Abstract
Trifluoromethoxylated molecules and selenylated compounds find a wide range of interesting applications, but separately. In order to combine the potential of these two motifs and to propose a new class of compounds, we have developed an electrophilic phenylseleno trifluoromethoxylation of alkenes, which leads to β-selenylated trifluoromethoxylated compounds or, upon subsequent reduction, to the trifluoromethoxylated ones.

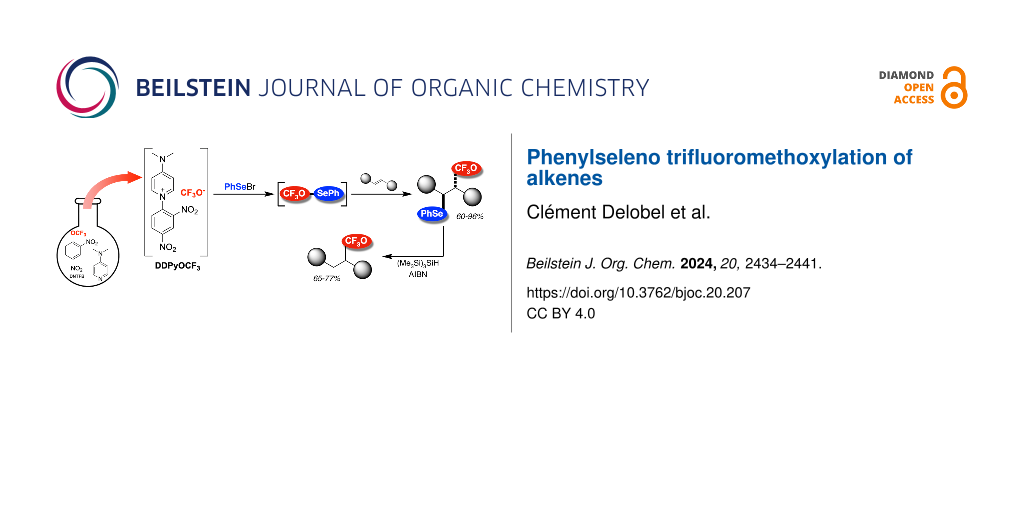
Graphical Abstract
Introduction
Due to the specific properties of the fluorine atom [1-3], fluorinated compounds are now present in a wide range of applications, from materials to life sciences [4-11]. In order to propose new molecules with specific properties for targeted applications, the development of new fluorinated moieties is an active research field [12]. Among these emerging fluorinated groups, the association of the CF3 moiety with chalcogens is an interesting approach. In particular, the trifluoromethoxy group (CF3O) possesses valuable properties such as electronegativity [13,14], lipophilicity [15,16], electronic effects [17,18], and conformation [19-21]. Some trifluoromethoxylated molecules can be used as drugs in the treatment of various pathologies (Figure 1) [22-29].
Figure 1: Examples of trifluoromethoxylated drugs.
Figure 1: Examples of trifluoromethoxylated drugs.
On the other hand, despite its toxicity at higher doses, selenium is also an essential trace element in human physiology [30,31]. Moreover, several selenylated molecules have found applications in various fields such as materials or bioactive compounds [32-43]. Some selenylated compounds exhibit fascinating biological properties.
Despite these separate converging interests, no methods have been described to synthesize both trifluoromethoxylated and phenylselenylated molecules.
The introduction of a CF3O moiety into organic molecules remains poorly described in the literature, especially the direct trifluoromethoxylation [44-46]. Only few radical trifluoromethoxylations of (hetero)aromatics [47-52], enol carbonates [53] or silyl enol ethers and allyl silanes [54] have been reported. Most of the methods described have used the trifluoromethoxide anion (CF3O−) [45]. Many sources of the CF3O− anion have been described, but with certain drawbacks such as their volatility, their tedious and expensive synthesis and the use of toxic reagents [55-65]. Recently, we reported the preparation of a stable solution of the CF3O− anion (DDPyOCF3) from the cheap and commercially available 2,4-dinitro(trifluoromethoxy)benzene (DNTFB) [66,67]. This DDPyOCF3 solution has shown a good reactivity to obtain various fluorinated compounds and especially trifluoromethoxylated molecules [68-71].
As another chapter of this research program, we propose here an easy and complementary access to CF3O-substituted alkyl compounds from alkenes and DDPyOCF3, more precisely to α-trifluoromethoxylated, β-phenylselenylated compounds.
Results and Discussion
The electrophilic addition of phenylselenyl halides to alkenes to form a selenonium intermediate that can be intercepted by an external nucleophile is a well-known method to obtain 1,2-disubstituted compounds [72-74]. Therefore, the reaction of alkenes with electrophilic sources of phenylselenyl in presence of DDPyOCF3 as a nucleophilic source of the CF3O group was studied (Table 1).
Table 1: Reaction of 1a with PhSeX and DDPyOCF3.a
|
|
|||||
| Entry | DNTFB (equiv) | DMAP (equiv) | PhSeX | Method | 2a (%) |
|---|---|---|---|---|---|
| 1 | 2 | 1 | PhSeCl | 1a then PhSeCl; 20 °C, 24 h | 12 |
| 2 | 3 | 2 | PhSeCl | 1a then PhSeCl; 20 °C, 24 h | 30 |
| 3 | 4 | 3 | PhSeCl | 1a then PhSeCl; 20 °C, 24 h | 32 |
| 4 | 3 | 2 | PhSeCl | 1a then PhSeCl; 0 °C, 24 h | 30 |
| 5 | 3 | 2 | PhSeCl |
1) PhSeCl, 15 min, 0 °C
2) 1a; 20 °C, 24 h |
44 |
| 6 | 3 | 2 | PhSeBr |
1) PhSeBr, 15 min, 0 °C
2) 1a; 20 °C, 24 h |
93 |
| 7 | 2 | 2 | PhSeBr |
1) PhSeBr, 15 min, 0 °C
2) 1a; 20 °C, 24 h |
85 |
| 8 | 2 | 2 | PhSeBr |
1) PhSeBr, 15 min, 0 °C
2) 1a; 20 °C, 2.5 h |
88 |
aYields determined by 19F NMR spectroscopy with PhCF3 as internal standard.
First, we started from the optimal conditions previously established for the trifluoromethoxylation by nucleophilic substitution, using an excess of DNTFB as a reservoir of CF3O− [68]. Thus, by adding cyclohexene (1a) to the preformed mixture of DNTFB (2 equiv) and DMAP (1 equiv), followed by the addition of PhSeCl, only a low yield of the expected α-trifluoromethoxylated,β-phenylselenylated compound 2a was observed (Table 1, entry 1). By increasing the amount of DNTFB and DMAP, the yield was doubled but remained low (entry 2 in Table 1) and did not evolve with a higher excess of reagents (entry 3). A reaction at lower temperature to possibly slow down the degradation of the CF3O− anion did not improve the results (Table 1, entry 4). A better yield was obtained by adding first the phenylselenyl chloride and stirring the mixture for 15 min at 0 °C before adding 1a (Table 1, entry 5). During all these reactions the formation of compound 3a was observed as by-product. This compound results from the competitive opening of the transient episelenonium by the more nucleophilic chloride anion competing with the less nucleophilic CF3O− anion.
To avoid this side reaction, the phenylselenyl chloride was replaced by phenylselenyl bromide, assuming that the bromide anion released is less nucleophilic than chloride in an aprotic solvent such as acetonitrile. Gratifyingly, an excellent yield was obtained (Table 1, entry 6) without detection of the brominated by-product. To facilitate purification, the amount of DNTFB was reduced to 2 equiv without significantly changing the result (Table 1, entry 7). Finally, the reaction time was reduced from 24 h to 2.5 h without affecting the yield (Table 1, entry 8).
In order to better understand the mechanism of the reaction, an NMR study of the premixing of DDPyOCF3 with PhSeBr was performed. The disappearance of the broad signal of CF3O− and the appearance of a new broad signal in the upper field were observed (Scheme 1). This suggests the formation of the highly reactive CF3OSePh species, which cannot be isolated. Furthermore, since only the trans stereomer 2a was observed, the transient formation of an episelenonium can be reasonably assumed. Consequently, the mechanism described in Scheme 1 can be proposed.
Scheme 1: Proposed mechanism of the reaction and 19F NMR of the DDPYOCF3/PhSeBr mixture.
Scheme 1: Proposed mechanism of the reaction and 19F NMR of the DDPYOCF3/PhSeBr mixture.
Under optimal conditions (Table 1, entry 8), various alkenes were functionalized (Scheme 2).
Scheme 2: Phenylseleno trifluoromethoxylation of various alkenes. Yields determined by 19F NMR spectroscopy with PhCF3 as internal standard (in parentheses isolated yields). a24 h. b48 h.
Scheme 2: Phenylseleno trifluoromethoxylation of various alkenes. Yields determined by 19F NMR spectroscopy w...
In general, the reaction gave good yields for both cyclic (2a–d, 2j) and aliphatic alkenes (2e–2i, 2k, l). Similar results were observed regardless of the position of the double bond in the molecule. Notably, a longer reaction time was required for less reactive or more hindered substrates. The reaction was stereoselective as only the anti products were obtained. A good regioselectivity was generally observed, as at least 80% of the main regioisomer were usually obtained when the substituents at the double bond differed significantly. When the substituent hindrance was less pronounced, the ratio was less significant (2f, 2j). Interestingly, a reverse regioselectivity was observed depending on the starting alkenes. For the terminal alkenes, the Markovnikov product (i.e. with the CF3O in the “internal” position) was predominant, whereas for the allylic alcohol derivatives, the anti-Markovnikov addition (i.e. with the CF3O in the “terminal” position) was predominant (2e vs 2g and 2h vs 2i). This could be rationalized by the electronic effect of the oxygen atom which disfavors the episelenonium opening with the CF3O− anion in the closest position to the oxygen atom. It is noteworthy that the regioisomeric ratio of terminal alkenes (2e, 2i) evolved with the reaction time. The amount of the kinetic terminal regioisomer (anti-Markovnikov – 2e’, 2i’) decreases with time in favor of the thermodynamic Markovnikov regioisomer (2e, 2i). This phenomenon cannot occur for products 2g and 2h because the kinetic and thermodynamic products are the same. This observation confirms the existence of an equilibrium between the episelenonium and the final products 2 (Scheme 1). It should be noted that the reaction with styrene gave low yields and the resulting products appeared very unstable. Finally, the tri-substituted alkene 1-methylcyclohexene did not give the expected products.
Finally, some more elaborated molecules such as the macrolactone 2j, the clofibrate derivative 2k, and the estrone derivative 2l were also successfully bis-functionalized.
Some products appeared to be sensitive during purification by chromatography on silica gel. Suspecting acid sensitivity, compound 2a was treated with trifluoroacetic acid to confirm this hypothesis (Scheme 3). The product resulting from the substitution of the CF3O group by the trifluoroacetoxy group was then observed. The activation of a fluorine atom from the CF3O group by H+ could be envisaged, which would then trigger the selenium attack to release difluorophosgene and HF, thus generating an episelenonium, which would finally be reopened by trifluoroacetate (Scheme 3).
Scheme 3: Degradation of 2a under acidic conditions.
Scheme 3: Degradation of 2a under acidic conditions.
Although the selenylated compounds 2 are of interest, the presence of the PhSe moiety allows other transformations to be considered. First, the oxidative elimination of the selenyl moiety to generate a double bond was first studied. However, regardless of the oxidative conditions used (mCPBA [75], H2O2 [75], selectfluor®/H2O [76], SO2Cl2/NaHCO3 (aq) [77,78]), in most cases a complex mixture was observed and no corresponding vinylic compound was detected by NMR.
The phenylselenyl moiety could also undergo radical reduction to produce trifluoromethoxylated molecules [79]. Using tris(trimethylsilyl)silane in the presence of AIBN [80], some compounds were successfully reduced to give the corresponding trifluoromethoxylated products with good yields (Scheme 4). This approach could be a complementary method to obtain trifluoromethoxylated compounds that are difficult to synthesize by nucleophilic substitution, such as products 5a and 5b [68].
Scheme 4: Radical deselenylation of 2. Yields determined by 19F NMR spectroscopy with PhCF3 as internal standard (in parentheses isolated yields).
Scheme 4: Radical deselenylation of 2. Yields determined by 19F NMR spectroscopy with PhCF3 as internal stand...
Conclusion
In this work, an efficient phenylseleno trifluoromethoxylation of alkenes has been developed to readily obtain β-selenylated trifluoromethoxylated compounds. These compounds can also undergo radical deselenylation to provide trifluoromethoxylated molecules that can be difficult to access by nucleophilic substitution. These results contribute to the further valorization of the DDPyOCF3 salt (arising from DNTFB/DMAP) as an efficient tool in organic fluorine chemistry.
Experimental
Typical procedure: Synthesis of 2. In a 10 mL vial, DNTFB (160 µL, 1 mmol, 2 equiv) is added in one portion to a stirred solution of DMAP (119 mg, 0.975 mmol, 1.95 equiv) in anhydrous MeCN (1.5 mL). The vial is closed and the reaction mixture is stirred in an ice bath for 15 minutes (the reaction rapidly turns orange after the addition of DNTFB and quickly turns yellow). Then, the tube is opened and PhSeBr (118 mg, 0.5 mmol, 1 equiv) is added in one portion. The resulting reaction mixture is stirred in the same ice bath for 15 minutes. Then, the tube is opened and the alkene (1, 0.5 mmol, 1 equiv) is added. The reaction is stirred at room temperature for 2.5 h (unless otherwise stated). Note that a yellowish precipitate is formed during the reaction for high yielding substrates. The reaction is monitored by 19F NMR (PhCF3 as internal standard). At the end of the reaction, the content of the vial is transferred to a separatory funnel and 10 mL of water are added. The aqueous layer is extracted three times with 10 mL of diethyl ether. The organic layers are combined and washed with 10 mL of water. The organic layer is dried with MgSO4, filtered, and concentrated under vacuum. Compounds 2 are obtained after purification by chromatography.
Supporting Information
| Supporting Information File 1: Additional experimental and analytical data and NMR spectra. | ||
| Format: PDF | Size: 4.3 MB | Download |
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2013. doi:10.1002/9783527651351
Return to citation in text: [1] -
Szabó, K. J.; Selander, N., Eds. Organofluorine Chemistry: Synthesis, Modeling, and Applications; Wiley: Weinheim, Germany, 2021. doi:10.1002/9783527825158
Return to citation in text: [1] -
Dolui, S.; Kumar, D.; Banerjee, S.; Ameduri, B. Acc. Mater. Res. 2021, 2, 242–251. doi:10.1021/accountsmr.1c00015
Return to citation in text: [1] -
Wang, Y.; Yang, X.; Meng, Y.; Wen, Z.; Han, R.; Hu, X.; Sun, B.; Kang, F.; Li, B.; Zhou, D.; Wang, C.; Wang, G. Chem. Rev. 2024, 124, 3494–3589. doi:10.1021/acs.chemrev.3c00826
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788
Return to citation in text: [1] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C. J.; Whittaker, A. K. Chem. Rev. 2022, 122, 167–208. doi:10.1021/acs.chemrev.1c00632
Return to citation in text: [1] -
Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S. S. Eur. J. Med. Chem. 2023, 260, 115758. doi:10.1016/j.ejmech.2023.115758
Return to citation in text: [1] -
Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467. doi:10.1016/j.isci.2020.101467
Return to citation in text: [1] -
Jeschke, P. Eur. J. Org. Chem. 2022, e202101513. doi:10.1002/ejoc.202101513
Return to citation in text: [1] -
Ma, J. A.; Cahard, D., Eds. Emerging Fluorinated Motifs: Synthesis, Properties and Applications; Wiley: Weinheim, Germany, 2020. doi:10.1002/9783527824342
Return to citation in text: [1] -
Huheey, J. E. J. Phys. Chem. 1965, 69, 3284–3291. doi:10.1021/j100894a011
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003
Return to citation in text: [1] -
Taft, R. W.; Price, E.; Fox, I. R.; Lewis, I. C.; Andersen, K. K.; Davis, G. T. J. Am. Chem. Soc. 1963, 85, 3146–3156. doi:10.1021/ja00903a022
Return to citation in text: [1] -
Taft, R. W.; Price, E.; Fox, I. R.; Lewis, I. C.; Andersen, K. K.; Davis, G. T. J. Am. Chem. Soc. 1963, 85, 709–724. doi:10.1021/ja00889a015
Return to citation in text: [1] -
Shishkov, I. F.; Khristenko, L. V.; Vilkov, L. V.; Oberhammer, H. J. Phys. Chem. A 2004, 108, 4966–4970. doi:10.1021/jp0492671
Return to citation in text: [1] -
Manteau, B.; Genix, P.; Brelot, L.; Vors, J.-P.; Pazenok, S.; Giornal, F.; Leuenberger, C.; Leroux, F. R. Eur. J. Org. Chem. 2010, 6043–6066. doi:10.1002/ejoc.201000958
Return to citation in text: [1] -
Kang, L.; Novick, S. E.; Gou, Q.; Spada, L.; Vallejo-López, M.; Caminati, W. J. Mol. Spectrosc. 2014, 297, 32–34. doi:10.1016/j.jms.2014.01.011
Return to citation in text: [1] -
Liu, J.; Lin, W.; Sorochinsky, A. E.; Butler, G.; Landa, A.; Han, J.; Soloshonok, V. A. J. Fluorine Chem. 2022, 257-258, 109978. doi:10.1016/j.jfluchem.2022.109978
Return to citation in text: [1] -
Bensimon, G.; Lacomblez, L.; Meininger, V. N. Engl. J. Med. 1994, 330, 585–591. doi:10.1056/nejm199403033300901
Return to citation in text: [1] -
Fang, T.; Al Khleifat, A.; Meurgey, J.-H.; Jones, A.; Leigh, P. N.; Bensimon, G.; Al-Chalabi, A. Lancet Neurol. 2018, 17, 416–422. doi:10.1016/s1474-4422(18)30054-1
Return to citation in text: [1] -
Stover, C. K.; Warrener, P.; VanDevanter, D. R.; Sherman, D. R.; Arain, T. M.; Langhorne, M. H.; Anderson, S. W.; Towell, J. A.; Yuan, Y.; McMurray, D. N.; Kreiswirth, B. N.; Barry, C. E.; Baker, W. R. Nature 2000, 405, 962–966. doi:10.1038/35016103
Return to citation in text: [1] -
Conradie, F.; Diacon, A. H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A. M.; Mendel, C. M.; Egizi, E.; Moreira, J.; Timm, J.; McHugh, T. D.; Wills, G. H.; Bateson, A.; Hunt, R.; Van Niekerk, C.; Li, M.; Olugbosi, M.; Spigelman, M. N. Engl. J. Med. 2020, 382, 893–902. doi:10.1056/nejmoa1901814
Return to citation in text: [1] -
Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; Yu, Q.; Ostrom, L.; Fordjour, P.; Anderson, D. L.; Monahan, J. E.; Kelleher, J. F.; Peukert, S.; Pan, S.; Wu, X.; Maira, S.-M.; García-Echeverría, C.; Briggs, K. J.; Watkins, D. N.; Yao, Y.-m.; Lengauer, C.; Warmuth, M.; Sellers, W. R.; Dorsch, M. Sci. Transl. Med. 2010, 2, 51r. doi:10.1126/scitranslmed.3001599
Return to citation in text: [1] -
Pan, S.; Wu, X.; Jiang, J.; Gao, W.; Wan, Y.; Cheng, D.; Han, D.; Liu, J.; Englund, N. P.; Wang, Y.; Peukert, S.; Miller-Moslin, K.; Yuan, J.; Guo, R.; Matsumoto, M.; Vattay, A.; Jiang, Y.; Tsao, J.; Sun, F.; Pferdekamper, A. C.; Dodd, S.; Tuntland, T.; Maniara, W.; Kelleher, J. F., III; Yao, Y.-m.; Warmuth, M.; Williams, J.; Dorsch, M. ACS Med. Chem. Lett. 2010, 1, 130–134. doi:10.1021/ml1000307
Return to citation in text: [1] -
Migden, M. R.; Guminski, A.; Gutzmer, R.; Dirix, L.; Lewis, K. D.; Combemale, P.; Herd, R. M.; Kudchadkar, R.; Trefzer, U.; Gogov, S.; Pallaud, C.; Yi, T.; Mone, M.; Kaatz, M.; Loquai, C.; Stratigos, A. J.; Schulze, H.-J.; Plummer, R.; Chang, A. L. S.; Cornélis, F.; Lear, J. T.; Sellami, D.; Dummer, R. Lancet Oncol. 2015, 16, 716–728. doi:10.1016/s1470-2045(15)70100-2
Return to citation in text: [1] -
European Food Safety Authority. EFSA Supporting Publ. 2017, 14, e15121E. doi:10.2903/sp.efsa.2017.e15121
Return to citation in text: [1] -
EFSA Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2014, 12, 3846. doi:10.2903/j.efsa.2014.3846
Return to citation in text: [1] -
Hoover, G. C.; Seferos, D. S. Chem. Sci. 2019, 10, 9182–9188. doi:10.1039/c9sc04279b
Return to citation in text: [1] -
Fan, B.; Lin, F.; Wu, X.; Zhu, Z.; Jen, A. K.-Y. Acc. Chem. Res. 2021, 54, 3906–3916. doi:10.1021/acs.accounts.1c00443
Return to citation in text: [1] -
Rayman, M. P. Lancet 2000, 356, 233–241. doi:10.1016/s0140-6736(00)02490-9
Return to citation in text: [1] -
Brown, K. M.; Arthur, J. R. Public Health Nutr. 2001, 4, 593–599. doi:10.1079/phn2001143
Return to citation in text: [1] -
Singh, N.; Halliday, A. C.; Thomas, J. M.; Kuznetsova, O. V.; Baldwin, R.; Woon, E. C. Y.; Aley, P. K.; Antoniadou, I.; Sharp, T.; Vasudevan, S. R.; Churchill, G. C. Nat. Commun. 2013, 4, 1332. doi:10.1038/ncomms2320
Return to citation in text: [1] -
Mousa, R.; Notis Dardashti, R.; Metanis, N. Angew. Chem., Int. Ed. 2017, 56, 15818–15827. doi:10.1002/anie.201706876
Return to citation in text: [1] -
Rocha, J. B. T.; Piccoli, B. C.; Oliveira, C. S. ARKIVOC 2017, No. ii, 457–491. doi:10.24820/ark.5550190.p009.784
Return to citation in text: [1] -
Jain, V. K.; Priyadarsini, K. I., Eds. Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry: Croydon, UK, 2017. doi:10.1039/9781788011907
Return to citation in text: [1] -
Chen, Z.; Lai, H.; Hou, L.; Chen, T. Chem. Commun. 2020, 56, 179–196. doi:10.1039/c9cc07683b
Return to citation in text: [1] -
Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Eur. J. Med. Chem. 2021, 223, 113621. doi:10.1016/j.ejmech.2021.113621
Return to citation in text: [1] -
Morán-Serradilla, C.; Plano, D.; Sanmartín, C.; Sharma, A. K. J. Med. Chem. 2024, 67, 7759–7787. doi:10.1021/acs.jmedchem.3c02426
Return to citation in text: [1] -
Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Org. Chem. Front. 2020, 7, 2815–2841. doi:10.1039/d0qo00640h
Return to citation in text: [1] -
Tang, P.; Jiang, X. Reagents for Direct Trifluoromethoxylation. In Emerging Fluorinated Motifs; Cahard, D.; Ma, J. A., Eds.; Wiley: Weinheim, Germany, 2020; pp 207–224. doi:10.1002/9783527824342.ch7
Return to citation in text: [1] -
Toulgoat, F.; Liger, F.; Billard, T. Chemistry of OCF3, SCF3, and SeCF3 Functional Groups. In Organofluorine Chemistry: Synthesis, Modeling, and Applications; Szabó, K.; Selander, N., Eds.; Wiley‐VCH: Weinheim, Germany, 2021; pp 49–97. doi:10.1002/9783527825158.ch3
Return to citation in text: [1] [2] -
Hao, B.-Y.; Han, Y.-P.; Zhang, Y.; Liang, Y.-M. Org. Biomol. Chem. 2023, 21, 4926–4954. doi:10.1039/d3ob00258f
Return to citation in text: [1] -
Jelier, B. J.; Tripet, P. F.; Pietrasiak, E.; Franzoni, I.; Jeschke, G.; Togni, A. Angew. Chem., Int. Ed. 2018, 57, 13784–13789. doi:10.1002/anie.201806296
Return to citation in text: [1] -
Zheng, W.; Morales-Rivera, C. A.; Lee, J. W.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 9645–9649. doi:10.1002/anie.201800598
Return to citation in text: [1] -
Zheng, W.; Lee, J. W.; Morales-Rivera, C. A.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 13795–13799. doi:10.1002/anie.201808495
Return to citation in text: [1] -
Lee, J. W.; Lee, K. N.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2019, 58, 11171–11181. doi:10.1002/anie.201902243
Return to citation in text: [1] -
Lee, J. W.; Lim, S.; Maienshein, D. N.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2020, 59, 21475–21480. doi:10.1002/anie.202009490
Return to citation in text: [1] -
Dix, S.; Golz, P.; Schmid, J. R.; Riedel, S.; Hopkinson, M. N. Chem. – Eur. J. 2021, 27, 11554–11558. doi:10.1002/chem.202101621
Return to citation in text: [1] -
Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Org. Lett. 2021, 23, 7088–7093. doi:10.1021/acs.orglett.1c02494
Return to citation in text: [1] -
Maas, L. M.; Fasting, C.; Voßnacker, P.; Limberg, N.; Golz, P.; Müller, C.; Riedel, S.; Hopkinson, M. N. Angew. Chem., Int. Ed. 2024, 63, e202317770. doi:10.1002/anie.202317770
Return to citation in text: [1] -
Billard, T. Methanesulfonic Acid, 1,1,1-Trifluoro-, Trifluoromethyl Ester. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd, 2016. doi:10.1002/047084289x.rn01930
Return to citation in text: [1] -
Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. J. Am. Chem. Soc. 2018, 140, 6801–6805. doi:10.1021/jacs.8b04000
Return to citation in text: [1] -
Lei, M.; Miao, H.; Wang, X.; Zhang, W.; Zhu, C.; Lu, X.; Shen, J.; Qin, Y.; Zhang, H.; Sha, S.; Zhu, Y. Tetrahedron Lett. 2019, 60, 1389–1392. doi:10.1016/j.tetlet.2019.04.033
Return to citation in text: [1] -
Li, Y.; Yang, Y.; Xin, J.; Tang, P. Nat. Commun. 2020, 11, 755. doi:10.1038/s41467-020-14598-1
Return to citation in text: [1] -
Newton, J. J.; Jelier, B. J.; Meanwell, M.; Martin, R. E.; Britton, R.; Friesen, C. M. Org. Lett. 2020, 22, 1785–1790. doi:10.1021/acs.orglett.0c00099
Return to citation in text: [1] -
Turksoy, A.; Scattolin, T.; Bouayad-Gervais, S.; Schoenebeck, F. Chem. – Eur. J. 2020, 26, 2183–2186. doi:10.1002/chem.202000116
Return to citation in text: [1] -
Jiang, X.; Tang, P. Chin. J. Chem. 2021, 39, 255–264. doi:10.1002/cjoc.202000465
Return to citation in text: [1] -
Lu, Z.; Kumon, T.; Hammond, G. B.; Umemoto, T. Angew. Chem., Int. Ed. 2021, 60, 16171–16177. doi:10.1002/anie.202104975
Return to citation in text: [1] -
Saiter, J.; Guérin, T.; Donnard, M.; Panossian, A.; Hanquet, G.; Leroux, F. R. Eur. J. Org. Chem. 2021, 3139–3147. doi:10.1002/ejoc.202100429
Return to citation in text: [1] -
Chen, L.-Y.; Pan, P.-F.; Lin, J.-H.; Jin, C.-M.; Xiao, J.-C. J. Org. Chem. 2023, 88, 3346–3352. doi:10.1021/acs.joc.2c03018
Return to citation in text: [1] -
Yuan, W.-J.; Tong, C.-L.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2023, 88, 4434–4441. doi:10.1021/acs.joc.2c03031
Return to citation in text: [1] -
Bonnefoy, C.; Chefdeville, E.; Panosian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2021, 27, 15986–15991. doi:10.1002/chem.202102809
Return to citation in text: [1] -
Duran-Camacho, G.; Ferguson, D. M.; Kampf, J. W.; Bland, D. C.; Sanford, M. S. Org. Lett. 2021, 23, 5138–5142. doi:10.1021/acs.orglett.1c01664
Return to citation in text: [1] -
Bonnefoy, C.; Panossian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2023, 29, e202301513. doi:10.1002/chem.202301513
Return to citation in text: [1] [2] [3] -
Bonnefoy, C.; Chefdeville, E.; Tourvieille, C.; Panossian, A.; Hanquet, G.; Leroux, F.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2022, 28, e202201589. doi:10.1002/chem.202201589
Return to citation in text: [1] -
Bonnefoy, C.; Gallego, A.; Delobel, C.; Raynal, B.; Decourt, M.; Chefdeville, E.; Hanquet, G.; Panossian, A.; Leroux, F. R.; Toulgoat, F.; Billard, T. Eur. J. Org. Chem. 2024, 27, e202400142. doi:10.1002/ejoc.202400142
Return to citation in text: [1] -
Wisson, L.; Hanquet, G.; Toulgoat, F.; Billard, T.; Panossian, A.; Leroux, F. R. Eur. J. Org. Chem. 2024, 27, e202400388. doi:10.1002/ejoc.202400388
Return to citation in text: [1] -
Paulmier, C. Selenium Reagents & Intermediates in Organic Synthesis; Pergamon Press: Oxford, 1986.
Return to citation in text: [1] -
Krief, A. Selenium. In Comprehensive Organometallic Chemistry II; Abel, E. W.; Stone, F. G. A.; Wilkinson, G., Eds.; Elsevier: Oxford, 1995; pp 515–569. doi:10.1016/b978-008046519-7.00104-0
Return to citation in text: [1] -
Preedy, V. R., Ed. Selenium: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: Cambridge, 2015. doi:10.1039/9781782622215
Return to citation in text: [1] -
Billard, T.; Langlois, B. R. Tetrahedron 1999, 55, 8065–8074. doi:10.1016/s0040-4020(99)00421-4
Return to citation in text: [1] [2] -
Guo, X.; Sun, X.; Jiang, M.; Zhao, Y. Synthesis 2022, 54, 1996–2004. doi:10.1055/a-1701-6700
Return to citation in text: [1] -
Engman, L. J. Org. Chem. 1987, 52, 4086–4094. doi:10.1021/jo00227a026
Return to citation in text: [1] -
Engman, L. Tetrahedron Lett. 1987, 28, 1463–1466. doi:10.1016/s0040-4039(00)95954-9
Return to citation in text: [1] -
Renaud, P. Radical Reactions Using Selenium Precursors. In Organoselenium Chemistry: Modern Developments in Organic Synthesis; Wirth, T., Ed.; Springer: Berlin, Heidelberg, 2000; pp 81–112. doi:10.1007/3-540-48171-0_4
Return to citation in text: [1] -
Ballestri, M.; Chatgilialoglu, C.; Clark, K. B.; Griller, D.; Giese, B.; Kopping, B. J. Org. Chem. 1991, 56, 678–683. doi:10.1021/jo00002a035
Return to citation in text: [1]
| 1. | Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x |
| 2. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2013. doi:10.1002/9783527651351 |
| 3. | Szabó, K. J.; Selander, N., Eds. Organofluorine Chemistry: Synthesis, Modeling, and Applications; Wiley: Weinheim, Germany, 2021. doi:10.1002/9783527825158 |
| 15. | Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001 |
| 16. | Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003 |
| 45. | Toulgoat, F.; Liger, F.; Billard, T. Chemistry of OCF3, SCF3, and SeCF3 Functional Groups. In Organofluorine Chemistry: Synthesis, Modeling, and Applications; Szabó, K.; Selander, N., Eds.; Wiley‐VCH: Weinheim, Germany, 2021; pp 49–97. doi:10.1002/9783527825158.ch3 |
| 13. | Huheey, J. E. J. Phys. Chem. 1965, 69, 3284–3291. doi:10.1021/j100894a011 |
| 14. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 55. | Billard, T. Methanesulfonic Acid, 1,1,1-Trifluoro-, Trifluoromethyl Ester. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd, 2016. doi:10.1002/047084289x.rn01930 |
| 56. | Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. J. Am. Chem. Soc. 2018, 140, 6801–6805. doi:10.1021/jacs.8b04000 |
| 57. | Lei, M.; Miao, H.; Wang, X.; Zhang, W.; Zhu, C.; Lu, X.; Shen, J.; Qin, Y.; Zhang, H.; Sha, S.; Zhu, Y. Tetrahedron Lett. 2019, 60, 1389–1392. doi:10.1016/j.tetlet.2019.04.033 |
| 58. | Li, Y.; Yang, Y.; Xin, J.; Tang, P. Nat. Commun. 2020, 11, 755. doi:10.1038/s41467-020-14598-1 |
| 59. | Newton, J. J.; Jelier, B. J.; Meanwell, M.; Martin, R. E.; Britton, R.; Friesen, C. M. Org. Lett. 2020, 22, 1785–1790. doi:10.1021/acs.orglett.0c00099 |
| 60. | Turksoy, A.; Scattolin, T.; Bouayad-Gervais, S.; Schoenebeck, F. Chem. – Eur. J. 2020, 26, 2183–2186. doi:10.1002/chem.202000116 |
| 61. | Jiang, X.; Tang, P. Chin. J. Chem. 2021, 39, 255–264. doi:10.1002/cjoc.202000465 |
| 62. | Lu, Z.; Kumon, T.; Hammond, G. B.; Umemoto, T. Angew. Chem., Int. Ed. 2021, 60, 16171–16177. doi:10.1002/anie.202104975 |
| 63. | Saiter, J.; Guérin, T.; Donnard, M.; Panossian, A.; Hanquet, G.; Leroux, F. R. Eur. J. Org. Chem. 2021, 3139–3147. doi:10.1002/ejoc.202100429 |
| 64. | Chen, L.-Y.; Pan, P.-F.; Lin, J.-H.; Jin, C.-M.; Xiao, J.-C. J. Org. Chem. 2023, 88, 3346–3352. doi:10.1021/acs.joc.2c03018 |
| 65. | Yuan, W.-J.; Tong, C.-L.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2023, 88, 4434–4441. doi:10.1021/acs.joc.2c03031 |
| 12. | Ma, J. A.; Cahard, D., Eds. Emerging Fluorinated Motifs: Synthesis, Properties and Applications; Wiley: Weinheim, Germany, 2020. doi:10.1002/9783527824342 |
| 53. | Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Org. Lett. 2021, 23, 7088–7093. doi:10.1021/acs.orglett.1c02494 |
| 4. | Dolui, S.; Kumar, D.; Banerjee, S.; Ameduri, B. Acc. Mater. Res. 2021, 2, 242–251. doi:10.1021/accountsmr.1c00015 |
| 5. | Wang, Y.; Yang, X.; Meng, Y.; Wen, Z.; Han, R.; Hu, X.; Sun, B.; Kang, F.; Li, B.; Zhou, D.; Wang, C.; Wang, G. Chem. Rev. 2024, 124, 3494–3589. doi:10.1021/acs.chemrev.3c00826 |
| 6. | Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788 |
| 7. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 8. | Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C. J.; Whittaker, A. K. Chem. Rev. 2022, 122, 167–208. doi:10.1021/acs.chemrev.1c00632 |
| 9. | Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S. S. Eur. J. Med. Chem. 2023, 260, 115758. doi:10.1016/j.ejmech.2023.115758 |
| 10. | Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467. doi:10.1016/j.isci.2020.101467 |
| 11. | Jeschke, P. Eur. J. Org. Chem. 2022, e202101513. doi:10.1002/ejoc.202101513 |
| 54. | Maas, L. M.; Fasting, C.; Voßnacker, P.; Limberg, N.; Golz, P.; Müller, C.; Riedel, S.; Hopkinson, M. N. Angew. Chem., Int. Ed. 2024, 63, e202317770. doi:10.1002/anie.202317770 |
| 30. | European Food Safety Authority. EFSA Supporting Publ. 2017, 14, e15121E. doi:10.2903/sp.efsa.2017.e15121 |
| 31. | EFSA Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2014, 12, 3846. doi:10.2903/j.efsa.2014.3846 |
| 44. | Tang, P.; Jiang, X. Reagents for Direct Trifluoromethoxylation. In Emerging Fluorinated Motifs; Cahard, D.; Ma, J. A., Eds.; Wiley: Weinheim, Germany, 2020; pp 207–224. doi:10.1002/9783527824342.ch7 |
| 45. | Toulgoat, F.; Liger, F.; Billard, T. Chemistry of OCF3, SCF3, and SeCF3 Functional Groups. In Organofluorine Chemistry: Synthesis, Modeling, and Applications; Szabó, K.; Selander, N., Eds.; Wiley‐VCH: Weinheim, Germany, 2021; pp 49–97. doi:10.1002/9783527825158.ch3 |
| 46. | Hao, B.-Y.; Han, Y.-P.; Zhang, Y.; Liang, Y.-M. Org. Biomol. Chem. 2023, 21, 4926–4954. doi:10.1039/d3ob00258f |
| 22. | Liu, J.; Lin, W.; Sorochinsky, A. E.; Butler, G.; Landa, A.; Han, J.; Soloshonok, V. A. J. Fluorine Chem. 2022, 257-258, 109978. doi:10.1016/j.jfluchem.2022.109978 |
| 23. | Bensimon, G.; Lacomblez, L.; Meininger, V. N. Engl. J. Med. 1994, 330, 585–591. doi:10.1056/nejm199403033300901 |
| 24. | Fang, T.; Al Khleifat, A.; Meurgey, J.-H.; Jones, A.; Leigh, P. N.; Bensimon, G.; Al-Chalabi, A. Lancet Neurol. 2018, 17, 416–422. doi:10.1016/s1474-4422(18)30054-1 |
| 25. | Stover, C. K.; Warrener, P.; VanDevanter, D. R.; Sherman, D. R.; Arain, T. M.; Langhorne, M. H.; Anderson, S. W.; Towell, J. A.; Yuan, Y.; McMurray, D. N.; Kreiswirth, B. N.; Barry, C. E.; Baker, W. R. Nature 2000, 405, 962–966. doi:10.1038/35016103 |
| 26. | Conradie, F.; Diacon, A. H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A. M.; Mendel, C. M.; Egizi, E.; Moreira, J.; Timm, J.; McHugh, T. D.; Wills, G. H.; Bateson, A.; Hunt, R.; Van Niekerk, C.; Li, M.; Olugbosi, M.; Spigelman, M. N. Engl. J. Med. 2020, 382, 893–902. doi:10.1056/nejmoa1901814 |
| 27. | Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; Yu, Q.; Ostrom, L.; Fordjour, P.; Anderson, D. L.; Monahan, J. E.; Kelleher, J. F.; Peukert, S.; Pan, S.; Wu, X.; Maira, S.-M.; García-Echeverría, C.; Briggs, K. J.; Watkins, D. N.; Yao, Y.-m.; Lengauer, C.; Warmuth, M.; Sellers, W. R.; Dorsch, M. Sci. Transl. Med. 2010, 2, 51r. doi:10.1126/scitranslmed.3001599 |
| 28. | Pan, S.; Wu, X.; Jiang, J.; Gao, W.; Wan, Y.; Cheng, D.; Han, D.; Liu, J.; Englund, N. P.; Wang, Y.; Peukert, S.; Miller-Moslin, K.; Yuan, J.; Guo, R.; Matsumoto, M.; Vattay, A.; Jiang, Y.; Tsao, J.; Sun, F.; Pferdekamper, A. C.; Dodd, S.; Tuntland, T.; Maniara, W.; Kelleher, J. F., III; Yao, Y.-m.; Warmuth, M.; Williams, J.; Dorsch, M. ACS Med. Chem. Lett. 2010, 1, 130–134. doi:10.1021/ml1000307 |
| 29. | Migden, M. R.; Guminski, A.; Gutzmer, R.; Dirix, L.; Lewis, K. D.; Combemale, P.; Herd, R. M.; Kudchadkar, R.; Trefzer, U.; Gogov, S.; Pallaud, C.; Yi, T.; Mone, M.; Kaatz, M.; Loquai, C.; Stratigos, A. J.; Schulze, H.-J.; Plummer, R.; Chang, A. L. S.; Cornélis, F.; Lear, J. T.; Sellami, D.; Dummer, R. Lancet Oncol. 2015, 16, 716–728. doi:10.1016/s1470-2045(15)70100-2 |
| 47. | Jelier, B. J.; Tripet, P. F.; Pietrasiak, E.; Franzoni, I.; Jeschke, G.; Togni, A. Angew. Chem., Int. Ed. 2018, 57, 13784–13789. doi:10.1002/anie.201806296 |
| 48. | Zheng, W.; Morales-Rivera, C. A.; Lee, J. W.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 9645–9649. doi:10.1002/anie.201800598 |
| 49. | Zheng, W.; Lee, J. W.; Morales-Rivera, C. A.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 13795–13799. doi:10.1002/anie.201808495 |
| 50. | Lee, J. W.; Lee, K. N.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2019, 58, 11171–11181. doi:10.1002/anie.201902243 |
| 51. | Lee, J. W.; Lim, S.; Maienshein, D. N.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2020, 59, 21475–21480. doi:10.1002/anie.202009490 |
| 52. | Dix, S.; Golz, P.; Schmid, J. R.; Riedel, S.; Hopkinson, M. N. Chem. – Eur. J. 2021, 27, 11554–11558. doi:10.1002/chem.202101621 |
| 19. | Shishkov, I. F.; Khristenko, L. V.; Vilkov, L. V.; Oberhammer, H. J. Phys. Chem. A 2004, 108, 4966–4970. doi:10.1021/jp0492671 |
| 20. | Manteau, B.; Genix, P.; Brelot, L.; Vors, J.-P.; Pazenok, S.; Giornal, F.; Leuenberger, C.; Leroux, F. R. Eur. J. Org. Chem. 2010, 6043–6066. doi:10.1002/ejoc.201000958 |
| 21. | Kang, L.; Novick, S. E.; Gou, Q.; Spada, L.; Vallejo-López, M.; Caminati, W. J. Mol. Spectrosc. 2014, 297, 32–34. doi:10.1016/j.jms.2014.01.011 |
| 17. | Taft, R. W.; Price, E.; Fox, I. R.; Lewis, I. C.; Andersen, K. K.; Davis, G. T. J. Am. Chem. Soc. 1963, 85, 3146–3156. doi:10.1021/ja00903a022 |
| 18. | Taft, R. W.; Price, E.; Fox, I. R.; Lewis, I. C.; Andersen, K. K.; Davis, G. T. J. Am. Chem. Soc. 1963, 85, 709–724. doi:10.1021/ja00889a015 |
| 32. | Hoover, G. C.; Seferos, D. S. Chem. Sci. 2019, 10, 9182–9188. doi:10.1039/c9sc04279b |
| 33. | Fan, B.; Lin, F.; Wu, X.; Zhu, Z.; Jen, A. K.-Y. Acc. Chem. Res. 2021, 54, 3906–3916. doi:10.1021/acs.accounts.1c00443 |
| 34. | Rayman, M. P. Lancet 2000, 356, 233–241. doi:10.1016/s0140-6736(00)02490-9 |
| 35. | Brown, K. M.; Arthur, J. R. Public Health Nutr. 2001, 4, 593–599. doi:10.1079/phn2001143 |
| 36. | Singh, N.; Halliday, A. C.; Thomas, J. M.; Kuznetsova, O. V.; Baldwin, R.; Woon, E. C. Y.; Aley, P. K.; Antoniadou, I.; Sharp, T.; Vasudevan, S. R.; Churchill, G. C. Nat. Commun. 2013, 4, 1332. doi:10.1038/ncomms2320 |
| 37. | Mousa, R.; Notis Dardashti, R.; Metanis, N. Angew. Chem., Int. Ed. 2017, 56, 15818–15827. doi:10.1002/anie.201706876 |
| 38. | Rocha, J. B. T.; Piccoli, B. C.; Oliveira, C. S. ARKIVOC 2017, No. ii, 457–491. doi:10.24820/ark.5550190.p009.784 |
| 39. | Jain, V. K.; Priyadarsini, K. I., Eds. Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry: Croydon, UK, 2017. doi:10.1039/9781788011907 |
| 40. | Chen, Z.; Lai, H.; Hou, L.; Chen, T. Chem. Commun. 2020, 56, 179–196. doi:10.1039/c9cc07683b |
| 41. | Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Eur. J. Med. Chem. 2021, 223, 113621. doi:10.1016/j.ejmech.2021.113621 |
| 42. | Morán-Serradilla, C.; Plano, D.; Sanmartín, C.; Sharma, A. K. J. Med. Chem. 2024, 67, 7759–7787. doi:10.1021/acs.jmedchem.3c02426 |
| 43. | Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Org. Chem. Front. 2020, 7, 2815–2841. doi:10.1039/d0qo00640h |
| 72. | Paulmier, C. Selenium Reagents & Intermediates in Organic Synthesis; Pergamon Press: Oxford, 1986. |
| 73. | Krief, A. Selenium. In Comprehensive Organometallic Chemistry II; Abel, E. W.; Stone, F. G. A.; Wilkinson, G., Eds.; Elsevier: Oxford, 1995; pp 515–569. doi:10.1016/b978-008046519-7.00104-0 |
| 74. | Preedy, V. R., Ed. Selenium: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: Cambridge, 2015. doi:10.1039/9781782622215 |
| 66. | Bonnefoy, C.; Chefdeville, E.; Panosian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2021, 27, 15986–15991. doi:10.1002/chem.202102809 |
| 67. | Duran-Camacho, G.; Ferguson, D. M.; Kampf, J. W.; Bland, D. C.; Sanford, M. S. Org. Lett. 2021, 23, 5138–5142. doi:10.1021/acs.orglett.1c01664 |
| 68. | Bonnefoy, C.; Panossian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2023, 29, e202301513. doi:10.1002/chem.202301513 |
| 69. | Bonnefoy, C.; Chefdeville, E.; Tourvieille, C.; Panossian, A.; Hanquet, G.; Leroux, F.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2022, 28, e202201589. doi:10.1002/chem.202201589 |
| 70. | Bonnefoy, C.; Gallego, A.; Delobel, C.; Raynal, B.; Decourt, M.; Chefdeville, E.; Hanquet, G.; Panossian, A.; Leroux, F. R.; Toulgoat, F.; Billard, T. Eur. J. Org. Chem. 2024, 27, e202400142. doi:10.1002/ejoc.202400142 |
| 71. | Wisson, L.; Hanquet, G.; Toulgoat, F.; Billard, T.; Panossian, A.; Leroux, F. R. Eur. J. Org. Chem. 2024, 27, e202400388. doi:10.1002/ejoc.202400388 |
| 80. | Ballestri, M.; Chatgilialoglu, C.; Clark, K. B.; Griller, D.; Giese, B.; Kopping, B. J. Org. Chem. 1991, 56, 678–683. doi:10.1021/jo00002a035 |
| 68. | Bonnefoy, C.; Panossian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2023, 29, e202301513. doi:10.1002/chem.202301513 |
| 77. | Engman, L. J. Org. Chem. 1987, 52, 4086–4094. doi:10.1021/jo00227a026 |
| 78. | Engman, L. Tetrahedron Lett. 1987, 28, 1463–1466. doi:10.1016/s0040-4039(00)95954-9 |
| 79. | Renaud, P. Radical Reactions Using Selenium Precursors. In Organoselenium Chemistry: Modern Developments in Organic Synthesis; Wirth, T., Ed.; Springer: Berlin, Heidelberg, 2000; pp 81–112. doi:10.1007/3-540-48171-0_4 |
| 75. | Billard, T.; Langlois, B. R. Tetrahedron 1999, 55, 8065–8074. doi:10.1016/s0040-4020(99)00421-4 |
| 76. | Guo, X.; Sun, X.; Jiang, M.; Zhao, Y. Synthesis 2022, 54, 1996–2004. doi:10.1055/a-1701-6700 |
| 68. | Bonnefoy, C.; Panossian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem. – Eur. J. 2023, 29, e202301513. doi:10.1002/chem.202301513 |
| 75. | Billard, T.; Langlois, B. R. Tetrahedron 1999, 55, 8065–8074. doi:10.1016/s0040-4020(99)00421-4 |
© 2024 Delobel et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.