J.-L.; Liu, X.-G.; Wu, J.-Q.; Huang, Z.-S.; Li, Q.; Wang, H. Org. Lett. 2021, 23, 1891–1897. doi:10.1021/acs.orglett.1c00309 |
| 34. | Miyazoe, H.; Yamago, S.; Yoshida, J.-i. Angew. Chem., Int. Ed. 2000, 39, 3669–3671. doi:10.1002/1521-3773(20001016)39:20<3669::aid-anie3669>3.0.co;2-4 |
| 35. | Yamago, S.; Miyazoe, H.; Goto, R.; Hashidume, M.; Sawazaki, T.; Yoshida, J.-i. J. Am. Chem. Soc. 2001, 123, 3697–3705. doi:10.1021/ja003879r |
| 36. | Yamago, S. Synlett 2004, 1875–1890. doi:10.1055/s-2004-830883 |
| 100. | Zhu, T.-H.; Wang, S.-Y.; Tao, Y.-Q.; Wei, T.-Q.; Ji, S.-J. Org. Lett. 2014, 16, 1260–1263. doi:10.1021/ol500286x |
| 31. | Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069 |
| 95. | Liu, Y.; Chen, X.-L.; Li, X.-Y.; Zhu, S.-S.; Li, S.-J.; Song, Y.; Qu, L.-B.; Yu, B. J. Am. Chem. Soc. 2021, 143, 964–972. doi:10.1021/jacs.0c11138 |
| 96. | Wu, H.-Y.; Cao, Z.; Li, S.-Q.; Fu, Y.-W.; Li, J.-M.; Li, X.-H.; He, C.-M.; Chen, J.-Y. J. Org. Chem. 2023, 88, 17322–17329. doi:10.1021/acs.joc.3c02152 |
| 97. | Liu, S.; Huang, Y.; Wang, J.; Qing, F.-L.; Xu, X.-H. J. Am. Chem. Soc. 2022, 144, 1962–1970. doi:10.1021/jacs.1c12467 |
| 30. | Mitamura, T.; Tsuboi, Y.; Iwata, K.; Tsuchii, K.; Nomoto, A.; Sonoda, M.; Ogawa, A. Tetrahedron Lett. 2007, 48, 5953–5957. doi:10.1016/j.tetlet.2007.06.126 |
| 98. | Singh, M.; Yadav, A. K.; Yadav, L. D. S.; Singh, R. K. P. Tetrahedron Lett. 2018, 59, 3198–3201. doi:10.1016/j.tetlet.2018.07.024 |
© 2024 Ogawa and Yamamoto; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.