Abstract
In this work, we describe the synthesis of halogenated pyran analogues of ᴅ-talose using a halo-divergent strategy from known 1,6-anhydro-2,3-dideoxy-2,3-difluoro-β-ᴅ-mannopyranose. In solution and in the solid-state, all analogues adopt standard 4C1-like conformations despite 1,3-diaxial repulsion between the F2 and the C4 halogen. Moreover, the solid-state conformational analysis of halogenated pyrans reveals deviation in the intra-annular torsion angles arising from repulsion between the axial fluorine at C2 and the axial halogen at C4, which increases with the size of the halogen at C4 (F < Cl < Br < I). Crystal packing arrangements of pyran inter-halides show hydrogen bond acceptor and nonbonding interactions for the halogen at C4. Finally, density functional theory (DFT) calculations corroborate the preference of talose analogues to adopt a 4C1-like conformation and a natural bonding orbital (NBO) analysis demonstrates the effects of hyperconjugation from C–F antibonding orbitals.

Graphical Abstract
Introduction
Polyfluorinated pyran analogues of carbohydrates have attracted attention over the years. This class of glycomimetics has great biological potential with useful applications [1-7]. What about other halogens? Pyran inter-halide analogues of carbohydrates were rarely investigated as new tools in glycobiology [8]. This is surprising since the incorporation of halogens can improve cellular uptakes and enhance membrane binding and permeation [9-11]. In addition, halogen bonding is an important interaction in biological systems [12-17] and the beneficial effect of the chloro substituent has been reviewed recently [18].
As a result, there is a lack of efficient synthetic strategies to access multivicinal inter-halide stereocenters (i.e., contiguous chiral halides: F, Cl, Br, I) [19]. Only a handful of natural product syntheses have been reported [20,21], despite the promising biological activity of these unique inter-halides [22]. For our part, we recently reported the synthesis of contiguous inter-halide-bearing stereocenters using a Chiron approach from levoglucosan 1 (Figure 1a) [23]. Allopyranose inter-halides 4 incorporating the 2,3-cis, 3,4-cis relationship for the halogens were prepared via intermediates 2 and 3 from levoglucosan (1). Compounds 4 were the starting point to complex 2,3,4-trihalohexanetriols and 2,3,4,5-tetrahalohexanediols. Conformational analysis and lipophilicities of the latter compounds were determined and trihalogenated alkanes were incorporated into piperidines of pitolisant [23]. This work was an extension of our synthetic routes to multivicinal organofluorines to unveil some of their unique properties [24-30], such as the solution-state conformation of diastereomeric polyfluorohexitols [31].
Figure 1: Synthesis of trihalogenated pyrans: a) Chiron approach to multivicinal inter-halide derived from allopyranoses; b) synthesis and conformational analysis of pyran inter-halide analogues of ᴅ-talose integrating the 2,3-cis, 3,4-cis relationship for the halogens (this work).
Figure 1: Synthesis of trihalogenated pyrans: a) Chiron approach to multivicinal inter-halide derived from al...
Herein, we report the synthesis of pyran inter-halide analogues of ᴅ-talopyranose 6, integrating also the 2,3-cis, 3,4-cis relationship for the halogens, from known intermediate 5 (Figure 1b) [24]. The solution and the solid-state conformational analysis were supplemented with DFT calculations to understand key conformational drivers. This study adds more data to the field of nuclear magnetic resonance (NMR) spectroscopy of polyhalogenated molecules. It should be noted that the NMR predictions of such compounds remain very challenging [32].
Results and Discussion
Our recent discovery that the nature of halogen atoms can have a large impact on conformation and lipophilicity motivated us to investigate novel pyran inter-halides [23]. We used a halo-divergent route starting from the known 1,6-anhydro-2,3-dideoxy-2,3-difluoro-β-ᴅ-mannopyranose (5) readily accessible form levoglucosan (1, Scheme 1) [24]. Activation of the C4 hydroxy group as triflate and direct treatment with a nucleophilic halogen furnished intermediates 8–11. The latter compounds proved to be difficult to purify, therefore we were compelled to proceed directly to the next step. Cleavage of the 1,6-anhydro bridges was achieved under acetolysis conditions providing halogenated talopyranoses 12–15 in good yield over 3 steps as α anomers.
Scheme 1: Synthesis of halogenated talopyranose analogues 13–15, and 17 that include a 2,3-cis, 3,4-cis relationship for the halogens.
Scheme 1: Synthesis of halogenated talopyranose analogues 13–15, and 17 that include a 2,3-cis, 3,4-cis relat...
Luckily, inter-halides 13–15 were crystalline, allowing the absolute configuration to be confirmed by single-crystal diffraction analysis (see below) [33]. Unfortunately, trifluorinated analogue 12 was not crystalline. Thus, we removed the acetyl protecting groups and generated the corresponding p-bromobenzoate derivative 17 to obtain suitable crystalline material [24,34].
In order to decipher the key physical properties of complex pyran inter-halides, we performed 19F NMR analysis of halogenated talose analogues 12–15 (Figure 2). First, all analogues adopt standard 4C1-like conformations. Comparison of the vicinal and geminal coupling constants for each organohalogen suggests that there is little change in the conformations (although there is an increasing chair distortion for larger halogens, see below). Because F3 is adjacent to the C4 halogen, 19F resonance of F3 occurs at lower field than F2 for analogues 13–15. There is a strong increase in chemical shift of F3 depending on the incorporated halogen on the pyran core at C4: −208.33 ppm for 12 (fluorine), −197.95 ppm for 13 (chlorine), −192.80 ppm for 14 (bromine), and −184.56 ppm for 15 (iodine). Similarly, the increase in chemical shift of F2 is smaller as exemplified with an upfield shift of −205.46 ppm for 12 to −200.55 ppm for 15. Talopyranose analogues 12–15 incorporate a 2,3-cis, 3,4-cis relationship for the halogens. We previously prepared a small set of trihalogenated allopyranose analogues that also included the 2,3-cis, 3,4-cis relationship for the halogens (Figure 1a) [23]. 19F NMR analysis of halogenated allopyranose analogues can be found in Supporting Information File 1.
![[1860-5397-20-208-2]](/bjoc/content/figures/1860-5397-20-208-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Direct comparison of 19F resonances of halogenated talose analogues 12–15 (19F NMR; 470 MHz, CDCl3).
Figure 2: Direct comparison of 19F resonances of halogenated talose analogues 12–15 (19F NMR; 470 MHz, CDCl3)....
Our interest in the conformation of organohalogens motivated us to compare the solid-state conformation of halogenated pyrans 13–15, and 17 [24] with α-ᴅ-talose 18 [35] (Figure 3). The crystallographic data and structural refinement details for the crystal structures can be found in Supporting Information File 1. As tosyl and benzoate groups are essential for the crystallinity of multivicinal organofluorines they influence the solid-state conformations [31,36-38]. Thus, information drawn from the crystallographic data of compound 17 might be influenced by benzoate groups. We included compound 17 in our comparative analysis in any case. All structures adopt a standard 4C1-like conformation in the solid-state. This conformation occurs despite 1,3-diaxial repulsion between the F2 and the C4 halogen. The 1,3-diaxial repulsion between 2 fluorine atoms have been reported in recent years [24,39], however, the 1,3-diaxial repulsion between fluorine and other halogens is quite uncommon [40,41]. As for the C5–C6 rotamer, all analogues exhibit a gt conformation except for trifluorinated 17, which possesses a tg conformation. An axial substituent at C4 generally leads to a gt conformation [42,43], with some exceptions [24]. Bond distances, bond angles, torsion angles, and key interatomic distances are listed in Tables 1–4. It is important to point out that these results compare well with previous analysis of polyfluorinated carbohydrates [44,45].
![[1860-5397-20-208-3]](/bjoc/content/figures/1860-5397-20-208-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: X-ray analysis of compound 13–15, 17, and α-ᴅ-talose 18. ORTEP diagram showing 50% thermal ellipsoid probability (except for 18): carbon (gray), oxygen (red), fluorine (green), chlorine (orange), bromine (dark red), iodine (purple), and hydrogen (white).
Figure 3: X-ray analysis of compound 13–15, 17, and α-ᴅ-talose 18. ORTEP diagram showing 50% thermal ellipsoi...
The C–C bond lengths within the pyran rings of halogenated analogues are between 1.50 and 1.54 Å, which is similar to native talose (18, 1.52–1.53 Å) (Table 1, entries 1–4). However, all specified bond lengths within the pyran rings are shorter for halogenated analogues compared to α-ᴅ-talose, except for the C3–C4 bond of compound 15. This can be explained by the adjacent repulsion between CF3 with the CI4 group. Next, it has been reported that the C1–O1 bond lengths are shorter than the O5–C1 for α anomers [42,46,47]. Talopyranose (18) follows this trend, but not the halogenated analogues (Table 1, entries 7 and 8). Also, the exocyclic C1–O1 bond lengths of compounds 13–15 and 17 are in average 0.033 Å longer than native talose (18). As expected, all the C–F bond lengths are shorter than the corresponding C–OH bond lengths (Table 1, entries 10–12) [48]. The C2–F2 bond lengths are in average 0.025 Å shorter than the C2–OH bond and the C3–F3 bond lengths are in average 0.027 Å shorter than the C3–OH bond. Similarly, for compound 13–15, the C4–X bond lengths are longer than the C4–OH bond of native talose (1.43 Å): C4–Cl: 1.80 Å, C4–Br: 1.96 Å, and C4–I: 2.11 Å.
Table 1: Selected bond distances for compounds 13–15, 17, and 18.
| Entry | Bonds | Distances (Å) | ||||
|---|---|---|---|---|---|---|
| Talose (18)a | 17 | 13 | 14 | 15 | ||
| 1 | C1–C2 | 1.5316 | 1.518(3) | 1.526(2) | 1.523(3) | 1.53(1) |
| 2 | C2–C3 | 1.5234 | 1.506(3) | 1.513(2) | 1.508(3) | 1.50(2) |
| 3 | C3–C4 | 1.5300 | 1.508(4) | 1.522(2) | 1.520(3) | 1.54(2) |
| 4 | C4–C5 | 1.5325 | 1.524(3) | 1.527(2) | 1.523(3) | 1.53(1) |
| 5 | C5–C6 | 1.5127 | 1.525(3) | 1.507(2) | 1.513(3) | 1.50(1) |
| 6 | C5–O5 | 1.4489 | 1.432(3) | 1.438(2) | 1.435(2) | 1.44(1) |
| 7 | O5–C1 | 1.4380 | 1.399(3) | 1.403(2) | 1.403(2) | 1.40(1) |
| 8 | C1–O1 | 1.4028 | 1.442(3) | 1.429(2) | 1.431(2) | 1.44(1) |
| 9 | O1–C(O) | na | 1.361(3) | 1.372(2) | 1.374(3) | 1.40(1) |
| 10 | C2–F2 | 1.4228b | 1.393(3) | 1.402(2) | 1.407(2) | 1.39(1) |
| 11 | C3–F3 | 1.4212c | 1.397(3) | 1.393(2) | 1.396(2) | 1.39(1) |
| 12 | C4–X4 | 1.4279d | 1.395(3) | 1.797(2) | 1.956(2) | 2.11(1) |
aReference [35]; bC2–O2; cC3–O3; dC4–O4.
Table 2 shows the selected bond angles for compounds 13–15, 17, and 18. All the bond angles of halogenated analogues are larger by 0.1–4.07° than talose (18). As such, the H2–C2–F2 bond angles are similar for compounds 13–15 and 17 (109.69–109.78°), but significantly larger than the H2–C2–O2 bond angle of talose (105.87°). Moreover, the H3–C3–F3 bond angles slightly decrease according to the nature of the atom at C4 (F: 107.97°; Cl: 107.67°; Br: 107.47°; I: 107.46°), as compared with talose (108.85°). As for the H4–C4–X4 bond angles, the angles are similar for talose and the trifluorinated analogues: 109.33° and 109.36°, respectively. However, there is a bond angle narrowing for the other analogues (Cl: 108.07°; Br: 107.94°; I: 107.78°). Finally, the angles involving the exo-anomeric oxygen (O1–C1–O5) are similar with a difference of about 1° between the larger (compound 17) and smaller (compound 14) angle.
Table 2: Selected bond angles for compounds 13–15, 17, and 18.
| Entry | Bonds | Angles (°) | ||||
|---|---|---|---|---|---|---|
| Talose (18)a | 17 | 13 | 14 | 15 | ||
| 1 | C1–C2–C3 | 109.51 | 110.2(2) | 111.9(1) | 111.9(2) | 111.9(9) |
| 2 | C2–C3–C4 | 110.43 | 113.7(2) | 114.0(1) | 114.5(2) | 114.1(9) |
| 3 | C3–C4–C5 | 107.80 | 109.9(2) | 108.3(1) | 108.3(2) | 107.9(8) |
| 4 | C4–C5–O5 | 109.92 | 111.8(2) | 111.6(1) | 112.2(1) | 112.0(8) |
| 5 | C5–O5–C1 | 113.68 | 114.9(2) | 114.7(1) | 114.7(1) | 113.9(8) |
| 6 | O5–C1–C2 | 110.29 | 113.0(2) | 112.2(1) | 112.3(2) | 112.7(9) |
| 7 | O1–C1–O5 | 111.87 | 111.1(2) | 111.3(1) | 110.9(2) | 111.4(8) |
| 8 | O1–C1–C2 | 107.98 | 105.5(2) | 106.8(1) | 106.9(2) | 106.6(8) |
| 9 | C1–O1–C(O) | na | 116.2(2) | 115.4(1) | 115.3(2) | 115.2(8) |
| 10 | C1–C2–F2 | 109.78b | 106.9(2) | 105.4(1) | 105.2(1) | 104.6(8) |
| 11 | C3–C2–F2 | 112.49c | 110.5(2) | 110.4(1) | 110.5(2) | 110.9(9) |
| 12 | C2–C3–F3 | 107.54d | 109.3(2) | 109.5(1) | 109.6(1) | 109.9(9) |
| 13 | C4–C3–F3 | 113.36e | 109.7(2) | 110.1(1) | 110.0(1) | 110.1(8) |
| 14 | C3–C4–X4 | 108.18f | 109.7(2) | 112.1(1) | 112.1(1) | 111.9(7) |
| 15 | C5–C4–X4 | 111.14g | 109.2(2) | 112.1(1) | 112.5(1) | 113.5(7) |
| 16 | H2–C2–F2 | 105.87h | 109.75 | 109.69 | 109.72 | 109.78 |
| 17 | H3–C3–F3 | 108.85i | 107.97 | 107.67 | 107.47 | 107.46 |
| 18 | H4–C4–X4 | 109.33j | 109.36 | 108.07 | 107.94 | 107.78 |
aReference [35]; bC1–C2–O2; cC3–C2–O2; dC2–C3–O3; eC4–C3–O3; fC3–C4–O4; gC5–C4–O4; hH2–C2–O2; iH3–C3–O3; jH4–C4–O4.
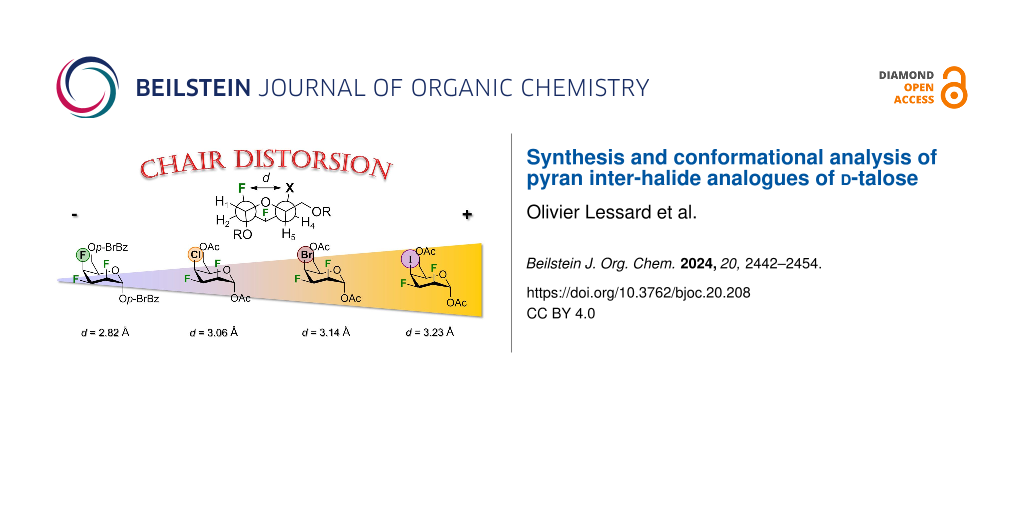
As stated above, all analogues exhibit a gt conformation except for compound 17, which is a tg conformer. This information could also be extracted from Table 3 by looking at the O5–C5–C6–O6 torsion angles (Table 3, entry 1). Table 3 also highlights that there are significant intra-annular torsion angles for halogenated analogues. There are reductions in the C1–C2–C3–C4 torsion angles for the halogenated pyrans as compared to compound 18 (–56.58°) (Table 3, entry 4). The decrease depends on the size of the halogen at C4 (F: –49.4°; Cl: –46.9°; Br: –46.5°; I: –46°). There is also a reduction in the C2–C3–C4–C5 torsion angles of about 8.4° in average for compound 13–15 and 17 falling outside the range of an ideal pyran ring (Table 3, entry 5) [35]. However, the C4–C5–O5–C1 torsion angles are similar for all compounds except for compound 17 (Table 3, entry 7). The deviation in the intra-annular torsion angles clearly arise from repulsion of the axial fluorine at C2 and the axial halogen at C4 as exemplified with the H3–C3–C2–F2 and the X4–C4–C3–H3 torsion angles being smaller than the expected 180° (Table 3, entry 16 and 17). The 1,3-diaxial repulsion leads to a deviation from parallel alignment as shown in Table 4. The Newman projections of the halogenated analogues show deviations from parallel alignment for the C2–F and C4–X substituents of 12.08° for 17, 17.08° for 13, 18.18° for 14, and 18.59° for 15 (talopyranose, 18: 5.92°). This deviation is responsible for the distance between F2 and the halogen at C4. Table 5 highlights key interatomic distances for all analogues. As such, the F2∙∙∙X4 distance increases with the size of the C4 halogen (F < Cl < Br < I): 2.82 Å for 17, 3.06 Å for 13, 3.14 Å for 14, and 3.23 Å for 15. Another interesting feature can be drawn from Table 5. As such, intramolecular F2···F3, F2···X4, and F3···X4 contacts are smaller than the sum of the Van der Walls radii [49]. Taking together, all these data clearly demonstrate that the nature of one halogen can have an impact on the solid-state conformation of halogenated pyrans.
Table 3: Selected torsion angles for compounds 13–15, 17, and 18.
| Entry | Bonds | Torsion angles (°) | ||||
|---|---|---|---|---|---|---|
| Talose 18a | 17 | 13 | 14 | 15 | ||
| 1 | O5–C5–C6–O6 | 70.35 | −178.3(2) | 75.4(1) | 74.9(2) | 75(1) |
| 2 | O5–C1–O1–C(O) | na | 94.7(2) | 89.0(2) | 88.7(2) | 88(1) |
| 3 | C2–C1–O1–C(O) | na | −142.5(2) | −148.1(1) | −148.5(2) | −148.4(9) |
| 4 | C1–C2–C3–C4 | −56.58 | −49.4(3) | −46.9(2) | −46.5(2) | −46(1) |
| 5 | C2–C3–C4–C5 | 57.88 | 50.1(3) | 50.0(2) | 49.0(2) | 49(1) |
| 6 | C3–C4–C5–O5 | −58.49 | −51.6(3) | −55.0(2) | −53.9(2) | −55(1) |
| 7 | C4–C5–O5–C1 | 60.88 | 56.4(2) | 60.5(2) | 59.8(2) | 61(1) |
| 8 | C5–O5–C1–C2 | −58.73 | −56.1(2) | −55.5(2) | −55.3(2) | −57(1) |
| 9 | O5–C1–C2–C3 | 55.31 | 51.0(3) | 47.6(2) | 47.6(2) | 48(1) |
| 10 | O5–C1–C2–F2 | −68.66b | −69.0(2) | −72.4(1) | −72.4(2) | −72(1) |
| 11 | C1–C2–C3–F3 | 179.26c | −172.3(2) | −170.7(1) | −170.7(1) | −170.6(8) |
| 12 | F2–C2–C3–C4 | 65.78d | 68.5(3) | 70.2(2) | 70.4(2) | 70(1) |
| 13 | F3–C3–C4–C5 | 178.62e | 172.8(2) | 173.5(1) | 173.0(1) | 173.1(8) |
| 14 | C2–C3–C4–X4 | −62.40f | −70.0(3) | −74.2(2) | −75.6(2) | −77(1) |
| 15 | X4–C4–C5–O5 | 59.91g | 68.8(2) | 69.2(1) | 70.5(2) | 70.0(9) |
| 16 | X4–C4–C3–H3 | 179.05h | 170.2 | 166.4 | 165.0 | 164.4 |
| 17 | H3–C3–C2–F2 | −175.98i | −171.7 | −170.4 | −170.3 | −170.9 |
aReference [35]; bO5–C1–C2–O2; cC1–C2–C3–O3; dO2–C2–C3–C4; eO3–C3–C4–C5; fC2–C3–C4–O4; gO4–C4–C5–O5; hO4–C4–C3–H3; iH3–C3–C2–O2.
Table 4: 1,3-Diaxial repulsion between C2–F and C4–X bonds for 13–15 and 17.a
|
|
|||||
| Entry | Angles (°) |
17
(X = F, R = p-BrBz) |
13
(X = Cl, R = Ac) |
14
(X = Br, R = Ac) |
15
(X = I, R = Ac) |
| 1 | α1 | 5.99 | 6.77 | 7.05 | 7.06 |
| 2 | α2 | 6.09 | 10.31 | 11.13 | 11.53 |
| 3 | α1 + α2 | 12.08 | 17.08 | 18.18 | 18.59 |
aFor α-talopyranose 18: α1 = 5.58°, α2 = 0.34°, and α1 + α2 = 5.92° (reference [35]).
Table 5: Key interatomic distances (intramolecular) for 13–15, 17, and 18.
| Entry | Atoms | Distances (Å) | ||||
|---|---|---|---|---|---|---|
| Talose (18)a | 17 | 13 | 14 | 15 | ||
| 1 | O1∙∙∙F2 | 3.6141b | 3.560(2) | 3.552(2) | 3.557(2) | 3.55(1) |
| 2 | O1∙∙∙F3 | 4.1700c | 4.194(2) | 4.288(2) | 4.285(2) | 4.28(1) |
| 3 | F2∙∙∙F3 | 2.8154d | 2.732(3) | 2.735(2) | 2.741(2) | 2.746(9) |
| 4 | F2∙∙∙X4 | 2.6546e | 2.817(2) | 3.056(1) | 3.143(1) | 3.228(6) |
| 5 | F3∙∙∙X4 | 2.8517f | 2.714(2) | 2.968(1) | 3.052(1) | 3.160(6) |
aReference [35]; bO1∙∙∙O2; cO1∙∙∙O3; dO2∙∙∙O3; eO2∙∙∙O4; fO3∙∙∙O4.
We also evaluated the Cremer–Pople ring puckering parameters (Table 6) [50]. For pyranoid rings, these parameters take the form of a spherical polar coordinate set, Q, θ, and φ, which define the point P, on a sphere of radius Q [51]. The smaller puckering amplitude (Q) values for the halogenated analogues indicate a flattened ring in comparison to the non-halogenated compound. The puckering amplitude for an ideal cyclohexane chair, with C–C bond lengths of 1.54 Å, is 0.63 Å [50]. The azimuthal angle (θ) represents the distortion of the ring. For pyranose rings, an azimuthal angle of θ = 0° represents a perfect 4C1 chair, and an angle of θ = 180° is the 1C4 chair. The distortion of the chair conformation increases with the size of the halogen at C4. Surprisingly, the trifluorinated analogue is less distorted than the non-halogenated talopyranose. The meridian angle (φ) indicates the nature of the distortion. The distortion of the trifluorinated analogue is in a direction somewhat between an E5 conformation (φ ≈ 300°) and a OH5 conformation (φ ≈ 330°). The other trihalogenated analogues are distorted towards an E5 conformation (φ ≈ 300°). The distortion of the non-halogenated talopyranose is in the direction on an 4E conformation (φ ≈ 240°).
The solid-state conformation of each of the pyran inter-halides 13–15 is unique. One would expect that compounds 13–15 would have distinct crystal packing arrangements based on the nature of the halogen. On the contrary, all analogues adopt a similar stacking pattern. Figure 4 shows the packing arrangement for compound 15 and the crystal packing of compound 13 and 14 can be found in the Supporting Information File 1. The halogens are on the same side of the pyran ring, thus increasing the overall molecular dipole moment (see Supporting Information File 1). This allows intermolecular C–X···H–C interactions responsible, in part, for the solid-state ordering [52]. Individual pyrans stack on top of one another in a manner consistent with electrostatic interactions with halogens facing H3, H4, and H5 (Figure 4a). As such, some intermolecular H···X bond distances and angles for compound 13–15 are listed in Table 7. Solid-state intermolecular interactions involving fluorine atoms have been well documented over the years for carbohydrate analogues [24,44,53] or other organofluorines [54]. In our case, there is a number of C–F···H–C interactions with F2 and H4 (13: d = 2.271 Å, 14: d = 2.270 Å, and 15: d = 2.356 Å) and with F3 and H4 (13: d = 2.867 Å, 14: d = 2.849 Å, and 15: d = 2.886 Å).
![[1860-5397-20-208-4]](/bjoc/content/figures/1860-5397-20-208-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Packing arrangement of compound compound 15; a) View down the b axis; b) proposed intermolecular interactions involving halogens. ORTEP diagram showing 50% thermal ellipsoid probability: carbon (gray), oxygen (red), fluorine (green), iodine (purple), and hydrogen (white).
Figure 4: Packing arrangement of compound compound 15; a) View down the b axis; b) proposed intermolecular in...
Table 7: Intermolecular X···H bond distances and angles for compound 13–15.
| Entry | Compound | D–H···A | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | a(D–H–A) (°) | a(C–X–H) (°) |
|---|---|---|---|---|---|---|---|
| 1 | 13 | F2···H3 | 0.980 | 3.704 | 3.826 | 89.69 | na |
| 2 | F2···H4 | 0.980 | 2.271 | 3.222 | 163.14 | na | |
| 3 | F3···H3 | 0.980 | 4.239 | 4.561 | 103.01 | na | |
| 4 | F3···H4 | 0.980 | 2.867 | 3.614 | 133.69 | na | |
| 5 | F3···H5 | 0.980 | 3.074 | 3.490 | 107.03 | na | |
| 6 | Cl4···H3 | 0.980 | 3.437 | 4.232 | 139.73 | 133.71 | |
| 7 | Cl4···H4 | 0.980 | 3.625 | 4.298 | 128.02 | 96.94 | |
| 8 | Cl4···H5 | 0.980 | 3.953 | 4.624 | 128.19 | 103.45 | |
| 9 | 14 | F2···H3 | 1.000 | 3.771 | 3.869 | 88.12 | na |
| 10 | F2···H4 | 1.000 | 2.270 | 3.233 | 161.19 | na | |
| 11 | F3···H3 | 1.000 | 4.290 | 4.599 | 101.79 | na | |
| 12 | F3···H4 | 1.000 | 2.849 | 3.626 | 135.07 | na | |
| 13 | F3···H5 | 1.000 | 3.130 | 3.533 | 105.61 | na | |
| 14 | Br4···H3 | 1.000 | 3.316 | 4.132 | 139.90 | 133.33 | |
| 15 | Br4···H4 | 1.000 | 3.526 | 4.213 | 127.67 | 95.15 | |
| 16 | Br4···H5 | 1.000 | 3.887 | 4.564 | 127.36 | 102.99 | |
| 17 | 15 | F2···H3 | 0.980 | 3.867 | 3.950 | 87.64 | na |
| 18 | F2···H4 | 0.981 | 2.356 | 3.293 | 159.70 | na | |
| 19 | F3···H3 | 0.980 | 4.382 | 4.673 | 101.24 | na | |
| 20 | F3···H4 | 0.981 | 2.886 | 3.668 | 137.42 | na | |
| 21 | F3···H5 | 0.980 | 3.248 | 3.612 | 103.92 | na | |
| 22 | I4···H3 | 0.980 | 3.285 | 4.086 | 140.25 | 132.28 | |
| 23 | I4···H4 | 0.981 | 3.521 | 4.192 | 127.59 | 93.77 | |
| 24 | I4···H5 | 0.980 | 3.905 | 4.559 | 126.76 | 102.70 | |
Does the halogen at C4 contribute to the stabilization within the crystal lattice? To answer this question, we have to look at the behavior of halogens as hydrogen bond acceptors (X···H) and nonbonding interactions (X···O/N/S). For C–X, a σ-hole arises when a valence electron is pulled into the σ-molecular orbital resulting in an electropositive crown and a flattening of the atomic radius, that accounts for the directionality of the interactions [55,56]. Thus, the halogen has an amphoteric character with an electropositive halogen bond ability along the σ-hole (C–X···O/N/S, a ≈ 180°) and an electronegative hydrogen bond acceptor perpendicular to the C–X bond (C–X···H, a ≈ 90°) [57-60]. Such halogen bonds have been detrimental in the understanding interactions of organic halogens in biological systems [61-65]. In our case, for compound 15, I4 interacts with H4 (d = 3.521 Å, a = 93.77°) and I4 also interact with the oxygen of the carbonyl of the acetate at C6 (I4···O, d = 3.147 Å; a = 179.70°) (Figure 4b and Table 7). This result is in line with hydrogen (C–I···H) and halogen (C–I···O) interactions that show remarkable differences in term of geometrical features [66]. It is important to point out that similar interactions are also present in the packing of compound 13 (Cl4···H4 (d = 3.625 Å, a = 96.94°) and Cl4···O (d = 3.203 Å, a = 174.03°)) and compound 14 (Br4···H4 (d = 3.526 Å, a = 95.15°) and Br4···O (d = 3.143 Å, a = 177.49°)) (Table 7) (and see Supporting Information File 1). To the best of our knowledge, this is the first application of halogen bonding in the context of solid-state ordering of pyran inter-halides.
Our interest in the synthesis and conformation of multivicinal inter-halides motivated us to use density functional theory (DFT) calculations to understand the preference of talose analogues to adopt 4C1-like conformations. DFT computations were performed using Gaussian 16 revision B.01 [67] with the CAM-B3LYP [68-70] functional and the Def2TZVP basis set [71], which includes effective core potentials for iodine. Empirical dispersion was accounted with Grimme’s D3 [72,73] correction including Becke–Johnson damping [74]. Computations were performed both in the gas phase (i.e., individual molecules with thermal corrections at 298.15 K based on ideal gas assumptions) and in a chloroform solution, using the polarizable continuum model (PCM) [75]. A natural bonding orbital (NBO) analysis was performed to study the effects of hyperconjugation from C–F antibonding orbitals [76].
First, dipole moments, enthalpy and Gibbs free energy differences between 1C4 and 4C1 chair structures are shown in Table 8. In the gas phase, there is little difference in enthalpy between the two structures. For Cl, Br, and I, the 1C4 structures are predicted to have slightly lower enthalpies. However, as these differences are much smaller than 1 kcal/mol, one should conclude that the structures are nearly degenerate. In solution, the picture is much clearer: the 4C1 structure is always lower in enthalpy and Gibbs free energy, which corresponds with the experimental measurements. One can see that the gap between the two structures tends to decrease as the halogen becomes larger (the minor exception being bromine). Dipole moments for the 4C1 structures are 2 Debye larger than for 1C4 structures, making them substantially more stable in chloroform.
Table 8: Dipole moments (Debye), enthalpy and Gibbs free energy differences (kcal/mol) computed (CAM-B3LYP-D3BJ/Def2TZVP) for 1C4 and 4C1 chair structures in the gas phase and in chloroform (PCM). Thermal corrections are reported at 298.15 K.
|
|
|||||||||
| Gas phase | Chloroform | ||||||||
| Entry | X | μ 1C4 | μ 4C1 | ΔH | ΔG | μ 1C4 | μ 4C1 | ΔH | ΔG |
| 1 | F | 4.55 | 6.48 | 0.98 | 2.51 | 5.95 | 8.09 | 2.47 | 3.90 |
| 2 | Cl | 4.45 | 6.34 | −0.03 | 1.31 | 5.93 | 7.93 | 1.29 | 2.21 |
| 3 | Br | 4.40 | 6.30 | −0.13 | 1.18 | 5.89 | 7.94 | 1.19 | 2.55 |
| 4 | I | 4.32 | 6.07 | −0.03 | 1.17 | 5.78 | 7.64 | 1.04 | 1.86 |
Both chair structures have multiple C–F bonds in gauche arrangements which are stable due to hyperconjugation: there is donation from C–H bonding orbitals to C–F (or C–X) antibonding orbitals that are aligned with one another. The principal difference between the two structures is that in the 1C4 structure there are two such interactions whereas in the 4C1 structure there are three. One can see that in the 4C1 structure the C5–H5 bond will donate to the antibonding orbital of the C4–X4 bond, and the C3–H3 bond will donate to the antibonding orbitals of the C4–X4 and the C2–F2 bonds. In the 1C4 structure, the C4–H4 and C2–H2 bonds will both donate to the antibonding orbital of the C3–F3 bond. These effects can be shown with an NBO analysis: the Kohn–Sham orbitals produced from DFT are localized to describe the system as one dominant resonance structure. As the Fock matrix is not diagonal in terms of the NBOs, coupling between orbitals can be quantified with second order perturbation theory. These couplings represent donation from an occupied NBO to an unoccupied NBO that would stabilize the system. The results are presented in Table 9. In both cases, there is also donation from the halogen lone pairs to C–H and C–C antibonding orbitals, but as these effects were near equivalent in both chair structures, they are omitted.
Table 9: Second order perturbation theory energies of the Fock matrix in the NBO basis for 4C1 and 1C4 structures, from CAM-B3LYP-D3BJ/Def2TZVP results. Only results from CHCl3 solvation (PCM) are reported as gas phase results showed no qualitative difference. Entries marked – are below the 0.50 kcal/mol threshold.
|
|
|
|||||||||
| Entry | Donor σ | Acceptor σ* | X = F | X = Cl | X = Br | X = I | X = F | X = Cl | X = Br | X = I |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C5–H5 | C4–X4 | 5.07 | 6.58 | 7.46 | 7.83 | – | – | – | – |
| 2 | C4–H4 | C4–X4 | 1.05 | 0.88 | 0.97 | 0.75 | 1.10 | 0.80 | 0.81 | 0.56 |
| 3 | C4–H4 | C3–F3 | – | – | – | – | 5.18 | 5.53 | 5.67 | 5.88 |
| 4 | C3–H3 | C4–X4 | 5.36 | 6.59 | 7.46 | 7.71 | 0.61 | – | – | – |
| 5 | C3–H3 | C3–F3 | 1.05 | 1.03 | 1.02 | 1.02 | 1.00 | 1.02 | 1.02 | 1.05 |
| 6 | C3–H3 | C2–F2 | 5.00 | 5.18 | 5.20 | 5.22 | 0.58 | 0.59 | 0.59 | 0.61 |
| 7 | C2–H2 | C3–F3 | 0.50 | 0.50 | 0.50 | 0.52 | 4.63 | 4.61 | 4.58 | 4.58 |
| 8 | C2–H2 | C2–F2 | 1.01 | 0.99 | 0.99 | 0.99 | 1.01 | 1.00 | 1.00 | 1.01 |
| 9 | C1–H1 | C2–F2 | 1.10 | 1.21 | 1.22 | 1.26 | 0.53 | 0.56 | 0.57 | 0.56 |
Conclusion
We described the synthesis and conformational analysis of halogenated pyran analogues of ᴅ-talose. All analogues adopt standard 4C1-like conformations both in solution and in the solid-state. The conformations were corroborated using DFT calculations by looking at the energy, enthalpy and Gibbs’ free energy differences between 1C4 and 4C1 chair structures. Crystallographic data of halogenated analogues shows intra-annular torsion angles demonstrated with the increasing distance between F2∙∙∙X4 in relation with the nature of the halogen at C4: F (d = 2.82 Å) < Cl (d = 3.06 Å) < Br (d = 3.14 Å) < I (d = 3.23 Å). Moreover, the Cremer–Pople ring puckering parameters show suitable differences in the distortion of the chair conformations. Crystal packing arrangements showed that the halogen at C4 contributed in the nonbonding (along the σ-hole) and hydrogen bond (perpendicular to the C–X bond) interactions. Finally, this study should be of general interest in the understanding of weak interactions that are now important to so many areas of chemistry, such as crystal engineering and supramolecular chemistry.
Supporting Information
| Supporting Information File 1: Experimental and analytical data, crystal structure determination and NMR spectra. | ||
| Format: PDF | Size: 1.5 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788
Return to citation in text: [1] -
Linclau, B.; Ardá, A.; Reichardt, N.-C.; Sollogoub, M.; Unione, L.; Vincent, S. P.; Jiménez-Barbero, J. Chem. Soc. Rev. 2020, 49, 3863–3888. doi:10.1039/c9cs00099b
Return to citation in text: [1] -
Huonnic, K.; Linclau, B. Chem. Rev. 2022, 122, 15503–15602. doi:10.1021/acs.chemrev.2c00086
Return to citation in text: [1] -
Lessard, O.; Lainé, D.; Giguère, D. Eur. J. Org. Chem. 2024, 27, e202400120. doi:10.1002/ejoc.202400120
Return to citation in text: [1] -
Govindaraj, V.; Ungati, H.; Jakka, S. R.; Bose, S.; Mugesh, G. Chem. – Eur. J. 2019, 25, 11180–11192. doi:10.1002/chem.201902243
Return to citation in text: [1] -
Ungati, H.; Govindaraj, V.; Nair, C. R.; Mugesh, G. Chem. – Eur. J. 2019, 25, 3391–3399. doi:10.1002/chem.201806122
Return to citation in text: [1] -
Gerebtzoff, G.; Li‐Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. ChemBioChem 2004, 5, 676–684. doi:10.1002/cbic.200400017
Return to citation in text: [1] -
Nunes, R. S.; Vila-Viçosa, D.; Costa, P. J. J. Am. Chem. Soc. 2021, 143, 4253–4267. doi:10.1021/jacs.0c12470
Return to citation in text: [1] -
Heidrich, J.; Sperl, L. E.; Boeckler, F. M. Front. Chem. (Lausanne, Switz.) 2019, 7, 9. doi:10.3389/fchem.2019.00009
Return to citation in text: [1] -
Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. J. Chem. Inf. Model. 2014, 54, 69–78. doi:10.1021/ci400539q
Return to citation in text: [1] -
Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Molecules 2017, 22, 1397. doi:10.3390/molecules22091397
Return to citation in text: [1] -
Hernandes, M. Z.; Cavalcanti, S. M. T.; Moreira, D. R. M.; de Azevedo Junior, W. F.; Leite, A. C. L. Curr. Drug Targets 2010, 11, 303–314. doi:10.2174/138945010790711996
Return to citation in text: [1] -
Wilcken, R.; Zimmermann, M. O.; Lange, A.; Joerger, A. C.; Boeckler, F. M. J. Med. Chem. 2013, 56, 1363–1388. doi:10.1021/jm3012068
Return to citation in text: [1] -
Chiodi, D.; Ishihara, Y. J. Med. Chem. 2023, 66, 5305–5331. doi:10.1021/acs.jmedchem.2c02015
Return to citation in text: [1] -
Tan, Y.; Luo, S.; Li, D.; Zhang, N.; Jia, S.; Liu, Y.; Qin, W.; Song, C. E.; Yan, H. J. Am. Chem. Soc. 2017, 139, 6431–6436. doi:10.1021/jacs.7b02076
Return to citation in text: [1] -
Bucher, C.; Deans, R. M.; Burns, N. Z. J. Am. Chem. Soc. 2015, 137, 12784–12787. doi:10.1021/jacs.5b08398
Return to citation in text: [1] -
Gribble, G. W. Mar. Drugs 2015, 13, 4044–4136. doi:10.3390/md13074044
Return to citation in text: [1] -
Gál, B.; Bucher, C.; Burns, N. Z. Mar. Drugs 2016, 14, 206. doi:10.3390/md14110206
Return to citation in text: [1] -
Lessard, O.; Lainé, D.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Org. Chem. Front. 2022, 9, 6566–6572. doi:10.1039/d2qo01433e
Return to citation in text: [1] [2] [3] [4] -
Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
Denavit, V.; St-Gelais, J.; Tremblay, T.; Giguère, D. Chem. – Eur. J. 2019, 25, 9272–9279. doi:10.1002/chem.201901346
Return to citation in text: [1] -
Denavit, V.; Lainé, D.; Bouzriba, C.; Shanina, E.; Gillon, É.; Fortin, S.; Rademacher, C.; Imberty, A.; Giguère, D. Chem. – Eur. J. 2019, 25, 4478–4490. doi:10.1002/chem.201806197
Return to citation in text: [1] -
St-Gelais, J.; Bouchard, M.; Denavit, V.; Giguère, D. J. Org. Chem. 2019, 84, 8509–8522. doi:10.1021/acs.joc.9b00795
Return to citation in text: [1] -
Lainé, D.; Denavit, V.; Lessard, O.; Carrier, L.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Beilstein J. Org. Chem. 2020, 16, 2880–2887. doi:10.3762/bjoc.16.237
Return to citation in text: [1] -
St-Gelais, J.; Côté, É.; Lainé, D.; Johnson, P. A.; Giguère, D. Chem. – Eur. J. 2020, 26, 13499–13506. doi:10.1002/chem.202002825
Return to citation in text: [1] -
Leclerc, C.; St-Gelais, J.; Cecioni, S.; Giguère, D. J. Fluorine Chem. 2024, 273, 110232. doi:10.1016/j.jfluchem.2023.110232
Return to citation in text: [1] -
Lainé, D.; Lessard, O.; St-Gelais, J.; Giguère, D. Chem. – Eur. J. 2021, 27, 3799–3805. doi:10.1002/chem.202004646
Return to citation in text: [1] [2] -
Passaglia, L.; Zanardi, M. M.; Sarotti, A. M. Org. Biomol. Chem. 2024, 22, 2435–2442. doi:10.1039/d3ob02077k
Return to citation in text: [1] -
Deposition number 2344127 (for 13), 2344124 (for 14), and 2344126 (for 15) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Return to citation in text: [1] -
Deposition number 1824902 (for 17) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Return to citation in text: [1] -
Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Hunter, L.; Slawin, A. M. Z.; Kirsch, P.; O’Hagan, D. Angew. Chem., Int. Ed. 2007, 46, 7887–7890. doi:10.1002/anie.200701988
Return to citation in text: [1] -
Hunter, L.; O’Hagan, D.; Slawin, A. M. Z. J. Am. Chem. Soc. 2006, 128, 16422–16423. doi:10.1021/ja066188p
Return to citation in text: [1] -
Bentler, P.; Erdeljac, N.; Bussmann, K.; Ahlqvist, M.; Knerr, L.; Bergander, K.; Daniliuc, C. G.; Gilmour, R. Org. Lett. 2019, 21, 7741–7745. doi:10.1021/acs.orglett.9b02662
Return to citation in text: [1] -
O’Hagan, D. Chem. – Eur. J. 2020, 26, 7981–7997. doi:10.1002/chem.202000178
Return to citation in text: [1] -
Marchand, A. P.; Sorokin, V. D.; Rajagopal, D.; Bott, S. G. Tetrahedron 1994, 50, 9933–9942. doi:10.1016/s0040-4020(01)89608-3
Return to citation in text: [1] -
Rodríguez‐Vázquez, N.; Salzinger, S.; Silva, L. F.; Amorín, M.; Granja, J. R. Eur. J. Org. Chem. 2013, 3477–3493. doi:10.1002/ejoc.201201565
Return to citation in text: [1] -
Jeffrey, G. A. Acta Crystallogr., Sect. B: Struct. Sci. 1990, 46, 89–103. doi:10.1107/s0108768189012449
Return to citation in text: [1] [2] -
Bock, K.; Duus, J. Ø. J. Carbohydr. Chem. 1994, 13, 513–543. doi:10.1080/07328309408011662
Return to citation in text: [1] -
Linclau, B.; Golten, S.; Light, M.; Sebban, M.; Oulyadi, H. Carbohydr. Res. 2011, 346, 1129–1139. doi:10.1016/j.carres.2011.04.007
Return to citation in text: [1] [2] -
Kim, H. W.; Rossi, P.; Shoemaker, R. K.; DiMagno, S. G. J. Am. Chem. Soc. 1998, 120, 9082–9083. doi:10.1021/ja9803714
Return to citation in text: [1] -
Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. J. Chem. Soc., Perkin Trans. 2 1987, S1–S19. doi:10.1039/p298700000s1
Return to citation in text: [1] -
Jeffrey, G. A.; Pople, J. A.; Radom, L. Carbohydr. Res. 1972, 25, 117–131. doi:10.1016/s0008-6215(00)82752-4
Return to citation in text: [1] -
O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Bondi, A. J. Phys. Chem. 1964, 68, 441–451. doi:10.1021/j100785a001
Return to citation in text: [1] -
Cremer, D.; Pople, J. A. J. Am. Chem. Soc. 1975, 97, 1354–1358. doi:10.1021/ja00839a011
Return to citation in text: [1] [2] -
Jeffrey, G. A.; Yates, J. H. Carbohydr. Res. 1979, 74, 319–322. doi:10.1016/s0008-6215(00)84786-2
Return to citation in text: [1] -
Steiner, T. Angew. Chem., Int. Ed. 2002, 41, 48–76. doi:10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u
Return to citation in text: [1] -
Bresciani, S.; Lebl, T.; Slawin, A. M. Z.; O’Hagan, D. Chem. Commun. 2010, 46, 5434–5436. doi:10.1039/c0cc01128b
Return to citation in text: [1] -
O’Hagan, D. Chem. Rec. 2023, 23, e202300027. doi:10.1002/tcr.202300027
Return to citation in text: [1] -
Clark, T.; Hennemann, M.; Murray, J. S.; Politzer, P. J. Mol. Model. 2007, 13, 291–296. doi:10.1007/s00894-006-0130-2
Return to citation in text: [1] -
Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k
Return to citation in text: [1] -
Scholfield, M. R.; Zanden, C. M. V.; Carter, M.; Ho, P. S. Protein Sci. 2013, 22, 139–152. doi:10.1002/pro.2201
Return to citation in text: [1] -
Nelyubina, Y. V.; Antipin, M. Y.; Dunin, D. S.; Kotov, V. Y.; Lyssenko, K. A. Chem. Commun. 2010, 46, 5325–5327. doi:10.1039/c0cc01094d
Return to citation in text: [1] -
Brammer, L.; Bruton, E. A.; Sherwood, P. Cryst. Growth Des. 2001, 1, 277–290. doi:10.1021/cg015522k
Return to citation in text: [1] -
Aakeröy, C. B.; Desper, J.; Helfrich, B. A.; Metrangolo, P.; Pilati, T.; Resnati, G.; Stevenazzi, A. Chem. Commun. 2007, 43, 4236–4238. doi:10.1039/b707458a
Return to citation in text: [1] -
Scholfield, M. R.; Ford, M. C.; Carlsson, A.-C. C.; Butta, H.; Mehl, R. A.; Ho, P. S. Biochemistry 2017, 56, 2794–2802. doi:10.1021/acs.biochem.7b00022
Return to citation in text: [1] -
Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. doi:10.1039/b926326h
Return to citation in text: [1] -
Auffinger, P.; Hays, F. A.; Westhof, E.; Ho, P. S. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 16789–16794. doi:10.1073/pnas.0407607101
Return to citation in text: [1] -
Lin, F.-Y.; MacKerell, A. D., Jr. J. Phys. Chem. B 2017, 121, 6813–6821. doi:10.1021/acs.jpcb.7b04198
Return to citation in text: [1] -
Lu, Y.; Wang, Y.; Xu, Z.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. J. Phys. Chem. B 2009, 113, 12615–12621. doi:10.1021/jp906352e
Return to citation in text: [1] -
Decato, D. A.; Sun, J.; Boller, M. R.; Berryman, O. B. Chem. Sci. 2022, 13, 11156–11162. doi:10.1039/d2sc03792k
Return to citation in text: [1] -
Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford CT, 2016.
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785
Return to citation in text: [1] -
Yanai, T.; Tew, D. P.; Handy, N. C. Chem. Phys. Lett. 2004, 393, 51–57. doi:10.1016/j.cplett.2004.06.011
Return to citation in text: [1] -
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a
Return to citation in text: [1] -
Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. J. Chem. Phys. 2010, 132, 154104. doi:10.1063/1.3382344
Return to citation in text: [1] -
Grimme, S.; Ehrlich, S.; Goerigk, L. J. Comput. Chem. 2011, 32, 1456–1465. doi:10.1002/jcc.21759
Return to citation in text: [1] -
Becke, A. D.; Johnson, E. R. J. Chem. Phys. 2006, 124, 014104. doi:10.1063/1.2139668
Return to citation in text: [1] -
Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999–3094. doi:10.1021/cr9904009
Return to citation in text: [1] -
Foster, J. P.; Weinhold, F. J. Am. Chem. Soc. 1980, 102, 7211–7218. doi:10.1021/ja00544a007
Return to citation in text: [1]
| 42. | Jeffrey, G. A. Acta Crystallogr., Sect. B: Struct. Sci. 1990, 46, 89–103. doi:10.1107/s0108768189012449 |
| 46. | Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. J. Chem. Soc., Perkin Trans. 2 1987, S1–S19. doi:10.1039/p298700000s1 |
| 47. | Jeffrey, G. A.; Pople, J. A.; Radom, L. Carbohydr. Res. 1972, 25, 117–131. doi:10.1016/s0008-6215(00)82752-4 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 50. | Cremer, D.; Pople, J. A. J. Am. Chem. Soc. 1975, 97, 1354–1358. doi:10.1021/ja00839a011 |
| 51. | Jeffrey, G. A.; Yates, J. H. Carbohydr. Res. 1979, 74, 319–322. doi:10.1016/s0008-6215(00)84786-2 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
| 50. | Cremer, D.; Pople, J. A. J. Am. Chem. Soc. 1975, 97, 1354–1358. doi:10.1021/ja00839a011 |
| 52. | Steiner, T. Angew. Chem., Int. Ed. 2002, 41, 48–76. doi:10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 44. | Linclau, B.; Golten, S.; Light, M.; Sebban, M.; Oulyadi, H. Carbohydr. Res. 2011, 346, 1129–1139. doi:10.1016/j.carres.2011.04.007 |
| 53. | Bresciani, S.; Lebl, T.; Slawin, A. M. Z.; O’Hagan, D. Chem. Commun. 2010, 46, 5434–5436. doi:10.1039/c0cc01128b |
| 68. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913 |
| 69. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 70. | Yanai, T.; Tew, D. P.; Handy, N. C. Chem. Phys. Lett. 2004, 393, 51–57. doi:10.1016/j.cplett.2004.06.011 |
| 71. | Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a |
| 66. | Decato, D. A.; Sun, J.; Boller, M. R.; Berryman, O. B. Chem. Sci. 2022, 13, 11156–11162. doi:10.1039/d2sc03792k |
| 57. | Scholfield, M. R.; Zanden, C. M. V.; Carter, M.; Ho, P. S. Protein Sci. 2013, 22, 139–152. doi:10.1002/pro.2201 |
| 58. | Nelyubina, Y. V.; Antipin, M. Y.; Dunin, D. S.; Kotov, V. Y.; Lyssenko, K. A. Chem. Commun. 2010, 46, 5325–5327. doi:10.1039/c0cc01094d |
| 59. | Brammer, L.; Bruton, E. A.; Sherwood, P. Cryst. Growth Des. 2001, 1, 277–290. doi:10.1021/cg015522k |
| 60. | Aakeröy, C. B.; Desper, J.; Helfrich, B. A.; Metrangolo, P.; Pilati, T.; Resnati, G.; Stevenazzi, A. Chem. Commun. 2007, 43, 4236–4238. doi:10.1039/b707458a |
| 61. | Scholfield, M. R.; Ford, M. C.; Carlsson, A.-C. C.; Butta, H.; Mehl, R. A.; Ho, P. S. Biochemistry 2017, 56, 2794–2802. doi:10.1021/acs.biochem.7b00022 |
| 62. | Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. doi:10.1039/b926326h |
| 63. | Auffinger, P.; Hays, F. A.; Westhof, E.; Ho, P. S. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 16789–16794. doi:10.1073/pnas.0407607101 |
| 64. | Lin, F.-Y.; MacKerell, A. D., Jr. J. Phys. Chem. B 2017, 121, 6813–6821. doi:10.1021/acs.jpcb.7b04198 |
| 65. | Lu, Y.; Wang, Y.; Xu, Z.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. J. Phys. Chem. B 2009, 113, 12615–12621. doi:10.1021/jp906352e |
| 55. | Clark, T.; Hennemann, M.; Murray, J. S.; Politzer, P. J. Mol. Model. 2007, 13, 291–296. doi:10.1007/s00894-006-0130-2 |
| 56. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 74. | Becke, A. D.; Johnson, E. R. J. Chem. Phys. 2006, 124, 014104. doi:10.1063/1.2139668 |
| 75. | Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999–3094. doi:10.1021/cr9904009 |
| 72. | Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. J. Chem. Phys. 2010, 132, 154104. doi:10.1063/1.3382344 |
| 73. | Grimme, S.; Ehrlich, S.; Goerigk, L. J. Comput. Chem. 2011, 32, 1456–1465. doi:10.1002/jcc.21759 |
| 1. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 2. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 3. | O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003 |
| 4. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 5. | Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788 |
| 6. | Linclau, B.; Ardá, A.; Reichardt, N.-C.; Sollogoub, M.; Unione, L.; Vincent, S. P.; Jiménez-Barbero, J. Chem. Soc. Rev. 2020, 49, 3863–3888. doi:10.1039/c9cs00099b |
| 7. | Huonnic, K.; Linclau, B. Chem. Rev. 2022, 122, 15503–15602. doi:10.1021/acs.chemrev.2c00086 |
| 18. | Chiodi, D.; Ishihara, Y. J. Med. Chem. 2023, 66, 5305–5331. doi:10.1021/acs.jmedchem.2c02015 |
| 23. | Lessard, O.; Lainé, D.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Org. Chem. Front. 2022, 9, 6566–6572. doi:10.1039/d2qo01433e |
| 12. | Nunes, R. S.; Vila-Viçosa, D.; Costa, P. J. J. Am. Chem. Soc. 2021, 143, 4253–4267. doi:10.1021/jacs.0c12470 |
| 13. | Heidrich, J.; Sperl, L. E.; Boeckler, F. M. Front. Chem. (Lausanne, Switz.) 2019, 7, 9. doi:10.3389/fchem.2019.00009 |
| 14. | Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. J. Chem. Inf. Model. 2014, 54, 69–78. doi:10.1021/ci400539q |
| 15. | Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Molecules 2017, 22, 1397. doi:10.3390/molecules22091397 |
| 16. | Hernandes, M. Z.; Cavalcanti, S. M. T.; Moreira, D. R. M.; de Azevedo Junior, W. F.; Leite, A. C. L. Curr. Drug Targets 2010, 11, 303–314. doi:10.2174/138945010790711996 |
| 17. | Wilcken, R.; Zimmermann, M. O.; Lange, A.; Joerger, A. C.; Boeckler, F. M. J. Med. Chem. 2013, 56, 1363–1388. doi:10.1021/jm3012068 |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 9. | Govindaraj, V.; Ungati, H.; Jakka, S. R.; Bose, S.; Mugesh, G. Chem. – Eur. J. 2019, 25, 11180–11192. doi:10.1002/chem.201902243 |
| 10. | Ungati, H.; Govindaraj, V.; Nair, C. R.; Mugesh, G. Chem. – Eur. J. 2019, 25, 3391–3399. doi:10.1002/chem.201806122 |
| 11. | Gerebtzoff, G.; Li‐Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. ChemBioChem 2004, 5, 676–684. doi:10.1002/cbic.200400017 |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 8. | Lessard, O.; Lainé, D.; Giguère, D. Eur. J. Org. Chem. 2024, 27, e202400120. doi:10.1002/ejoc.202400120 |
| 32. | Passaglia, L.; Zanardi, M. M.; Sarotti, A. M. Org. Biomol. Chem. 2024, 22, 2435–2442. doi:10.1039/d3ob02077k |
| 23. | Lessard, O.; Lainé, D.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Org. Chem. Front. 2022, 9, 6566–6572. doi:10.1039/d2qo01433e |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 25. | Denavit, V.; St-Gelais, J.; Tremblay, T.; Giguère, D. Chem. – Eur. J. 2019, 25, 9272–9279. doi:10.1002/chem.201901346 |
| 26. | Denavit, V.; Lainé, D.; Bouzriba, C.; Shanina, E.; Gillon, É.; Fortin, S.; Rademacher, C.; Imberty, A.; Giguère, D. Chem. – Eur. J. 2019, 25, 4478–4490. doi:10.1002/chem.201806197 |
| 27. | St-Gelais, J.; Bouchard, M.; Denavit, V.; Giguère, D. J. Org. Chem. 2019, 84, 8509–8522. doi:10.1021/acs.joc.9b00795 |
| 28. | Lainé, D.; Denavit, V.; Lessard, O.; Carrier, L.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Beilstein J. Org. Chem. 2020, 16, 2880–2887. doi:10.3762/bjoc.16.237 |
| 29. | St-Gelais, J.; Côté, É.; Lainé, D.; Johnson, P. A.; Giguère, D. Chem. – Eur. J. 2020, 26, 13499–13506. doi:10.1002/chem.202002825 |
| 30. | Leclerc, C.; St-Gelais, J.; Cecioni, S.; Giguère, D. J. Fluorine Chem. 2024, 273, 110232. doi:10.1016/j.jfluchem.2023.110232 |
| 22. | Gál, B.; Bucher, C.; Burns, N. Z. Mar. Drugs 2016, 14, 206. doi:10.3390/md14110206 |
| 31. | Lainé, D.; Lessard, O.; St-Gelais, J.; Giguère, D. Chem. – Eur. J. 2021, 27, 3799–3805. doi:10.1002/chem.202004646 |
| 20. | Bucher, C.; Deans, R. M.; Burns, N. Z. J. Am. Chem. Soc. 2015, 137, 12784–12787. doi:10.1021/jacs.5b08398 |
| 21. | Gribble, G. W. Mar. Drugs 2015, 13, 4044–4136. doi:10.3390/md13074044 |
| 76. | Foster, J. P.; Weinhold, F. J. Am. Chem. Soc. 1980, 102, 7211–7218. doi:10.1021/ja00544a007 |
| 19. | Tan, Y.; Luo, S.; Li, D.; Zhang, N.; Jia, S.; Liu, Y.; Qin, W.; Song, C. E.; Yan, H. J. Am. Chem. Soc. 2017, 139, 6431–6436. doi:10.1021/jacs.7b02076 |
| 23. | Lessard, O.; Lainé, D.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Org. Chem. Front. 2022, 9, 6566–6572. doi:10.1039/d2qo01433e |
| 23. | Lessard, O.; Lainé, D.; Fecteau, C.-É.; Johnson, P. A.; Giguère, D. Org. Chem. Front. 2022, 9, 6566–6572. doi:10.1039/d2qo01433e |
| 33. | Deposition number 2344127 (for 13), 2344124 (for 14), and 2344126 (for 15) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service. |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 34. | Deposition number 1824902 (for 17) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service. |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 44. | Linclau, B.; Golten, S.; Light, M.; Sebban, M.; Oulyadi, H. Carbohydr. Res. 2011, 346, 1129–1139. doi:10.1016/j.carres.2011.04.007 |
| 45. | Kim, H. W.; Rossi, P.; Shoemaker, R. K.; DiMagno, S. G. J. Am. Chem. Soc. 1998, 120, 9082–9083. doi:10.1021/ja9803714 |
| 40. | Marchand, A. P.; Sorokin, V. D.; Rajagopal, D.; Bott, S. G. Tetrahedron 1994, 50, 9933–9942. doi:10.1016/s0040-4020(01)89608-3 |
| 41. | Rodríguez‐Vázquez, N.; Salzinger, S.; Silva, L. F.; Amorín, M.; Granja, J. R. Eur. J. Org. Chem. 2013, 3477–3493. doi:10.1002/ejoc.201201565 |
| 42. | Jeffrey, G. A. Acta Crystallogr., Sect. B: Struct. Sci. 1990, 46, 89–103. doi:10.1107/s0108768189012449 |
| 43. | Bock, K.; Duus, J. Ø. J. Carbohydr. Chem. 1994, 13, 513–543. doi:10.1080/07328309408011662 |
| 31. | Lainé, D.; Lessard, O.; St-Gelais, J.; Giguère, D. Chem. – Eur. J. 2021, 27, 3799–3805. doi:10.1002/chem.202004646 |
| 36. | Hunter, L.; Slawin, A. M. Z.; Kirsch, P.; O’Hagan, D. Angew. Chem., Int. Ed. 2007, 46, 7887–7890. doi:10.1002/anie.200701988 |
| 37. | Hunter, L.; O’Hagan, D.; Slawin, A. M. Z. J. Am. Chem. Soc. 2006, 128, 16422–16423. doi:10.1021/ja066188p |
| 38. | Bentler, P.; Erdeljac, N.; Bussmann, K.; Ahlqvist, M.; Knerr, L.; Bergander, K.; Daniliuc, C. G.; Gilmour, R. Org. Lett. 2019, 21, 7741–7745. doi:10.1021/acs.orglett.9b02662 |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 39. | O’Hagan, D. Chem. – Eur. J. 2020, 26, 7981–7997. doi:10.1002/chem.202000178 |
| 24. | Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P. A.; Giguère, D. Nat. Commun. 2018, 9, 4721. doi:10.1038/s41467-018-06901-y |
| 35. | Hansen, L. K.; Hordvik, A. Acta Chem. Scand., Ser. A 1977, 31, 187–191. doi:10.3891/acta.chem.scand.31a-0187 |
© 2024 Lessard et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.