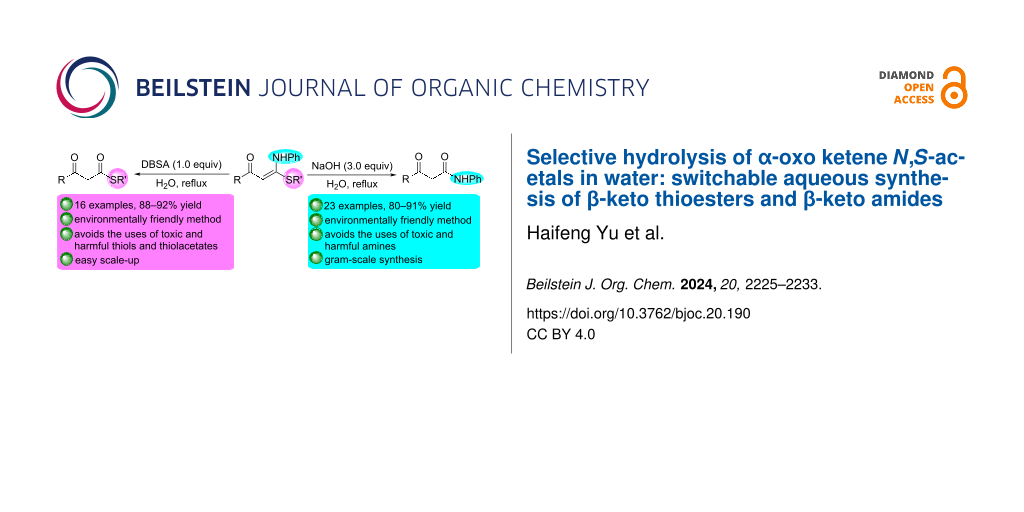
Abstract
An eco-friendly selective hydrolysis of chain α-oxo ketene N,S-acetals in water for the switchable synthesis of β-keto thioesters and β-keto amides is reported. In refluxing water, the hydrolysis reactions of α-oxo ketene N,S-acetals in the presence of 1.0 equiv of dodecylbenzenesulfonic acid effectively afforded β-keto thioesters in excellent yield, while β-keto amides were successfully obtained in excellent yield when the hydrolysis reactions were carried out in the presence of 3.0 equiv of NaOH. The green approach to β-keto thioesters and β-keto amides avoids the use of harmful organic solvents, thiols and thiolacetates as well as amines, which could result in serious environmental and safety issues.
Graphical Abstract
Introduction
In the past decades, the application of easily available and stable α-oxo ketene N,S-acetals as significant synthons has received more and more attention in organic synthesis due to their unique structural characters and multiple good reactivities [1-4]. Both β-keto thioesters [5-12] and β-keto amides [13-22] have served as useful synthetic intermediates for the synthesis of a range of potent natural products. Therefore, much effort has focused on their synthesis in the past decades [23-30]. These methods for the synthesis of β-keto thioesters include the mercaptolysis of diketene (Scheme 1a, path 1) [23], 2,2,6-trimethyl-4H-1,3-dioxin-4-one (Scheme 1a, path 2) [24], acylated Meldrum’s acids (Scheme 1a, path 3) [25] or β-keto esters (Scheme 1a, path 4) [26,27], the aldol reaction between aldehydes and S-ethyl acetothioate followed by oxidation with Dess–Martin periodinane (Scheme 1a, path 5) [28], the hydrolysis of α-oxo ketene dithioacetals (Scheme 1a, path 6) [29] and MgBr2·OEt2-catalyzed acylation of thioesters and acyl chlorides (Scheme 1a, path 7) [30]. For β-keto amides, they could be efficiently synthesized from the nucleophilic substitution reactions of amines with β-keto acids (Scheme 1b, path 1) [31-33], β-keto esters (Scheme 1b, path 2) [34] and the nucleophilic addition reactions of amines with diketenes (Scheme 1b, path 3) [35] as well as isocyanates with various nucleophilic reagents (Scheme 1b, path 4), such as silyl enol ethers [36], enamines [37], α-acylphosphonium ylides [38] and lithium enolates [39]. Recently, the hydrolysis of α-oxo ketene N,S-acetals was developed to prepare both β-keto thioesters and β-keto amides [40,41]. Li and co-authors achieved the synthesis of β-keto thioesters by CF3SO3H-promoted hydrolysis of α-oxo ketene N,S-acetals with an amino leaving group (Scheme 1a, path 8) [40]. Subsequently, using NaOH-promoted hydrolysis of α-oxo ketene N,S-acetals, we efficiently prepared β-keto amides (Scheme 1b, path 5) [41]. Despite great progress, all the reported reactions were performed in organic medium, such as CH3CN, CH2Cl2 and CF3CH2OH, which can result in serious environmental and safety problems. Therefore, the development of an environmentally compliant synthetic method for the preparation of β-keto thioesters and β-keto amides remains imperative.
Scheme 1: Synthesis of α-keto thioesters and β-keto amides.
Scheme 1: Synthesis of α-keto thioesters and β-keto amides.
Organic reactions in water are an important and exciting research topic of green chemistry because water as a solvent exhibits fascinating features, such as low cost, good environmental compatibility, nontoxicity and nonflammability. In addition, the use of water solvent can reduce the discharge of harmful organic solvents [42-50]. In the past 10 years, while expanding the synthesis and application of α-oxo ketene dithioacetal derivatives [51-64], we conducted research on their organic reactions in water and reported some good results, such as thioacetalization using ketene dithioacetals as odorless thiol equivalent [65], Friedel–Crafts alkylation of cyclic ketene dithioacetals with alcohols [66], the hydrolysis of chain α-oxo ketene dithioacetals [67] or β-ethylthio-β-indolyl-substituted α,β-unsaturated ketones [68], the cyclocondensation reaction of β-ethylthio-β-indolyl-substituted α,β-unsaturated ketones with hydrazines/hydroxylamine [69-71] and the tandem [5C + 1C/1N]-cycloaromatization of α-alkenoyl ketene dithioacetals and nitroethane/amines [72,73]. As part of our continuous research in this context, more recently we investigated the selective hydrolysis of α-oxo ketene N,S-acetals in water to gain an environmentally compliant synthetic method for β-keto thioesters and β-keto amides (Scheme 1c). Herein, we report our findings.
Results and Discussion
At the outset of our studies, to optimize the reaction conditions for the selective synthesis of β-keto thioesters and β-keto amides, we explored the hydrolysis reaction of (E)-3-(ethylthio)-1-phenyl-3-(phenylamino)prop-2-en-1-one (1a, 0.25 mmol, 70.8 mg) in water under different conditions (Table 1). We initially tested the reaction in the presence of dodecylbenzenesulfonic acid (DBSA) in boiling water and found that the amount of DBSA has a dramatic influence on this reaction. Using 1.0 equiv of DBSA, the reaction efficiently gave the desired S-ethyl 3-oxo-3-phenylpropanethioate (2a) in 91% yield (Table 1, entry 1). However, increasing the amount of DBSA to 2.0 equiv, the yield of 2a did not improve remarkably (Table 1, entry 2), and reducing the amount of DBSA resulted in a lower yield of 2a (Table 1, entry 3). Additionally, when DBSA was replaced by other acids such as H2SO4 and CF3SO3H, the reaction showed poor effectiveness due to the poor solubility of 1a in water (Table 1, entries 4 and 5). Most notably, 2a was an inseparable mixture of keto and enol isomers, reaching a keto/enol ratio of 5:4 as determined by 1H NMR spectroscopy. Thus, the optimized reaction conditions for the synthesis of 2a were determined to be 1.0 equiv of DBSA as catalyst and reflux temperature (conditions A). Subsequently, we turned our attention to the hydrolysis reaction in the presence of hydroxide for the preparation of 3-oxo-N,3-diphenylpropanamide (3a). Firstly, we chose NaOH to optimize the reaction conditions. Apparently, in the absence of a solubilizer, no reaction occurred due to poor solubility of 1a in boiling water (Table 1, entry 6). Macrogol 400 (PEG-400) is emerging as an environmentally friendly nonionic solubilizer due to its unique merits, such as nontoxicity, inexpensiveness, nonflammability, low volatility and good water solubility, which are consistent with the concept of green chemistry [74,75]. Therefore, we tested the reaction in the presence of PEG-400 (Table 1, entries 7–9). It was found that the reaction uniquely produced 3a in 48% yield when using 3.0 equiv of PEG-400 as solubilizer (Table 1, entry 8), and further increasing the PEG-400 loading could not remarkably improve the yield of 3a (Table 1, entry 9). With this in mind, we selected 3.0 equiv of PEG-400 as solubilizer in our study. Next, we examined the influence of the amount of NaOH on the reaction (Table 1, entries 10–12). The reaction obviously showed dependence on the amount of NaOH, and 3a was obtained in 90% yield when the reaction ran for 24 h in the presence of 3.0 equiv of NaOH (Table 1, entry 11). However, when lowering the reaction temperature to 90 °C, the reaction efficiency significantly decreased (Table 1, entry 13). Alike 2a, the keto isomer of 3a was the dominant isomer, with a keto/enol ratio of 3:1. Then, we tested the effects of different bases on the reaction and found that the strong bases NaOH and KOH gave 3a in high yield (Table 1, entries 11 and 14), while the reaction afforded 3a in low yield in the presence of weak bases such as Na2CO3 and Et3N (Table 1, entries 15 and 16). Accordingly, the optimal reaction conditions for the synthesis of 3a were 3.0 equiv of NaOH as catalyst and reflux temperature (conditions B).
Table 1: Optimization of the reaction conditions.
|
|
|||||
| entry | catalyst (equiv) | PEG-400 (equiv) | time (h) | yield (%)b | |
| 2a | 3a | ||||
| 1 | DBSA (1.0) | — | 5 | 91 | 5 |
| 2 | DBSA (2.0) | — | 2 | 89 | 3 |
| 3 | DBSA (0.5) | — | 8 | 70 | 24 |
| 4 | H2SO4 (1.0) | — | 12 | 61 | 18 (12)c |
| 5 | CF3SO3H (1.0) | — | 12 | 56 | 15 (22)c |
| 6 | NaOH (1.0) | — | 24 | 0 | 0 (94)c |
| 7 | NaOH (1.0) | 2.0 | 24 | 0 | 30 (60)c |
| 8 | NaOH (1.0) | 3.0 | 24 | 0 | 48 (47)c |
| 9 | NaOH (1.0) | 4.0 | 24 | 0 | 49 (42)c |
| 10 | NaOH (2.0) | 3.0 | 24 | 0 | 72 (21)c |
| 11 | NaOH (3.0) | 3.0 | 24 | 0 | 90 |
| 12 | NaOH (4.0) | 3.0 | 22 | 0 | 89 |
| 13d | NaOH (3.0) | 3.0 | 24 | 0 | 74 (18)c |
| 14 | KOH (3.0) | 3.0 | 24 | 0 | 88 |
| 15 | Na2CO3 (3.0) | 3.0 | 24 | 0 | 31 (60)c |
| 16 | Et3N (3.0) | 3.0 | 24 | 0 | 20 (72)c |
aConditions: 1a (0.25 mmol, 70.8 mg), H2O (1 mL), in air. bIsolated yield. cRecovery rate of 1a. dReaction at 90 °C.
With the optimal reaction conditions in hand, we next examined the scope of the two hydrolysis reactions (Scheme 2). Initially, the hydrolysis reaction for the synthesis of β-keto thioesters 2 was investigated under conditions A, and the results are shown in Scheme 2. (E)-3-(Ethylthio)-1-aryl-3-(phenylamino)prop-2-en-1-ones 1a–l were smoothly hydrolyzed to produce a series of S-ethyl 3-oxo-3-arylpropanethioates 2a–l in excellent yield, wherein the electronic effects of electron-donating and electron-withdrawing groups on the aromatic ring adjacent to the carbonyl group did not impact the formation of the products 2a–l. A variety of valuable functional groups on the benzene ring of 1b–j, such as methyl, methoxy, trifluoromethyl, and halogen atoms (F, Cl, Br, I), were well compatible with reaction conditions A. Most notably, unlike work by Li et al. [40], S-ethyl 3-oxo-3-(4-(trifluoromethyl)phenyl)propanethioate (2j) could be obtained in 91% yield. In a similar fashion, S-alkyl 3-oxo-3-phenylpropanethioates 2m–p were also efficiently prepared in excellent yield from the hydrolysis reaction of (E)-3-(alkylthio)-1-aryl-3-(phenylamino)prop-2-en-1-ones 1m–p under conditions A.
Scheme 2: Synthesis of β-keto thioesters 2. Reaction conditions A: 1 (0.25 mmol), DBSA (87.9 mg, 0.25 mmol), H2O (1 mL), reflux, 5 h. Isolated yield is stated. Keto/enol ratio of 2 was determined by 1H NMR spectroscopy.
Scheme 2: Synthesis of β-keto thioesters 2. Reaction conditions A: 1 (0.25 mmol), DBSA (87.9 mg, 0.25 mmol), H...
Next, the generality of the synthesis of β-keto amides 3 under conditions B was investigated (Scheme 3). 3-Oxo-N-phenyl-3-arylpropanamides 3a–l and 3-oxo-N-aryl-3-phenylpropanamides 3m–v could be produced in excellent yield from the hydrolysis of α-oxo ketene N,S-acetals under conditions B. The results showed that electron-donating as well as electron-withdrawing substituents on the two phenyl rings in compounds 1, such as methyl, methoxy, halogen atoms (F, Cl, Br, I), CF3 and SO2CH3, were well tolerated, and their electronic effects insignificantly impacted the formation of 3. Similarly, N-benzyl-3-oxo-3-phenylpropanamide (3w) could also be obtained in 80% yield when the hydrolysis reaction of (E)-3-(benzylamino)-3-(ethylthio)-1-phenylprop-2-en-1-one (1aa) was carried out under conditions B.
Scheme 3: Synthesis of β-keto amides 3. Reaction conditions B: 1 (0.25 mmol), NaOH (0.75 mmol, 30 mg), H2O (1 mL), reflux, 24 h. Isolated yield is stated. Keto/enol ratio of 3 was determined by 1H NMR spectroscopy.
Scheme 3: Synthesis of β-keto amides 3. Reaction conditions B: 1 (0.25 mmol), NaOH (0.75 mmol, 30 mg), H2O (1...
Furthermore, to explore the synthetic practicality of the two chemical processes, the synthesis of 2a and 3a on a gram scale was tested using the hydrolysis reaction of 1a under conditions A and B, respectively. When the hydrolysis reactions of 1a were performed on a 5 mmol scale, 0.915 g of 2a and 1.028 g of 3a were obtained in 88% and 86% yield, respectively (Scheme 4).
Scheme 4: Gram-scale hydrolysis reactions of 1a.
Scheme 4: Gram-scale hydrolysis reactions of 1a.
Next, the EcoScale value of the hydrolysis reactions of 1a under conditions A and B was calculated [76] to evaluate the green metrics of the two aqueous reactions (Table 2). It was found that the two procedures showed EcoScale values of 82.5 and 77.0, respectively, which indicated that the two procedures exhibited good environmental friendliness.
Table 2: EcoScale calculations and results for the synthesis of 2a and 3a [75].
| synthesis of 2a | ||
| parameter | parameter details | penalty points |
| yield | 91% | 4.5 |
| price of reaction components to obtain 10 mmol of product | 1a | 0 |
| DBSA | 0 | |
| safety | DBSA | 0 |
| technical setup | common setup | 0 |
| temperature, time | heating, >1 h | 3 |
| workup and purification | classical chromatography | 10 |
| EcoScale score | 82.5 | |
| synthesis of 3a | ||
| parameter | parameter details | penalty points |
| yield | 90% | 5 |
| price of reaction components to obtain 10 mmol of product | 1a | 0 |
| PEG-400 | 0 | |
| NaOH | 0 | |
| safety | PEG-400 | 0 |
| NaOH | 5 (dangerous for environment) | |
| technical setup | common setup | 0 |
| temperature, time | heating, >1 h | 3 |
| workup and purification | classical chromatography | 10 |
| EcoScale score | 77.0 | |
Based on the results above and on literature precedents [40,41], a plausible mechanistic pathway for the formation of 2 and 3 is shown in Scheme 5 (with the reaction of 1a as an example). In the presence of DBSA, the protonation of 1a results in the carbocation intermediate I. Then, the nucleophilic attack of H2O at the carbocation of I produces intermediate II, which converts into intermediate III through a deprotonation–protonation process. Finally, the elimination of PhNH2 from intermediate III occurs to afford the desired product 2a. In the presence of NaOH, the Michael addition between 1a and base initially occurs to form adduct I', which is then transformed into intermediate II' by elimination of ethanethiolate. Subsequently, β-keto amide 3a is obtained when II' releases H+.
Scheme 5: Proposed mechanism for formation of β-keto thioesters 2 and β-keto amides 3.
Scheme 5: Proposed mechanism for formation of β-keto thioesters 2 and β-keto amides 3.
Conclusion
In summary, we have successfully developed an environmentally friendly method for the selective aqueous synthesis of β-keto thioesters and β-keto amides by simply changing reaction conditions. These features, including a good substrate scope, excellent yield and selectivity and ease of scale-up, rendered the green hydrolysis reaction very environment-friendly, practical, and attractive.
Experimental
1H and 13C{1H} NMR spectra were recorded on a Bruker DRX-600 spectrometer and all chemical shift values are referenced to TMS (δ = 0.00 ppm for 1H) and CDCl3 (δ = 77.16 ppm for 13C). HRMS analysis was achieved with a Bruck microTof using the ESI method. All melting points are uncorrected. Analytical TLC plates (Sigma-Aldrich silica gel 60 F200) were analyzed under UV light (254 nm). Chromatographic purifications were performed on SDZF silica gel 160.
The starting α-oxo ketene N,S-acetals 1 are known compounds [40,41] and easily prepared according to References [40,58].
Typical procedure for the preparation of β-keto thioesters 2 (2a as an example)
A mixture of (E)-3-(ethylthio)-1-phenyl-3-(phenylamino)prop-2-en-1-one (1a, 70.8 mg, 0.25 mmol) and DBSA (87.9 mg, 0.25 mmol) in water (2 mL) was stirred at reflux in an oil bath under air for 5 h until 1a was completely consumed, as confirmed by TLC monitoring. Then, the pH value of the reaction mixture was adjusted to neutral using a saturated NaHCO3 solution, and the reaction mixture was extracted with CH2Cl2 (3 × 20 mL). The organic solution was dried with anhydrous Na2SO4, and the crude product was purified by column chromatography on 300–400 mesh silica gel (petroleum ether (60–90 °C)/ethyl acetate 60:1, v/v) to give 2a (47.3 mg, 91%).
Typical procedure for the preparation of β-keto amides 3 (3a as an example)
A mixture of (E)-3-(ethylthio)-1-phenyl-3-(phenylamino)prop-2-en-1-one (1a, 70.8 mg, 0.25 mmol), NaOH (30 mg, 0.75 mmol) and PEG-400 (0.236 mL, 0.75 mmol) in water (2 mL) was stirred at reflux in an oil bath under air for 24 h until 1a was completely consumed, as confirmed by TLC monitoring. Then, the pH value of the reaction mixture was adjusted to neutral using a 10% CH3COOH solution, and the reaction mixture was extracted with CH2Cl2 (3 × 20 mL). The organic solution was dried with anhydrous Na2SO4, and the crude product was purified by column chromatography on 30–400 mesh silica gel (petroleum ether (60–90 °C)/ethyl acetate 20:1, v/v) to give 3a (53.8 mg, 90%).
Supporting Information
| Supporting Information File 1: Analytic data and copies of 1H and 13C NMR spectra of compounds 2 and 3. | ||
| Format: PDF | Size: 17.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Zhang, L.; Dong, J.; Xu, X.; Liu, Q. Chem. Rev. 2016, 116, 287–322. doi:10.1021/acs.chemrev.5b00360
Return to citation in text: [1] -
Liu, Z.; Huang, F.; Wu, P.; Wang, Q.; Yu, Z. J. Org. Chem. 2018, 83, 5731–5750. doi:10.1021/acs.joc.8b00775
Return to citation in text: [1] -
Huang, F.; Wu, P.; Yu, Z. J. Org. Chem. 2020, 85, 4373–4385. doi:10.1021/acs.joc.0c00093
Return to citation in text: [1] -
Wu, P.; He, Y.; Wang, H.; Zhou, Y.-G.; Yu, Z. Org. Lett. 2020, 22, 310–315. doi:10.1021/acs.orglett.9b04335
Return to citation in text: [1] -
Maity, R.; Naskar, S.; Mal, K.; Biswas, S.; Das, I. Adv. Synth. Catal. 2017, 359, 4405–4410. doi:10.1002/adsc.201701020
Return to citation in text: [1] -
Maity, R.; Naskar, S.; Das, I. J. Org. Chem. 2018, 83, 2114–2124. doi:10.1021/acs.joc.7b03054
Return to citation in text: [1] -
Jiang, Y.; Stornetta, A.; Villalta, P. W.; Wilson, M. R.; Boudreau, P. D.; Zha, L.; Balbo, S.; Balskus, E. P. J. Am. Chem. Soc. 2019, 141, 11489–11496. doi:10.1021/jacs.9b02453
Return to citation in text: [1] -
Zhou, S.; Malet, N. R.; Song, L.; Corre, C.; Challis, G. L. Chem. Commun. 2020, 56, 14443–14446. doi:10.1039/d0cc05658h
Return to citation in text: [1] -
Bae, D.; Lee, J.; Jin, H.; Ryu, D. H. J. Org. Chem. 2021, 86, 6001–6014. doi:10.1021/acs.joc.1c00323
Return to citation in text: [1] -
Takeuchi, Y.; Akagawa, K.; Kudo, K. J. Org. Chem. 2021, 86, 17307–17317. doi:10.1021/acs.joc.1c02441
Return to citation in text: [1] -
Chisuga, T.; Nagai, A.; Miyanaga, A.; Goto, E.; Kishikawa, K.; Kudo, F.; Eguchi, T. ACS Chem. Biol. 2022, 17, 198–206. doi:10.1021/acschembio.1c00856
Return to citation in text: [1] -
Kopp, J.; Brückner, R. Eur. J. Org. Chem. 2024, 27, e202301001. doi:10.1002/ejoc.202301001
Return to citation in text: [1] -
Chen, Z.; Liu, J.; Jin, C.; Tan, Q.; Ye, M. Tetrahedron Lett. 2019, 60, 1265–1267. doi:10.1016/j.tetlet.2019.04.001
Return to citation in text: [1] -
Mourelle-Insua, Á.; Méndez-Sánchez, D.; Galman, J. L.; Slabu, I.; Turner, N. J.; Gotor-Fernández, V.; Lavandera, I. Catal. Sci. Technol. 2019, 9, 4083–4090. doi:10.1039/c9cy01004a
Return to citation in text: [1] -
Li, B.; Cheng, X.; Guan, Z.-Y.; Li, S.-Y.; Huo, T.; Cheng, G.; Fan, Y.-H.; Zhou, F.-S.; Deng, Q.-H. Org. Chem. Front. 2020, 7, 3509–3514. doi:10.1039/d0qo01022g
Return to citation in text: [1] -
Fulo, H. F.; Rueb, N. J.; Gaston, R., Jr.; Batsomboon, P.; Ahmed, K. T.; Barrios, A. M.; Dudley, G. B. Org. Biomol. Chem. 2021, 19, 10596–10600. doi:10.1039/d1ob02106k
Return to citation in text: [1] -
Zhang, Z.; Cao, X.; Wang, G.; Zhang, G.; Zhang, X. Green Chem. 2022, 24, 3035–3041. doi:10.1039/d2gc00395c
Return to citation in text: [1] -
Zhang, X.; Yu, Y.; Li, W.; Shi, L.; Li, H. J. Org. Chem. 2022, 87, 16263–16275. doi:10.1021/acs.joc.2c01839
Return to citation in text: [1] -
Xu, L.; Ma, Z.; Hu, X.; Zhang, X.; Gao, S.; Liang, D.; Wang, B.; Li, W.; Li, Y. Org. Biomol. Chem. 2022, 20, 1013–1018. doi:10.1039/d1ob02379a
Return to citation in text: [1] -
Tan, Q.; Chen, Q.; Zhu, Z.; Liu, X. Chem. Commun. 2022, 58, 9686–9689. doi:10.1039/d2cc03443c
Return to citation in text: [1] -
Zhao, X.; Yang, Z.; Cheng, Y.; Huang, A.; Hu, F.; Ling, F.; Zhong, W. Adv. Synth. Catal. 2022, 364, 3074–3080. doi:10.1002/adsc.202200524
Return to citation in text: [1] -
Wei, Y.; Wang, G.; Zhang, Z.; Li, M.; Ma, N.; Wu, H.; Zhang, G. J. Org. Chem. 2024, 89, 1127–1139. doi:10.1021/acs.joc.3c02319
Return to citation in text: [1] -
Duus, F.; Jakobsen, P.; Lawesson, S.-O. Tetrahedron 1968, 24, 5323–5336. doi:10.1016/s0040-4020(01)96327-6
Return to citation in text: [1] [2] -
Xin, D.; Burgess, K. Org. Lett. 2014, 16, 2108–2110. doi:10.1021/ol5005643
Return to citation in text: [1] [2] -
Davis, J. B.; Bailey, J. D.; Sello, J. K. Org. Lett. 2009, 11, 2984–2987. doi:10.1021/ol9009893
Return to citation in text: [1] [2] -
Ponde, D. E.; Deshpande, V. H.; Bulbule, V. J.; Sudalai, A.; Gajare, A. S. J. Org. Chem. 1998, 63, 1058–1063. doi:10.1021/jo971404l
Return to citation in text: [1] [2] -
Wunderlich, J.; Roß, T.; Schröder, M.; Hahn, F. Org. Lett. 2020, 22, 4955–4959. doi:10.1021/acs.orglett.0c01348
Return to citation in text: [1] [2] -
Hayashi, Y.; Miyamoto, Y.; Shoji, M. Tetrahedron Lett. 2002, 43, 4079–4082. doi:10.1016/s0040-4039(02)00675-5
Return to citation in text: [1] [2] -
Yu, H.; Zhao, L.; Diao, Q.; Li, T.; Liao, P.; Hou, D.; Xin, G. Synlett 2017, 28, 1828–1834. doi:10.1055/s-0036-1588982
Return to citation in text: [1] [2] -
Aderibigbe, S. O.; Coltart, D. M. J. Org. Chem. 2019, 84, 9770–9777. doi:10.1021/acs.joc.9b00397
Return to citation in text: [1] [2] -
Anwar, M.; Moloney, M. G. Tetrahedron Lett. 2007, 48, 7259–7262. doi:10.1016/j.tetlet.2007.08.052
Return to citation in text: [1] -
Wang, J.; Yuan, Y.; Xiong, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2012, 14, 2210–2213. doi:10.1021/ol300418h
Return to citation in text: [1] -
Yuan, Y.; Yang, R.; Zhang-Negrerie, D.; Wang, J.; Du, Y.; Zhao, K. J. Org. Chem. 2013, 78, 5385–5392. doi:10.1021/jo400541s
Return to citation in text: [1] -
Yadlapalli, R. K.; Chourasia, O. P.; Vemuri, K.; Sritharan, M.; Perali, R. S. Bioorg. Med. Chem. Lett. 2012, 22, 2708–2711. doi:10.1016/j.bmcl.2012.02.101
Return to citation in text: [1] -
Kaslow, C. E.; Sommer, N. B. J. Am. Chem. Soc. 1946, 68, 644–647. doi:10.1021/ja01208a035
Return to citation in text: [1] -
Ojima, I.; Inaba, S.; Nagai, Y. Tetrahedron Lett. 1973, 14, 4271–4272. doi:10.1016/s0040-4039(01)87167-7
Return to citation in text: [1] -
Hünig, S.; Hübner, K.; Benzing, E. Chem. Ber. 1962, 95, 926–936. doi:10.1002/cber.19620950417
Return to citation in text: [1] -
Bestmann, H. J.; Kumar, K. Chem. Ber. 1983, 116, 2708–2710. doi:10.1002/cber.19831160725
Return to citation in text: [1] -
Hendi, S. B.; Hendi, M. S.; Wolfe, J. F. Synth. Commun. 1987, 17, 13–18. doi:10.1080/00397918708063898
Return to citation in text: [1] -
Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Li, L.; Jiang, C.; Peng, T.; Zhang, N.; Ji, J.; Yu, H.; Zhao, X. Synth. Commun. 2024, 54, 312–320. doi:10.1080/00397911.2023.2300643
Return to citation in text: [1] [2] [3] [4] -
Ballmann, M.; Ruer, P. C.; Hofnagel, O.; Hiller, W.; Krause, N. ACS Sustainable Chem. Eng. 2022, 10, 7288–7298. doi:10.1021/acssuschemeng.2c00713
Return to citation in text: [1] -
Shimoyama, Y.; Ishizuka, T.; Kotani, H.; Kojima, T. ACS Catal. 2019, 9, 671–678. doi:10.1021/acscatal.8b04004
Return to citation in text: [1] -
Cui, X.-l.; Tian, H.-y.; Wang, S.-j.; Chen, X.; Kong, D.-l. J. Org. Chem. 2023, 88, 8576–8582. doi:10.1021/acs.joc.3c00499
Return to citation in text: [1] -
Shen, D.; Li, L.; Ren, T.; Chen, K.; Zhang, X.; Zhang, H.; Zhang, S.; Gong, P.; Zhang, F.; Chao, M. J. Org. Chem. 2024, 89, 2691–2702. doi:10.1021/acs.joc.3c02762
Return to citation in text: [1] -
Jia, Y.; Jiang, P.; Wang, X.; Ablajan, K. J. Org. Chem. 2024, 89, 835–843. doi:10.1021/acs.joc.3c01421
Return to citation in text: [1] -
Sun, X.; Zheng, N.; Liu, G.; Wu, Q.; Song, W. Chem. Commun. 2022, 58, 13234–13237. doi:10.1039/d2cc04352a
Return to citation in text: [1] -
Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. Chem. – Eur. J. 2022, 28, e202104524. doi:10.1002/chem.202104524
Return to citation in text: [1] -
Tian, Y.-M.; Hofmann, E.; Silva, W.; Pu, X.; Touraud, D.; Gschwind, R. M.; Kunz, W.; König, B. Angew. Chem., Int. Ed. 2023, 62, e202218775. doi:10.1002/anie.202218775
Return to citation in text: [1] -
Kitanosono, T.; Lu, F.; Masuda, K.; Yamashita, Y.; Kobayashi, S. Angew. Chem., Int. Ed. 2022, 61, e202202335. doi:10.1002/anie.202202335
Return to citation in text: [1] -
Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. doi:10.1002/anie.201002737
Return to citation in text: [1] -
Yu, H.-F. Synth. Commun. 2013, 43, 1280–1286. doi:10.1080/00397911.2011.631075
Return to citation in text: [1] -
Yu, H. Chin. J. Chem. 2012, 30, 367–371. doi:10.1002/cjoc.201100096
Return to citation in text: [1] -
Zhao, H.; Diao, Q.; Yu, H.; Li, T.; Liao, P.; Hou, D. Chem. Res. Chin. Univ. 2017, 33, 746–752. doi:10.1007/s40242-017-7082-1
Return to citation in text: [1] -
Zhao, X.-b.; Wang, Y.-m.; Yu, H.-f.; Lv, Y.-c.; Jiang, S.-a.; Wang, N. Tetrahedron 2021, 97, 132427. doi:10.1016/j.tet.2021.132427
Return to citation in text: [1] -
Yu, H.; Mo, J.; Niu, X.; Luo, D.; Che, G. Adv. Synth. Catal. 2023, 365, 3118–3128. doi:10.1002/adsc.202300559
Return to citation in text: [1] -
Yu, H.; Zhang, Y.; Li, T.; Liao, P.; Diao, Q.; Xin, G.; Meng, Q.; Hou, D. RSC Adv. 2015, 5, 11293–11296. doi:10.1039/c4ra14626c
Return to citation in text: [1] -
Yu, H.; Zhang, X.; Li, L.; Luo, H.; Che, G. Org. Chem. Front. 2023, 10, 686–698. doi:10.1039/d2qo01716d
Return to citation in text: [1] [2] -
Yu, H.; Yu, Z. Angew. Chem., Int. Ed. 2009, 48, 2929–2933. doi:10.1002/anie.200900278
Return to citation in text: [1] -
Yu, H.; Li, T.; Liao, P. Synthesis 2012, 44, 3743–3756. doi:10.1055/s-0032-1317691
Return to citation in text: [1] -
Yu, H.-F.; Wang, W.-J. Synth. Commun. 2020, 50, 1133–1140. doi:10.1080/00397911.2019.1681001
Return to citation in text: [1] -
Wang, W.-J.; Yu, H.-F. Synth. Commun. 2019, 49, 377–385. doi:10.1080/00397911.2018.1555851
Return to citation in text: [1] -
Yu, H.; Liao, P.; Mei, Z. Synthesis 2020, 52, 2111–2120. doi:10.1055/s-0040-1707999
Return to citation in text: [1] -
Yu, H.; Wang, K.; Zhang, X.; Wang, W. Synthesis 2021, 53, 1989–1999. doi:10.1055/s-0040-1706658
Return to citation in text: [1] -
Dong, D.; Ouyang, Y.; Yu, H.; Liu, Q.; Liu, J.; Wang, M.; Zhu, J. J. Org. Chem. 2005, 70, 4535–4537. doi:10.1021/jo050271y
Return to citation in text: [1] -
Yu, H.; Liao, P. Tetrahedron Lett. 2016, 57, 2868–2872. doi:10.1016/j.tetlet.2016.05.062
Return to citation in text: [1] -
Qi, F.; Yu, H.-F.; Wang, Y.-N.; Lv, Y.; Li, Y.-X.; Han, L.; Wang, R.; Feng, X.-N. Synth. Commun. 2017, 47, 2220–2224. doi:10.1080/00397911.2017.1368084
Return to citation in text: [1] -
Hu, X.; Yu, H.; Wang, W.; Jiang, S.; Liu, Q.; He, J. Chin. J. Org. Chem. 2019, 39, 3183–3189. doi:10.6023/cjoc201904002
Return to citation in text: [1] -
Zhao, Y.; Yu, H.; Liao, P.; Wang, W. Chem. Res. Chin. Univ. 2020, 36, 847–852. doi:10.1007/s40242-019-0011-8
Return to citation in text: [1] -
Zhao, X.-B.; Jiang, S.-A.; Wang, N.; Yu, H.-F. Synth. Commun. 2020, 50, 3404–3412. doi:10.1080/00397911.2020.1801747
Return to citation in text: [1] -
Jia, J.; Su, Y.; Yu, H.-F.; Du, H.-T.; Mei, Z.-M. Synth. Commun. 2021, 51, 1581–1587. doi:10.1080/00397911.2021.1894339
Return to citation in text: [1] -
Yu, H.; Zhang, Z.; Zhang, X.; Xu, Y.; Huo, D.; Zhang, L.; Wang, W. J. Org. Chem. 2022, 87, 2985–2996. doi:10.1021/acs.joc.1c02825
Return to citation in text: [1] -
Wang, W.; Zhang, Z.; Zhang, X.; Yu, H.; Luo, H.; Huo, D.; Xu, Y.; Zhao, X. Chin. J. Org. Chem. 2023, 43, 742–750. doi:10.6023/cjoc202207032
Return to citation in text: [1] -
Dickerson, T. J.; Reed, N. N.; Janda, K. D. Chem. Rev. 2002, 102, 3325–3344. doi:10.1021/cr010335e
Return to citation in text: [1] -
Zhao, H.; Zhang, T.; Yan, T.; Cai, M. J. Org. Chem. 2015, 80, 8849–8855. doi:10.1021/acs.joc.5b01388
Return to citation in text: [1] [2] -
Van Aken, K.; Strekowski, L.; Patiny, L. Beilstein J. Org. Chem. 2006, 2, 3–9. doi:10.1186/1860-5397-2-3
Return to citation in text: [1]
| 69. | Zhao, Y.; Yu, H.; Liao, P.; Wang, W. Chem. Res. Chin. Univ. 2020, 36, 847–852. doi:10.1007/s40242-019-0011-8 |
| 70. | Zhao, X.-B.; Jiang, S.-A.; Wang, N.; Yu, H.-F. Synth. Commun. 2020, 50, 3404–3412. doi:10.1080/00397911.2020.1801747 |
| 71. | Jia, J.; Su, Y.; Yu, H.-F.; Du, H.-T.; Mei, Z.-M. Synth. Commun. 2021, 51, 1581–1587. doi:10.1080/00397911.2021.1894339 |
| 72. | Yu, H.; Zhang, Z.; Zhang, X.; Xu, Y.; Huo, D.; Zhang, L.; Wang, W. J. Org. Chem. 2022, 87, 2985–2996. doi:10.1021/acs.joc.1c02825 |
| 73. | Wang, W.; Zhang, Z.; Zhang, X.; Yu, H.; Luo, H.; Huo, D.; Xu, Y.; Zhao, X. Chin. J. Org. Chem. 2023, 43, 742–750. doi:10.6023/cjoc202207032 |
| 74. | Dickerson, T. J.; Reed, N. N.; Janda, K. D. Chem. Rev. 2002, 102, 3325–3344. doi:10.1021/cr010335e |
| 75. | Zhao, H.; Zhang, T.; Yan, T.; Cai, M. J. Org. Chem. 2015, 80, 8849–8855. doi:10.1021/acs.joc.5b01388 |
| 1. | Zhang, L.; Dong, J.; Xu, X.; Liu, Q. Chem. Rev. 2016, 116, 287–322. doi:10.1021/acs.chemrev.5b00360 |
| 2. | Liu, Z.; Huang, F.; Wu, P.; Wang, Q.; Yu, Z. J. Org. Chem. 2018, 83, 5731–5750. doi:10.1021/acs.joc.8b00775 |
| 3. | Huang, F.; Wu, P.; Yu, Z. J. Org. Chem. 2020, 85, 4373–4385. doi:10.1021/acs.joc.0c00093 |
| 4. | Wu, P.; He, Y.; Wang, H.; Zhou, Y.-G.; Yu, Z. Org. Lett. 2020, 22, 310–315. doi:10.1021/acs.orglett.9b04335 |
| 23. | Duus, F.; Jakobsen, P.; Lawesson, S.-O. Tetrahedron 1968, 24, 5323–5336. doi:10.1016/s0040-4020(01)96327-6 |
| 36. | Ojima, I.; Inaba, S.; Nagai, Y. Tetrahedron Lett. 1973, 14, 4271–4272. doi:10.1016/s0040-4039(01)87167-7 |
| 23. | Duus, F.; Jakobsen, P.; Lawesson, S.-O. Tetrahedron 1968, 24, 5323–5336. doi:10.1016/s0040-4020(01)96327-6 |
| 24. | Xin, D.; Burgess, K. Org. Lett. 2014, 16, 2108–2110. doi:10.1021/ol5005643 |
| 25. | Davis, J. B.; Bailey, J. D.; Sello, J. K. Org. Lett. 2009, 11, 2984–2987. doi:10.1021/ol9009893 |
| 26. | Ponde, D. E.; Deshpande, V. H.; Bulbule, V. J.; Sudalai, A.; Gajare, A. S. J. Org. Chem. 1998, 63, 1058–1063. doi:10.1021/jo971404l |
| 27. | Wunderlich, J.; Roß, T.; Schröder, M.; Hahn, F. Org. Lett. 2020, 22, 4955–4959. doi:10.1021/acs.orglett.0c01348 |
| 28. | Hayashi, Y.; Miyamoto, Y.; Shoji, M. Tetrahedron Lett. 2002, 43, 4079–4082. doi:10.1016/s0040-4039(02)00675-5 |
| 29. | Yu, H.; Zhao, L.; Diao, Q.; Li, T.; Liao, P.; Hou, D.; Xin, G. Synlett 2017, 28, 1828–1834. doi:10.1055/s-0036-1588982 |
| 30. | Aderibigbe, S. O.; Coltart, D. M. J. Org. Chem. 2019, 84, 9770–9777. doi:10.1021/acs.joc.9b00397 |
| 37. | Hünig, S.; Hübner, K.; Benzing, E. Chem. Ber. 1962, 95, 926–936. doi:10.1002/cber.19620950417 |
| 13. | Chen, Z.; Liu, J.; Jin, C.; Tan, Q.; Ye, M. Tetrahedron Lett. 2019, 60, 1265–1267. doi:10.1016/j.tetlet.2019.04.001 |
| 14. | Mourelle-Insua, Á.; Méndez-Sánchez, D.; Galman, J. L.; Slabu, I.; Turner, N. J.; Gotor-Fernández, V.; Lavandera, I. Catal. Sci. Technol. 2019, 9, 4083–4090. doi:10.1039/c9cy01004a |
| 15. | Li, B.; Cheng, X.; Guan, Z.-Y.; Li, S.-Y.; Huo, T.; Cheng, G.; Fan, Y.-H.; Zhou, F.-S.; Deng, Q.-H. Org. Chem. Front. 2020, 7, 3509–3514. doi:10.1039/d0qo01022g |
| 16. | Fulo, H. F.; Rueb, N. J.; Gaston, R., Jr.; Batsomboon, P.; Ahmed, K. T.; Barrios, A. M.; Dudley, G. B. Org. Biomol. Chem. 2021, 19, 10596–10600. doi:10.1039/d1ob02106k |
| 17. | Zhang, Z.; Cao, X.; Wang, G.; Zhang, G.; Zhang, X. Green Chem. 2022, 24, 3035–3041. doi:10.1039/d2gc00395c |
| 18. | Zhang, X.; Yu, Y.; Li, W.; Shi, L.; Li, H. J. Org. Chem. 2022, 87, 16263–16275. doi:10.1021/acs.joc.2c01839 |
| 19. | Xu, L.; Ma, Z.; Hu, X.; Zhang, X.; Gao, S.; Liang, D.; Wang, B.; Li, W.; Li, Y. Org. Biomol. Chem. 2022, 20, 1013–1018. doi:10.1039/d1ob02379a |
| 20. | Tan, Q.; Chen, Q.; Zhu, Z.; Liu, X. Chem. Commun. 2022, 58, 9686–9689. doi:10.1039/d2cc03443c |
| 21. | Zhao, X.; Yang, Z.; Cheng, Y.; Huang, A.; Hu, F.; Ling, F.; Zhong, W. Adv. Synth. Catal. 2022, 364, 3074–3080. doi:10.1002/adsc.202200524 |
| 22. | Wei, Y.; Wang, G.; Zhang, Z.; Li, M.; Ma, N.; Wu, H.; Zhang, G. J. Org. Chem. 2024, 89, 1127–1139. doi:10.1021/acs.joc.3c02319 |
| 34. | Yadlapalli, R. K.; Chourasia, O. P.; Vemuri, K.; Sritharan, M.; Perali, R. S. Bioorg. Med. Chem. Lett. 2012, 22, 2708–2711. doi:10.1016/j.bmcl.2012.02.101 |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 41. | Li, L.; Jiang, C.; Peng, T.; Zhang, N.; Ji, J.; Yu, H.; Zhao, X. Synth. Commun. 2024, 54, 312–320. doi:10.1080/00397911.2023.2300643 |
| 5. | Maity, R.; Naskar, S.; Mal, K.; Biswas, S.; Das, I. Adv. Synth. Catal. 2017, 359, 4405–4410. doi:10.1002/adsc.201701020 |
| 6. | Maity, R.; Naskar, S.; Das, I. J. Org. Chem. 2018, 83, 2114–2124. doi:10.1021/acs.joc.7b03054 |
| 7. | Jiang, Y.; Stornetta, A.; Villalta, P. W.; Wilson, M. R.; Boudreau, P. D.; Zha, L.; Balbo, S.; Balskus, E. P. J. Am. Chem. Soc. 2019, 141, 11489–11496. doi:10.1021/jacs.9b02453 |
| 8. | Zhou, S.; Malet, N. R.; Song, L.; Corre, C.; Challis, G. L. Chem. Commun. 2020, 56, 14443–14446. doi:10.1039/d0cc05658h |
| 9. | Bae, D.; Lee, J.; Jin, H.; Ryu, D. H. J. Org. Chem. 2021, 86, 6001–6014. doi:10.1021/acs.joc.1c00323 |
| 10. | Takeuchi, Y.; Akagawa, K.; Kudo, K. J. Org. Chem. 2021, 86, 17307–17317. doi:10.1021/acs.joc.1c02441 |
| 11. | Chisuga, T.; Nagai, A.; Miyanaga, A.; Goto, E.; Kishikawa, K.; Kudo, F.; Eguchi, T. ACS Chem. Biol. 2022, 17, 198–206. doi:10.1021/acschembio.1c00856 |
| 12. | Kopp, J.; Brückner, R. Eur. J. Org. Chem. 2024, 27, e202301001. doi:10.1002/ejoc.202301001 |
| 35. | Kaslow, C. E.; Sommer, N. B. J. Am. Chem. Soc. 1946, 68, 644–647. doi:10.1021/ja01208a035 |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 58. | Yu, H.; Zhang, X.; Li, L.; Luo, H.; Che, G. Org. Chem. Front. 2023, 10, 686–698. doi:10.1039/d2qo01716d |
| 28. | Hayashi, Y.; Miyamoto, Y.; Shoji, M. Tetrahedron Lett. 2002, 43, 4079–4082. doi:10.1016/s0040-4039(02)00675-5 |
| 30. | Aderibigbe, S. O.; Coltart, D. M. J. Org. Chem. 2019, 84, 9770–9777. doi:10.1021/acs.joc.9b00397 |
| 75. | Zhao, H.; Zhang, T.; Yan, T.; Cai, M. J. Org. Chem. 2015, 80, 8849–8855. doi:10.1021/acs.joc.5b01388 |
| 26. | Ponde, D. E.; Deshpande, V. H.; Bulbule, V. J.; Sudalai, A.; Gajare, A. S. J. Org. Chem. 1998, 63, 1058–1063. doi:10.1021/jo971404l |
| 27. | Wunderlich, J.; Roß, T.; Schröder, M.; Hahn, F. Org. Lett. 2020, 22, 4955–4959. doi:10.1021/acs.orglett.0c01348 |
| 31. | Anwar, M.; Moloney, M. G. Tetrahedron Lett. 2007, 48, 7259–7262. doi:10.1016/j.tetlet.2007.08.052 |
| 32. | Wang, J.; Yuan, Y.; Xiong, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2012, 14, 2210–2213. doi:10.1021/ol300418h |
| 33. | Yuan, Y.; Yang, R.; Zhang-Negrerie, D.; Wang, J.; Du, Y.; Zhao, K. J. Org. Chem. 2013, 78, 5385–5392. doi:10.1021/jo400541s |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 41. | Li, L.; Jiang, C.; Peng, T.; Zhang, N.; Ji, J.; Yu, H.; Zhao, X. Synth. Commun. 2024, 54, 312–320. doi:10.1080/00397911.2023.2300643 |
| 25. | Davis, J. B.; Bailey, J. D.; Sello, J. K. Org. Lett. 2009, 11, 2984–2987. doi:10.1021/ol9009893 |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 29. | Yu, H.; Zhao, L.; Diao, Q.; Li, T.; Liao, P.; Hou, D.; Xin, G. Synlett 2017, 28, 1828–1834. doi:10.1055/s-0036-1588982 |
| 76. | Van Aken, K.; Strekowski, L.; Patiny, L. Beilstein J. Org. Chem. 2006, 2, 3–9. doi:10.1186/1860-5397-2-3 |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 41. | Li, L.; Jiang, C.; Peng, T.; Zhang, N.; Ji, J.; Yu, H.; Zhao, X. Synth. Commun. 2024, 54, 312–320. doi:10.1080/00397911.2023.2300643 |
| 38. | Bestmann, H. J.; Kumar, K. Chem. Ber. 1983, 116, 2708–2710. doi:10.1002/cber.19831160725 |
| 39. | Hendi, S. B.; Hendi, M. S.; Wolfe, J. F. Synth. Commun. 1987, 17, 13–18. doi:10.1080/00397918708063898 |
| 67. | Qi, F.; Yu, H.-F.; Wang, Y.-N.; Lv, Y.; Li, Y.-X.; Han, L.; Wang, R.; Feng, X.-N. Synth. Commun. 2017, 47, 2220–2224. doi:10.1080/00397911.2017.1368084 |
| 68. | Hu, X.; Yu, H.; Wang, W.; Jiang, S.; Liu, Q.; He, J. Chin. J. Org. Chem. 2019, 39, 3183–3189. doi:10.6023/cjoc201904002 |
| 65. | Dong, D.; Ouyang, Y.; Yu, H.; Liu, Q.; Liu, J.; Wang, M.; Zhu, J. J. Org. Chem. 2005, 70, 4535–4537. doi:10.1021/jo050271y |
| 66. | Yu, H.; Liao, P. Tetrahedron Lett. 2016, 57, 2868–2872. doi:10.1016/j.tetlet.2016.05.062 |
| 42. | Ballmann, M.; Ruer, P. C.; Hofnagel, O.; Hiller, W.; Krause, N. ACS Sustainable Chem. Eng. 2022, 10, 7288–7298. doi:10.1021/acssuschemeng.2c00713 |
| 43. | Shimoyama, Y.; Ishizuka, T.; Kotani, H.; Kojima, T. ACS Catal. 2019, 9, 671–678. doi:10.1021/acscatal.8b04004 |
| 44. | Cui, X.-l.; Tian, H.-y.; Wang, S.-j.; Chen, X.; Kong, D.-l. J. Org. Chem. 2023, 88, 8576–8582. doi:10.1021/acs.joc.3c00499 |
| 45. | Shen, D.; Li, L.; Ren, T.; Chen, K.; Zhang, X.; Zhang, H.; Zhang, S.; Gong, P.; Zhang, F.; Chao, M. J. Org. Chem. 2024, 89, 2691–2702. doi:10.1021/acs.joc.3c02762 |
| 46. | Jia, Y.; Jiang, P.; Wang, X.; Ablajan, K. J. Org. Chem. 2024, 89, 835–843. doi:10.1021/acs.joc.3c01421 |
| 47. | Sun, X.; Zheng, N.; Liu, G.; Wu, Q.; Song, W. Chem. Commun. 2022, 58, 13234–13237. doi:10.1039/d2cc04352a |
| 48. | Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. Chem. – Eur. J. 2022, 28, e202104524. doi:10.1002/chem.202104524 |
| 49. | Tian, Y.-M.; Hofmann, E.; Silva, W.; Pu, X.; Touraud, D.; Gschwind, R. M.; Kunz, W.; König, B. Angew. Chem., Int. Ed. 2023, 62, e202218775. doi:10.1002/anie.202218775 |
| 50. | Kitanosono, T.; Lu, F.; Masuda, K.; Yamashita, Y.; Kobayashi, S. Angew. Chem., Int. Ed. 2022, 61, e202202335. doi:10.1002/anie.202202335 |
| 51. | Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. doi:10.1002/anie.201002737 |
| 52. | Yu, H.-F. Synth. Commun. 2013, 43, 1280–1286. doi:10.1080/00397911.2011.631075 |
| 53. | Yu, H. Chin. J. Chem. 2012, 30, 367–371. doi:10.1002/cjoc.201100096 |
| 54. | Zhao, H.; Diao, Q.; Yu, H.; Li, T.; Liao, P.; Hou, D. Chem. Res. Chin. Univ. 2017, 33, 746–752. doi:10.1007/s40242-017-7082-1 |
| 55. | Zhao, X.-b.; Wang, Y.-m.; Yu, H.-f.; Lv, Y.-c.; Jiang, S.-a.; Wang, N. Tetrahedron 2021, 97, 132427. doi:10.1016/j.tet.2021.132427 |
| 56. | Yu, H.; Mo, J.; Niu, X.; Luo, D.; Che, G. Adv. Synth. Catal. 2023, 365, 3118–3128. doi:10.1002/adsc.202300559 |
| 57. | Yu, H.; Zhang, Y.; Li, T.; Liao, P.; Diao, Q.; Xin, G.; Meng, Q.; Hou, D. RSC Adv. 2015, 5, 11293–11296. doi:10.1039/c4ra14626c |
| 58. | Yu, H.; Zhang, X.; Li, L.; Luo, H.; Che, G. Org. Chem. Front. 2023, 10, 686–698. doi:10.1039/d2qo01716d |
| 59. | Yu, H.; Yu, Z. Angew. Chem., Int. Ed. 2009, 48, 2929–2933. doi:10.1002/anie.200900278 |
| 60. | Yu, H.; Li, T.; Liao, P. Synthesis 2012, 44, 3743–3756. doi:10.1055/s-0032-1317691 |
| 61. | Yu, H.-F.; Wang, W.-J. Synth. Commun. 2020, 50, 1133–1140. doi:10.1080/00397911.2019.1681001 |
| 62. | Wang, W.-J.; Yu, H.-F. Synth. Commun. 2019, 49, 377–385. doi:10.1080/00397911.2018.1555851 |
| 63. | Yu, H.; Liao, P.; Mei, Z. Synthesis 2020, 52, 2111–2120. doi:10.1055/s-0040-1707999 |
| 64. | Yu, H.; Wang, K.; Zhang, X.; Wang, W. Synthesis 2021, 53, 1989–1999. doi:10.1055/s-0040-1706658 |
| 40. | Xu, Q.; Zheng, B.; Pan, L.; Liu, Q.; Li, Y. Eur. J. Org. Chem. 2019, 3704–3710. doi:10.1002/ejoc.201900634 |
| 41. | Li, L.; Jiang, C.; Peng, T.; Zhang, N.; Ji, J.; Yu, H.; Zhao, X. Synth. Commun. 2024, 54, 312–320. doi:10.1080/00397911.2023.2300643 |
© 2024 Yu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.