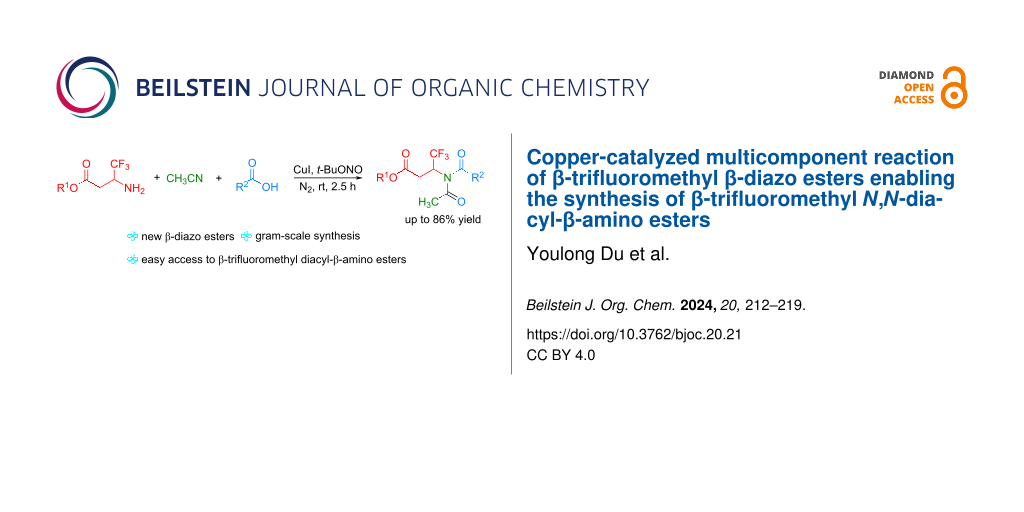
Abstract
An efficient multicomponent reaction of newly designed β-trifluoromethyl β-diazo esters, acetonitrile, and carboxylic acids via an interrupted esterification process under copper-catalyzed conditions has been developed, which affords various unsymmetrical β-trifluoromethyl N,N-diacyl-β-amino esters in good to excellent yields. The reaction features mild conditions, a wide scope of β-amino esters and carboxylic acids, and also applicability to large-scale synthesis, thus providing an efficient way for the synthesis of β-trifluoromethyl β-diacylamino esters. Furthermore, this reaction represents the first example of a Mumm rearrangement of β-trifluoromethyl β-diazo esters.
Graphical Abstract
Introduction
The insertion of fluorine-containing components frequently confers desirable physical and biological properties to organic molecules, and the development of fluorine-containing drugs is an important field of research [1-9]. It is estimated that 30% of drugs and over 50% of agricultural chemicals contain at least one fluorine atom, among which architectural motifs containing fluorine and amino acid residues are a fast-growing segment of modern pharmaceuticals [10-13].
Fluoroalkyldiazo compounds belong to the most versatile and valuable reagents in organic synthesis, as they can be used as diazo intermediates or carbene precursors for the rapid construction of complex molecules along with the introduction of fluoroalkyl groups [14-16]. Although the reaction of trifluorodiazoethane [17-27] as well as α-diazo esters [28-30] have been widely explored, β-trifluoromethyl β-diazo esters have been less investigated, mainly due to the instability of such structures. Therefore, methods for the synthesis of β-trifluoromethyl β-diazo esters and their applications in organic synthesis are needed but remain challenging.
On the other hand, several interesting transformations of nitrile ylides from diazo compounds have been developed in the past years [31-38]. In particular, acylglycine esters could be easily constructed with ester-containing diazo compounds as the starting materials. For example, Wan and co-workers developed a cascade reaction of α-diazo esters, nitriles, and carboxylic acids via the generation of nitrile ylides and Mumm rearrangement affording unsymmetric diacyl α-amino acid esters as products (Scheme 1a) [39]. In 2017, Zhang, Hu, and co-workers developed a Cu-catalyzed reaction of CF3CHN2 with carboxylic acids and acetonitrile via a similar process to afford a series of N-trifluoroethylimides (Scheme 1b) [40,41]. Inspired by these elegant works [31-41] and based on our continuous interest in reactions of fluoroalkyldiazo compounds [42-49], we sought to develop reactions of the unexplored β-trifluoromethyl β-diazo esters. We hypothesized that nitrile ylides, in situ generated from nitriles and β-trifluoromethyl β-amino esters, could also react with carboxylic acids to give nitriliums, which then could undergo a Mumm rearrangement to provide unsymmetrical β-trifluoromethyl diacyl-β-amino esters as products (Scheme 1c). Herein, we report our results on the design of β-trifluoromethyl β-diazo esters and their application in a three-component reaction with nitriles and carboxylic acids under mild conditions. A variety of unnatural unsymmetrical β-trifluoromethyl diacyl-β-amino esters were obtained in good yields, which are useful synthetic scaffolds [50-52] but difficult to obtain by other methods [53-57]. This work is the first example of the reaction of β-trifluoromethyl β-diazo esters, which enriches the studied content of fluoroalkyl diazo compounds.
Scheme 1: Mumm-type rearrangement of diazo compounds.
Scheme 1: Mumm-type rearrangement of diazo compounds.
Results and Discussion
Due to the instability of β-carbonyl diazo compounds and the occurrence of possible side reactions [58-61], screening of reaction conditions to optimize this conversion and inhibit the occurrence of side reactions was carried out with benzyl 3-amino-4,4,4-trifluorobutanoate (1a) and benzoic acid (3a) as model substrates. The initial reaction of amine 1a and acid 3a in acetonitrile in the presence of diazotization reagent tert-butyl nitrite with CuI (10 mol %) as catalysts for 2.5 h at room temperature proceeded to afford the desired unsymmetrical β-trifluoromethyl diacyl-β-amino ester 4a in 54% yield (Table 1, entry 1). The loading amount of catalyst CuI plays a crucial role in the formation of the desired product 4a. Increasing the loading amount of CuI, the yield could be raised to 66% when 20 mol % of CuI was used as catalyst (Table 1, entries 2 and 3). However, further increasing the amount of the catalyst led to an obvious decrease in the yield of product 4a (Table 1, entries 4 and 5). Variation on the reaction temperature also afforded the corresponding product 4a but failed to bring any improvement on the reaction outcome (Table 1, entries 6 and 7). Further optimization of the reaction conditions focused on the variation of the amounts of amine 1a and tert-butyl nitrite (Table 1, entries 8–12). Considering the instability of the diazo structure generated from amine 1a, we increased the amount of amine 1a and tert-butyl nitrite to 4 equivalents. Pleasingly, the yield of product 4a was further increased to 74% (Table 1, entry 12). Furthermore, we optimized the reaction time and found that shortening the reaction time resulted in a decreased yield (Table 1, entry 13). Increasing the reaction time to 3 h also did not lead to any better result mainly due to the decomposition of product 4a (Table 1, entry 14).
Table 1: Optimization of reaction conditions.a
|
|
||||||
| Entry | 1a (equiv) | CuI (mol %) | t-BuONO (equiv) | T (°C) | Time (h) | Yieldb (%) |
| 1 | 2 | 10 | 2 | rt | 2.5 | 54 |
| 2 | 2 | 15 | 2 | rt | 2.5 | 57 |
| 3 | 2 | 20 | 2 | rt | 2.5 | 66 |
| 4 | 2 | 30 | 2 | rt | 2.5 | 32 |
| 5 | 2 | 40 | 2 | rt | 2.5 | trace |
| 6 | 2 | 20 | 2 | 0 | 2.5 | 20 |
| 7 | 2 | 20 | 2 | 60 | 2.5 | 38 |
| 8 | 2 | 20 | 1 | rt | 2.5 | 38 |
| 9 | 2 | 20 | 3 | rt | 2.5 | 41 |
| 10 | 1 | 20 | 2 | rt | 2.5 | 27 |
| 11 | 3 | 20 | 2 | rt | 2.5 | 43 |
| 12 | 4 | 20 | 4 | rt | 2.5 | 74 |
| 13 | 4 | 20 | 4 | rt | 1.5 | 43 |
| 14 | 4 | 20 | 4 | rt | 3 | 60 |
aReaction conditions: amine 1a (0.4 mmol), benzoic acid 3a (0.1 mmol), CuI (20 mol %), t-BuONO (0.4 mmol) and CH3CN (2 mL) under nitrogen atmosphere. bIsolated yield based on acid 3a.
With the optimized reaction conditions in hand, we next evaluated the substrate scope by using a variety of structurally diverse carboxylic acids 3 to react with β-trifluoromethyl β-amino esters 1. As shown in Scheme 2, all the substituted benzoic acids 3 tested were well tolerated in this reaction, and the corresponding product 4 was successfully prepared at moderate to excellent yields (4a–e, 4h–m, 31–86% yields). The benzoic acids featuring a wide range of functional groups, including alkyl (4a–e), halogen (4h–l), and phenyl (4m), were all suitable substrates for this reaction. However, the benzoic acid with ortho-substituent did not afford the expected product (4f) mainly due to the steric hindrance effect. Notably, substrates with electron-withdrawing groups (4h–l, 76–86% yields) provided better chemical yields in this reaction compared with those containing electron-donating groups (4b–e, 31–84% yields). For the case with a strong electron-donating group (methoxy, 3g) only traces of 4g were produced. Besides benzoic acid, the current Cu-catalyzed reaction was also applicable to other aromatic acid substrates. Using 2-naphthoic and 1-naphthoic acid as substrates, the corresponding products 4n and 4o were produced well with yields of 78% and 54%, respectively. Unfortunately, the tested aliphatic acid, such as cyclohexanecarboxylic acid, did not work in the system to produce the expected product (4p). In addition, the β-trifluoromethyl β-amino benzyl ester substrates 1 with different ester groups were tried to react with benzoic acid (3a) to further extend the substrate range. To our delight, both the amines with electron-donating groups (4q and 4r) and electron-withdrawing groups (4s and 4t) could generate the target products with moderate to good yields (46–71%). We also tried another nitrile substrate, such as cyclopropyl acetonitrile, which yielded only very small amounts of the expected product (4u).
Scheme 2: Substrate scope study of this Cu-catalyzed reaction.
Scheme 2: Substrate scope study of this Cu-catalyzed reaction.
To gain insight into the mechanism of this reaction, several control experiments were performed. First, the reaction was conducted under the optimized conditions without the addition of CuI. The conversion of the starting substrates to the desired product 4a was decreased and only 14% yield of 4a was obtained (Scheme 3a). However, as shown in entry 1 of Table 1, 54% yield product 4a was produced by this reaction in the presence of 10 mol % CuI. These results demonstrate that copper catalysis plays a crucial role in the generation of the desired product 4a. Moreover, we performed this reaction without the addition of tert-butyl nitrite (Scheme 3b). The expected three-component tandem reaction did not occur, and the target 4a was not observed with almost all of the starting amine 1a remaining. This result indicates the reaction proceeds through the diazo intermediate.
According to the above experimental results and literature reports [39-41,58,59], a possible mechanism for this Cu-catalyzed reaction of β-trifluoromethyl β-amino esters was proposed in Scheme 4. Initially, β-trifluoromethyl β-amino ester 1a reacts with tert-butyl nitrite to form trifluoromethylated β-carbonyl diazo intermediate A. Then, the diazo intermediate A reacts with the copper catalyst generating the Cu-carbene intermediate B, which undergoes nucleophilic attack by acetonitrile to form the intermediate C. Subsequently, nucleophilic addition of benzoic acid to intermediate C affords the acetimidic anhydride D with the release of CuI catalyst for the next catalytic cycle. Finally, the acetimidic anhydride D undergoes a Mumm rearrangement to furnish the desired β-trifluoromethyl diacylamino ester 4a.
The final goal of this work is the examination of the scale-up applicability of this three-component tandem reaction (Scheme 5). To our delight, the reaction also proceeded smoothly when the amount of β-trifluoromethyl β-amino ester 1a was increased ten-fold to 988.8 mg. The corresponding β-trifluoromethyl diacylamino ester 4a was obtained in 58% chemical yield. This result indicates the wide synthesis utility of the reactions reported in this work.
Conclusion
In summary, a series of new β-trifluoromethyl β-diazo esters have been designed, which are applied for the first time in a cascade reaction through an interrupted esterification with nitrile ylides as the key intermediates under copper-catalysis conditions. Varieties of unsymmetric trifluoromethyl diacyl β-amino esters can be easily constructed with good chemical yields. The reaction is conducted under mild conditions and shows good applicability to different series of substrates, which provides an efficient way for the preparation of unsymmetric trifluoromethyl diacyl β-amino esters.
Experimental
General procedure for copper-catalyzed multicomponent reaction of β-amino esters
Into a flask were added amines 1 (0.4 mmol), acids 3 (0.1 mmol), CuI (20 mol %), and CH3CN (2 mL). Then, the mixture was stirred at room temperature under a nitrogen atmosphere and t-BuONO (0.4 mmol) was added dropwise. Stirring was continued at room temperature for 2.5 h and the solvent was removed in vacuum. Products 4 were purified on a TLC plate of 20 cm × 20 cm using petroleum ether/ethyl acetate 7:1 (v/v) as eluent.
Supporting Information
| Supporting Information File 1: Experimental details and spectral data. | ||
| Format: PDF | Size: 2.2 MB | Download |
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013–1029. doi:10.1016/j.jfluchem.2006.06.007
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992–1012. doi:10.1016/j.jfluchem.2006.05.006
Return to citation in text: [1] -
O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003
Return to citation in text: [1] -
He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A. E.; Butler, G.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2023, 34, 107578. doi:10.1016/j.cclet.2022.06.001
Return to citation in text: [1] -
Yu, Y.; Liu, A.; Dhawan, G.; Mei, H.; Zhang, W.; Izawa, K.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2021, 32, 3342–3354. doi:10.1016/j.cclet.2021.05.042
Return to citation in text: [1] -
Mei, H.; Remete, A. M.; Zou, Y.; Moriwaki, H.; Fustero, S.; Kiss, L.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2020, 31, 2401–2413. doi:10.1016/j.cclet.2020.03.050
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Mei, H.; Han, J.; White, S.; Graham, D. J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N. A.; Soloshonok, V. A. Chem. – Eur. J. 2020, 26, 11349–11390. doi:10.1002/chem.202000617
Return to citation in text: [1] -
Mei, H.; Han, J.; Klika, K. D.; Izawa, K.; Sato, T.; Meanwell, N. A.; Soloshonok, V. A. Eur. J. Med. Chem. 2020, 186, 111826. doi:10.1016/j.ejmech.2019.111826
Return to citation in text: [1] -
Wang, Q.; Han, J.; Sorochinsky, A.; Landa, A.; Butler, G.; Soloshonok, V. A. Pharmaceuticals 2022, 15, 999. doi:10.3390/ph15080999
Return to citation in text: [1] -
Wang, N.; Mei, H.; Dhawan, G.; Zhang, W.; Han, J.; Soloshonok, V. A. Molecules 2023, 28, 3651. doi:10.3390/molecules28093651
Return to citation in text: [1] -
Mykhailiuk, P. K.; Koenigs, R. M. Chem. – Eur. J. 2019, 25, 6053–6063. doi:10.1002/chem.201804953
Return to citation in text: [1] -
Mertens, L.; Koenigs, R. M. Org. Biomol. Chem. 2016, 14, 10547–10556. doi:10.1039/c6ob01618a
Return to citation in text: [1] -
Ollevier, T.; Carreras, V. ACS Org. Inorg. Au 2022, 2, 83–98. doi:10.1021/acsorginorgau.1c00027
Return to citation in text: [1] -
Mykhailiuk, P. K. Chem. Rev. 2020, 120, 12718–12755. doi:10.1021/acs.chemrev.0c00406
Return to citation in text: [1] -
Britton, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2017, 56, 8823–8827. doi:10.1002/anie.201704529
Return to citation in text: [1] -
Hock, K. J.; Mertens, L.; Metze, F. K.; Schmittmann, C.; Koenigs, R. M. Green Chem. 2017, 19, 905–909. doi:10.1039/c6gc03187k
Return to citation in text: [1] -
Chen, Y.-J.; Zhang, F.-G.; Ma, J.-A. Org. Lett. 2021, 23, 6062–6066. doi:10.1021/acs.orglett.1c02139
Return to citation in text: [1] -
Jonker, S. J. T.; Jayarajan, R.; Kireilis, T.; Deliaval, M.; Eriksson, L.; Szabó, K. J. J. Am. Chem. Soc. 2020, 142, 21254–21259. doi:10.1021/jacs.0c09923
Return to citation in text: [1] -
Zhang, X.; Liu, Z.; Yang, X.; Dong, Y.; Virelli, M.; Zanoni, G.; Anderson, E. A.; Bi, X. Nat. Commun. 2019, 10, 284. doi:10.1038/s41467-018-08253-z
Return to citation in text: [1] -
Li, J.; Zhang, D.; Chen, J.; Ma, C.; Hu, W. ACS Catal. 2020, 10, 4559–4565. doi:10.1021/acscatal.0c00972
Return to citation in text: [1] -
Gao, C.-F.; Zhou, Y.; Ma, H.; Zhang, Y.; Nie, J.; Zhang, F.-G.; Ma, J.-A. CCS Chem. 2022, 4, 3693–3704. doi:10.31635/ccschem.022.202201923
Return to citation in text: [1] -
Liu, Y.; Pang, T.; Yao, W.; Zhong, F.; Wu, G. Org. Lett. 2023, 25, 1958–1962. doi:10.1021/acs.orglett.3c00464
Return to citation in text: [1] -
Dhami, A.; Chandrasekharan, S. P.; Mohanan, K. Org. Lett. 2023, 25, 3018–3022. doi:10.1021/acs.orglett.3c00801
Return to citation in text: [1] -
Schaus, L.; Das, A.; Knight, A. M.; Jimenez-Osés, G.; Houk, K. N.; Garcia-Borràs, M.; Arnold, F. H.; Huang, X. Angew. Chem., Int. Ed. 2023, 62, e202208936. doi:10.1002/anie.202208936
Return to citation in text: [1] -
Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981–10080. doi:10.1021/acs.chemrev.5b00121
Return to citation in text: [1] -
Xia, Y.; Qiu, D.; Wang, J. Chem. Rev. 2017, 117, 13810–13889. doi:10.1021/acs.chemrev.7b00382
Return to citation in text: [1] -
Che, J.; Niu, L.; Jia, S.; Xing, D.; Hu, W. Nat. Commun. 2020, 11, 1511. doi:10.1038/s41467-020-15345-2
Return to citation in text: [1] -
Horneff, T.; Chuprakov, S.; Chernyak, N.; Gevorgyan, V.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 14972–14974. doi:10.1021/ja805079v
Return to citation in text: [1] [2] -
Austeri, M.; Rix, D.; Zeghida, W.; Lacour, J. Org. Lett. 2011, 13, 1394–1397. doi:10.1021/ol2000815
Return to citation in text: [1] [2] -
Zibinsky, M.; Fokin, V. V. Org. Lett. 2011, 13, 4870–4872. doi:10.1021/ol201949h
Return to citation in text: [1] [2] -
Billedeau, R. J.; Klein, K. R.; Kaplan, D.; Lou, Y. Org. Lett. 2013, 15, 1421–1423. doi:10.1021/ol400062w
Return to citation in text: [1] [2] -
Lonzi, G.; López, L. A. Adv. Synth. Catal. 2013, 355, 1948–1954. doi:10.1002/adsc.201300346
Return to citation in text: [1] [2] -
Karad, S. N.; Liu, R.-S. Angew. Chem., Int. Ed. 2014, 53, 5444–5448. doi:10.1002/anie.201403015
Return to citation in text: [1] [2] -
Li, H.; Wu, X.; Hao, W.; Li, H.; Zhao, Y.; Wang, Y.; Lian, P.; Zheng, Y.; Bao, X.; Wan, X. Org. Lett. 2018, 20, 5224–5227. doi:10.1021/acs.orglett.8b02172
Return to citation in text: [1] [2] -
Cai, B.-G.; Yao, W.-Z.; Li, L.; Xuan, J. Org. Lett. 2022, 24, 6647–6652. doi:10.1021/acs.orglett.2c02671
Return to citation in text: [1] [2] -
Chen, J.; Shao, Y.; Ma, L.; Ma, M.; Wan, X. Org. Biomol. Chem. 2016, 14, 10723–10732. doi:10.1039/c6ob02037b
Return to citation in text: [1] [2] [3] -
Peng, S.-Q.; Zhang, X.-W.; Zhang, L.; Hu, X.-G. Org. Lett. 2017, 19, 5689–5692. doi:10.1021/acs.orglett.7b02866
Return to citation in text: [1] [2] [3] -
Hu, X.-G.; Qiu, X.-F.; Liu, D.-Y.; Zhang, W.-F. Synthesis 2021, 53, 961–970. doi:10.1055/a-1339-3227
Return to citation in text: [1] [2] [3] -
Mei, H.; Wang, L.; Pajkert, R.; Wang, Q.; Xu, J.; Liu, J.; Röschenthaler, G.-V.; Han, J. Org. Lett. 2021, 23, 1130–1134. doi:10.1021/acs.orglett.1c00150
Return to citation in text: [1] -
Mei, H.; Liu, J.; Pajkert, R.; Wang, L.; Röschenthaler, G.-V.; Han, J. Org. Chem. Front. 2021, 8, 767–772. doi:10.1039/d0qo01394c
Return to citation in text: [1] -
Liu, J.; Xu, J.; Pajkert, R.; Mei, H.; Röschenthaler, G.-V.; Han, J. Acta Chim. Sin. (Chin. Ed.) 2021, 79, 747–750. doi:10.6023/a21030096
Return to citation in text: [1] -
Liu, J.; Pajkert, R.; Wang, L.; Mei, H.; Röschenthaler, G.-V.; Han, J. Chin. Chem. Lett. 2022, 33, 2429–2432. doi:10.1016/j.cclet.2021.10.066
Return to citation in text: [1] -
Wang, Q.; Liu, J.; Mei, H.; Pajkert, R.; Röschenthaler, G.-V.; Han, J. Adv. Synth. Catal. 2023, 365, 2883–2887. doi:10.1002/adsc.202300595
Return to citation in text: [1] -
Wang, Q.; Liu, J.; Mei, H.; Pajkert, R.; Kessler, M.; Röschenthaler, G.-V.; Han, J. Org. Lett. 2022, 24, 8036–8040. doi:10.1021/acs.orglett.2c03268
Return to citation in text: [1] -
Wang, Q.; Liu, J.; Wang, N.; Pajkert, R.; Mei, H.; Röschenthaler, G.-V.; Han, J. Adv. Synth. Catal. 2022, 364, 1969–1974. doi:10.1002/adsc.202200330
Return to citation in text: [1] -
Xu, J.; Liu, J.; Mei, H.; Soloshonok, V. A.; Han, J. Chem. Heterocycl. Compd. 2023, 59, 465–471. doi:10.1007/s10593-023-03217-8
Return to citation in text: [1] -
Meng, G.; Zhang, J.; Szostak, M. Chem. Rev. 2021, 121, 12746–12783. doi:10.1021/acs.chemrev.1c00225
Return to citation in text: [1] -
Chen, J.; Xia, Y.; Lee, S. Org. Lett. 2020, 22, 3504–3508. doi:10.1021/acs.orglett.0c00958
Return to citation in text: [1] -
Ferreira, P. M. T.; Maia, H. L. S.; Monteiro, L. S. Eur. J. Org. Chem. 2003, 2635–2644. doi:10.1002/ejoc.200300103
Return to citation in text: [1] -
Soloshonok, V. A.; Ono, T.; Soloshonok, I. V. J. Org. Chem. 1997, 62, 7538–7539. doi:10.1021/jo9710238
Return to citation in text: [1] -
Soloshonok, V. A.; Kukhar, V. P. Tetrahedron 1996, 52, 6953–6964. doi:10.1016/0040-4020(96)00300-6
Return to citation in text: [1] -
Zhou, S.; Wang, J.; Chen, X.; Aceña, J. L.; Soloshonok, V. A.; Liu, H. Angew. Chem., Int. Ed. 2014, 53, 7883–7886. doi:10.1002/anie.201403556
Return to citation in text: [1] -
Shibata, N.; Nishimine, T.; Shibata, N.; Tokunaga, E.; Kawada, K.; Kagawa, T.; Sorochinsky, A. E.; Soloshonok, V. A. Chem. Commun. 2012, 48, 4124–4126. doi:10.1039/c2cc30627a
Return to citation in text: [1] -
Soloshonok, V. A.; Kirilenko, A. G.; Kukhar', V. P.; Resnati, G. Tetrahedron Lett. 1993, 34, 3621–3624. doi:10.1016/s0040-4039(00)73652-5
Return to citation in text: [1] -
Wang, N.; Qiao, Y.; Du, Y.; Mei, H.; Han, J. Org. Biomol. Chem. 2022, 20, 7467–7471. doi:10.1039/d2ob01391f
Return to citation in text: [1] [2] -
Mei, H.; Wang, N.; Li, Z.; Han, J. Org. Lett. 2022, 24, 2258–2263. doi:10.1021/acs.orglett.2c00738
Return to citation in text: [1] [2] -
Jiang, L.; Wang, Z.; Armstrong, M.; Suero, M. G. Angew. Chem., Int. Ed. 2021, 60, 6177–6184. doi:10.1002/anie.202015077
Return to citation in text: [1] -
Barluenga, J.; Lonzi, G.; Riesgo, L.; Tomás, M.; López, L. A. J. Am. Chem. Soc. 2011, 133, 18138–18141. doi:10.1021/ja208965b
Return to citation in text: [1]
| 1. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 2. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 3. | Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013–1029. doi:10.1016/j.jfluchem.2006.06.007 |
| 4. | Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992–1012. doi:10.1016/j.jfluchem.2006.05.006 |
| 5. | O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003 |
| 6. | He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A. E.; Butler, G.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2023, 34, 107578. doi:10.1016/j.cclet.2022.06.001 |
| 7. | Yu, Y.; Liu, A.; Dhawan, G.; Mei, H.; Zhang, W.; Izawa, K.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2021, 32, 3342–3354. doi:10.1016/j.cclet.2021.05.042 |
| 8. | Mei, H.; Remete, A. M.; Zou, Y.; Moriwaki, H.; Fustero, S.; Kiss, L.; Soloshonok, V. A.; Han, J. Chin. Chem. Lett. 2020, 31, 2401–2413. doi:10.1016/j.cclet.2020.03.050 |
| 9. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 28. | Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981–10080. doi:10.1021/acs.chemrev.5b00121 |
| 29. | Xia, Y.; Qiu, D.; Wang, J. Chem. Rev. 2017, 117, 13810–13889. doi:10.1021/acs.chemrev.7b00382 |
| 30. | Che, J.; Niu, L.; Jia, S.; Xing, D.; Hu, W. Nat. Commun. 2020, 11, 1511. doi:10.1038/s41467-020-15345-2 |
| 17. | Mykhailiuk, P. K. Chem. Rev. 2020, 120, 12718–12755. doi:10.1021/acs.chemrev.0c00406 |
| 18. | Britton, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2017, 56, 8823–8827. doi:10.1002/anie.201704529 |
| 19. | Hock, K. J.; Mertens, L.; Metze, F. K.; Schmittmann, C.; Koenigs, R. M. Green Chem. 2017, 19, 905–909. doi:10.1039/c6gc03187k |
| 20. | Chen, Y.-J.; Zhang, F.-G.; Ma, J.-A. Org. Lett. 2021, 23, 6062–6066. doi:10.1021/acs.orglett.1c02139 |
| 21. | Jonker, S. J. T.; Jayarajan, R.; Kireilis, T.; Deliaval, M.; Eriksson, L.; Szabó, K. J. J. Am. Chem. Soc. 2020, 142, 21254–21259. doi:10.1021/jacs.0c09923 |
| 22. | Zhang, X.; Liu, Z.; Yang, X.; Dong, Y.; Virelli, M.; Zanoni, G.; Anderson, E. A.; Bi, X. Nat. Commun. 2019, 10, 284. doi:10.1038/s41467-018-08253-z |
| 23. | Li, J.; Zhang, D.; Chen, J.; Ma, C.; Hu, W. ACS Catal. 2020, 10, 4559–4565. doi:10.1021/acscatal.0c00972 |
| 24. | Gao, C.-F.; Zhou, Y.; Ma, H.; Zhang, Y.; Nie, J.; Zhang, F.-G.; Ma, J.-A. CCS Chem. 2022, 4, 3693–3704. doi:10.31635/ccschem.022.202201923 |
| 25. | Liu, Y.; Pang, T.; Yao, W.; Zhong, F.; Wu, G. Org. Lett. 2023, 25, 1958–1962. doi:10.1021/acs.orglett.3c00464 |
| 26. | Dhami, A.; Chandrasekharan, S. P.; Mohanan, K. Org. Lett. 2023, 25, 3018–3022. doi:10.1021/acs.orglett.3c00801 |
| 27. | Schaus, L.; Das, A.; Knight, A. M.; Jimenez-Osés, G.; Houk, K. N.; Garcia-Borràs, M.; Arnold, F. H.; Huang, X. Angew. Chem., Int. Ed. 2023, 62, e202208936. doi:10.1002/anie.202208936 |
| 14. | Mykhailiuk, P. K.; Koenigs, R. M. Chem. – Eur. J. 2019, 25, 6053–6063. doi:10.1002/chem.201804953 |
| 15. | Mertens, L.; Koenigs, R. M. Org. Biomol. Chem. 2016, 14, 10547–10556. doi:10.1039/c6ob01618a |
| 16. | Ollevier, T.; Carreras, V. ACS Org. Inorg. Au 2022, 2, 83–98. doi:10.1021/acsorginorgau.1c00027 |
| 58. | Wang, N.; Qiao, Y.; Du, Y.; Mei, H.; Han, J. Org. Biomol. Chem. 2022, 20, 7467–7471. doi:10.1039/d2ob01391f |
| 59. | Mei, H.; Wang, N.; Li, Z.; Han, J. Org. Lett. 2022, 24, 2258–2263. doi:10.1021/acs.orglett.2c00738 |
| 60. | Jiang, L.; Wang, Z.; Armstrong, M.; Suero, M. G. Angew. Chem., Int. Ed. 2021, 60, 6177–6184. doi:10.1002/anie.202015077 |
| 61. | Barluenga, J.; Lonzi, G.; Riesgo, L.; Tomás, M.; López, L. A. J. Am. Chem. Soc. 2011, 133, 18138–18141. doi:10.1021/ja208965b |
| 10. | Mei, H.; Han, J.; White, S.; Graham, D. J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N. A.; Soloshonok, V. A. Chem. – Eur. J. 2020, 26, 11349–11390. doi:10.1002/chem.202000617 |
| 11. | Mei, H.; Han, J.; Klika, K. D.; Izawa, K.; Sato, T.; Meanwell, N. A.; Soloshonok, V. A. Eur. J. Med. Chem. 2020, 186, 111826. doi:10.1016/j.ejmech.2019.111826 |
| 12. | Wang, Q.; Han, J.; Sorochinsky, A.; Landa, A.; Butler, G.; Soloshonok, V. A. Pharmaceuticals 2022, 15, 999. doi:10.3390/ph15080999 |
| 13. | Wang, N.; Mei, H.; Dhawan, G.; Zhang, W.; Han, J.; Soloshonok, V. A. Molecules 2023, 28, 3651. doi:10.3390/molecules28093651 |
| 39. | Chen, J.; Shao, Y.; Ma, L.; Ma, M.; Wan, X. Org. Biomol. Chem. 2016, 14, 10723–10732. doi:10.1039/c6ob02037b |
| 40. | Peng, S.-Q.; Zhang, X.-W.; Zhang, L.; Hu, X.-G. Org. Lett. 2017, 19, 5689–5692. doi:10.1021/acs.orglett.7b02866 |
| 41. | Hu, X.-G.; Qiu, X.-F.; Liu, D.-Y.; Zhang, W.-F. Synthesis 2021, 53, 961–970. doi:10.1055/a-1339-3227 |
| 58. | Wang, N.; Qiao, Y.; Du, Y.; Mei, H.; Han, J. Org. Biomol. Chem. 2022, 20, 7467–7471. doi:10.1039/d2ob01391f |
| 59. | Mei, H.; Wang, N.; Li, Z.; Han, J. Org. Lett. 2022, 24, 2258–2263. doi:10.1021/acs.orglett.2c00738 |
| 31. | Horneff, T.; Chuprakov, S.; Chernyak, N.; Gevorgyan, V.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 14972–14974. doi:10.1021/ja805079v |
| 32. | Austeri, M.; Rix, D.; Zeghida, W.; Lacour, J. Org. Lett. 2011, 13, 1394–1397. doi:10.1021/ol2000815 |
| 33. | Zibinsky, M.; Fokin, V. V. Org. Lett. 2011, 13, 4870–4872. doi:10.1021/ol201949h |
| 34. | Billedeau, R. J.; Klein, K. R.; Kaplan, D.; Lou, Y. Org. Lett. 2013, 15, 1421–1423. doi:10.1021/ol400062w |
| 35. | Lonzi, G.; López, L. A. Adv. Synth. Catal. 2013, 355, 1948–1954. doi:10.1002/adsc.201300346 |
| 36. | Karad, S. N.; Liu, R.-S. Angew. Chem., Int. Ed. 2014, 53, 5444–5448. doi:10.1002/anie.201403015 |
| 37. | Li, H.; Wu, X.; Hao, W.; Li, H.; Zhao, Y.; Wang, Y.; Lian, P.; Zheng, Y.; Bao, X.; Wan, X. Org. Lett. 2018, 20, 5224–5227. doi:10.1021/acs.orglett.8b02172 |
| 38. | Cai, B.-G.; Yao, W.-Z.; Li, L.; Xuan, J. Org. Lett. 2022, 24, 6647–6652. doi:10.1021/acs.orglett.2c02671 |
| 39. | Chen, J.; Shao, Y.; Ma, L.; Ma, M.; Wan, X. Org. Biomol. Chem. 2016, 14, 10723–10732. doi:10.1039/c6ob02037b |
| 40. | Peng, S.-Q.; Zhang, X.-W.; Zhang, L.; Hu, X.-G. Org. Lett. 2017, 19, 5689–5692. doi:10.1021/acs.orglett.7b02866 |
| 41. | Hu, X.-G.; Qiu, X.-F.; Liu, D.-Y.; Zhang, W.-F. Synthesis 2021, 53, 961–970. doi:10.1055/a-1339-3227 |
| 50. | Meng, G.; Zhang, J.; Szostak, M. Chem. Rev. 2021, 121, 12746–12783. doi:10.1021/acs.chemrev.1c00225 |
| 51. | Chen, J.; Xia, Y.; Lee, S. Org. Lett. 2020, 22, 3504–3508. doi:10.1021/acs.orglett.0c00958 |
| 52. | Ferreira, P. M. T.; Maia, H. L. S.; Monteiro, L. S. Eur. J. Org. Chem. 2003, 2635–2644. doi:10.1002/ejoc.200300103 |
| 40. | Peng, S.-Q.; Zhang, X.-W.; Zhang, L.; Hu, X.-G. Org. Lett. 2017, 19, 5689–5692. doi:10.1021/acs.orglett.7b02866 |
| 41. | Hu, X.-G.; Qiu, X.-F.; Liu, D.-Y.; Zhang, W.-F. Synthesis 2021, 53, 961–970. doi:10.1055/a-1339-3227 |
| 53. | Soloshonok, V. A.; Ono, T.; Soloshonok, I. V. J. Org. Chem. 1997, 62, 7538–7539. doi:10.1021/jo9710238 |
| 54. | Soloshonok, V. A.; Kukhar, V. P. Tetrahedron 1996, 52, 6953–6964. doi:10.1016/0040-4020(96)00300-6 |
| 55. | Zhou, S.; Wang, J.; Chen, X.; Aceña, J. L.; Soloshonok, V. A.; Liu, H. Angew. Chem., Int. Ed. 2014, 53, 7883–7886. doi:10.1002/anie.201403556 |
| 56. | Shibata, N.; Nishimine, T.; Shibata, N.; Tokunaga, E.; Kawada, K.; Kagawa, T.; Sorochinsky, A. E.; Soloshonok, V. A. Chem. Commun. 2012, 48, 4124–4126. doi:10.1039/c2cc30627a |
| 57. | Soloshonok, V. A.; Kirilenko, A. G.; Kukhar', V. P.; Resnati, G. Tetrahedron Lett. 1993, 34, 3621–3624. doi:10.1016/s0040-4039(00)73652-5 |
| 39. | Chen, J.; Shao, Y.; Ma, L.; Ma, M.; Wan, X. Org. Biomol. Chem. 2016, 14, 10723–10732. doi:10.1039/c6ob02037b |
| 31. | Horneff, T.; Chuprakov, S.; Chernyak, N.; Gevorgyan, V.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 14972–14974. doi:10.1021/ja805079v |
| 32. | Austeri, M.; Rix, D.; Zeghida, W.; Lacour, J. Org. Lett. 2011, 13, 1394–1397. doi:10.1021/ol2000815 |
| 33. | Zibinsky, M.; Fokin, V. V. Org. Lett. 2011, 13, 4870–4872. doi:10.1021/ol201949h |
| 34. | Billedeau, R. J.; Klein, K. R.; Kaplan, D.; Lou, Y. Org. Lett. 2013, 15, 1421–1423. doi:10.1021/ol400062w |
| 35. | Lonzi, G.; López, L. A. Adv. Synth. Catal. 2013, 355, 1948–1954. doi:10.1002/adsc.201300346 |
| 36. | Karad, S. N.; Liu, R.-S. Angew. Chem., Int. Ed. 2014, 53, 5444–5448. doi:10.1002/anie.201403015 |
| 37. | Li, H.; Wu, X.; Hao, W.; Li, H.; Zhao, Y.; Wang, Y.; Lian, P.; Zheng, Y.; Bao, X.; Wan, X. Org. Lett. 2018, 20, 5224–5227. doi:10.1021/acs.orglett.8b02172 |
| 38. | Cai, B.-G.; Yao, W.-Z.; Li, L.; Xuan, J. Org. Lett. 2022, 24, 6647–6652. doi:10.1021/acs.orglett.2c02671 |
| 42. | Mei, H.; Wang, L.; Pajkert, R.; Wang, Q.; Xu, J.; Liu, J.; Röschenthaler, G.-V.; Han, J. Org. Lett. 2021, 23, 1130–1134. doi:10.1021/acs.orglett.1c00150 |
| 43. | Mei, H.; Liu, J.; Pajkert, R.; Wang, L.; Röschenthaler, G.-V.; Han, J. Org. Chem. Front. 2021, 8, 767–772. doi:10.1039/d0qo01394c |
| 44. | Liu, J.; Xu, J.; Pajkert, R.; Mei, H.; Röschenthaler, G.-V.; Han, J. Acta Chim. Sin. (Chin. Ed.) 2021, 79, 747–750. doi:10.6023/a21030096 |
| 45. | Liu, J.; Pajkert, R.; Wang, L.; Mei, H.; Röschenthaler, G.-V.; Han, J. Chin. Chem. Lett. 2022, 33, 2429–2432. doi:10.1016/j.cclet.2021.10.066 |
| 46. | Wang, Q.; Liu, J.; Mei, H.; Pajkert, R.; Röschenthaler, G.-V.; Han, J. Adv. Synth. Catal. 2023, 365, 2883–2887. doi:10.1002/adsc.202300595 |
| 47. | Wang, Q.; Liu, J.; Mei, H.; Pajkert, R.; Kessler, M.; Röschenthaler, G.-V.; Han, J. Org. Lett. 2022, 24, 8036–8040. doi:10.1021/acs.orglett.2c03268 |
| 48. | Wang, Q.; Liu, J.; Wang, N.; Pajkert, R.; Mei, H.; Röschenthaler, G.-V.; Han, J. Adv. Synth. Catal. 2022, 364, 1969–1974. doi:10.1002/adsc.202200330 |
| 49. | Xu, J.; Liu, J.; Mei, H.; Soloshonok, V. A.; Han, J. Chem. Heterocycl. Compd. 2023, 59, 465–471. doi:10.1007/s10593-023-03217-8 |
© 2024 Du et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.