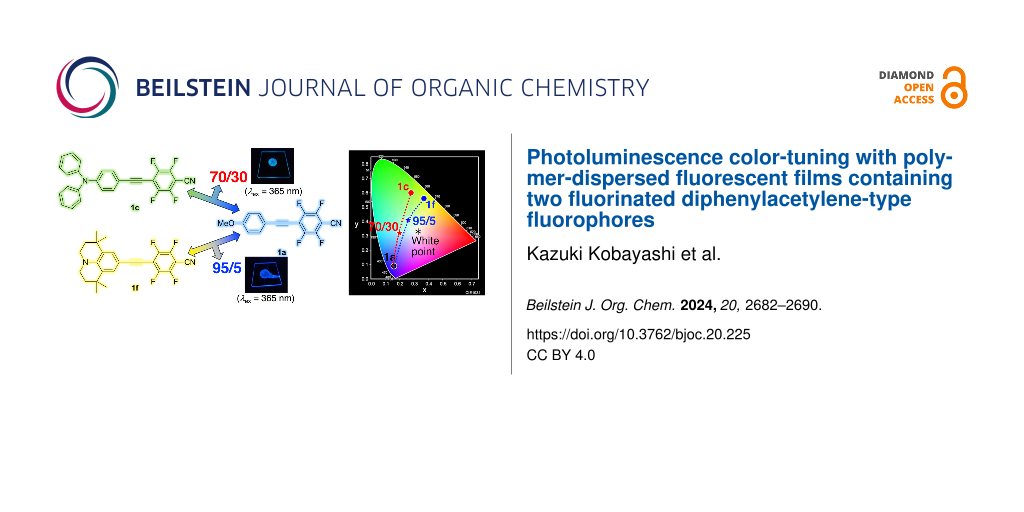
Abstract
The development of organic light-emitting devices has driven demand for new luminescent materials, particularly after the 2001 discovery of aggregation-induced emission. This study focuses on fluorinated diphenylacetylene-based luminescent molecules, revealing that specific molecular modifications can enhance fluorescence and achieve a wide range of photoluminescence colors. A simple and effective luminescence color-tuning method is proposed to investigate the photoluminescence behavior of two-component polymer dispersion films blended with two types of fluorinated diphenylacetylenes, namely blue- and yellow- or red-fluorescent fluorinated diphenylacetylenes. It is confirmed that if blue and green–yellow or yellow fluorophores are blended in appropriate ratios, a binary blend with color coordinates (0.20, 0.32) can be achieved, which approaches the white point of pure white emission. These findings contribute to the development of effective lighting and display devices as new white-light-emitting materials.

Graphical Abstract
Introduction
Luminescent materials in lighting and display devices have become indispensable in daily life [1-3]. In recent years, organic electroluminescent devices have attracted significant attention as lightweight and energy-saving optical devices, and there has been a strong demand for the development of luminescent materials. Until now, the design of solid-state light-emitting materials has not been established, and therefore, their development has been severely delayed [4-6]. However, since Tang et al. first reported the aggregation-induced emission phenomenon in 2001 [7], the development of solid-state light-emitting materials has accelerated significantly [8-10].
Many photoluminescent materials that emit blue, green, and yellow photoluminescence (PL) have been developed, whereas red PL with PL wavelengths in the long wavelength region is considered difficult to achieve owing to the energy gap law [11-13]. Over the past few decades, our group has been vigorously pursuing the exploration of functional molecules with a linear diphenylacetylene structure as the π-conjugated core. As a part of our research projects, we have begun to explore diphenylacetylene-based luminescent molecules despite diphenylacetylene not exhibiting fluorescence at room temperature because it undergoes a πσ* excited state that rapidly forms a trans-bend structure (Figure 1a) [14-16].
Figure 1: (a) Reported photophysics of diphenylacetylene after photoexcitation. (b) Our molecular design to suppress internal conversion.
Figure 1: (a) Reported photophysics of diphenylacetylene after photoexcitation. (b) Our molecular design to s...
Our extensive efforts have revealed that introducing electron-donating alkoxy and electron-withdrawing cyano groups at both ends of the diphenylacetylene scaffold slows the internal conversion to the πσ* excited state. Further incorporating four fluoro substituents in the short-axis direction of the electron-deficient aromatic ring significantly retards the internal conversion via the formation of H···F hydrogen bonds, leading to a marked blue fluorescence in the crystalline state (Figure 1b) [17-20]. Recently, the introduction of N,N-disubstituted amino groups as electron-donating groups was shown to promote intramolecular charge transfer (ICT) and shifted the PL wavelength to longer wavelengths, resulting in yellow or orange fluorescence in the solid state [21,22]. In addition, cross-linking between the amino group and attached benzene ring effectively suppresses the formation of the twisted ICT state, resulting in red fluorescence even in the solid state (Figure 2) [23].
Figure 2: Relationship between the molecular structure of fluorinated diphenylacetylenes and photoluminescence (PL) color in their crystalline states.
Figure 2: Relationship between the molecular structure of fluorinated diphenylacetylenes and photoluminescenc...
The precise tuning of molecular and electronic structures has made it possible to produce a wide range of PL colors. However, the development of white-light-emitting materials, which are especially indispensable for our affluent life, is more difficult than the development of the blue-, yellow-, and red-light-emitting molecules mentioned above [24-26]. Therefore, to achieve white luminescence covering the entire spectral range of the visible light region, two or more colors of fluorescence or phosphorescence from different luminescent centers in the polymer matrix should be combined, and the PL color can be precisely tuned by controlling the ratio of the PL luminescent materials [27-29]. In this study, we prepared polymer dispersion fluorescent films containing two compounds from our fluorinated diphenylacetylene library that exhibit different PL characteristics from blue to red in the solid state, as shown in Figure 2, and investigated their PL behavior and PL color-tuning potential.
Results and Discussion
Photoluminescence behavior of poly(methyl methacrylate) (PMMA) films
Initially, we tested the PL behavior of polymer dispersion films containing fluorinated diphenylacetylenes 1a–g as a single component, as shown in Figure 3, because the PL behavior of fluorescent molecules dispersed in a polymer matrix generally differs from that in a dilute solution or the crystalline state. The PL wavelengths (λPL), fluorescence quantum yields (ΦPL), fluorescence lifetimes (τPL), and Commission Internationale de l'Eclairage (CIE) chromaticity coordinates of the PMMA dispersion films containing 1 wt % of compounds 1a–g are summarized in Table 1.
![[1860-5397-20-225-3]](/bjoc/content/figures/1860-5397-20-225-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) PL spectra of the donor–π–acceptor (D–π–A)-type diphenylacetylene compounds 1a–g contained in a poly(methyl methacrylate) (PMMA) dispersion film. (b) Photographs of the PMMA dispersion films under ultraviolet (UV) irradiation (λex = 365 nm). (c) A PL color diagram defined by the Commission Internationale de l'Eclairage (CIE).
Figure 3: (a) PL spectra of the donor–π–acceptor (D–π–A)-type diphenylacetylene compounds 1a–g contained in a...
Table 1: Photophysical data of compounds 1a–g contained in a PMMA dispersion film.
| λex [nm] | λPL [nm] (ΦPL)a | τPL [ns] | coordinate (x, y)b | |
| 1a | 310 | 415 (0.76) | 4.68 | (0.16, 0.09) |
| 1b | 300 | 479 (1.00) | 3.21 | (0.19, 0.33) |
| 1c | 300 | 506 (0.84) | 3.19 | (0.28, 0.60) |
| 1d | 400 | 512 (0.66) | 3.89 | (0.28, 0.51) |
| 1e | 440 | 517 (0.54) | 2.26 | (0.30, 0.53) |
| 1f | 440 | 534 (0.66) | 3.73 | (0.37, 0.56) |
| 1g | 450 | 544 (0.03) | 4.84 | (0.41, 0.54) |
aMeasured using an integrating sphere; bchromaticity coordinates defined by the CIE.
The PMMA dispersion films containing 1 wt % of compounds 1a–g all exhibited a single PL band with λPLs in the range of 415–544 nm, and their PL colors varied from dark blue to yellow with (x, y) coordinates of (0.16, 0.09) and (0.41, 0.54), respectively (Figure 3). A blueshift in the λPL ranging from 28 nm to a maximum of 126 nm was observed for all compounds contained in a PMMA film, based on a comparison with the λPL of the crystalline state shown in Figure 2. A decrease in the ΦPL was observed for the PMMA dispersion films containing 1a with a methoxy substituent, 1c with a diphenylamino group, or 1g with a phenothiazine unit. Judging from the fact that compounds 1a and 1c form a tight molecular packing via intermolecular H···F hydrogen bonds which suppress non-radiative deactivation in the crystalline state [20,21], we speculated that the polymer dispersion state had lost the intermolecular interactions, which accelerated the non-radiative deactivation process. On the other hand, the other derivatives, namely 1b and 1d–f, showed increased ΦPL values in the PMMA films compared with those in the crystalline state, presumably due to a suppression of the formation of non-fluorescent twisted intramolecular charge transfer (TICT) states caused by the large ICT characteristics.
Photoluminescence behavior of PMMA dispersion fluorescent films containing two fluorinated diphenylacetylenes
Based on the solid-state fluorescent molecule library 1a–g developed by our group [20-23], we expected that white photoluminescent devices could be developed by precisely controlling the two-component mixture system of blue- and yellow-fluorescent molecules. From the perspective of both the PL color and ΦPL, we selected the methoxy-substituted compound 1a as an effective blue-fluorescent molecule for use in a two-component mixing system. Among diphenylamino-substituted 1c with chromaticity (x, y) coordinates of (0.28, 0.60), 1f containing a tetramethyljulolidine unit with (x, y) coordinates of (0.37, 0.56), and 1g containing a phenothiazine unit with (x, y) coordinates of (0.41, 0.54), 1c and 1f were finally selected as candidates for yellow-fluorescent molecules from the viewpoint of their ΦPL. Therefore, we investigated the PL behavior of PMMA dispersion films containing blue-fluorescent 1a, green–yellow fluorescent 1c, and yellow-fluorescent 1f.
Photoluminescence behavior of PMMA dispersion films containing a mixture of blue fluorophore 1a and green–yellow fluorophore 1c
PMMA dispersion films containing 1 wt % of blue fluorophore 1a and green–yellow fluorophore 1c in various weight ratios were prepared. Their PL spectra and photophysical data are depicted in Figure 4 and Table 2 summarizes the collected photophysical data.
![[1860-5397-20-225-4]](/bjoc/content/figures/1860-5397-20-225-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) PL spectra of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and green–yellow fluorophore 1c in various weight ratios. (b) Photographs of the PMMA dispersion films under UV irradiation (λex = 365 nm). (c) A CIE color diagram of the PL color of the PMMA dispersion films containing 1a and 1c in various ratios.
Figure 4: (a) PL spectra of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and green–yellow f...
Table 2: Photophysical data of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and green–yellow fluorophore 1c in various weight ratios.
| Ratio of 1a:1c | λPL [nm] (ΦPL)a | τPL [ns] | coordinate (x, y)b | EFRET [%]c |
| 100:0 | 415 (0.76) | 4.68 | (0.16, 0.09) | – |
| 95:5 | 404 (0.97) | 2.50 | (0.17, 0.14) | 47 |
| 91:9 | 404, 473 (0.92) | 2.52 | (0.18, 0.20) | 46 |
| 80:20 | 400, 480 (0.93) | 1.58 | (0.19, 0.27) | 66 |
| 70:30 | 392, 482 (0.76) | 1.35 | (0.20, 0.32) | 71 |
| 60:40 | 496 (0.95) | 2.73 | (0.24, 0.43) | 42 |
| 50:50 | 390, 504 (1.0) | 2.79 | (0.24, 0.45) | 40 |
| 0:100 | 506 (0.84) | 3.19 | (0.28, 0.60) | – |
aMeasured using an integrating sphere; bPL color coordinate defined by the CIE; cfluorescence resonance energy transfer (FRET) efficiency (EFRET) = 1 − (τDA/τD).
As mentioned above, the PMMA film containing 1 wt % of fluorophore 1a with a methoxy group exhibited a single PL band with a λPL at approximately 415 nm and dark blue PL with chromaticity coordinates of (0.16, 0.09). In contrast, the PMMA film containing 1 wt % of fluorophore 1c with a diphenylamino group showed green–yellow PL with a λPL at approximately 506 nm and chromaticity coordinates of (0.28, 0.60). The PL behavior of the 1 wt % PMMA film blended with blue fluorophore 1a and green–yellow fluorophore 1c in a 50:50 ratio was evaluated, and two PL bands appeared, namely major and minor PL bands with λPLs at approximately 504 and 390 nm, respectively. The fluorescent color of the PMMA film mixed in a 50:50 ratio showed light green–yellow PL with chromaticity coordinates of (0.24, 0.45), which suggests a rapid energy transfer of the excitation energy from blue fluorophore 1a to green–yellow fluorophore 1c. The absorption wavelengths and spectral shapes in the ultraviolet (UV)–visible absorption spectra of the PMMA films containing 1 wt % of 1a and 1c or 1f as representative examples (Figure S2 in Supporting Information File 1) were similar to those in the corresponding excitation spectra, which clearly indicates that the PL originates from a single fluorophore. Using a 1 wt % PMMA film blended with 1a and 1c in an 80:20 ratio (Figure S2g in Supporting Information File 1), the excitation spectrum obtained by monitoring the long PL wavelength derived from 1c was also in good agreement with the absorption spectrum of 1a in the two-component film. This result also clearly suggests an energy transfer from 1a to 1c.
The fluorescence resonance energy transfer efficiency (EFRET) [30,31] was calculated from the ratio of the fluorescence lifetimes of the two- and high-energy-component films (Equation 1):
where τDA and τD are the PL lifetimes of the two- and single-component films, respectively. An EFRET of 40% for the two-component PMMA film blended in a 50:50 ratio indicates that the excitation energy was transferred relatively efficiently from 1a to 1c.
Next, the PL behavior of a PMMA film with a higher ratio of 1a was investigated by mixing 1a:1c in a ratio of 95:5. Contrary to the above result, a single PL band with a λPL at approximately 404 nm and a shoulder peak in the long-wavelength region were observed. The τPL of the PMMA film blended in a 95:5 ratio was 2.50 ns with an EFRET of 47%, indicating that the PL band of 1c was very small because of non-radiative deactivation, in addition to the low PL component of 1c. The PL color of the PMMA film blended in a 95:5 ratio was dark blue, with color coordinates of (0.17, 0.14).
Based on these results, various mixing ratios of 1a and 1c ranging from 50:50 to 95:5 were investigated (91:9, 80:20, 70:30, and 60:40). Thus, a PMMA film mixed with 1a and 1c in a ratio of 91:9 exhibited two PL bands with λPLs at approximately 404 and 473 nm, respectively. The PL bands on the short- and long-wavelength sides were considered to be derived from the emissions of 1a and 1c, respectively. For the PL of the PMMA film blended in a 91:9 ratio, the τPL and EFRET values were 2.52 ns and 46%, respectively. Although the PMMA film blended in a 91:9 ratio was observed to have two distinct PL bands, the PL color turned blue with coordinates of (0.18, 0.20). Furthermore, when the mixing ratio of 1a to 1c was changed to 80:20, 70:30, and 60:40, the relative intensity of the long-wavelength PL band originating from the PL of 1c relative to that of 1a increased with the increasing mixing ratio of 1c. The τPL values were in the range of 1.35–2.73 ns in these blends and the EFRET values were in the range of 42–71%. These results indicate a good energy transfer for all 1a and 1c mixtures. Based on the PL spectra of the PMMA films blended in each mixing ratio, the PL colors of the 80:20 and 70:30 blends were light blue with coordinates of (0.19, 0.27) and (0.20, 0.32), respectively, and the PL color of the 60:40 blend was green–blue with coordinates of (0.24, 0.43). The PL color of the PMMA films containing the 1a/1c mixture prepared in each mixing ratio varied along a straight line connecting the color coordinates of the PMMA films containing the individual component 1a or 1c. In other words, tuning the PL color was possible by controlling the mixing ratio. In addition, when the mixing ratio of 1a to 1c was 70:30, the chromaticity coordinates were (0.20, 0.32), which are relatively close to the ideal color coordinates of (0.33, 0.33) for white. The results for the PMMA film containing a mixture of 1a and 1c indicate that the use of fluorophores with PL bands on the longer-wavelength side is more effective in approaching the ideal white color than green–yellow fluorophore 1c.
Photoluminescence behavior of PMMA dispersion films containing a mixture of blue fluorophore 1a and yellow fluorophore 1f
Based on our investigation of the PL behavior of PMMA dispersion films containing a mixture of 1a and 1c, we investigated the PL properties of PMMA dispersion films containing a mixture of blue fluorophore 1a and yellow fluorophore 1f (Figure 5). The collected photophysical data are summarized in Table 3.
![[1860-5397-20-225-5]](/bjoc/content/figures/1860-5397-20-225-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: (a) PL spectra of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and yellow fluorophore 1f in various weight ratios. (b) Photographs of their PMMA dispersion films under UV irradiation (λex = 365 nm). (c) A CIE color diagram of the PL color of PMMA dispersion films containing 1a and 1f in various weight ratios.
Figure 5: (a) PL spectra of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and yellow fluorop...
Table 3: Photophysical data of PMMA dispersion films containing 1 wt % of blue fluorophore 1a and yellow fluorophore 1f in various weight ratios.
| Ratio of 1a:1f | λPL [nm] (ΦPL)a | τPL [ns] | coordinate (x, y)b | EFRET [%]c |
| 100:0 | 415 (0.76) | 4.68 | (0.16, 0.09) | – |
| 98:2 | 407 (0.76) | 1.96 | (0.17, 0.16) | 58 |
| 97:3 | 417 (0.82) | 2.04 | (0.18, 0.17) | 56 |
| 95:5 | 401, 520 (0.94) | 2.48 | (0.26, 0.41) | 47 |
| 80:20 | 384, 530 (1.0) | 2.53 | (0.35, 0.53) | 46 |
| 50:50 | 392, 541 (1.0) | 2.12 | (0.38, 0.54) | 55 |
| 0:100 | 534 (0.66) | 3.73 | (0.37, 0.56) | – |
aMeasured using an integrating sphere; bPL color coordinates defined by the CIE; cEFRET = 1 – (τDA/τD).
Unlike the PMMA film containing green–yellow fluorophore 1c, the 1 wt % PMMA film containing 1f with a tetramethyljulolidine backbone exhibited yellow PL with a λPL at approximately 534 nm and chromaticity coordinates of (0.37, 0.56). When blue fluorophore 1a and yellow fluorophore 1f were blended in ratios of 50:50 and 80:20, the 1 wt % PMMA films containing this mixture showed a major PL band derived from 1f with a λPL at 530–541 nm, along with a minor PL band derived from 1a at 384–392 nm. The fluorescent color of the PMMA film containing this blend was yellow with chromaticity coordinates of (0.38, 0.54) for the blend in a 50:50 ratio, and green–yellow with chromaticity coordinates of (0.35, 0.53) for the blend in an 80:20 ratio. The τPL values of the PMMA films containing blends with ratios of 50:50 and 80:20 were 2.12 and 2.53 ns, respectively, and their EFRET values were 55% and 46%. Both mixtures showed a relatively fast energy transfer, which likely caused the PL of 1f to be the major component. Furthermore, the excitation spectrum obtained by monitoring the long PL wavelength originating from 1f using a 1 wt % PMMA film blended with 1a and 1f in an 80:20 ratio was also consistent with the corresponding absorption spectrum of 1a (Figure S2h in Supporting Information File 1). This result clearly indicates an energy transfer from 1a to 1f. Next, we evaluated the PL properties of the PMMA film blended in a 95:5 ratio, which had a higher weight ratio of 1a. As a result, two distinct PL bands with λPLs at approximately 401 and 520 nm appeared, which correspond to the PL derived from blue-fluorescent 1a and yellow-fluorescent 1f, respectively. The τPL was measured to be 2.48 ns, indicating an increase in the short-lived 1a component. The EFRET was also calculated to be 47%, which indicates that energy transfer occurred smoothly from 1a to 1f. The PL color of the PMMA film blended in a 95:5 ratio was pale green–yellow with chromaticity coordinates of (0.26, 0.41).
Further studies were conducted on PMMA films with increased content of 1a and blends of 1a and 1f in ratios of 97:3 and 98:2. Compared with the PL spectrum of the PMMA film blended in a 95:5 ratio, the PL intensity of the short-wavelength PL band derived from the emission of 1a increased with increasing contents of 1a. The τPL values of the PMMA films blended in ratios of 97:3 and 98:2 were 2.04 and 1.96 ns, respectively, and their EFRET values were 56% and 58%. These results indicate that a relatively efficient transfer of energy occurred from 1a to 1f. However, the PL color of the PMMA films containing the blend was light purple with chromaticity coordinates of (0.18, 0.17) for the 97:3 ratio, and blue with chromaticity coordinates of (0.17, 0.16) for the 98:2 ratio. Similar to the PL color trend in the PMMA films blended with 1a and 1c, the PL colors of the PMMA films blended with 1a and 1f were located on a line connecting them, suggesting that tuning the PL color from blue to yellow is possible by precisely adjusting the mixing ratios of 1a and 1f. When the mixing ratio of 1a to 1f was 95:5, the chromaticity coordinates were (0.26, 0.41). The PL of the PMMA films blended with the 1a/1f binary mixture was relatively close to white, although it was slightly different from the ideal white point.
Conclusion
In conclusion, we prepared PMMA dispersion films with a single component of fluorinated diphenylacetylene or a blend of two fluorinated diphenylacetylenes at a concentration of 1 wt % and investigated their PL behavior in detail. Among the fluorinated diphenylacetylene libraries with excellent solid-state luminescent properties, the PMMA dispersion film containing a methoxy-substituted fluorinated diphenylacetylene exhibited dark blue PL, the PMMA film containing a diphenylamino-substituted fluorinated diphenylacetylene exhibited green–yellow PL, and the PMMA film containing a fluorinated diphenylacetylene with a tetramethyljulolidine skeleton exhibited yellow PL. Intensive investigation of the PL behavior of these PMMA films blended as a binary mixture of blue and green–yellow or yellow fluorophores showed smooth energy transfer from the high-energy fluorescent component, that is, the methoxy-substituted fluorinated diphenylacetylene, to the yellow–green or yellow fluorophore, respectively. The PL behavior of each PMMA film blended as a binary mixture was investigated by varying the mixing ratio, and a wide range of PL colors, including dark blue, green–yellow, and yellow with chromaticity coordinates of (0.16, 0.09), (0.28, 0.60), and (0.37, 0.56), respectively, were obtained. In addition, both PMMA films containing the binary blends had color coordinates of (0.20, 0.32) and (0.26, 0.41) by precisely controlling the mixing ratio, and an approach to the white point (0.33, 0.33) of pure white emission could be observed. Further fine-tuning of the mixing ratios and PMMA films containing red–green–blue ternary mixtures holds promise for developing white-light-emitting materials with higher color purities and more diverse PL color tunings.
Experimental
Fabrication of PMMA dispersion films
PMMA films containing one of two fluorinated diphenylacetylenes were prepared using an Opticoat MS-B100 spin coater (MIKASA, Japan). The mother liquor for the thin-film deposition was prepared as follows: it was mounted on a thoroughly cleaned glass slide and clamped to the upper rotating plate of the spin coater. PMMA (100 mg) and the fluorophore (1.0 wt %) were dissolved in CHCl3 (3 mL), and a few drops of the solution were dropped onto a glass substrate. The top plate of the spin coater was rotated sequentially at 500 rpm for 5 s, 750 rpm for 5 s, and 1200 rpm for 10 s. For photophysical measurements, thin and smooth films were prepared by spin-coating followed by solvent evaporation.
Photophysical properties
The PL spectra and quantum yields were measured using a Quantaurus-QY C11347-01 absolute PL quantum yield spectrometer (Hamamatsu Photonics, Japan). The PL lifetime was measured using a Quantaurus-Tau C11367-34 fluorescence lifetime spectrometer (Hamamatsu Photonics, Japan).
Supporting Information
| Supporting Information File 1: PL spectra and PL decay profiles of PMMA films containing 1 wt % of compounds or binary mixtures. | ||
| Format: PDF | Size: 3.1 MB | Download |
Funding
This research was partially funded by the Murata Science Foundation and Shorai Foundation for Science and Technology. Research equipment from the MEXT Project was used in this study to promote the public utilization of an advanced research infrastructure, which is a program for supporting the introduction of the new sharing system (grant number: JPMXS0421800223).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Bispo-Jr, A. G.; Saraiva, L. F.; Lima, S. A. M.; Pires, A. M.; Davolos, M. R. J. Lumin. 2021, 237, 118167. doi:10.1016/j.jlumin.2021.118167
Return to citation in text: [1] -
Zhang, Q.; Wang, X.; Wang, Y. Inorg. Chem. Front. 2020, 7, 1034–1045. doi:10.1039/c9qi01428d
Return to citation in text: [1] -
Jüstel, T.; Nikol, H.; Ronda, C. Angew. Chem., Int. Ed. 1998, 37, 3084–3103. doi:10.1002/(sici)1521-3773(19981204)37:22<3084::aid-anie3084>3.0.co;2-w
Return to citation in text: [1] -
Birks, J. B. Photophysics of Aromatic Molecules; John Wiley & Sons: New York, NY, USA, 1970.
Return to citation in text: [1] -
Thomas, S. W.; Joly, G. D.; Swager, T. M. Chem. Rev. 2007, 107, 1339–1386. doi:10.1021/cr0501339
Return to citation in text: [1] -
Chen, C.-T. Chem. Mater. 2004, 16, 4389–4400. doi:10.1021/cm049679m
Return to citation in text: [1] -
Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 1740–1741. doi:10.1039/b105159h
Return to citation in text: [1] -
Xu, L. Coord. Chem. Rev. 2024, 506, 215701. doi:10.1016/j.ccr.2024.215701
Return to citation in text: [1] -
Xie, Y.; Li, Z.; Zhao, C.; Lv, R.; Li, Y.; Zhang, Z.; Teng, M.; Wan, Q. Luminescence 2024, 39, e4621. doi:10.1002/bio.4621
Return to citation in text: [1] -
Huang, G.; Du, X.; Bo, H.; Li, B. S. Mater. Chem. Front. 2024, 8, 104–132. doi:10.1039/d3qm00621b
Return to citation in text: [1] -
Yoon, S.; Teets, T. S. Chem. Commun. 2021, 57, 1975–1988. doi:10.1039/d0cc08067e
Return to citation in text: [1] -
Englman, R.; Jortner, J. Mol. Phys. 1970, 18, 145–164. doi:10.1080/00268977000100171
Return to citation in text: [1] -
Englman, R.; Jortner, J. J. Lumin. 1970, 1–2, 134–142. doi:10.1016/0022-2313(70)90029-3
Return to citation in text: [1] -
Saltiel, J.; Kumar, V. K. R. J. Phys. Chem. A 2012, 116, 10548–10558. doi:10.1021/jp307896c
Return to citation in text: [1] -
Zgierski, M. Z.; Lim, E. C. Chem. Phys. Lett. 2004, 387, 352–355. doi:10.1016/j.cplett.2004.02.029
Return to citation in text: [1] -
Ferrante, C.; Kensy, U.; Dick, B. J. Phys. Chem. 1993, 97, 13457–13463. doi:10.1021/j100153a008
Return to citation in text: [1] -
Yamada, S.; Konno, T. Chem. Rec. 2023, 23, e202300094. doi:10.1002/tcr.202300094
Return to citation in text: [1] -
Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2022, 46, 4562–4569. doi:10.1039/d1nj05539a
Return to citation in text: [1] -
Morita, M.; Yamada, S.; Konno, T. Molecules 2021, 26, 2274. doi:10.3390/molecules26082274
Return to citation in text: [1] -
Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2020, 44, 6704–6708. doi:10.1039/d0nj01268h
Return to citation in text: [1] [2] [3] -
Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. CrystEngComm 2022, 24, 936–941. doi:10.1039/d1ce01671g
Return to citation in text: [1] [2] [3] -
Yamada, S.; Kobayashi, K.; Konno, T. Molecules 2022, 27, 5782. doi:10.3390/molecules27185782
Return to citation in text: [1] [2] -
Kobayashi, K.; Yamada, S.; Sakurai, T.; Yasui, M.; Konno, T. J. Mater. Chem. C submitted.
Return to citation in text: [1] [2] -
Kumari, B.; Dahiwadkar, R.; Kanvah, S. Aggregate 2022, 3, e191. doi:10.1002/agt2.191
Return to citation in text: [1] -
Panigrahi, K.; Nag, A. J. Phys. Chem. C 2022, 126, 8553–8564. doi:10.1021/acs.jpcc.2c01679
Return to citation in text: [1] -
Chen, Z.; Ho, C.-L.; Wang, L.; Wong, W.-Y. Adv. Mater. (Weinheim, Ger.) 2020, 32, e1903269. doi:10.1002/adma.201903269
Return to citation in text: [1] -
Furukawa, S.; Shono, H.; Mutai, T.; Araki, K. ACS Appl. Mater. Interfaces 2014, 6, 16065–16070. doi:10.1021/am503956t
Return to citation in text: [1] -
Sato, T.; Pandey, R. K.; Higuchi, M. Dalton Trans. 2013, 42, 16036–16042. doi:10.1039/c3dt51354h
Return to citation in text: [1] -
Sun, M.; Li, Y.; Zhao, L.; Zhang, X.; Zhang, C.; Jin, X.; Shan, D.; Li, G. ACS Appl. Polym. Mater. 2021, 3, 2998–3008. doi:10.1021/acsapm.1c00162
Return to citation in text: [1] -
Chung, H. S.; Louis, J. M.; Gopich, I. V. J. Phys. Chem. B 2016, 120, 680–699. doi:10.1021/acs.jpcb.5b11351
Return to citation in text: [1] -
Becker, W. J. Microsc. (Oxford, U. K.) 2012, 247, 119–136. doi:10.1111/j.1365-2818.2012.03618.x
Return to citation in text: [1]
| 1. | Bispo-Jr, A. G.; Saraiva, L. F.; Lima, S. A. M.; Pires, A. M.; Davolos, M. R. J. Lumin. 2021, 237, 118167. doi:10.1016/j.jlumin.2021.118167 |
| 2. | Zhang, Q.; Wang, X.; Wang, Y. Inorg. Chem. Front. 2020, 7, 1034–1045. doi:10.1039/c9qi01428d |
| 3. | Jüstel, T.; Nikol, H.; Ronda, C. Angew. Chem., Int. Ed. 1998, 37, 3084–3103. doi:10.1002/(sici)1521-3773(19981204)37:22<3084::aid-anie3084>3.0.co;2-w |
| 11. | Yoon, S.; Teets, T. S. Chem. Commun. 2021, 57, 1975–1988. doi:10.1039/d0cc08067e |
| 12. | Englman, R.; Jortner, J. Mol. Phys. 1970, 18, 145–164. doi:10.1080/00268977000100171 |
| 13. | Englman, R.; Jortner, J. J. Lumin. 1970, 1–2, 134–142. doi:10.1016/0022-2313(70)90029-3 |
| 8. | Xu, L. Coord. Chem. Rev. 2024, 506, 215701. doi:10.1016/j.ccr.2024.215701 |
| 9. | Xie, Y.; Li, Z.; Zhao, C.; Lv, R.; Li, Y.; Zhang, Z.; Teng, M.; Wan, Q. Luminescence 2024, 39, e4621. doi:10.1002/bio.4621 |
| 10. | Huang, G.; Du, X.; Bo, H.; Li, B. S. Mater. Chem. Front. 2024, 8, 104–132. doi:10.1039/d3qm00621b |
| 7. | Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 1740–1741. doi:10.1039/b105159h |
| 20. | Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2020, 44, 6704–6708. doi:10.1039/d0nj01268h |
| 21. | Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. CrystEngComm 2022, 24, 936–941. doi:10.1039/d1ce01671g |
| 22. | Yamada, S.; Kobayashi, K.; Konno, T. Molecules 2022, 27, 5782. doi:10.3390/molecules27185782 |
| 23. | Kobayashi, K.; Yamada, S.; Sakurai, T.; Yasui, M.; Konno, T. J. Mater. Chem. C submitted. |
| 4. | Birks, J. B. Photophysics of Aromatic Molecules; John Wiley & Sons: New York, NY, USA, 1970. |
| 5. | Thomas, S. W.; Joly, G. D.; Swager, T. M. Chem. Rev. 2007, 107, 1339–1386. doi:10.1021/cr0501339 |
| 6. | Chen, C.-T. Chem. Mater. 2004, 16, 4389–4400. doi:10.1021/cm049679m |
| 30. | Chung, H. S.; Louis, J. M.; Gopich, I. V. J. Phys. Chem. B 2016, 120, 680–699. doi:10.1021/acs.jpcb.5b11351 |
| 31. | Becker, W. J. Microsc. (Oxford, U. K.) 2012, 247, 119–136. doi:10.1111/j.1365-2818.2012.03618.x |
| 23. | Kobayashi, K.; Yamada, S.; Sakurai, T.; Yasui, M.; Konno, T. J. Mater. Chem. C submitted. |
| 27. | Furukawa, S.; Shono, H.; Mutai, T.; Araki, K. ACS Appl. Mater. Interfaces 2014, 6, 16065–16070. doi:10.1021/am503956t |
| 28. | Sato, T.; Pandey, R. K.; Higuchi, M. Dalton Trans. 2013, 42, 16036–16042. doi:10.1039/c3dt51354h |
| 29. | Sun, M.; Li, Y.; Zhao, L.; Zhang, X.; Zhang, C.; Jin, X.; Shan, D.; Li, G. ACS Appl. Polym. Mater. 2021, 3, 2998–3008. doi:10.1021/acsapm.1c00162 |
| 21. | Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. CrystEngComm 2022, 24, 936–941. doi:10.1039/d1ce01671g |
| 22. | Yamada, S.; Kobayashi, K.; Konno, T. Molecules 2022, 27, 5782. doi:10.3390/molecules27185782 |
| 20. | Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2020, 44, 6704–6708. doi:10.1039/d0nj01268h |
| 21. | Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. CrystEngComm 2022, 24, 936–941. doi:10.1039/d1ce01671g |
| 17. | Yamada, S.; Konno, T. Chem. Rec. 2023, 23, e202300094. doi:10.1002/tcr.202300094 |
| 18. | Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2022, 46, 4562–4569. doi:10.1039/d1nj05539a |
| 19. | Morita, M.; Yamada, S.; Konno, T. Molecules 2021, 26, 2274. doi:10.3390/molecules26082274 |
| 20. | Morita, M.; Yamada, S.; Konno, T. New J. Chem. 2020, 44, 6704–6708. doi:10.1039/d0nj01268h |
| 14. | Saltiel, J.; Kumar, V. K. R. J. Phys. Chem. A 2012, 116, 10548–10558. doi:10.1021/jp307896c |
| 15. | Zgierski, M. Z.; Lim, E. C. Chem. Phys. Lett. 2004, 387, 352–355. doi:10.1016/j.cplett.2004.02.029 |
| 16. | Ferrante, C.; Kensy, U.; Dick, B. J. Phys. Chem. 1993, 97, 13457–13463. doi:10.1021/j100153a008 |
| 24. | Kumari, B.; Dahiwadkar, R.; Kanvah, S. Aggregate 2022, 3, e191. doi:10.1002/agt2.191 |
| 25. | Panigrahi, K.; Nag, A. J. Phys. Chem. C 2022, 126, 8553–8564. doi:10.1021/acs.jpcc.2c01679 |
| 26. | Chen, Z.; Ho, C.-L.; Wang, L.; Wong, W.-Y. Adv. Mater. (Weinheim, Ger.) 2020, 32, e1903269. doi:10.1002/adma.201903269 |
© 2024 Kobayashi et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.