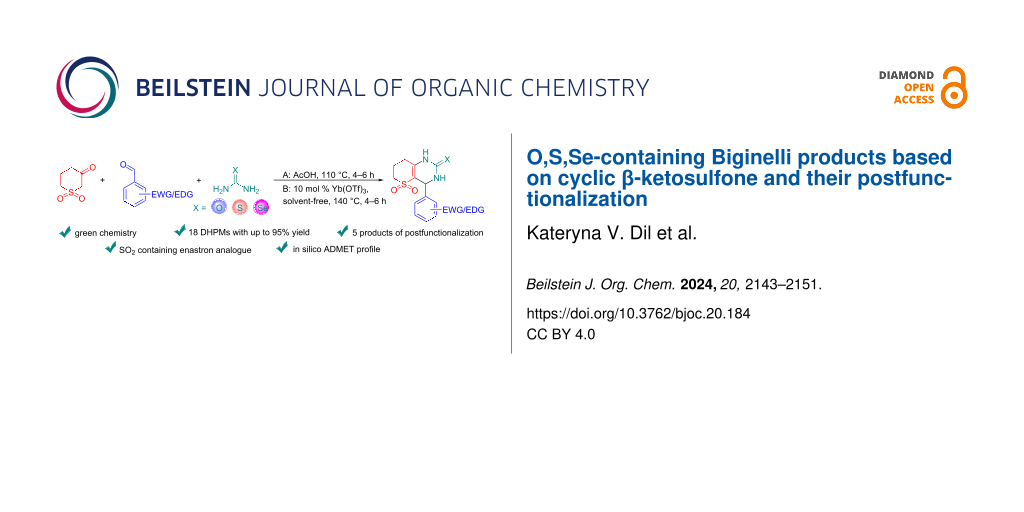
Abstract
A one-pot three-component Biginelli synthesis of dihydropyrimidinones/thiones/selenones via acetic acid or solvent-free Yb(OTf)3-catalyzed tandem reaction of β-ketosulfone (dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide), an appropriate urea, and arylaldehyde has been developed. The reaction proceeds with high chemo- and regioselectivity to give diverse DHPMs in reasonable yields up to 95%. Moreover, an SO2-containing analogue of anticancer drug-candidate enastron (SO2 vs C=O) was obtained by using the here reported method in gram scale. We also demonstrate the reactivity of the Biginelli product in various directions – synthesis of condensed thiazoles and tetrazoles. In silico assessment of ADMET parameters shows that most compounds meet the lead-likeness requirements. The biological profiles of new compounds demonstrate high probability levels of activity against the following pathogens/diseases: Candida albicans, Alphis gossypii, Tripomastigote Chagas, Tcruzi amastigota, Tcruzi epimastigota, Leishmania amazonensis, and Dengue larvicida.
Graphical Abstract
Introduction
Multicomponent reactions (MCRs) are the key methodology to access valuable heterocycles for medicinal chemistry projects. The classical Biginelli reaction (1893) is an acid-catalyzed, three-component reaction between an aldehyde, β-ketoester, and urea that produces 3,4-dihydropyrimidin-2(1H)-ones, also known as DHPMs (Scheme 1A). This reaction is believed to be one of the most famous MCRs with >2000 papers published in the last 20 years (according to Scopus database). These MCRs allow the direct synthesis of known DHMP drugs such as monastrol, piperastrol, enastron, fluorastrol etc. (Scheme 1B) and dozens of highly bioactive compounds with anticancer, antihypertensive, antiinflammatory, antioxidant, antimicrobial, antifungal, antimalarial, antitubercular, antidiabetic, antifilarial, anti-Alzheimer, antiepileptics and other activities [1-11]. The concept of "privileged structures" in medicinal chemistry highlights derivatives capable of interacting with multiple receptors or enzymes, making them ideal candidates for drug discovery. Dihydropyrimidinones (DHPMs) and their derivatives are particularly noteworthy within this category. Accordingly, their synthesis is of significant interest for organic and medicinal chemists. DHPMs are found in a variety of marine-sourced alkaloids, which are essential for creating biologically active natural products [10]. Some of the DHPM derivatives are also known as functional polymers, adhesives, and fabric dyes [8,12].
Scheme 1: The general Biginelli reaction (A) and examples of DHMP (B) and thiopyran-1,1-dioxide (C) containing drugs.
Scheme 1: The general Biginelli reaction (A) and examples of DHMP (B) and thiopyran-1,1-dioxide (C) containin...
In recent decades, the scope of the original Biginelli reaction shown in Scheme 1A was significantly extended by variation of the 1,3-dicarbonyl-containing building blocks. Many groups have elegantly demonstrated the synthetic versatility of numerous enolizable carbonyl components, including β-keto esters, cyclic/acyclic β-diketones, β-keto amides, coumarins, alicyclic ketones, β-ketophosphonates, α-nitroketones, curcumin, and barbituric acid derivatives [1,2,8,9]. We analyzed a number of Biginelli-type products and publications and concluded that Se-containing DHPMs among the rarest examples and, in addition to this, ketosulfones have never been used as enolizable carbonyl component in this chemistry. To the best of our knowledge only one compound from the target group was published (2003) [13] before this work, however, without any spectral evidence (Figure 1).
Figure 1: Number of aryl-substituted Biginelli-type products and publications as analyzed by Reaxys database. The search was performed using depicted substructures “on all atoms” (May 2024).
Figure 1: Number of aryl-substituted Biginelli-type products and publications as analyzed by Reaxys database....
Considering our constant interest in the development of methods for the synthesis of new S-heterocyclic systems we tried to combine the broad synthetic potential of Biginelli condensation and high reactivity β-ketosulfone 1 (dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide) in various condensation reactions [14-20]. It was also worth mentioning that the thiopyran-1,1-dioxide motif is presented in a number of biologically active compounds including important market drugs as antiglaucoma agent dorzolamide [21], diuretic/anticancer meticrane [22] and antiherpesvirus agent amenamevir (ASP-2151) [23] which was recently synthesized by Ugi-4CR [24] (Scheme 1C).
Results and Discussion
Reaction optimization
Over the past two decades more than 300 various catalytic systems have been proposed for Biginelli chemistry, e.g., simple inorganic and organic acids, metal salts, metal oxides, ionic liquids, phosphines, nanocatalysts, organocatalysts, ion exchange resins [1,4,9] or even visible light-driven methods [25-28].
We started our study from the optimization of the reaction conditions using β-ketosulfone 1, benzaldehyde and thiourea as model reaction. According to the literature, the reaction has been shown to work best and most efficiently under acidic conditions since such conditions enhance the selectivity, so various catalysts, mainly acidic, were tested for the model Biginelli reaction and the results are shown in Table 1.
Table 1: Optimization of reaction.
|
|
|||
| Entry | Conditionsа | Isolated yield 2a + 2a’, % |
Isomer ratio,
2a:2a’ |
| 1 | 0.8 equiv NaI, 0.8 equiv TMSCl, MeCN, 22 °C, 4 h | traces | n.d. |
| 2 | 4 equiv TMSCl, DMF, 60 °C, 14 h | traces | n.d. |
| 3 | 4 equiv TMSCl, DMF, −20 °C, 5 days | 23 | 2:1 |
| 4 | 4 equiv TMSCl, DMF, 22 °C, 3 days | 59 | 2:1.2 |
| 5 | 4 equiv TMSCl, DMF/MeCN (1:1), 22 °C, 2 days | 51 | 3:1 |
| 6b | 3 equiv TMSCl, DMF, 22 °C, 5 days | 72 | 2:1 |
| 7 | 3 equiv HСl, EtOH, 82 °C, 6 h | 23 | 1:0 |
| 8 | 10 mol % p-TsOH, MeOH, 82 °C, 36 h | 35 | 1:0 |
| 9 | 10 mol % CAN, EtOH, 82 °C, 18 h | 32 | 1:0 |
| 10 | 10 mol % SrCl2·6H2O, EtOH, 82 °C, 18 h | 8 | 1:0 |
| 11 | 10 mol % La(NO2)3·6H2O, solvent-free, 82 °С, 18 h | 61 | 1:0 |
| 12c | 10 mol % Yb(OTf)3, solvent-free, 140 °C, 6 h | 65 | 1:0 |
| 13c | AcOH, 110 °C, 14 h | 68 | 1:0 |
| 14 | AcOH, 82 °C, 14 h | 40 | 1:0 |
| 15 | AcOH, 110 °C, 4 h | 71 | 1:0 |
| 16c | AcOH, 110 °C, 4 h | 74 | 1:0 |
| 17d | TFA, 110 °C, 14 h | 45 | 1:0 |
| 18c | AcOH, MW (300 W), 120 °С, 20 min | 36 | 1:0 |
aUnless specifically stated the ratio of ketosulfone/benzaldehyde/thiourea 1:1:1 (1 mmol scale); bketosulfone (1 mmol, 1 equiv), benzaldehyde (2 mmol, 2 equiv), thiourea (1 mmol, 1 equiv); cketosulfone (1 mmol, 1 equiv), benzaldehyde (1 mmol, 1 equiv), thiourea (1.2 mmol, 1.2 equiv); dketosulfone (1 mmol, 1 equiv), benzaldehyde (1 mmol, 1 equiv), thiourea (3 mmol, 3 equiv).
One of the most effective promoters for this type of reaction is TMSCl [29-31] and we also tried to involve TMSCl in our study (Table 1, entries 1–6), but in any case, we received a mixture of regioisomeric products 2a/2a’ (3:1 to 2:1) confirmed by 2D NMR spectroscopy (see Supporting Information File 1, Figures S1–S6). Classical conditions for Biginelli reaction (reflux in ethanolic HCl) gave only 23% yield (Table 1, entry 7). Reflux in alcoholic media in the presence of p-toluenesulfonic acid or CAN (ceric ammonium nitrate) was also not particularly successful (Table 1, entries 8 and 9, yield 32–35%). We subsequently explored a range of reaction conditions to improve overall yield and selectivity. We experimented with Lewis acids (Table 1, entries 10–12) to catalyze the reaction and observe low conversion with SrCl2. The reaction was significantly better with both La(NO2)3 and Yb(OTf)3 under solvent-free conditions (Table 1, entries 11 and 12, yield 61–65%). The most promising results were obtained by using simplest heating in acetic acid (Table 1, entries 13–16). After some playing with temperature, reaction time, and ratio of starting reagents we ultimately found conditions (AcOH, 110 oC, 4 h) suitable best for our chemistry and leading to the yield of the target product 2a 74% (Table 1, entry 16). However, increasing acidity and using trifluoroacetic acid (Table 1, entry 17) did not improve the overall yield. We also tried microwave activation conditions since this is a known technique for reactions of this type [32], but unfortunately, we did not find any improvement in the yield (Table 1, entry 18).
Reaction scope
We then used optimized reaction conditions from Table 1, entry 16 (method A) and 12 (method B) to further explore the scope of the reaction (Scheme 2). By employing various EWG/EDG-substituted benzaldehydes and urea/thiourea/selenourea we synthesized novel Biginelli products 2a–q with up to 95% yield. The use of selenourea has been shown to give low yields of products (up to 33%). In fact, we have thus expanded the range of available selenium-containing DHPMs in addition to the work of other authors [33-35].
Scheme 2: Scope of the obtained Biginelli products 2a–q.
Scheme 2: Scope of the obtained Biginelli products 2a–q.
We also attempted to replace urea/thiourea/selenourea with N-alkyl/aryl-substituted analogues, and aldehyde component switch to heteroaromatic (2-pyridinaldehyde) and aliphatic (iPrCHO, cinnamaldehyde). Unfortunately, we failed in both replacements and were unable to obtain any reasonable products. The list of unsuccessful reagents is shown in Figure S7 (see Supporting Information File 1).
Next, we paid attention to testing the conditions we developed for the synthesis of the SO2-containing analogue 2r of potent anticancer drug enastron in gram scale (Scheme 3). Enastron is a novel dihydropyrimidine-based mitotic kinesin spindle protein KSP/Eg5 inhibitor [36]. We hope that compound 2r and its analogues obtained in this work can be further deeply studied by in silico and in vitro methods to discover the compound most suitable for clinical trials.
Scheme 3: Synthesis of SO2-containing enastron analogue 2r.
Scheme 3: Synthesis of SO2-containing enastron analogue 2r.
The structures of the synthesized compounds 2a–r were confirmed by spectral data. The 1H NMR spectra of the obtained products are characterized by the following signals: aromatic ring protons (6.25–8.23 ppm), Ar–CH group proton singlet (5.13–5.44 ppm), broadened NH singlets (7.78–10.89 ppm), as well as the corresponding signals of successive 3 × CH2 groups of the sulfone fragment (2.18–3.34 ppm). Such a set of signals clearly corresponds to the heterocycles depicted above. Figure S8 (see Supporting Information File 1) shows 1H NMR spectra for Ph-substituted O,S,Se-DHPMs 2a,h,o. It can be seen from them that when going from oxygen to selenium (2h→2a→2o), the signals of both NH groups shift to down field (7.87/9.26→9.75/10.50→10.26/10.85 ppm) and become more equivalent (Δδ = 1.39→0.75→0.59 ppm accordingly). The key signal in the 13C NMR spectra is located in the regions 174.04–174.69 ppm (C=S), 151.44–151.86 ppm (C=O), and 170.15–170.91 ppm (C=Se) accordingly.
Utilization of reaction products
The Biginelli reaction is the traditional method for synthesizing DHPM scaffolds, but it faces limitations in product diversity. To overcome these challenges, two main strategies have been developed. The first strategy involves modifying the conventional components of the Biginelli chemistry, while the second focuses on the postmodification of the Biginelli products [2]. Both approaches were tested in this work. We used Hantzsch-type thiazole synthesis for postmodification of product 2a. By employing 2-bromoacetophenone, bromomalononitrile and 2-bromo-1-tetralone we obtained condensed thiazoles 3–5 in 67–88% yields using slightly modified methods described [37-39]. We also applied desulfurization to obtain products 6 and 7 [40,41] (Scheme 4).
Scheme 4: Postmodification of the Biginelli product 2a.
Scheme 4: Postmodification of the Biginelli product 2a.
While the Hantzsch thiazole synthesis is well documented from a synthetic and mechanistic point [42,43] and do not need discussion, more desulfurizations and oxidations of rare Biginelli products are discussed in Supporting Information File 1 using compound 2a as an example.
In silico evaluation of ADMET parameters and biological profile
We performed in silico screening of the biological properties of Biginelli products 2a–r and their postfunctionalized derivatives 3–7 using SwissADME (http://www.swissadme.ch) [44], ProTox 3.0 (https://tox-new.charite.de) [45], and MolPredictX (https://www.molpredictx.ufpb.br) [46] free online software. Considering ADMET [47] and other crucial properties, we found that all compounds (except for the nitro derivative 2e) do not violate the Lipinski, Ghose, Veber, Egan, and Muegge rules and PAINS filter [48-52]. The lipophilicity (estimated as log Po/w) for all compounds was shown to be in a wide range from 0.43 to 4.11. The topological polar surface area (TPSA), which is important for oral bioavailability, was found to be 67–129 Å2 for all compounds except products 4 and 2e. None of the compounds penetrate the BBB (blood–brain barrier) except for seleno-2p and desulfurized product 7. Regarding water solubility, we can conclude that all compounds are either soluble or moderately soluble. For more details, see Supporting Information File 1 (Table S1). To visualize the lead-likeness of compounds 2a–r, 3–7, we utilized the free online software LLAMA (https://llama.leeds.ac.uk) [53], which showed that 70% of the products (16 of 23) fall within the specific lead-like space (Figure 2).
![[1860-5397-20-184-2]](/bjoc/content/figures/1860-5397-20-184-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Distribution of compounds 2a–r, 3–7 (log P (y)–MW (x)) through LLAMA software. The chemical structure of a representative compound 2m is shown.
Figure 2: Distribution of compounds 2a–r, 3–7 (log P (y)–MW (x)) through LLAMA software. The chemical structu...
We then used the free online software ProTox 3.0 for computational toxicity assessment of the products as their LD50 values. The tested compounds mainly belong to the 4th class of acute oral toxicity with 300 < LD50 ≤ 2000 mg/kg. In addition, we used MolPredictX (https://www.molpredictx.ufpb.br) [46] to evaluate potential biotargets (pathogens, species, diseases) for new synthesized compounds. If we focus on high levels of biological activity probability (80% and above), the following pathogens and diseases may be potential areas of interest: Alphis gossypii, Tripomastigote Chagas, Candida albicans, Tcruzi amastigota, Leishmania amazonensis, Tcruzi epimastigota, Dengue larvicida, and for selected cases Alzheimer and Sars-COVID. For more details, see Table S1 (Supporting Information File 1).
Conclusion
In summary, we have demonstrated the catalytic regioselective Biginelli synthesis of new S-heterocyclic systems ‒ 4-aryl-4,6,7,8-tetrahydro-1H-thiopyrano[3,2-d]pyrimidine-2(3H)-one/thione/selenone 5,5-dioxides and some of their derivatives. Furthermore, this methodology was successfully applied for the synthesis of the SO2-containing analogue of the anticancer drug-candidate enastron (SO2 vs C=O), and we believe a multitude of other sulfones of both synthetic and biological importance can be obtained by using the in this work reported efficient, multicomponent and green protocol. We postfunctionalized the typical Biginelli product using Hantzsch-type thiazole chemistry and desulfurization. Assessing drug-likeness, we found that most of the synthesized compounds correspond to the parameters established by the Lipinski, Ghose, Veber, Egan, and Muegge rules. In silico screening of their biological profiles indicated that these new derivatives fall into the 4th class of acute toxicity. Additionally, they exhibit potential high activity against diseases associated with these species: Alphis gossypii, Tripomastigote Chagas, Candida albicans, Tcruzi amastigota, Leishmania amazonensis, Tcruzi epimastigota, Dengue larvicida, and for selected cases Alzheimer and Sars-COVID.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of new compounds. | ||
| Format: PDF | Size: 12.9 MB | Download |
Acknowledgements
The authors are grateful to Enamine Ltd. (Kyiv, Ukraine) for NMR/HRMS support. The authors also thank Charite University of Medicine, Germany (Institute for Physiology, Structural Bioinformatics Group), Federal University of Paraíba, Brazil (Laboratory of Cheminformatics), University of Lausanne, Switzerland (SIB Swiss Institute of Bioinformatics), and the University of Leeds, UK (School of Chemistry) for free online cheminformatics software. V.A.P. would like to express special thanks to the Matsumae International Foundation for financial support for the presentation of this work at the 15th International Kyoto Conference on New Aspects of Organic Chemistry (IKCOC-15, November 20–23, 2023, Kyoto, Japan). In addition, last but not least, we thank all brave defenders of Ukraine that made finalizing this work possible.
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Faizan, S.; Roohi, T. F.; Raju, R. M.; Sivamani, Y.; BR, P. K. J. Mol. Struct. 2023, 1291, 136020. doi:10.1016/j.molstruc.2023.136020
Return to citation in text: [1] [2] [3] -
Sánchez-Sancho, F.; Escolano, M.; Gaviña, D.; Csáky, A. G.; Sánchez-Roselló, M.; Díaz-Oltra, S.; del Pozo, C. Pharmaceuticals 2022, 15, 948. doi:10.3390/ph15080948
Return to citation in text: [1] [2] [3] -
Marinescu, M. Molecules 2021, 26, 6022. doi:10.3390/molecules26196022
Return to citation in text: [1] -
Chopda, L. V.; Dave, P. N. ChemistrySelect 2020, 5, 5552–5572. doi:10.1002/slct.202000742
Return to citation in text: [1] [2] -
Costanzo, P.; Nardi, M.; Oliverio, M. Eur. J. Org. Chem. 2020, 3954–3964. doi:10.1002/ejoc.201901923
Return to citation in text: [1] -
Mohammadi, B.; Behbahani, F. K. Mol. Diversity 2018, 22, 405–446. doi:10.1007/s11030-017-9806-z
Return to citation in text: [1] -
Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M. K.; Rawal, R. K. Eur. J. Med. Chem. 2017, 132, 108–134. doi:10.1016/j.ejmech.2017.03.025
Return to citation in text: [1] -
Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135–5149. doi:10.1016/j.tetlet.2016.09.047
Return to citation in text: [1] [2] [3] -
Suresh; Sandhu, J. S. ARKIVOC 2012, No. i, 66–133. doi:10.3998/ark.5550190.0013.103
Return to citation in text: [1] [2] [3] -
Matos, L. H. S.; Masson, F. T.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779–1789. doi:10.1016/j.ejmech.2017.10.073
Return to citation in text: [1] [2] -
Palchykov, V.; Manko, N.; Finiuk, N.; Pokhodylo, N. Ukr. Biochem. Zh. 2022, 94, 64–74. doi:10.15407/ubj94.01.064
Return to citation in text: [1] -
Zhao, Y.; Wu, H.; Wang, Z.; Wei, Y.; Wang, Z.; Tao, L. Sci. China: Chem. 2016, 59, 1541–1547. doi:10.1007/s11426-016-0219-4
Return to citation in text: [1] -
Abelman, M. M.; Smith, S. C.; James, D. R. Tetrahedron Lett. 2003, 44, 4559–4562. doi:10.1016/s0040-4039(03)00985-7
Return to citation in text: [1] -
Palchykov, V. A.; Dil, K. V.; Okovytyy, S. I. J. Chem. Technol. 2023, 31, 411–418. doi:10.15421/jchemtech.v31i2.126304
Return to citation in text: [1] -
Dil, K. V.; Kozyriev, Y. K.; Palchykov, V. A. Chem. Pap. 2023, 77, 7249–7254. doi:10.1007/s11696-023-03006-9
Return to citation in text: [1] -
Pokhodylo, N. T.; Tupychak, M. A.; Palchykov, V. A. Synth. Commun. 2020, 50, 1835–1844. doi:10.1080/00397911.2020.1757113
Return to citation in text: [1] -
Shyyka, O. Y.; Pokhodylo, N. T.; Palchykov, V. A.; Finiuk, N. S.; Stoika, R. S.; Obushak, M. D. Chem. Heterocycl. Compd. 2020, 56, 793–799. doi:10.1007/s10593-020-02732-2
Return to citation in text: [1] -
Kozirev, E. K.; Palchykov, V. A. Chem. Heterocycl. Compd. 2019, 55, 349–351. doi:10.1007/s10593-019-02463-z
Return to citation in text: [1] -
Chabanenko, R. M.; Mykolenko, S. Yu.; Kozirev, E. K.; Palchykov, V. A. Synth. Commun. 2018, 48, 2198–2205. doi:10.1080/00397911.2018.1486427
Return to citation in text: [1] -
Palchykov, V. A.; Chabanenko, R. M.; Konshin, V. V.; Dotsenko, V. V.; Krivokolysko, S. G.; Chigorina, E. A.; Horak, Y. I.; Lytvyn, R. Z.; Vakhula, A. A.; Obushak, M. D.; Mazepa, A. V. New J. Chem. 2018, 42, 1403–1412. doi:10.1039/c7nj03846a
Return to citation in text: [1] -
Fekri, S.; Rabiei, A.; Hooshmandi, S.; Nouri, H.; Abtahi, S.-H. Int. Ophthalmol. 2024, 44, 101. doi:10.1007/s10792-024-03005-z
Return to citation in text: [1] -
Wang, Y.; Sharma, A.; Ge, F.; Chen, P.; Yang, Y.; Liu, H.; Liu, H.; Zhao, C.; Mittal, L.; Asthana, S.; Schmidt-Wolf, I. G. H. Front. Oncol. 2023, 13, 1157366. doi:10.3389/fonc.2023.1157366
Return to citation in text: [1] -
Kawashima, M.; Watanabe, D.; Fujio, K.; Komazaki, H. J. Dermatol. 2023, 50, 311–318. doi:10.1111/1346-8138.16608
Return to citation in text: [1] -
Li, X.; Zarganes-Tzitzikas, T.; Kurpiewska, K.; Dömling, A. Green Chem. 2023, 25, 1322–1325. doi:10.1039/d2gc04869h
Return to citation in text: [1] -
Mohamadpour, F. Sci. Rep. 2023, 13, 10262. doi:10.1038/s41598-023-37526-x
Return to citation in text: [1] -
Mohamadpour, F. ACS Omega 2022, 7, 8429–8436. doi:10.1021/acsomega.1c05808
Return to citation in text: [1] -
Gadkari, Y. U.; Hatvate, N. T.; Takale, B. S.; Telvekar, V. N. New J. Chem. 2020, 44, 8167–8170. doi:10.1039/d0nj01351j
Return to citation in text: [1] -
Devthade, V.; Kamble, G.; Ghugal, S. G.; Chikhalia, K. H.; Umare, S. S. ChemistrySelect 2018, 3, 4009–4014. doi:10.1002/slct.201800591
Return to citation in text: [1] -
Zhu, Y.; Pan, Y.; Huang, S. Synth. Commun. 2004, 34, 3167–3174. doi:10.1081/scc-200028607
Return to citation in text: [1] -
Ryabukhin, S. V.; Plaskon, A. S.; Ostapchuk, E. N.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2007, 417–427. doi:10.1055/s-2007-965881
Return to citation in text: [1] -
Zhu, Y.-l.; Huang, S.-l.; Pan, Y.-j. Eur. J. Org. Chem. 2005, 2354–2367. doi:10.1002/ejoc.200400845
Return to citation in text: [1] -
Heravi, M. M.; Ghavidel, M.; Heidari, B. Curr. Org. Synth. 2016, 13, 569–600. doi:10.2174/1570179413666151218202307
Return to citation in text: [1] -
Fouda, A. M.; Assiri, M. A.; Ali, T. E. Phosphorus, Sulfur Silicon Relat. Elem. 2020, 195, 324–330. doi:10.1080/10426507.2019.1694023
Return to citation in text: [1] -
Mohammadi, B.; Behbahani, F. K.; Marandi, G. B.; Mirza, B. Phosphorus, Sulfur Silicon Relat. Elem. 2021, 196, 54–60. doi:10.1080/10426507.2020.1800702
Return to citation in text: [1] -
Chen, P.; Tu, M. Tetrahedron Lett. 2018, 59, 987–990. doi:10.1016/j.tetlet.2018.01.070
Return to citation in text: [1] -
Bouzina, A.; Bouone, Y. O.; Sekiou, O.; Aissaoui, M.; Ouk, T.-S.; Djemel, A.; Mansouri, R.; Ibrahim-Ouali, M.; Bouslama, Z.; Aouf, N.-E. RSC Adv. 2023, 13, 19567–19584. doi:10.1039/d3ra02904b
Return to citation in text: [1] -
Nemr, M. T. M.; Teleb, M.; AboulMagd, A. M.; El-Naggar, M. E.; Gouda, N.; Abdel-Ghany, A. A.; Elshaier, Y. A. M. M. J. Mol. Struct. 2023, 1272, 134216. doi:10.1016/j.molstruc.2022.134216
Return to citation in text: [1] -
Nagarajaiah, H. M.; Khazi, I. A.; Begum, N. S. J. Chem. Sci. 2015, 127, 467–479. doi:10.1007/s12039-015-0797-y
Return to citation in text: [1] -
Banothu, J.; Khanapur, M.; Basavoju, S.; Bavantula, R.; Narra, M.; Abbagani, S. RSC Adv. 2014, 4, 22866–22874. doi:10.1039/c4ra02514h
Return to citation in text: [1] -
Wang, X.-C.; Wei, Y.; Da, Y.-X.; Zhang, Z.; Quan, Z.-J. Heterocycles 2011, 83, 2811–2822. doi:10.3987/com-11-12351
Return to citation in text: [1] -
Kim, S. S.; Choi, B. S.; Lee, J. H.; Lee, K. K.; Lee, T. H.; Kim, Y. H.; Shin, H. Synlett 2009, 599–602. doi:10.1055/s-0028-1087920
Return to citation in text: [1] -
Ali, S. H.; Sayed, A. R. Synth. Commun. 2021, 51, 670–700. doi:10.1080/00397911.2020.1854787
Return to citation in text: [1] -
Duc, D. X.; Chung, N. T. Curr. Org. Synth. 2022, 19, 702–730. doi:10.2174/1570179419666220216122637
Return to citation in text: [1] -
Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 42717. doi:10.1038/srep42717
Return to citation in text: [1] -
Banerjee, P.; Eckert, A. O.; Schrey, A. K.; Preissner, R. Nucleic Acids Res. 2018, 46, W257–W263. doi:10.1093/nar/gky318
Return to citation in text: [1] -
Tullius Scotti, M.; Herrera‐Acevedo, C.; Barros de Menezes, R. P.; Martin, H.-J.; Muratov, E. N.; Ítalo de Souza Silva, Á.; Faustino Albuquerque, E.; Ferreira Calado, L.; Coy‐Barrera, E.; Scotti., L. Mol. Inf. 2022, 41, 10.1002/minf.202200133. doi:10.1002/minf.202200133
Return to citation in text: [1] [2] -
Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. Med. Chem. Commun. 2019, 10, 148–157. doi:10.1039/c8md00472b
Return to citation in text: [1] -
Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Adv. Drug Delivery Rev. 2001, 46, 3–26. doi:10.1016/s0169-409x(00)00129-0
Return to citation in text: [1] -
Ghose, A. K.; Viswanadhan, V. N.; Wendoloski, J. J. J. Comb. Chem. 1999, 1, 55–68. doi:10.1021/cc9800071
Return to citation in text: [1] -
Veber, D. F.; Johnson, S. R.; Cheng, H.-Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. J. Med. Chem. 2002, 45, 2615–2623. doi:10.1021/jm020017n
Return to citation in text: [1] -
Egan, W. J.; Merz, K. M.; Baldwin, J. J. J. Med. Chem. 2000, 43, 3867–3877. doi:10.1021/jm000292e
Return to citation in text: [1] -
Muegge, I.; Heald, S. L.; Brittelli, D. J. Med. Chem. 2001, 44, 1841–1846. doi:10.1021/jm015507e
Return to citation in text: [1] -
Colomer, I.; Empson, C. J.; Craven, P.; Owen, Z.; Doveston, R. G.; Churcher, I.; Marsden, S. P.; Nelson, A. Chem. Commun. 2016, 52, 7209–7212. doi:10.1039/c6cc03244c
Return to citation in text: [1]
| 1. | Faizan, S.; Roohi, T. F.; Raju, R. M.; Sivamani, Y.; BR, P. K. J. Mol. Struct. 2023, 1291, 136020. doi:10.1016/j.molstruc.2023.136020 |
| 2. | Sánchez-Sancho, F.; Escolano, M.; Gaviña, D.; Csáky, A. G.; Sánchez-Roselló, M.; Díaz-Oltra, S.; del Pozo, C. Pharmaceuticals 2022, 15, 948. doi:10.3390/ph15080948 |
| 3. | Marinescu, M. Molecules 2021, 26, 6022. doi:10.3390/molecules26196022 |
| 4. | Chopda, L. V.; Dave, P. N. ChemistrySelect 2020, 5, 5552–5572. doi:10.1002/slct.202000742 |
| 5. | Costanzo, P.; Nardi, M.; Oliverio, M. Eur. J. Org. Chem. 2020, 3954–3964. doi:10.1002/ejoc.201901923 |
| 6. | Mohammadi, B.; Behbahani, F. K. Mol. Diversity 2018, 22, 405–446. doi:10.1007/s11030-017-9806-z |
| 7. | Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M. K.; Rawal, R. K. Eur. J. Med. Chem. 2017, 132, 108–134. doi:10.1016/j.ejmech.2017.03.025 |
| 8. | Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135–5149. doi:10.1016/j.tetlet.2016.09.047 |
| 9. | Suresh; Sandhu, J. S. ARKIVOC 2012, No. i, 66–133. doi:10.3998/ark.5550190.0013.103 |
| 10. | Matos, L. H. S.; Masson, F. T.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779–1789. doi:10.1016/j.ejmech.2017.10.073 |
| 11. | Palchykov, V.; Manko, N.; Finiuk, N.; Pokhodylo, N. Ukr. Biochem. Zh. 2022, 94, 64–74. doi:10.15407/ubj94.01.064 |
| 13. | Abelman, M. M.; Smith, S. C.; James, D. R. Tetrahedron Lett. 2003, 44, 4559–4562. doi:10.1016/s0040-4039(03)00985-7 |
| 33. | Fouda, A. M.; Assiri, M. A.; Ali, T. E. Phosphorus, Sulfur Silicon Relat. Elem. 2020, 195, 324–330. doi:10.1080/10426507.2019.1694023 |
| 34. | Mohammadi, B.; Behbahani, F. K.; Marandi, G. B.; Mirza, B. Phosphorus, Sulfur Silicon Relat. Elem. 2021, 196, 54–60. doi:10.1080/10426507.2020.1800702 |
| 35. | Chen, P.; Tu, M. Tetrahedron Lett. 2018, 59, 987–990. doi:10.1016/j.tetlet.2018.01.070 |
| 1. | Faizan, S.; Roohi, T. F.; Raju, R. M.; Sivamani, Y.; BR, P. K. J. Mol. Struct. 2023, 1291, 136020. doi:10.1016/j.molstruc.2023.136020 |
| 2. | Sánchez-Sancho, F.; Escolano, M.; Gaviña, D.; Csáky, A. G.; Sánchez-Roselló, M.; Díaz-Oltra, S.; del Pozo, C. Pharmaceuticals 2022, 15, 948. doi:10.3390/ph15080948 |
| 8. | Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135–5149. doi:10.1016/j.tetlet.2016.09.047 |
| 9. | Suresh; Sandhu, J. S. ARKIVOC 2012, No. i, 66–133. doi:10.3998/ark.5550190.0013.103 |
| 36. | Bouzina, A.; Bouone, Y. O.; Sekiou, O.; Aissaoui, M.; Ouk, T.-S.; Djemel, A.; Mansouri, R.; Ibrahim-Ouali, M.; Bouslama, Z.; Aouf, N.-E. RSC Adv. 2023, 13, 19567–19584. doi:10.1039/d3ra02904b |
| 8. | Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135–5149. doi:10.1016/j.tetlet.2016.09.047 |
| 12. | Zhao, Y.; Wu, H.; Wang, Z.; Wei, Y.; Wang, Z.; Tao, L. Sci. China: Chem. 2016, 59, 1541–1547. doi:10.1007/s11426-016-0219-4 |
| 29. | Zhu, Y.; Pan, Y.; Huang, S. Synth. Commun. 2004, 34, 3167–3174. doi:10.1081/scc-200028607 |
| 30. | Ryabukhin, S. V.; Plaskon, A. S.; Ostapchuk, E. N.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2007, 417–427. doi:10.1055/s-2007-965881 |
| 31. | Zhu, Y.-l.; Huang, S.-l.; Pan, Y.-j. Eur. J. Org. Chem. 2005, 2354–2367. doi:10.1002/ejoc.200400845 |
| 10. | Matos, L. H. S.; Masson, F. T.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779–1789. doi:10.1016/j.ejmech.2017.10.073 |
| 32. | Heravi, M. M.; Ghavidel, M.; Heidari, B. Curr. Org. Synth. 2016, 13, 569–600. doi:10.2174/1570179413666151218202307 |
| 23. | Kawashima, M.; Watanabe, D.; Fujio, K.; Komazaki, H. J. Dermatol. 2023, 50, 311–318. doi:10.1111/1346-8138.16608 |
| 1. | Faizan, S.; Roohi, T. F.; Raju, R. M.; Sivamani, Y.; BR, P. K. J. Mol. Struct. 2023, 1291, 136020. doi:10.1016/j.molstruc.2023.136020 |
| 4. | Chopda, L. V.; Dave, P. N. ChemistrySelect 2020, 5, 5552–5572. doi:10.1002/slct.202000742 |
| 9. | Suresh; Sandhu, J. S. ARKIVOC 2012, No. i, 66–133. doi:10.3998/ark.5550190.0013.103 |
| 22. | Wang, Y.; Sharma, A.; Ge, F.; Chen, P.; Yang, Y.; Liu, H.; Liu, H.; Zhao, C.; Mittal, L.; Asthana, S.; Schmidt-Wolf, I. G. H. Front. Oncol. 2023, 13, 1157366. doi:10.3389/fonc.2023.1157366 |
| 25. | Mohamadpour, F. Sci. Rep. 2023, 13, 10262. doi:10.1038/s41598-023-37526-x |
| 26. | Mohamadpour, F. ACS Omega 2022, 7, 8429–8436. doi:10.1021/acsomega.1c05808 |
| 27. | Gadkari, Y. U.; Hatvate, N. T.; Takale, B. S.; Telvekar, V. N. New J. Chem. 2020, 44, 8167–8170. doi:10.1039/d0nj01351j |
| 28. | Devthade, V.; Kamble, G.; Ghugal, S. G.; Chikhalia, K. H.; Umare, S. S. ChemistrySelect 2018, 3, 4009–4014. doi:10.1002/slct.201800591 |
| 21. | Fekri, S.; Rabiei, A.; Hooshmandi, S.; Nouri, H.; Abtahi, S.-H. Int. Ophthalmol. 2024, 44, 101. doi:10.1007/s10792-024-03005-z |
| 14. | Palchykov, V. A.; Dil, K. V.; Okovytyy, S. I. J. Chem. Technol. 2023, 31, 411–418. doi:10.15421/jchemtech.v31i2.126304 |
| 15. | Dil, K. V.; Kozyriev, Y. K.; Palchykov, V. A. Chem. Pap. 2023, 77, 7249–7254. doi:10.1007/s11696-023-03006-9 |
| 16. | Pokhodylo, N. T.; Tupychak, M. A.; Palchykov, V. A. Synth. Commun. 2020, 50, 1835–1844. doi:10.1080/00397911.2020.1757113 |
| 17. | Shyyka, O. Y.; Pokhodylo, N. T.; Palchykov, V. A.; Finiuk, N. S.; Stoika, R. S.; Obushak, M. D. Chem. Heterocycl. Compd. 2020, 56, 793–799. doi:10.1007/s10593-020-02732-2 |
| 18. | Kozirev, E. K.; Palchykov, V. A. Chem. Heterocycl. Compd. 2019, 55, 349–351. doi:10.1007/s10593-019-02463-z |
| 19. | Chabanenko, R. M.; Mykolenko, S. Yu.; Kozirev, E. K.; Palchykov, V. A. Synth. Commun. 2018, 48, 2198–2205. doi:10.1080/00397911.2018.1486427 |
| 20. | Palchykov, V. A.; Chabanenko, R. M.; Konshin, V. V.; Dotsenko, V. V.; Krivokolysko, S. G.; Chigorina, E. A.; Horak, Y. I.; Lytvyn, R. Z.; Vakhula, A. A.; Obushak, M. D.; Mazepa, A. V. New J. Chem. 2018, 42, 1403–1412. doi:10.1039/c7nj03846a |
| 24. | Li, X.; Zarganes-Tzitzikas, T.; Kurpiewska, K.; Dömling, A. Green Chem. 2023, 25, 1322–1325. doi:10.1039/d2gc04869h |
| 40. | Wang, X.-C.; Wei, Y.; Da, Y.-X.; Zhang, Z.; Quan, Z.-J. Heterocycles 2011, 83, 2811–2822. doi:10.3987/com-11-12351 |
| 41. | Kim, S. S.; Choi, B. S.; Lee, J. H.; Lee, K. K.; Lee, T. H.; Kim, Y. H.; Shin, H. Synlett 2009, 599–602. doi:10.1055/s-0028-1087920 |
| 2. | Sánchez-Sancho, F.; Escolano, M.; Gaviña, D.; Csáky, A. G.; Sánchez-Roselló, M.; Díaz-Oltra, S.; del Pozo, C. Pharmaceuticals 2022, 15, 948. doi:10.3390/ph15080948 |
| 37. | Nemr, M. T. M.; Teleb, M.; AboulMagd, A. M.; El-Naggar, M. E.; Gouda, N.; Abdel-Ghany, A. A.; Elshaier, Y. A. M. M. J. Mol. Struct. 2023, 1272, 134216. doi:10.1016/j.molstruc.2022.134216 |
| 38. | Nagarajaiah, H. M.; Khazi, I. A.; Begum, N. S. J. Chem. Sci. 2015, 127, 467–479. doi:10.1007/s12039-015-0797-y |
| 39. | Banothu, J.; Khanapur, M.; Basavoju, S.; Bavantula, R.; Narra, M.; Abbagani, S. RSC Adv. 2014, 4, 22866–22874. doi:10.1039/c4ra02514h |
| 53. | Colomer, I.; Empson, C. J.; Craven, P.; Owen, Z.; Doveston, R. G.; Churcher, I.; Marsden, S. P.; Nelson, A. Chem. Commun. 2016, 52, 7209–7212. doi:10.1039/c6cc03244c |
| 46. | Tullius Scotti, M.; Herrera‐Acevedo, C.; Barros de Menezes, R. P.; Martin, H.-J.; Muratov, E. N.; Ítalo de Souza Silva, Á.; Faustino Albuquerque, E.; Ferreira Calado, L.; Coy‐Barrera, E.; Scotti., L. Mol. Inf. 2022, 41, 10.1002/minf.202200133. doi:10.1002/minf.202200133 |
| 47. | Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. Med. Chem. Commun. 2019, 10, 148–157. doi:10.1039/c8md00472b |
| 48. | Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Adv. Drug Delivery Rev. 2001, 46, 3–26. doi:10.1016/s0169-409x(00)00129-0 |
| 49. | Ghose, A. K.; Viswanadhan, V. N.; Wendoloski, J. J. J. Comb. Chem. 1999, 1, 55–68. doi:10.1021/cc9800071 |
| 50. | Veber, D. F.; Johnson, S. R.; Cheng, H.-Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. J. Med. Chem. 2002, 45, 2615–2623. doi:10.1021/jm020017n |
| 51. | Egan, W. J.; Merz, K. M.; Baldwin, J. J. J. Med. Chem. 2000, 43, 3867–3877. doi:10.1021/jm000292e |
| 52. | Muegge, I.; Heald, S. L.; Brittelli, D. J. Med. Chem. 2001, 44, 1841–1846. doi:10.1021/jm015507e |
| 45. | Banerjee, P.; Eckert, A. O.; Schrey, A. K.; Preissner, R. Nucleic Acids Res. 2018, 46, W257–W263. doi:10.1093/nar/gky318 |
| 46. | Tullius Scotti, M.; Herrera‐Acevedo, C.; Barros de Menezes, R. P.; Martin, H.-J.; Muratov, E. N.; Ítalo de Souza Silva, Á.; Faustino Albuquerque, E.; Ferreira Calado, L.; Coy‐Barrera, E.; Scotti., L. Mol. Inf. 2022, 41, 10.1002/minf.202200133. doi:10.1002/minf.202200133 |
| 42. | Ali, S. H.; Sayed, A. R. Synth. Commun. 2021, 51, 670–700. doi:10.1080/00397911.2020.1854787 |
| 43. | Duc, D. X.; Chung, N. T. Curr. Org. Synth. 2022, 19, 702–730. doi:10.2174/1570179419666220216122637 |
| 44. | Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 42717. doi:10.1038/srep42717 |
© 2024 Dil and Palchykov; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.