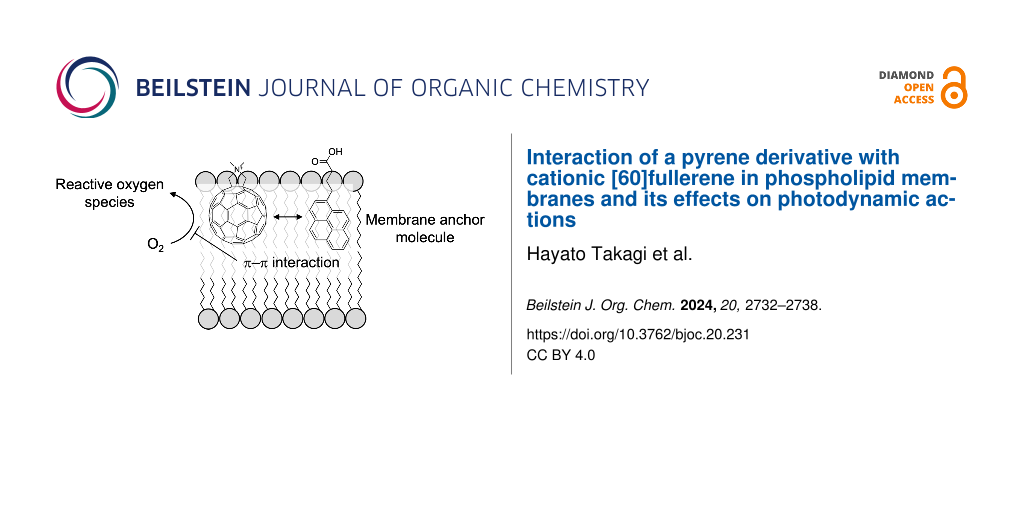
Abstract
We have reported that upon visible light irradiation, ferrocene-porphyrin-[60]fullerene triad molecules yield long-lived charge-separated states, enabling the control of the plasma membrane potential (Vm) in living cells. These previous studies indicated that the localization of the triad molecules in a specific intra-membrane orientation and the suppression of the photodynamic actions of the [60]fullerene (C60) moiety are likely important to achieve fast and safe control of Vm, respectively. In this study, by mimicking our previous system of triad molecules and living cells, we report a simplified model system with a cationic C60 derivative (catC60) and a liposome with embedded 1-pyrenebutyric acid (PyBA) to demonstrate that the addition of PyBA was important to achieve fast and safer control of Vm.

Graphical Abstract
Introduction
The [60]fullerene (C60) is known as an excellent electron acceptor [1,2] and is commonly used in organic solar cell applications [3]. Taking advantage of the fact that C60 can be an acceptor in photoinduced charge-separation systems, we have previously employed ferrocene-porphyrin-C60 triad molecules (Figure 1a) in a biological system to control the plasma membrane potential (Vm) of living mammalian neuronal cells under photoirradiation [4-6]. Generally, Vm originates from a difference in electric charge on the two sides of the plasma membrane (approximately 5 nm thickness), with a slight excess of the positive ions inside relative to the negative ions outside. Our ferrocene-porphyrin-C60 triad molecule exhibited long-lived charge-separated states under visible light irradiation [7], with the C60 species becoming negatively charged while the ferrocene moiety became positively charged (Figure 1a). This charge-separated state can be used to initiate nanoscale electric fields, e.g., Vm. The design of the triad molecules may also help to keep their orientation within the plasma membrane to have the C60 moiety located near the outer membrane surface and the ferrocene moiety near the inner membrane surface (Figure 1b). With this favorable arrangement of the molecules, it was expected to trigger a photoinduced change of the Vm that occurs at very fast time scales (less than milliseconds), leading to the (partial) cancellation of the Vm. However, in reality, the change occurred on a minute time scale, indicating that the favorable arrangement was not sufficiently achieved in the plasma membrane.
![[1860-5397-20-231-1]](/bjoc/content/figures/1860-5397-20-231-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) Chemical structure and schematic illustration of the charge-separated state of a triad molecule and (b) control of the membrane potential by the illuminated molecule with a favourable arrangement. (c) Strategy of the present study for controlling both the location and photodynamic actions of a cationic derivative of C60 (catC60), a simple model compound of the triad molecules, in a membrane via π–π interactions with 1-pyrenebutyric acid (PyBA). (d–f) Chemical structures of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) (d), catC60 (e), and PyBA (f).
Figure 1: (a) Chemical structure and schematic illustration of the charge-separated state of a triad molecule...
One of the concerns with our previous triad molecules was photoinduced generation of reactive oxygen species (ROS) [8]. In our more recent study, the reversal of Vm after stopping photoirradiation of our triad molecule was associated with the renewal of the plasma membrane through endocytosis in living cells [6]. These results suggested that the photoinduced change in Vm was caused by some modification – most likely oxidation – of the plasma membrane by the photoexcited triad molecule. Taken together, for the realization of rapid control of Vm using such C60-based molecules in the membrane, the suppression of ROS generation is an important consideration. In this study, we aim to develop a system to achieve a quick Vm control without damaging the membranes by using a C60 derivative and a pyrene derivative as a model system for the triad molecules.
C60 has been reported to be incorporated into the phospholipid bilayers at the central part of membrane due to its hydrophobicity [9,10]. In contrast, to achieve the favorable arrangement as described above, the C60 moiety of the triad molecule needs to be located near the outer membrane surface. To facilitate this arrangement, in this study, we utilized a simplified system (Figure 1c) consisting of (i) liposomes of 1,2-dimyristoyl-sn-glyreco-3-phosphocholine (DMPC, Figure 1d), a well-known model of the plasma membrane, (ii) a cationic derivative of C60 (catC60, Figure 1e) as a replacement of the triad molecules, and (iii) 1-pyrenebutyric acid (PyBA, Figure 1f) as an anchor molecule for catC60 to be localized near the surface of phospholipid membranes [11,12]. With this model system, we aimed to examine whether both the intramembrane localization and the photodynamic actions of catC60 can be modulated by PyBA.
Results and Discussion
The catC60-loaded liposomes (catC60-lip) were prepared by hydration of a catC60-embedded DMPC film [13] and compared with C60-loaded liposomes (C60-lip) by physicochemical characterizations. When catC60 or C60 was added to DMPC (in a 1:1 molar ratio to DMPC) the zeta potential of the catC60-lip was higher (16 mV) than that of C60-lip (–0.3 mV). Based on the experiments of differential scanning calorimetry analyses with varied amount of catC60 or C60 (Figure 2a), the addition of catC60 caused the disappearance of phase transition of DMPC liposomes in a dose-dependent manner and more efficiently than the case with C60. These results suggested that catC60 was more likely to localize near the surface of the lipid bilayer of catC60-lip than the C60 in C60-lip [14]. Similarly, the incorporation of PyBA into the pre-prepared liposomes was tested by zeta potential analysis (–15 mV) and differential scanning calorimetry analysis (Figure 2b), showing a clear dose dependency on the amount of PyBA added.
![[1860-5397-20-231-2]](/bjoc/content/figures/1860-5397-20-231-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Differential scanning calorimetry analysis for the phase transition of liposomes (1 mM phospholipid). (a) Effect of the addition of C60 (left) or a cationic derivative of C60 (catC60) (right) at various molar equivalents (mol equiv) to the phospholipid of liposomes. (b) Effect of 1-pyrenebutyric acid (PyBA) addition at various concentrations to liposomes without catC60 and C60. The gel-to-liquid crystalline phase transition for 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes was observed at 25 °C. All the measurements were performed with liposome samples dispersed in phosphate-buffered saline (PBS(–)).
Figure 2: Differential scanning calorimetry analysis for the phase transition of liposomes (1 mM phospholipid...
The absorption spectra of catC60-lip and C60-lip were compared in PBS(–) (Figure 3). At two different concentrations, no significant change was observed in catC60-lip, whereas broadening and a red shift were observed in C60-lip at higher concentrations (10 mol equiv). These results indicate that catC60 was better dispersed in the DMPC membrane than C60. The results also provided some insight into the situation of our previous triad molecule – how the undesired aggregate formation of the triad molecules is reduced during solubilization and cell studies in physiologically relevant media [15].
![[1860-5397-20-231-3]](/bjoc/content/figures/1860-5397-20-231-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: UV–vis absorption spectra of liposomes (1 mM phospholipid) with C60 (a) or a cationic derivative of C60 (catC60) (b) added at various molar equivalents (mol equiv) to phospholipid. The equivalent of C60 added in C60-loaded liposomes (C60-lip) was 0.1, 1 and 10 mol equiv, and the equivalent of catC60 added to catC60-loaded liposomes (catC60-lip) was 0.1 and 1 mol equiv. Spectra of 10 mol equiv of C60 in (a) and 1 mol equiv of catC60 in (b) were measured after 10-fold dilution. The absorption peak positions for C60 and catC60 are indicated with dotted lines. Liposome samples were dispersed in phosphate-buffered saline (PBS(–)).
Figure 3: UV–vis absorption spectra of liposomes (1 mM phospholipid) with C60 (a) or a cationic derivative of...
The interaction between catC60 and PyBA in the liposomes was assessed by the fluorescence spectra of PyBA in catC60-lip [16]. The catC60-lip containing catC60 at 0, 5.4, and 54 µM were mixed with PyBA (50 µM) in PBS(–), and the fluorescence spectra were measured. As shown in Figure 4a, the intensity decreased upon increasing the concentration of the catC60 in the liposomes, showing the quench of PyBA fluorescence by catC60, presumably by interacting in the liposome membrane. The incomplete quenching after the addition of PyBA at a concentration comparable to that of catC60 may be attributed to the presence of unembedded PyBA in the dispersion. To this PyBA-embedded catC60-lip system, methanol was added to completely destroy the liposome structures, resulting in the regain of the fluorescence intensity (Figure 4b). The results clearly demonstrate that PyBA interacts with catC60 in the DMPC membrane near the surface, at least to some extent, indicating the potential of PyBA acting an anchor molecule to catC60 in the liposome membrane. Nevertheless, further study is necessary to gain more insight into their location in the membrane.
![[1860-5397-20-231-4]](/bjoc/content/figures/1860-5397-20-231-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Fluorescence spectra of 1-pyrenebutyric acid (PyBA) in cationic derivative of C60 (catC60)-loaded liposomes (catC60-lip, 1 mM phospholipid) containing catC60 at various concentrations. (a) Effect of catC60 in liposomes on the fluorescence intensity of PyBA, with concentrations of catC60 at 0, 5.4, 54 µM, and PyBA at 50 µM. (b) Fluorescence spectra of catC60-lip with 0 and 54 µM catC60, treated with 50 µM PyBA, after addition of methanol. Liposome samples were dispersed in phosphate-buffered saline (PBS(–)).
Figure 4: Fluorescence spectra of 1-pyrenebutyric acid (PyBA) in cationic derivative of C60 (catC60)-loaded l...
The results above indicated the interaction of catC60 with PyBA in the DMPC liposome membrane. We anticipated some effect of PyBA on the photoinduced generation of ROS by catC60 due to such interaction within the liposome membrane. To investigate such effects, we employed an electron spin resonance (ESR) spin-trapping method to evaluate the generation of ROS by catC60 in the absence or presence of PyBA. As spin trapping reagents for the singlet oxygen (1O2), hydroxyl radical (•OH) and superoxide radical anion (O2•–); 2,2,6,6,-tetramethyl-4-piperidone (4-oxo-TEMP), 3,4-dihydro-2,3-dimethyl-2H-pyrrole 1-oxide (DMPO), and 5-(diethoxyphosphoryl)-5-methyl-1-pyrrolidone-N-oxide (DEPMPO) were respectively used (schemes in Figure 5). Our previous study demonstrated that both 1O2 and O2•– were generated under irradiation of triad molecules in DMSO/H2O [8].
![[1860-5397-20-231-5]](/bjoc/content/figures/1860-5397-20-231-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Photoinduced generation of reactive oxygen species (ROS) by cationic derivative of C60 (catC60)-loaded liposomes (catC60-lip) (5 µM catC60) in the absence and presence of 1-pyrenebutyric acid (PyBA, 50 µM). (a) X-band electron spin resonance (ESR) spectra of 2,2,6,6,-tetramethyl-4-piperidone (4-oxo-TEMP) adduct with 1O2 generated by catC60 under irradiation by a blue LED. Experimental conditions: (i) catC60 5 µM and 4-oxo-TEMP 100 μM, in phosphate-buffered saline (PBS(–)) under dark conditions. (ii) catC60 5 µM and 4-oxo-TEMP 100 μM in PBS(–) under irradiation for 30 min by blue LED lamp. (iii) catC60 5 µM, PyBA 67 µM, and 4-oxo-TEMP 100 μM, in PBS(–) under irradiation for 30 min by blue LED lamp. (b) X-band ESR spectra of 3,4-dihydro-2,3-dimethyl-2H-pyrrole 1-oxide (DMPO) adduct with •OH generated by catC60 under irradiation by a blue LED. Experimental conditions: (i) catC60 5 µM, β-nicotinamide adenine dinucleotide, reduced disodium salt hydrate (NADH) 8 mM, Fe(II)-diethylenetriaminepentaacetic acid (DETAPAC) 1 mM, and DMPO 100 mM in PBS(–) under irradiation for 30 min by blue LED lamp. (ii) catC60 5 µM, NADH 8 mM, Fe(II)-DETAPAC 1 mM, and DMPO 100 mM in PBS(–) under irradiation for 30 min by blue LED lamp. (iii) catC60 5 µM, PyBA 67 µM, NADH 8 mM, Fe(II)-DETAPAC 1 mM, and DMPO 100 mM in PBS(–) under irradiation for 30 min by blue LED lamp. (c) X-band ESR spectra of 5-(diethoxyphosphoryl)-5-methyl-1-pyrrolidone-N-oxide (DEPMPO) adduct with undefined radicals (i, ii) or •CH3 (iii, iv) generated by catC60 under irradiation by a blue LED. Experimental conditions: (i) catC60 5 µM, NADH 8 mM, DETAPAC 1 mM, and DEPMPO 100 mM in PBS(–) under irradiation for 30 min by blue LED lamp. (ii) catC60 5 µM, PyBA 67 µM, NADH 8 mM, DETAPAC 1 mM, and DEPMPO 100 mM in PBS(–) under irradiation for 30 min by blue LED lamp. (iii) catC60 5 µM, NADH 8 mM, DETAPAC 1 mM, and DEPMPO 100 mM in a 4-to-1 (v/v) mixture of PBS(–) and dimethyl sulfoxide (DMSO) under irradiation for 30 min by blue LED lamp. (iv) catC60 5 µM, PyBA 67 µM, NADH 8 mM, DETAPAC 1 mM, and DEPMPO 100 mM in a 4-to-1 (v/v) mixture of PBS(–) and DMSO under irradiation for 30 min by blue LED lamp. In the ESR spectra, signals corresponding to the adducts are indicated with red (4-oxo-TEMPO in a), blue (DMPO-OH in b), and green (DEPMPO-CH3 in c) arrows.
Figure 5: Photoinduced generation of reactive oxygen species (ROS) by cationic derivative of C60 (catC60)-loa...
Under irradiation by a blue LED (464–477 nm, 23 lm·W–1), significant ESR signals corresponding to the 1O2 adduct of 4-oxo-TEMP (4-oxo-TEMPO) were observed in the dispersion of catC60-lip ([catC60] = 5 µM) in PBS(–) showing an evidence of energy transfer reaction by the photoexcited catC60 (Figure 5a(ii)). In the presence of electron donor (NADH) under photoirradiation, •OH generation was observed as a •OH adduct of DMPO (DMPO-OH, Figure 5b(ii)) revealing that electron transfer reaction was also occurring. Using DEPMPO as a spin trapping reagent, detection of O2•– was tried and some radical adducts were detected, but without being clearly identified (Figure 5c(i), (ii)). The reason of the inability of O2•– detection is not known at present. Upon addition of dimethyl sulfoxide (DMSO) to this system, an adduct of DEPMPO and •CH3 (DEPMPO-CH3), which was presumably generated from the reaction of •OH and DMSO, was clearly observed, further confirming the generation of •OH (Figure 5b(iii)). At the same time, unusually fast conversion of O2•– to •OH was also suggested in this system.
The results above suggest that catC60-lip generated both types of ROS (1O2 and •OH) via energy transfer and electron transfer mechanisms. The present results are in line with previous studies of photoinduced ROS generation by C60 and its derivatives [17-19]. The most important: upon the addition of PyBA to catC60-lip, the signal intensities of both types of ROS (1O2 and •OH) were decreased (Figure 5a(iii), b(iii), c(iv)). These results indicate that PyBA suppresses ROS generation by catC60-lip in liposome environment, which would be advantageous for the nanoscale control of Vm by the triad molecules.
Conclusion
In summary, our findings indicate that PyBA can interact with catC60 within DMPC liposomes and modestly inhibit the photoinduced generation of ROS by catC60. These insights offer valuable guidance for the photocontrol of the plasma membrane potential (Vm) using fullerene-containing triad molecules on a millisecond scale.
Experimental
Preparation of liposomes with catC60 (catC60-lip) or C60 (C60-lip)
Liposomes were prepared using a thin-film hydration method. DMPC (NOF AMERICA Corporation, White Plains, NY, USA) was solubilized in ethanol (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and catC60, which was synthesized according to a previous report [20], or C60 (NOF AMERICA Corporation, White Plains, NY, USA) was solubilized in a 1:4 (vol:vol) mixture of DMSO (Nacalai Tesque Inc., Kyoto, Japan) and toluene (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). DMPC in ethanol and catC60 or C60 in DMSO/toluene were mixed in molar ratios of 1:0, 1:0.1, 1:1, or 1:10, and the solvent was removed using a rotary evaporator (Rotavapor R-300, BÜCHI Labortechnik AG, Switzerland) at 40 °C to prepare the lipid films. The lipid films were then dried overnight in vacuo. Then, the films were hydrated with PBS(–) (137 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4) so that the theoretical value of DMPC concentration was 3 mg/mL, and the resulting suspension was sonicated at 30 °C until the lipid membrane had completely peeled off from the flask. To remove free catC60 and C60, the resulting suspension was centrifuged at 20,000g at room temperature for 10 min. The supernatant was collected and subjected to more than 20 extrusions using a Mini-Extruder equipped with a 100 nm-pore-size membrane (Croda International Plc. Avanti Polar Lipids, Inc.).
Differential scanning calorimetry (DSC)
DSC was performed using a MicroCalTMPEAQ-DSC System (Malvern Panalytical, Ltd., Malvern, U.K.). Liposomal suspensions of DMPC with or without catC60 or C60 were dispersed in PBS(–) (1 mM DMPC). Measurements were performed following equilibration at 10 °C at a scan rate of 180 °C/h. Measurements were also performed after mixing of 50, 100 or 500 µM PyBA (Sigma-Aldrich, St. Louis, MI, USA) and DMPC liposomes without catC60 or C60 followed by dialysis of the mixture against 3 L PBS for 2 h to remove free PyBA. Data analysis, including calculation of the phase transition temperature, was performed using the MicroCal PEAQ-DSC Software.
UV–vis absorption measurement
UV–vis spectra of DMPC liposomes (1 mM DMPC) with or without catC60 or C60 were measured in PBS(–) using a UV-3600 Plus absorption spectrometer (Shimadzu Corporation, Kyoto, Japan).
Fluorescence measurement
DMPC liposomes containing 0, 5.4, or 54 µM catC60 were mixed with PyBA (final concentration of 50 µM) in PBS(–), and the mixture was dialysed against 3 L PBS for 2 h to remove free PyBA. Fluorescence spectra were measured using an RF-6000 spectrofluorometer (Shimadzu Corporation, Kyoto, Japan) (excitation at 341 nm, emission at 360–500 nm) to evaluate the interaction between catC60 and PyBA in DMPC membranes. Measurements were also performed after the addition of 10 times the volume of methanol to the liposome samples to liberate catC60 and PyBA from the membranes.
ESR measurements for photoinduced 1O2 and O2•– generation
ESR spectra were recorded on a Bruker EMX, Continuous Wave X-Band EPR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). Suprasil® ESR tubes with a diameter of 4 mm, length of 250 mm and a wall thickness of 0.8 mm were used (SP Wilmad-LabGlass, New Jersey, US). 4-Oxo-TEMP was purchased from ABCR (Karlsruhe, Germany) and purified by sublimation prior to use. The 50 µL Blaubrand® intraMark capillaries were used in the EPR measurements (Brand GMBH, Wertheim, Germany). DEPMPO was bought from Enzo Life Sciences AG (Farmingdale, NY, USA). FeSO4, DETAPAC and NADH was bought from Sigma-Aldrich (St. Louis, Missouri, USA). DMPO was bought from TCI (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan). Irradiation was performed by blue LED light (464–477 nm, 23 lm·W–1) from Lumiflex300 Pro RGB LED Stripes (LUMITRONIX LED-Technik GmbH, Hechingen, Germany) containing 120 LED lamps assembled in an aluminium cylindrical container with a diameter of 8.5 cm. ESR measurements conditions: microwave frequency 9.78 GHz, microwave power 10 mW, receiver gain 5.02 × 104, modulation amplitude 1.00 G, modulation frequency 100 kHz, 3 scan average, sweep time 83.89 s.
1O2 Generation: All measurements were performed in PBS(–). Ten µL of catC60 sample solution (25 µM), 10 µL of 4-oxo TEMP solution (500 mM) and 30 µL of PBS(–) were mixed in a 0.5 mL Eppendorf tube. For the measurement in the presence of PyBA, 10 µL of PyBA solution (335 µM) was added instead of 10 µM of PBS(–). The solution was subjected to O2 bubbling for 30 seconds and then taken into 50 µL capillary and sealed. The solution was then irradiated with blue LED light for 30 minutes. The capillary was taken into the ESR tube for measurement at room temperature.
•OH Generation: All measurements were performed in PBS(–). Ten µL of catC60 sample solution (25 µM), 10 µL of Fe(II)-DETAPAC solution (5 mM), 10 µL of DMPO solution (500 mM), 10 µL of NADH (40 mM) and 10 µL PBS(–) were mixed in a 0.5 mL Eppendorf tube. For the measurement in the presence of PyBA, 10 µL of PyBA solution (335 µM) was added instead of 10 µL of PBS(–). The solution was subjected to O2 bubbling for 30 seconds and then taken into 50 µL capillary and sealed. The solution was irradiated with blue LED light for 30 minutes. The capillary was taken into the ESR tube and ESR spectra were recorded at room temperature.
O2•– Generation: Measurements were performed in a mixture of DMSO and PBS(–) (1-to-4, v/v). Ten µL of catC60 sample solution (25 µM), 10 µL of DETAPAC solution (5 mM), 10 µL of DEPMPO solution (500 mM), 10 µL of NADH (40 mM) and 10 µL of PBS(–) were mixed in a 0.5 mL Eppendorf tube. For the measurement in the presence of PyBA, 10 µL of PyBA solution (335 µM) was added instead of 10 µL of PBS(–). The solution was subjected to O2 bubbling for 30 seconds and then taken into 50 µL capillary and sealed. The solution was irradiated with blue LED light for 30 minutes. The capillary was taken into the ESR tube and ESR spectra were recorded at room temperature.
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Sariciftci, N. S.; Smilowitz, L.; Heeger, A. J.; Wudl, F. Science 1992, 258, 1474–1476. doi:10.1126/science.258.5087.1474
Return to citation in text: [1] -
Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789–1791. doi:10.1126/science.270.5243.1789
Return to citation in text: [1] -
Imahori, H.; Kobori, Y.; Kaji, H. Acc. Mater. Res. 2021, 2, 501–514. doi:10.1021/accountsmr.1c00045
Return to citation in text: [1] -
Cai, N.; Takano, Y.; Numata, T.; Inoue, R.; Mori, Y.; Murakami, T.; Imahori, H. J. Phys. Chem. C 2017, 121, 17457–17465. doi:10.1021/acs.jpcc.7b04466
Return to citation in text: [1] -
Takano, Y.; Miyake, K.; Sobhanan, J.; Biju, V.; Tkachenko, N. V.; Imahori, H. Chem. Commun. 2020, 56, 12562–12565. doi:10.1039/d0cc05326k
Return to citation in text: [1] -
Numata, T.; Fukuda, R.; Hirano, M.; Yamaguchi, K.; Sato-Numata, K.; Imahori, H.; Murakami, T. Cell. Physiol. Biochem. 2020, 54, 899–916. doi:10.33594/000000277
Return to citation in text: [1] [2] -
Imahori, H.; Tamaki, K.; Guldi, D. M.; Luo, C.; Fujitsuka, M.; Ito, O.; Sakata, Y.; Fukuzumi, S. J. Am. Chem. Soc. 2001, 123, 2607–2617. doi:10.1021/ja003346i
Return to citation in text: [1] -
Takano, Y.; Numata, T.; Fujishima, K.; Miyake, K.; Nakao, K.; Grove, W. D.; Inoue, R.; Kengaku, M.; Sakaki, S.; Mori, Y.; Murakami, T.; Imahori, H. Chem. Sci. 2016, 7, 3331–3337. doi:10.1039/c5sc04135j
Return to citation in text: [1] [2] -
Qiao, R.; Roberts, A. P.; Mount, A. S.; Klaine, S. J.; Ke, P. C. Nano Lett. 2007, 7, 614–619. doi:10.1021/nl062515f
Return to citation in text: [1] -
Ikeda, A.; Kiguchi, K.; Shigematsu, T.; Nobusawa, K.; Kikuchi, J.-i.; Akiyama, M. Chem. Commun. 2011, 47, 12095–12097. doi:10.1039/c1cc14650e
Return to citation in text: [1] -
Jablonski, A. E.; Kawakami, T.; Ting, A. Y.; Payne, C. K. J. Phys. Chem. Lett. 2010, 1, 1312–1315. doi:10.1021/jz100248c
Return to citation in text: [1] -
Takeuchi, T.; Kosuge, M.; Tadokoro, A.; Sugiura, Y.; Nishi, M.; Kawata, M.; Sakai, N.; Matile, S.; Futaki, S. ACS Chem. Biol. 2006, 1, 299–303. doi:10.1021/cb600127m
Return to citation in text: [1] -
Bangham, A. D.; Standish, M. M.; Watkins, J. C. J. Mol. Biol. 1965, 13, 238–252. doi:10.1016/s0022-2836(65)80093-6
Return to citation in text: [1] -
Ikeda, A.; Mae, T.; Ueda, M.; Sugikawa, K.; Shigeto, H.; Funabashi, H.; Kuroda, A.; Akiyama, M. Chem. Commun. 2017, 53, 2966–2969. doi:10.1039/c7cc00302a
Return to citation in text: [1] -
Numata, T.; Murakami, T.; Kawashima, F.; Morone, N.; Heuser, J. E.; Takano, Y.; Ohkubo, K.; Fukuzumi, S.; Mori, Y.; Imahori, H. J. Am. Chem. Soc. 2012, 134, 6092–6095. doi:10.1021/ja3007275
Return to citation in text: [1] -
Sluch, M. I.; Samuel, I. D. W.; Petty, M. C. Chem. Phys. Lett. 1997, 280, 315–320. doi:10.1016/s0009-2614(97)01159-7
Return to citation in text: [1] -
Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. J. Am. Chem. Soc. 2003, 125, 12803–12809. doi:10.1021/ja0355574
Return to citation in text: [1] -
Serda, M.; Szewczyk, G.; Krzysztyńska-Kuleta, O.; Korzuch, J.; Dulski, M.; Musioł, R.; Sarna, T. ACS Biomater. Sci. Eng. 2020, 6, 5930–5940. doi:10.1021/acsbiomaterials.0c00932
Return to citation in text: [1] -
Liosi, K.; Stasyuk, A. J.; Masero, F.; Voityuk, A. A.; Nauser, T.; Mougel, V.; Solà, M.; Yamakoshi, Y. JACS Au 2021, 1, 1601–1611. doi:10.1021/jacsau.1c00239
Return to citation in text: [1] -
Guo, Q.; Ghalei, B.; Qin, D.; Mizutani, D.; Joko, I.; Al-Aziz, H.; Higashino, T.; Ito, M. M.; Imahori, H.; Sivaniah, E. Chem. Commun. 2023, 59, 10012–10015. doi:10.1039/d3cc02175k
Return to citation in text: [1]
| 1. | Sariciftci, N. S.; Smilowitz, L.; Heeger, A. J.; Wudl, F. Science 1992, 258, 1474–1476. doi:10.1126/science.258.5087.1474 |
| 2. | Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789–1791. doi:10.1126/science.270.5243.1789 |
| 8. | Takano, Y.; Numata, T.; Fujishima, K.; Miyake, K.; Nakao, K.; Grove, W. D.; Inoue, R.; Kengaku, M.; Sakaki, S.; Mori, Y.; Murakami, T.; Imahori, H. Chem. Sci. 2016, 7, 3331–3337. doi:10.1039/c5sc04135j |
| 20. | Guo, Q.; Ghalei, B.; Qin, D.; Mizutani, D.; Joko, I.; Al-Aziz, H.; Higashino, T.; Ito, M. M.; Imahori, H.; Sivaniah, E. Chem. Commun. 2023, 59, 10012–10015. doi:10.1039/d3cc02175k |
| 7. | Imahori, H.; Tamaki, K.; Guldi, D. M.; Luo, C.; Fujitsuka, M.; Ito, O.; Sakata, Y.; Fukuzumi, S. J. Am. Chem. Soc. 2001, 123, 2607–2617. doi:10.1021/ja003346i |
| 4. | Cai, N.; Takano, Y.; Numata, T.; Inoue, R.; Mori, Y.; Murakami, T.; Imahori, H. J. Phys. Chem. C 2017, 121, 17457–17465. doi:10.1021/acs.jpcc.7b04466 |
| 5. | Takano, Y.; Miyake, K.; Sobhanan, J.; Biju, V.; Tkachenko, N. V.; Imahori, H. Chem. Commun. 2020, 56, 12562–12565. doi:10.1039/d0cc05326k |
| 6. | Numata, T.; Fukuda, R.; Hirano, M.; Yamaguchi, K.; Sato-Numata, K.; Imahori, H.; Murakami, T. Cell. Physiol. Biochem. 2020, 54, 899–916. doi:10.33594/000000277 |
| 8. | Takano, Y.; Numata, T.; Fujishima, K.; Miyake, K.; Nakao, K.; Grove, W. D.; Inoue, R.; Kengaku, M.; Sakaki, S.; Mori, Y.; Murakami, T.; Imahori, H. Chem. Sci. 2016, 7, 3331–3337. doi:10.1039/c5sc04135j |
| 3. | Imahori, H.; Kobori, Y.; Kaji, H. Acc. Mater. Res. 2021, 2, 501–514. doi:10.1021/accountsmr.1c00045 |
| 17. | Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. J. Am. Chem. Soc. 2003, 125, 12803–12809. doi:10.1021/ja0355574 |
| 18. | Serda, M.; Szewczyk, G.; Krzysztyńska-Kuleta, O.; Korzuch, J.; Dulski, M.; Musioł, R.; Sarna, T. ACS Biomater. Sci. Eng. 2020, 6, 5930–5940. doi:10.1021/acsbiomaterials.0c00932 |
| 19. | Liosi, K.; Stasyuk, A. J.; Masero, F.; Voityuk, A. A.; Nauser, T.; Mougel, V.; Solà, M.; Yamakoshi, Y. JACS Au 2021, 1, 1601–1611. doi:10.1021/jacsau.1c00239 |
| 13. | Bangham, A. D.; Standish, M. M.; Watkins, J. C. J. Mol. Biol. 1965, 13, 238–252. doi:10.1016/s0022-2836(65)80093-6 |
| 15. | Numata, T.; Murakami, T.; Kawashima, F.; Morone, N.; Heuser, J. E.; Takano, Y.; Ohkubo, K.; Fukuzumi, S.; Mori, Y.; Imahori, H. J. Am. Chem. Soc. 2012, 134, 6092–6095. doi:10.1021/ja3007275 |
| 11. | Jablonski, A. E.; Kawakami, T.; Ting, A. Y.; Payne, C. K. J. Phys. Chem. Lett. 2010, 1, 1312–1315. doi:10.1021/jz100248c |
| 12. | Takeuchi, T.; Kosuge, M.; Tadokoro, A.; Sugiura, Y.; Nishi, M.; Kawata, M.; Sakai, N.; Matile, S.; Futaki, S. ACS Chem. Biol. 2006, 1, 299–303. doi:10.1021/cb600127m |
| 16. | Sluch, M. I.; Samuel, I. D. W.; Petty, M. C. Chem. Phys. Lett. 1997, 280, 315–320. doi:10.1016/s0009-2614(97)01159-7 |
| 9. | Qiao, R.; Roberts, A. P.; Mount, A. S.; Klaine, S. J.; Ke, P. C. Nano Lett. 2007, 7, 614–619. doi:10.1021/nl062515f |
| 10. | Ikeda, A.; Kiguchi, K.; Shigematsu, T.; Nobusawa, K.; Kikuchi, J.-i.; Akiyama, M. Chem. Commun. 2011, 47, 12095–12097. doi:10.1039/c1cc14650e |
| 6. | Numata, T.; Fukuda, R.; Hirano, M.; Yamaguchi, K.; Sato-Numata, K.; Imahori, H.; Murakami, T. Cell. Physiol. Biochem. 2020, 54, 899–916. doi:10.33594/000000277 |
| 14. | Ikeda, A.; Mae, T.; Ueda, M.; Sugikawa, K.; Shigeto, H.; Funabashi, H.; Kuroda, A.; Akiyama, M. Chem. Commun. 2017, 53, 2966–2969. doi:10.1039/c7cc00302a |
© 2024 Takagi et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.