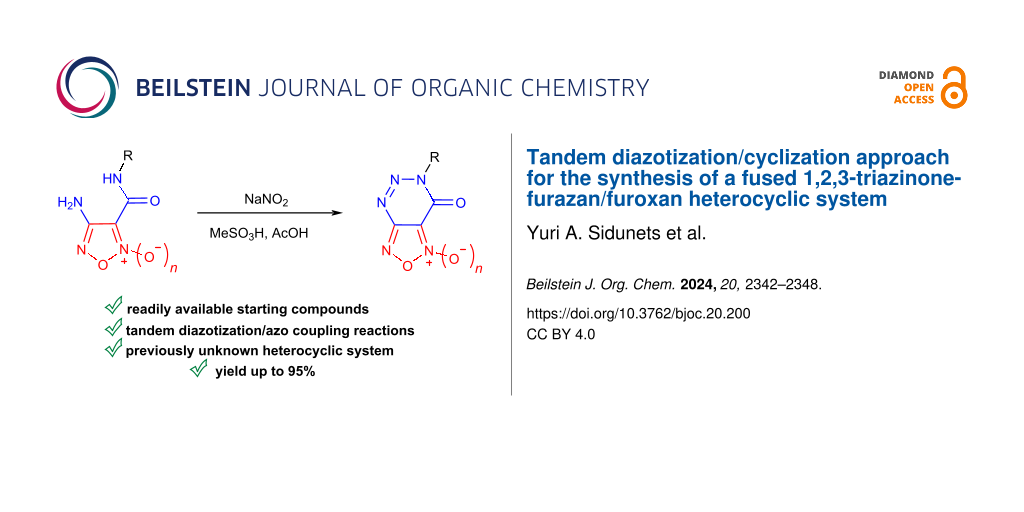
Abstract
A straightforward protocol for the synthesis of a previously unknown [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-one heterocyclic system was developed. The described approach is based on tandem diazotization/azo coupling reactions of (1,2,5-oxadiazolyl)carboxamide derivatives bearing both aromatic and aliphatic substituents. The NO-donor ability of the synthesized furoxano[3,4-d][1,2,3]triazin-7(6H)-ones was additionally evaluated. The elaborated method provides access to novel nitrogen heterocyclic compounds with potential applications as drug candidates or thermostable components of functional organic materials.
Graphical Abstract
Introduction
Nitrogen heterocycles are a significant and broad class of organic substances included in the structure of various natural products and pharmacologically active molecules. For example, nucleic acids, proteins and enzymes, hormones and vitamins, essential for the functioning of a living organism, also contain nitrogen frameworks [1,2]. Besides that, nitrogen-containing compounds are widely used in medicine as antibiotics, anticancer, non-steroidal anti-inflammatory, antihypertensive, antipsychotic, anxiolytic and in other pharmaceuticals [3-5]. Therefore, considering the diversity of biological properties, development of reliable approaches for the synthesis of new nitrogen heterocyclic systems is a highly urgent goal.
1,2,5-Oxadiazoles (furazans) and their N-oxides (furoxans) are important representatives of nitrogen heterocycles due to their wide applications in various fields of medicine, chemistry, and materials science [6,7]. For example, these heterocycles serve as valuable building blocks for the synthesis of high-energy materials [8-13]. Moreover, furazan derivatives possess antiproliferative, antibacterial, antiparasitic and antiviral activity [14-16]. On the other hand, furoxans referred to as unique heterocyclic compounds that exhibit NO-releasing properties under physiological conditions and do not demonstrate nitrate tolerance. Nitric oxide (NO) is a signaling molecule that plays a key role in numerous physiologic and pathologic processes. Thus, NO regulates blood flow and tissue oxygenation, so disruption of the production and transport of NO in the vascular system leads to various diseases [17-20]. Therefore, due to their NO-releasing abilities, furoxan derivatives also demonstrate anticancer, antiplatelet, antiviral and antiparasitic properties [21-32].
Another valuable nitrogen heterocyclic scaffold in medicinal chemistry is 1,2,3-triazin-4-one. Such compounds exhibit a wide variety of biological activities including antitumor, anticonvulsant, diuretic, anesthetic and sedative effects [33-36]. Also, several commercially available pharmaceuticals used as herbicidal, antibacterial, fungicidal and insecticidal agents contain a 1,2,3-triazine ring [37-39]. The structures of some bioactive 1,2,3-triazin-4-one derivatives are shown in Figure 1. Hence, one can assume that molecular hybridization of the 1,2,3-triazin-4-one moiety with the 1,2,5-oxadiazole core can lead to a significant modification of the pharmacological properties and may find application in the design of new promising medications.
Figure 1: Examples of bioactive compounds containing the 1,2,3-triazin-4-one core.
Figure 1: Examples of bioactive compounds containing the 1,2,3-triazin-4-one core.
Herein, we present a convenient synthetic approach for the preparation of previously unknown [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-one heterocyclic systems containing both a furoxan/furazan fragment condensed to a 1,2,3-triazin-4-one core. The proposed method is based on tandem diazotization/azo coupling reactions of the corresponding amides (Scheme 1). In addition, application perspectives of thus prepared heterocyclic entities as thermally stable components of functional organic materials or NO-donor drug candidates are also unveiled.
Scheme 1: Tandem diazotization/azo coupling reactions of (1,2,5-oxadiazolyl)carboxamides containing an amino functionality.
Scheme 1: Tandem diazotization/azo coupling reactions of (1,2,5-oxadiazolyl)carboxamides containing an amino ...
Results and Discussion
We started our investigations toward the development of the desired synthetic approach to [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-one 2-oxides 1 using functionalized furoxans 2 and 3 as suitable substrates. The starting amide precursors 2 were synthesized via the reaction of the readily available 4-amino-3-(azidocarbonyl)-1,2,5-oxadiazole 2-oxide (3) with various amines, following a previously described procedure (see Supporting Information File 1 for details) [40,41]. Subsequently, we investigated the possibility of tandem diazotization/azo coupling reactions of the obtained compounds 2. It should be emphasized that amino-1,2,5-oxadiazoles correspond to very weak nucleophiles due to the highly electron-withdrawing effect of the heterocycle. Our previous efforts achieved a certain result indicating that (1,2,5-oxadiazolyl)diazonium salts, whether isolated or generated in situ, may undergo various controlled transformations [42]. However, previously, we failed to introduce amino-1,2,5-oxadiazoles bearing an amide functionality into the diazotization protocol, arguably due to an increased electron-withdrawing effect and elevated instability of thus generated diazonium salts. In this regard, amide 2a and mesitylene were selected as model objects to optimize the reaction conditions, since azo coupling of (1,2,5-oxadiazolyl)diazonium salts with electron-donating arenes is known to proceed quantitatively [42]. We varied the diazotization reagents, solvents and temperature, and the obtained results are summarized in Table 1.
Table 1: Optimization of the diazotization of amide 2aa.
|
|
||||
| № | [NO+] | Solvent | T oC | Yield, % b |
|---|---|---|---|---|
| 1 | NaNO2 | TFA | 0–5 | 18 |
| 2 | NOBF4 | TFA | 0–5 | 20 |
| 3 | NaNO2 | TFA | −10–0 | 30 |
| 4 | NOBF4 | TFA | −10–0 | 33 |
| 5 | NaNO2 | TFA + AcOH [1:1] | −10–0 | 35 |
| 6 | NOBF4 | TFA + AcOH [1:1] | −10–0 | 49 |
| 7 | NaNO2 | MeSO3H + AcOH [1:1] | −10–0 | 86 |
| 8 | NOBF4 | MeSO3H + AcOH [1:1] | −10–0 | 12 |
aReaction conditions: 2a (0.5 mmol, 0.12 g), nitrosating reagent (0.53 mmol), solvent (3 mL), stirring at the indicated temperature for 20 min, then mesitylene (0.5 mmol, 0.07 mL), stirring at 20 oC for 10 min. bIsolated yield.
Initially, NaNO2 and NOBF4 were chosen as nitrosating reagents in TFA solution. In all cases (Table 1, entries 1–4), the formation of the target product 4 was observed, but the yield did not exceed 33%. Apparently, such low yield of 4-(mesityldiazenyl)-3-(p-tolylcarbamoyl)-1,2,5-oxadiazole 2-oxide (4) is likely due to the moderate solubility of the starting amide 2a in TFA. To improve the solubility of compound 2a, we tested mixtures of acids as solvent (Table 1, entries 5–8). Thus, the best yield of product 4 was obtained using NaNO2 in an AcOH + MeSO3H [1:1] solution (Table 1, entry 7). Organic solvents (CH2Cl2, MeCN) were not applied due to a known rapid decomposition of the generated (1,2,5-oxadiazolyl)diazonium salts [42].
The optimized conditions were applied for the preparation of [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-one 2-oxides 1 (Scheme 2). Note, that triazinones 1a–d bearing aryl substituents at position 6 were obtained in high yields, however, in the case of the 2-methoxyphenyl derivative the yield of target triazinone 1e was somewhat lower arguably due to steric hindrance. To our delight, furoxancarboxamides 2f–h bearing aliphatic substituents or amino acid residues also smoothly underwent the studied tandem protocol and the corresponding biheterocyclic compounds 1f–h were obtained in yields of 45–77%.
Scheme 2: Synthesis of target furoxanotriazinones 1a–h.
Scheme 2: Synthesis of target furoxanotriazinones 1a–h.
After having developed a general method for the synthesis of target furoxanotriazinones 1a–h, we extended this approach to amides 5 containing a furazan ring that were obtained via the reaction of readily available 4-amino-3-furazancarboxylic acid 6 with various amines using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate (TBTU) as a coupling reagent (Scheme 3; see Supporting Information File 1 for details) [43]. As expected, amides 5 also undergo tandem diazotization/azo coupling reaction to form the target [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-ones 7. It should also be noted, that compounds 7 were obtained in similar yields as the corresponding furoxan analogues, indicating that the developed tandem protocol does not depend on the presence of the N-oxide moiety in the parent heterocycle.
Scheme 3: The synthesis of furazanotriazinones 7a–h.
Scheme 3: The synthesis of furazanotriazinones 7a–h.
All synthesized triazinones 1 and 7 were fully characterized by IR, 1H and 13C NMR spectroscopy, and high-resolution mass spectrometry. The structure of compounds 1b and 7h was additionally confirmed by X-ray diffraction (see Supporting Information File 1 for details) (Figure 2).
Figure 2: The X-ray structure of compound 1b (CCDC 2363621) and 7h (CCDC 2363622).
Figure 2: The X-ray structure of compound 1b (CCDC 2363621) and 7h (CCDC 2363622).
To confirm the reaction mechanism, we performed diazotization followed by azo coupling of amide 2a using labeled Na15NO2 as the nitrosating reagent (Scheme 4). As a result, 15N-labeled triazinone 8 was obtained. Thus, we have demonstrated that the terminal nitrogen atom in the diazonium fragment of intermediate 9 becomes the N5 atom of compound 8 (corresponding 15N NMR spectra are provided in Supporting Information File 1).
Scheme 4: Control experiment with Na15NO2.
Scheme 4: Control experiment with Na15NO2.
To explore the potential application of the obtained compounds 1 and 7, we conducted a series of studies. Thus, due to the presence of a furoxan fragment, triazinones 1 can act as NO-donors. To assess their NO-release capability, compounds 1 were kept for 1 hour under physiological conditions (pH 7.4, 37 °C), then Griess reagent was added and studied by spectrophotometry (this reagent detects nitrite formed by the enzymatic oxidation of NO) [44,45]. As shown in Figure 3, compounds 1a–e containing an aryl substituent at position 6 exhibited low NO-donor ability (0.3–4.5%). In contrast, compounds 1f–h with an aliphatic fragment showed moderate activity, with the maximum value recorded for compound 1f – 36.9%. Therefore, the synthesized triazinones 1 exhibit a wide range of NO-releasing properties and could be considered as potential drug candidates.
Additionally, thermal stability of the obtained triazinones 1 and 7 was evaluated by differential scanning calorimetry. The experiments demonstrated that derivatives 1a–f and 7a–e are thermally stable substances with a melting point range of 150–224 °C (DSC curves are provided in Supporting Information File 1), and could be used as components of functional organic materials.
Conclusion
In summary, we have developed a convenient and straightforward approach for the synthesis of previously unknown [1,2,5]oxadiazolo[3,4-d][1,2,3]triazin-7(6H)-one derivatives based on tandem diazotization/azo coupling reactions of readily available (1,2,5-oxadiazolyl)carboxamides containing an amino functionality. The developed protocol was found to be suitable for the preparation of a library of new biheterocyclic molecules bearing aromatic and aliphatic substituents as well as incorporating amino acid residues. The obtained furoxanotriazinones have demonstrated a moderate NO-releasing ability across a wide range of concentrations under physiological conditions. Moreover, the target bicyclic compounds were shown to be thermostable substances and could be used in various fields of materials science.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data of all products, copies of 1H, 13C NMR, 15N spectra of new compounds, DSC curves,X-ray crystallographic data and copies of IR spectra. | ||
| Format: PDF | Size: 7.9 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Walsh, C. T. Tetrahedron Lett. 2015, 56, 3075–3081. doi:10.1016/j.tetlet.2014.11.046
Return to citation in text: [1] -
Kabir, E.; Uzzaman, M. Results Chem. 2022, 4, 100606. doi:10.1016/j.rechem.2022.100606
Return to citation in text: [1] -
Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g
Return to citation in text: [1] -
Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909
Return to citation in text: [1] -
Kumar, A.; Singh, A. K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J. P.; Pathak, P.; Grishina, M.; Verma, A.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Kumar, P. Pharmaceuticals 2023, 16, 299. doi:10.3390/ph16020299
Return to citation in text: [1] -
Fershtat, L. L.; Teslenko, F. E. Synthesis 2021, 53, 3673–3682. doi:10.1055/a-1529-7678
Return to citation in text: [1] -
Kumar, G.; Kumar, R.; Mazumder, A.; Salahuddin; Kumar, U. Chem. Biol. Drug Des. 2023, 102, 907–920. doi:10.1111/cbdd.14276
Return to citation in text: [1] -
Fershtat, L. L.; Makhova, N. N. ChemPlusChem 2020, 85, 13–42. doi:10.1002/cplu.201900542
Return to citation in text: [1] -
Larin, A. A.; Degtyarev, D. D.; Ananyev, I. V.; Pivkina, A. N.; Fershtat, L. L. Chem. Eng. J. 2023, 470, 144144. doi:10.1016/j.cej.2023.144144
Return to citation in text: [1] -
Wang, S.; Xu, Y.; Jiang, S.; Yang, F.; Li, D.; Wang, P.; Lin, Q.; Lu, M. Chem. Eng. J. 2023, 454, 140358. doi:10.1016/j.cej.2022.140358
Return to citation in text: [1] -
Shaferov, A. V.; Fershtat, L. L. Russ. Chem. Rev. 2024, 93, RCR5109. doi:10.59761/rcr5109
Return to citation in text: [1] -
Deltsov, I. D.; Ananyev, I. V.; Meerov, D. B.; Fershtat, L. L. J. Org. Chem. 2024, 89, 174–182. doi:10.1021/acs.joc.3c01858
Return to citation in text: [1] -
Zhang, J.; Zhou, J.; Bi, F.; Wang, B. Chin. Chem. Lett. 2020, 31, 2375–2394. doi:10.1016/j.cclet.2020.01.026
Return to citation in text: [1] -
Mancini, R. S.; Barden, C. J.; Weaver, D. F.; Reed, M. A. J. Med. Chem. 2021, 64, 1786–1815. doi:10.1021/acs.jmedchem.0c01901
Return to citation in text: [1] -
Hermann, T.; Hochegger, P.; Dolensky, J.; Seebacher, W.; Saf, R.; Kaiser, M.; Mäser, P.; Weis, R. Pharmaceuticals 2021, 14, 412. doi:10.3390/ph14050412
Return to citation in text: [1] -
Levinson, F. S.; Evgen'ev, M. I.; Ermolaeva, E. A.; Efimov, S. I.; Falyakhov, I. F.; Garipov, T. V.; Karimova, R. G. Pharm. Chem. J. 2003, 37, 522–525. doi:10.1023/b:phac.0000014855.66913.83
Return to citation in text: [1] -
Murad, F. Biosci. Rep. 2004, 24, 452–474. doi:10.1007/s10540-005-2741-8
Return to citation in text: [1] -
Heinrich, T. A.; da Silva, R. S.; Miranda, K. M.; Switzer, C. H.; Wink, D. A.; Fukuto, J. M. Br. J. Pharmacol. 2013, 169, 1417–1429. doi:10.1111/bph.12217
Return to citation in text: [1] -
Xu, W.; Liu, L. Z.; Loizidou, M.; Ahmed, M.; Charles, I. G. Cell Res. 2002, 12, 311–320. doi:10.1038/sj.cr.7290133
Return to citation in text: [1] -
Andrabi, S. M.; Sharma, N. S.; Karan, A.; Shahriar, S. M. S.; Cordon, B.; Ma, B.; Xie, J. Adv. Sci. 2023, 10, e2303259. doi:10.1002/advs.202303259
Return to citation in text: [1] -
Cerecetto, H.; Porcal, W. Mini-Rev. Med. Chem. 2005, 5, 57–71. doi:10.2174/1389557053402864
Return to citation in text: [1] -
Abu Yousef, M.; Matsubara, R. RSC Adv. 2023, 13, 5228–5248. doi:10.1039/d3ra00189j
Return to citation in text: [1] -
Hwang, K. J.; Park, Y. C.; Kim, H. J.; Lee, J. H. Biosci., Biotechnol., Biochem. 1998, 62, 1693–1697. doi:10.1271/bbb.62.1693
Return to citation in text: [1] -
Schiefer, I. T.; VandeVrede, L.; Fa’, M.; Arancio, O.; Thatcher, G. R. J. J. Med. Chem. 2012, 55, 3076–3087. doi:10.1021/jm201504s
Return to citation in text: [1] -
Fershtat, L. L.; Makhova, N. N. ChemMedChem 2017, 12, 622–638. doi:10.1002/cmdc.201700113
Return to citation in text: [1] -
Li, X.; Wang, X.; Xu, C.; Huang, J.; Wang, C.; Wang, X.; He, L.; Ling, Y. MedChemComm 2015, 6, 1130–1136. doi:10.1039/c5md00158g
Return to citation in text: [1] -
Fernandes, G. F. d. S.; de Souza, P. C.; Marino, L. B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M. C.; Pavan, F. R.; dos Santos, J. L. Eur. J. Med. Chem. 2016, 123, 523–531. doi:10.1016/j.ejmech.2016.07.039
Return to citation in text: [1] -
Makhova, N. N.; Rakitin, O. A. Chem. Heterocycl. Compd. 2017, 53, 849–851. doi:10.1007/s10593-017-2135-4
Return to citation in text: [1] -
Kulikov, A. S.; Epishina, M. A.; Zhilin, E. S.; Shuvaev, A. D.; Fershtat, L. L.; Makhova, N. N. Mendeleev Commun. 2021, 31, 42–45. doi:10.1016/j.mencom.2021.01.012
Return to citation in text: [1] -
Zhilin, E. S.; Ustyuzhanina, N. E.; Fershtat, L. L.; Nifantiev, N. E.; Makhova, N. N. Chem. Biol. Drug Des. 2022, 100, 1017–1024. doi:10.1111/cbdd.13918
Return to citation in text: [1] -
Fershtat, L. L.; Shuvaev, A. D.; Zhilin, E. S. Synthesis 2023, 55, 1863–1874. doi:10.1055/a-2011-7264
Return to citation in text: [1] -
Chaplygin, D. A.; Gorbunov, Y. K.; Fershtat, L. L. Asian J. Org. Chem. 2021, 10, 2644–2653. doi:10.1002/ajoc.202100475
Return to citation in text: [1] -
Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. Eur. J. Med. Chem. 2017, 142, 74–86. doi:10.1016/j.ejmech.2017.06.003
Return to citation in text: [1] -
Kumar, R.; Singh, A. D.; Singh, J.; Singh, H.; Roy, R. K.; Chaudhary, A. Mini-Rev. Med. Chem. 2014, 14, 72–83. doi:10.2174/1389557513666140103111017
Return to citation in text: [1] -
Seela, F.; Lindner, M.; Glaçon, V.; Lin, W. J. Org. Chem. 2004, 69, 4695–4700. doi:10.1021/jo040150i
Return to citation in text: [1] -
Shiva Kumar, K.; Adepu, R.; Sandra, S.; Rambabu, D.; Rama Krishna, G.; Malla Reddy, C.; Misra, P.; Pal, M. Bioorg. Med. Chem. Lett. 2012, 22, 1146–1150. doi:10.1016/j.bmcl.2011.11.096
Return to citation in text: [1] -
Roy, K.; Paul, S. J. Mol. Model. 2010, 16, 137–153. doi:10.1007/s00894-009-0528-8
Return to citation in text: [1] -
Hunt, J. C. A.; Briggs, E.; Clarke, E. D.; Whittingham, W. G. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5226. doi:10.1016/j.bmcl.2007.06.076
Return to citation in text: [1] -
Migawa, M. T.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 2005, 48, 3840–3851. doi:10.1021/jm0402014
Return to citation in text: [1] -
Larin, A. A.; Fershtat, L. L.; Ustyuzhanina, N. E.; Gening, M. L.; Nifantiev, N. E.; Makhova, N. N. Mendeleev Commun. 2018, 28, 595–597. doi:10.1016/j.mencom.2018.11.010
Return to citation in text: [1] -
Ovchinnikov, I. V.; Kulikov, A. S.; Makhova, N. N.; Tosco, P.; Di Stilo, A.; Fruttero, R.; Gasco, A. Farmaco 2003, 58, 677–681. doi:10.1016/s0014-827x(03)00106-x
Return to citation in text: [1] -
Zhilin, E. S.; Fershtat, L. L.; Bystrov, D. M.; Kulikov, A. S.; Dmitrienko, A. O.; Ananyev, I. V.; Makhova, N. N. Eur. J. Org. Chem. 2019, 4248–4259. doi:10.1002/ejoc.201900622
Return to citation in text: [1] [2] [3] -
Yue, E. W.; Douty, B.; Wayland, B.; Bower, M.; Liu, X.; Leffet, L.; Wang, Q.; Bowman, K. J.; Hansbury, M. J.; Liu, C.; Wei, M.; Li, Y.; Wynn, R.; Burn, T. C.; Koblish, H. K.; Fridman, J. S.; Metcalf, B.; Scherle, P. A.; Combs, A. P. J. Med. Chem. 2009, 52, 7364–7367. doi:10.1021/jm900518f
Return to citation in text: [1] -
Moorcroft, M. J.; Davis, J.; Compton, R. G. Talanta 2001, 54, 785–803. doi:10.1016/s0039-9140(01)00323-x
Return to citation in text: [1] -
Stebletsova, I. A.; Larin, A. A.; Ananyev, I. V.; Fershtat, L. L. Molecules 2023, 28, 6969. doi:10.3390/molecules28196969
Return to citation in text: [1]
| 1. | Walsh, C. T. Tetrahedron Lett. 2015, 56, 3075–3081. doi:10.1016/j.tetlet.2014.11.046 |
| 2. | Kabir, E.; Uzzaman, M. Results Chem. 2022, 4, 100606. doi:10.1016/j.rechem.2022.100606 |
| 14. | Mancini, R. S.; Barden, C. J.; Weaver, D. F.; Reed, M. A. J. Med. Chem. 2021, 64, 1786–1815. doi:10.1021/acs.jmedchem.0c01901 |
| 15. | Hermann, T.; Hochegger, P.; Dolensky, J.; Seebacher, W.; Saf, R.; Kaiser, M.; Mäser, P.; Weis, R. Pharmaceuticals 2021, 14, 412. doi:10.3390/ph14050412 |
| 16. | Levinson, F. S.; Evgen'ev, M. I.; Ermolaeva, E. A.; Efimov, S. I.; Falyakhov, I. F.; Garipov, T. V.; Karimova, R. G. Pharm. Chem. J. 2003, 37, 522–525. doi:10.1023/b:phac.0000014855.66913.83 |
| 44. | Moorcroft, M. J.; Davis, J.; Compton, R. G. Talanta 2001, 54, 785–803. doi:10.1016/s0039-9140(01)00323-x |
| 45. | Stebletsova, I. A.; Larin, A. A.; Ananyev, I. V.; Fershtat, L. L. Molecules 2023, 28, 6969. doi:10.3390/molecules28196969 |
| 8. | Fershtat, L. L.; Makhova, N. N. ChemPlusChem 2020, 85, 13–42. doi:10.1002/cplu.201900542 |
| 9. | Larin, A. A.; Degtyarev, D. D.; Ananyev, I. V.; Pivkina, A. N.; Fershtat, L. L. Chem. Eng. J. 2023, 470, 144144. doi:10.1016/j.cej.2023.144144 |
| 10. | Wang, S.; Xu, Y.; Jiang, S.; Yang, F.; Li, D.; Wang, P.; Lin, Q.; Lu, M. Chem. Eng. J. 2023, 454, 140358. doi:10.1016/j.cej.2022.140358 |
| 11. | Shaferov, A. V.; Fershtat, L. L. Russ. Chem. Rev. 2024, 93, RCR5109. doi:10.59761/rcr5109 |
| 12. | Deltsov, I. D.; Ananyev, I. V.; Meerov, D. B.; Fershtat, L. L. J. Org. Chem. 2024, 89, 174–182. doi:10.1021/acs.joc.3c01858 |
| 13. | Zhang, J.; Zhou, J.; Bi, F.; Wang, B. Chin. Chem. Lett. 2020, 31, 2375–2394. doi:10.1016/j.cclet.2020.01.026 |
| 6. | Fershtat, L. L.; Teslenko, F. E. Synthesis 2021, 53, 3673–3682. doi:10.1055/a-1529-7678 |
| 7. | Kumar, G.; Kumar, R.; Mazumder, A.; Salahuddin; Kumar, U. Chem. Biol. Drug Des. 2023, 102, 907–920. doi:10.1111/cbdd.14276 |
| 42. | Zhilin, E. S.; Fershtat, L. L.; Bystrov, D. M.; Kulikov, A. S.; Dmitrienko, A. O.; Ananyev, I. V.; Makhova, N. N. Eur. J. Org. Chem. 2019, 4248–4259. doi:10.1002/ejoc.201900622 |
| 3. | Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g |
| 4. | Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909 |
| 5. | Kumar, A.; Singh, A. K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J. P.; Pathak, P.; Grishina, M.; Verma, A.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Kumar, P. Pharmaceuticals 2023, 16, 299. doi:10.3390/ph16020299 |
| 43. | Yue, E. W.; Douty, B.; Wayland, B.; Bower, M.; Liu, X.; Leffet, L.; Wang, Q.; Bowman, K. J.; Hansbury, M. J.; Liu, C.; Wei, M.; Li, Y.; Wynn, R.; Burn, T. C.; Koblish, H. K.; Fridman, J. S.; Metcalf, B.; Scherle, P. A.; Combs, A. P. J. Med. Chem. 2009, 52, 7364–7367. doi:10.1021/jm900518f |
| 37. | Roy, K.; Paul, S. J. Mol. Model. 2010, 16, 137–153. doi:10.1007/s00894-009-0528-8 |
| 38. | Hunt, J. C. A.; Briggs, E.; Clarke, E. D.; Whittingham, W. G. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5226. doi:10.1016/j.bmcl.2007.06.076 |
| 39. | Migawa, M. T.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 2005, 48, 3840–3851. doi:10.1021/jm0402014 |
| 42. | Zhilin, E. S.; Fershtat, L. L.; Bystrov, D. M.; Kulikov, A. S.; Dmitrienko, A. O.; Ananyev, I. V.; Makhova, N. N. Eur. J. Org. Chem. 2019, 4248–4259. doi:10.1002/ejoc.201900622 |
| 33. | Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. Eur. J. Med. Chem. 2017, 142, 74–86. doi:10.1016/j.ejmech.2017.06.003 |
| 34. | Kumar, R.; Singh, A. D.; Singh, J.; Singh, H.; Roy, R. K.; Chaudhary, A. Mini-Rev. Med. Chem. 2014, 14, 72–83. doi:10.2174/1389557513666140103111017 |
| 35. | Seela, F.; Lindner, M.; Glaçon, V.; Lin, W. J. Org. Chem. 2004, 69, 4695–4700. doi:10.1021/jo040150i |
| 36. | Shiva Kumar, K.; Adepu, R.; Sandra, S.; Rambabu, D.; Rama Krishna, G.; Malla Reddy, C.; Misra, P.; Pal, M. Bioorg. Med. Chem. Lett. 2012, 22, 1146–1150. doi:10.1016/j.bmcl.2011.11.096 |
| 42. | Zhilin, E. S.; Fershtat, L. L.; Bystrov, D. M.; Kulikov, A. S.; Dmitrienko, A. O.; Ananyev, I. V.; Makhova, N. N. Eur. J. Org. Chem. 2019, 4248–4259. doi:10.1002/ejoc.201900622 |
| 21. | Cerecetto, H.; Porcal, W. Mini-Rev. Med. Chem. 2005, 5, 57–71. doi:10.2174/1389557053402864 |
| 22. | Abu Yousef, M.; Matsubara, R. RSC Adv. 2023, 13, 5228–5248. doi:10.1039/d3ra00189j |
| 23. | Hwang, K. J.; Park, Y. C.; Kim, H. J.; Lee, J. H. Biosci., Biotechnol., Biochem. 1998, 62, 1693–1697. doi:10.1271/bbb.62.1693 |
| 24. | Schiefer, I. T.; VandeVrede, L.; Fa’, M.; Arancio, O.; Thatcher, G. R. J. J. Med. Chem. 2012, 55, 3076–3087. doi:10.1021/jm201504s |
| 25. | Fershtat, L. L.; Makhova, N. N. ChemMedChem 2017, 12, 622–638. doi:10.1002/cmdc.201700113 |
| 26. | Li, X.; Wang, X.; Xu, C.; Huang, J.; Wang, C.; Wang, X.; He, L.; Ling, Y. MedChemComm 2015, 6, 1130–1136. doi:10.1039/c5md00158g |
| 27. | Fernandes, G. F. d. S.; de Souza, P. C.; Marino, L. B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M. C.; Pavan, F. R.; dos Santos, J. L. Eur. J. Med. Chem. 2016, 123, 523–531. doi:10.1016/j.ejmech.2016.07.039 |
| 28. | Makhova, N. N.; Rakitin, O. A. Chem. Heterocycl. Compd. 2017, 53, 849–851. doi:10.1007/s10593-017-2135-4 |
| 29. | Kulikov, A. S.; Epishina, M. A.; Zhilin, E. S.; Shuvaev, A. D.; Fershtat, L. L.; Makhova, N. N. Mendeleev Commun. 2021, 31, 42–45. doi:10.1016/j.mencom.2021.01.012 |
| 30. | Zhilin, E. S.; Ustyuzhanina, N. E.; Fershtat, L. L.; Nifantiev, N. E.; Makhova, N. N. Chem. Biol. Drug Des. 2022, 100, 1017–1024. doi:10.1111/cbdd.13918 |
| 31. | Fershtat, L. L.; Shuvaev, A. D.; Zhilin, E. S. Synthesis 2023, 55, 1863–1874. doi:10.1055/a-2011-7264 |
| 32. | Chaplygin, D. A.; Gorbunov, Y. K.; Fershtat, L. L. Asian J. Org. Chem. 2021, 10, 2644–2653. doi:10.1002/ajoc.202100475 |
| 17. | Murad, F. Biosci. Rep. 2004, 24, 452–474. doi:10.1007/s10540-005-2741-8 |
| 18. | Heinrich, T. A.; da Silva, R. S.; Miranda, K. M.; Switzer, C. H.; Wink, D. A.; Fukuto, J. M. Br. J. Pharmacol. 2013, 169, 1417–1429. doi:10.1111/bph.12217 |
| 19. | Xu, W.; Liu, L. Z.; Loizidou, M.; Ahmed, M.; Charles, I. G. Cell Res. 2002, 12, 311–320. doi:10.1038/sj.cr.7290133 |
| 20. | Andrabi, S. M.; Sharma, N. S.; Karan, A.; Shahriar, S. M. S.; Cordon, B.; Ma, B.; Xie, J. Adv. Sci. 2023, 10, e2303259. doi:10.1002/advs.202303259 |
| 40. | Larin, A. A.; Fershtat, L. L.; Ustyuzhanina, N. E.; Gening, M. L.; Nifantiev, N. E.; Makhova, N. N. Mendeleev Commun. 2018, 28, 595–597. doi:10.1016/j.mencom.2018.11.010 |
| 41. | Ovchinnikov, I. V.; Kulikov, A. S.; Makhova, N. N.; Tosco, P.; Di Stilo, A.; Fruttero, R.; Gasco, A. Farmaco 2003, 58, 677–681. doi:10.1016/s0014-827x(03)00106-x |
© 2024 Sidunets et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.





![[1860-5397-20-200-3]](/bjoc/content/figures/1860-5397-20-200-3.png?scale=2.0&max-width=1024&background=FFFFFF)