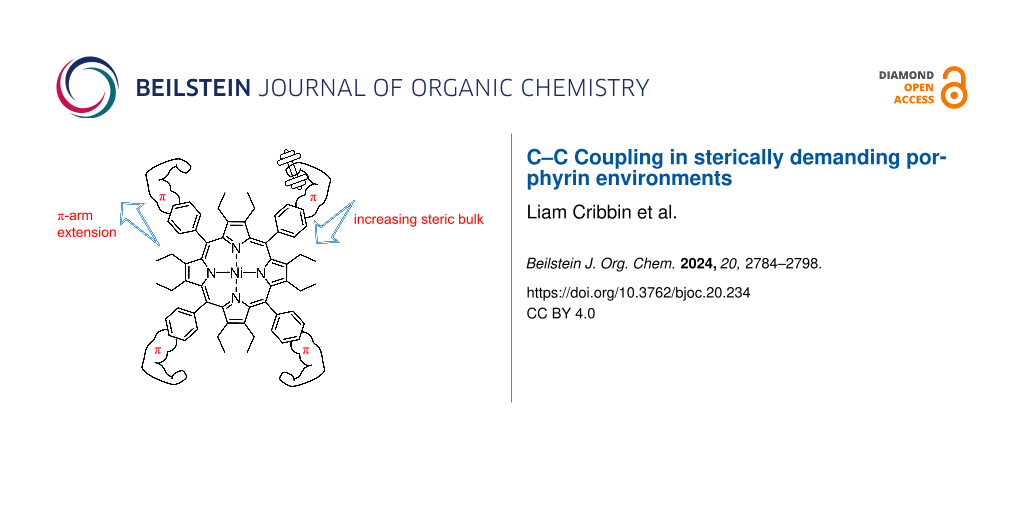
Abstract
Unlike their planar counterparts, classic synthetic protocols for C–C bond forming reactions on nonplanar porphyrins are underdeveloped. The development of C–C bond forming reactions on nonplanar porphyrins is critical in advancing this field of study for more complex porphyrin architectures, which could be used in supramolecular assemblies, catalysis, or sensing. In this work a library of arm-extended dodecasubstituted porphyrins was synthesized through the optimization of the classic Suzuki–Miyaura coupling of peripheral haloaryl substituents with a range of boronic acids. We report on palladium-catalyzed coupling attempts on the ortho-, meta-, and para-meso-phenyl position of sterically demanding dodecasubstituted saddle-shaped porphyrins. While para- and meta-substitutions could be achieved, ortho-functionalization in these systems remains elusive. Furthermore, borylation of a dodecasubstituted porphyrin’s meso-phenyl position was explored and a subsequent C–C coupling showed the polarity of the reaction can be reversed resulting in higher yields. X-ray analysis of the target compounds revealed the formation of supramolecular assemblies, capable of accommodating substrates in their void.
Graphical Abstract
Introduction
Porphyrins are tetrapyrrolic macrocycles that perform essential processes in nature, such as oxygen transport in hemoglobin and photosynthesis [1]. Porphyrins are often described as planar 18π aromatic macrocycles; however, molecular structure analysis frequently reveals nonplanar ring distortion [2,3]. In fact, porphyrins with nonplanar ring distortions are vital for many natural processes to occur, e.g., nonplanarity can alter oxygen affinity of the metal iron core [4,5]. Nonplanar porphyrins offer a marked difference in chemical and physical properties when compared to their planar compatriots [6], with relatively smaller HOMO–LUMO gaps resulting in an observed bathochromic shift in the UV–vis absorption spectrum [7]. The phenomenon of nonplanarity results from the porphyrin ring deforming from the mean porphyrin plane either by steric repulsion in the core of the macrocycle or by bulky substituents at the porphyrin periphery [3]. This affords four principle distortion modes, saddle, dome, ruffle or wave [8], which can be quantified by the normal-coordinate structural decomposition (NSD) method developed by Shelnutt and co-workers [9] and further implemented and visualized by us [8,10]. Of the four main quantifiable distortion modes, saddle-shaped porphyrins can be afforded by peri-interactions between β-substituents and the meso-substituents [3,11], or alternatively by core protonation, whereby all four-core nitrogen atoms are protonated to produce the diacid [12,13]; these diacids can tilt the pyrrole rings 20–40° [14] from the mean-porphyrin plane. Norvaiša et al. showed that a saddle-shaped porphyrin as a dodecasubstituted diacid can bind anions via two independent faces and trap anions such as pyrophosphate [15]. Saddle-shaped porphyrins have also been exploited by researchers for the use in organocatalysis as bifunctional system [16,17]. Dodecasubstitution of porphyrin, as seen in Figure 1, often results in saddle-shaped distortion; however, ruffled [18] and almost planar [19] dodecasubstituted porphyrins have been reported.
Figure 1: (A) Structures of tetrasubstituted 5,10,15,20-tetraphenylporphyrin (TPP, 1), dodecasubstituted 2,3,7,8,12,13,17,18-octaethyl-5,10,15,20-tetraphenylporphyrin (OETPP, 2), and octasubstituted 2,3,7,8,12,13,17,18-octaethylporphyrin (OEP, 3). (B) Aim of this work, the arm extension of the meso-phenyl position of dodecasubstituted porphyrins.
Figure 1: (A) Structures of tetrasubstituted 5,10,15,20-tetraphenylporphyrin (TPP, 1), dodecasubstituted 2,3,...
Despite the increasing interest in the chemical and physical properties of nonplanar porphyrins only limited synthetic methods are available for the functionalization of these macrocycles [6]. An attractive approach to accomplish further substitution directly on the meso- or a meso-phenyl ortho/meta/para positions of a porphyrin, is the introduction of C–C bond forming chemistry. This is typically achieved using palladium and/or another transition-metal catalyst [20]. Sonagashira [21], Suzuki–Miyaura [22], Heck [23], Stille [24,25], Negishi [26], and Kumada [27] coupling reactions, as well as modern iridium and rhodium-based coupling techniques [28], are just some examples of the C–C bond formations that have been implemented to achieve complex substitution patterns and functional arrangements on porphyrins.
Of these named coupling reactions, Suzuki–Miyaura couplings are known to be a robust tool when functionalizing porphyrins [29,30]. Many complex porphyrinoid architectures have been synthesized in this manner, from functional porphyrin arrays [31-33] to sterically challenging meso-substituted aryl bis-pocket porphyrins [34] and tetrabromoanthracenyl porphyrins [35]. In general, the halogen atom needed for the Suzuki coupling reaction resides on the porphyrin; however, Suzuki–Miyaura reactivity has also been shown to be reversed whereby the synthesis of borolanylporphyrins leads to a different approach to reactivity [36]. Borolanylporphyrins can be synthesized by Miyaura-borylation of the halogenated porphyrin [24,37]. There are also reported instances of borolanylporphyrins being synthesized under condensation conditions [36,38]. Despite the many synthetic advancements for the decoration of porphyrins, many of these strategies are utilized only with planar porphyrins. Apart from the arylation of the β-position of 2,3,5,7,8,10,12,13,15,17,18,20-dodecaarylporphyrins, developed by Smith and co-workers [39] few reports on synthetic techniques for dodecasubstituted nonplanar porphyrins can be found in literature. In light of the promise of appropriately designed nonplanar porphyrins as receptors and catalysts we report here on our efforts to use the Suzuki–Miyaura reaction for the modification of the o,m,p-phenyl positions in 5,10,15,20-tetraryl-2,3,7,8,12,13,17,18-octaethylporphyrins.
Results and Discussion
Investigation of the Suzuki coupling reaction at the meso-phenyl position of dodecasubstituted porphyrins
Synthesis of porphyrin precursors
To investigate the Suzuki coupling at the ortho-, meta- and para-position of a dodecasubstituted saddle-shaped porphyrin, first the precursor porphyrins 11, 12, and 13 had to be synthesized (Scheme 1). The synthetic route to achieve OET-xBrPPs (2,3,7,8,12,13,17,18-octaethyl-5,10,15,20-tetra(x-bromo)phenylporphyrin, where x = ortho/meta/para) pyrrole 7 was synthesized through literature procedures [40,41]. Pyrrole 7 was then subjected to condensation with aldehydes 8, 9, and 10 under Lindsey conditions [42] utilizing BF3·OEt2 and DDQ [43] to achieve porphyrins 4, 5, and 6, which were not isolated and instead reacted immediately.
Scheme 1: Reaction scheme for the synthesis of OET-xBrPPs and subsequent Ni(II) metalation.
Scheme 1: Reaction scheme for the synthesis of OET-xBrPPs and subsequent Ni(II) metalation.
Ni(II)porphyrins 11, 12, and 13 were prepared by reacting porphyrins 4, 5, and 6 in toluene for 18 hours using Ni(II)(acac)2 under an inert atmosphere [43] attaining a 18%, 28%, and 29% yield for porphyrins 11, 12, and 13, respectively, over two steps. Porphyrins 6 and 13 had previously been described in literature [43].
Coupling at the meso-para-phenyl position
The exploration of aryl substitution of OET-xBrPPs using the Suzuki coupling began with investigating first the Suzuki reaction compatibility of boronic acid 14 with porphyrin 13. Porphyrin 13 and phenylboronic acid (14) were subjected to coupling at 85 °C for 48 hours using Pd2dba3/SPhos as a catalyst/ligand giving porphyrin 26 in a 32% yield, based on a literature procedure [35]. With initial success in the synthesis porphyrin 26, this Suzuki coupling reaction was performed on 13, for a range of boronic acids/esters as shown in Figure 2 and Scheme 2. Boronic acids/esters were chosen based on their electronic properties (activating/deactivating) as well as their steric bulk (e.g., 9-anthracenylboronic acid (15)). Table 1 lists all attempts at the meso-para-phenyl position.
Figure 2: Substrates used for the investigations for the Suzuki–Miyaura coupling reactions.
Figure 2: Substrates used for the investigations for the Suzuki–Miyaura coupling reactions.
Scheme 2: Scope of arm-extended dodecasubstituted porphyrins synthesized via modification of the meso-para-phenyl position of porphyrin 13.
Scheme 2: Scope of arm-extended dodecasubstituted porphyrins synthesized via modification of the meso-para-ph...
Table 1: Optimization table for the Suzuki–Miyaura coupling reactions with porphyrin 13.
| Entry |
Catalyst/ligand
SPhos (1 equiv) |
Cat. mol % per C–Br |
Base
(24 equiv) |
Temperature | Time |
Boronic acid/ester
(3 equiv per C–Br) |
Yield %
(porphyrin) |
| 1 | Pd2dba3/Sphos | 6.25% | K3PO4 | 85 °C | 48 h | 14 | 32% (26) |
| 2 | Pd2dba3/Sphos | 6.25% | K3PO4 | 85 °C | 48 h | 15 | trace |
| 3 | Pd2dba3/SPhos | 6.25% | K3PO4 | 110 °C | 48 h | 15 | 39% (27) |
| 4 | Pd2dba3/Sphos | 6.25% | K3PO4 | 85 °C | 48 h | 16a | 0 |
| 5 | Pd2dba3/Sphos | 6.25% | Cs2CO3 | 85 °C | 48 h | 16a | 0 |
| 6 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 16b | 8% (30) |
| 7 | Pd2dba3/SPhos | 6.25% | K3PO4 | 110 °C | 48 h | 17 | 48% (28) |
| 8 | Pd2dba3/SPhos | 12.5% | K3PO4 | 110 °C | 48 h | 18a | 0 |
| 9 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 18b | 72% (29) |
| 10 | Pd2dba3/Sphos | 6.25% | K3PO4 | 85 °C | 48 h | 19 | 0 |
| 11 | Pd2dba3/SPhos | 6.25% | K3PO4 | 110 °C | 48 h | 19a | trace |
| 12 | Pd2dba3/SPhos | 12.5% | K3PO4 | 110 °C | 24 h | 20 | 25% (34) |
| 13 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 110 °C | 24 h | 21 | 56% (33) |
| 14 | Pd2dba3/SPhos | 6.25% | K3PO4 | 110 °C | 48 h | 23 | trace |
| 15 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 23 | trace |
| 16 | Pd(PPh3)4 | 10% | Na2CO3 | 100 °C | 1 h | 23b | 0 |
| 17 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 24 h | 23 | trace |
| 18 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 110 °C | 24 h | 23 | trace |
| 19 | Pd2dba3/SPhos | 25% | Cs2CO3 | 110 °C | 24 h | 23 | 16% (31) |
| 20 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 110 °C | 24 h | 24 | 58% (35) |
| 21 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 24 h | 25 | 47% (32) |
a5 equiv of boronic acid used in this reaction per C–Br. bMicrowave irradiation instead of conventional heating was used.
When attempting the synthesis of tetra(p-phenylanthracene)porphyrin (27) the conditions used before (Table 1, entry 1) resulted only in trace amounts of porphyrin 27 (Table 1, entry 2).
The reaction temperature was increased to 110 °C, affording the desired porphyrin 27 in a 39% yield (Table 1, entry 3). A temperature of 110 °C was also used for the synthesis of terphenylporphyrin 28 using boronic acid 17, affording terphenylporphyrin 28 in 48% yield (Table 1, entry 7).
Boronic acids with heteroatoms and activating/deactivating electronic properties were investigated next. Attempts to introduce electron-withdrawing groups at the para-position with substrate boronic acid 16a (Table 1, entries 4 and 5) yielded no tetracoupled product. Similarly, coupling with 18a resulted in most of the starting material porphyrin 13 being left unreacted. On switching the substrate from boronic acid to the boronic acid ester and opting for the weaker base Cs2CO3 instead of K3PO4, a significant difference in reactivity was observed with a 72% yield accomplished in the synthesis of porphyrin 29 (Table 1, entry 9), bearing a methoxycarbonyl electron-withdrawing group utilizing boronic acid pinacol ester 18b. Following on from these results porphyrin 30 was synthesized in an 8% yield, when switching to weaker base Cs2CO3 using pinacol ester 16b (Table 1, entry 6). Switching the base to a weaker one, may have slowed down the protodeboronation process, as substrates with electron-withdrawing groups are postulated to increase the Lewis acidity of the boronic acid, which may allow an increased incidence of protodeboronation to occur. It is also known that aryl–B(Pin) complexes have a greater stability than boronic acids and other employed esters as the four methyl groups protect the boron center from attack of water [44,45], preventing protodeboronation from the hydrolysis route. However, protodeboronation can be complex when it comes to pKa considerations, for example, 3,5-dinitrophenylboronic acid has a marginally lower pKa than pentafluorophenyl boronic acid [46]; however, it undergoes protodeboronation, several orders of magnitude slower [47].
The synthesis of porphyrin 31 with a benzothiophene moiety, proved challenging (Table 1, entries 14–19). Use of a microwave-assisted procedure [48], switching catalyst to Pd(PPh)3, and base to Na2CO3 (Table 1, entry 16) gave no product.
Ultimately, an increased catalyst loading of 25 mol % per C–Br bond gave the desired porphyrin in a 16% yield when using Cs2CO3 as base. The synthesis of other heterocycle-appended dodecasubstituted porphyrins, achieved porphyrins 32 and 33 in a 47% and 56% yield, respectively (Table 1, entries 13 and 21), using Cs2CO3 as the base. Electron-withdrawing sulfur-containing heterocyclic substrates 21 and 23 do not readily undergo protodeboronation even at high pH [44,47] making the yields of porphyrins 31 and 33 higher than expected considering the electronic similarities between substrates 4-nitrophenylboronic acid and 3-thiaphenylboronic acid (16a and 21) and the yields obtained when coupling. The weakly electron-withdrawing boronic acid 24 when coupled with porphyrin 13, resulted in porphyrin 35 in a 58% yield (Table 1, entry 20). Reactivity with the electron-donating 4-methylphenylboronic acid (34) was established using K3PO4 at 110 °C (Table 1, entry 12). No product was obtained in the coupling of electron-donating (4-(dimethylamino)phenyl)boronic acid (19), even upon increasing the number of equivalents of boronic acid (Table 1, entries 10 and 11).
Coupling at the meso-meta-phenyl position
Optimization of conditions for OET-meta-BrPPs 12 (Scheme 3) were investigated next. Table 2 summarizes the reaction conditions used to synthesize a library of OET-meta-arylPPs as shown in Scheme 3. As a starting point initial conditions used in the synthesis for porphyrin 26 were used (Table 1, entry 1). This gave biphenylporphyrin 36 in a 16% yield (Table 2, entry 1). The lower yield is expected due to the increased steric demand at the meta-positions. Coupling of sterically bulky 9-anthracenylboronic acid (15) and porphyrin 12 gave no conversion when the base was switched from K3PO4 to Cs2CO3 (Table 2, entry 2). K3PO4 was reimplemented in the reaction and trace reactivity was observed by TLC (Table 2, entry 3). Next, the catalyst loading was increased to 12.5 mol % (Table 2, entry 4). Formation of palladium black was observed but product formation was also indicated by TLC and 1H NMR. For a final attempt at establishing reactivity with boronic acid 15 the temperature was increased to 110 °C and gave the desired anthracenylporphyrin 37 in a 32% yield. In the case of boronic acids with larger π-systems, e.g., 15, K3PO4 was required to achieve the tetra-coupled product. This trend is consistent in reactivity observed with porphyrins 12 and 13. Similarly, no terphenyl product was formed in the coupling reaction between 12 and 17 (Table 2, entry 6) when using Cs2CO3. Similar to the reactivity observed with 9-anthracenylboronic acid (15), no conversion to the desired product was established. Increasing the temperature and catalyst loading (Table 2, entry 5) gave the terphenylporphyrin 38 in a 7% yield (Table 2, entry 7).
Scheme 3: Scope of arm-extended dodecasubstituted porphyrins synthesized via reaction at the meso-meta-phenyl position of porphyrin 12.
Scheme 3: Scope of arm-extended dodecasubstituted porphyrins synthesized via reaction at the meso-meta-phenyl...
Table 2: Optimization table for the Suzuki-coupling reaction on porphyrin 12.
| Entry |
Catalyst/ligand
SPhos (1 equiv) |
Cat. mol % per C–Br |
Base
(24 equiv) |
Temp. | Time |
Boronic acid/ester
(3 equiv per C–Br) |
Yield % (porphyrin) |
| 1 | Pd2dba3/SPhos | 6.25% | K3PO4 | 85 °C | 48 h | 14 | 16% (36) |
| 2 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 15 | 0 |
| 3 | Pd2dba3/SPhos | 6.25% | K3PO4 | 85 °C | 48 h | 15 | trace |
| 4 | Pd2dba3/SPhos | 12.5% | K3PO4 | 85 °C | 48 h | 15 | trace |
| 5 | Pd2dba3/SPhos | 12.5% | K3PO4 | 110 °C | 48 h | 15 | 32% (37) |
| 6 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 17 | 0 |
| 7 | Pd2dba3/SPhos | 12.5% | K3PO4 | 110 °C | 24 h | 17 | 7% (38) |
| 8 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 18b | 0 |
| 9 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 85 °C | 48 h | 18b | 23% (39) |
| 10 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 85 °C | 24 h | 16b | 4% (40) |
| 11 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 48 h | 20 | 0 |
| 12 | Pd2dba3/SPhos | 12.5% | K3PO4 | 110 °C | 24 h | 20 | 30% (44) |
| 13 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 110 °C | 24 h | 21 | 10% (43) |
| 14 | Pd2dba3/SPhos | 25% | Cs2CO3 | 110 °C | 24 h | 23 | 4% (41) |
| 15 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 110 °C | 24 h | 24 | 31% (45) |
| 16 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 110 °C | 24 h | 25 | 30% (42) |
| 17 | Pd2dba3/SPhos | 12.5% | NaOAc | 110 °C | 24 h | 16a | 18% (40) |
The use of Cs2CO3 is still required for boronic acids bearing electron-withdrawing functionalities at the meta-phenyl position (Table 2, entries 9 and 10). However, an increase in catalyst loading to 12.5% was required per C–Br bond when coupling at the meso-meta-phenyl position in 12 compared to the corresponding para- position in 13 (Table 2, entry 8). Porphyrins 39 and initially 40 were synthesized in a 23% and 4% yield, respectively. This is significantly lower than for the corresponding para-products. Porphyrin 45 was synthesized in a 31% yield (Table 2, entry 15), with a 3-methoxy electron-withdrawing group, again a lower yield compared to the para-analogue porphyrin 35. Use of the electron-donating p-tolylboronic acid (20), resulted in a 30% yield (Table 2, entry 12) again requiring an increase of catalyst loading and a change of base to K3PO4. Heterocyclic boronic acids/esters were again investigated for coupling reactivity with a consistent trend of lower yields experienced for porphyrins 41, 42, and 43 of 4%, 30%, and 10%, respectively. Overall, a general trend of decreased yield moving from the para- to meta-position was observed, also a general increased catalyst concentration was needed for reactivity to occur at the meta-position.
Lastly, decreasing the basicity of the solution further by switching to sodium acetate as a basic source increased the yield of porphyrin 40 from 4% with Cs2CO3 to 18% using sodium acetate (Table 2, entry 17). This indicates that a further decrease in basicity may improve yields.
Coupling at the meso-ortho-phenyl position
Unlike the success achieved in the synthesis of OET-meta/para-arylPPs, the ortho-position on the meso-phenyl proved much more intractable (Scheme 4). Table 3 provides a summary of the attempts made to achieve Suzuki-coupling reactivity in OET-ortho-BrPPs.
Scheme 4: Attempts of arm-extension of dodecasubstituted porphyrins at the meso-ortho-phenyl position.
Scheme 4: Attempts of arm-extension of dodecasubstituted porphyrins at the meso-ortho-phenyl position.
Table 3: Optimization table for the Suzuki-coupling reaction on porphyrin 11.
| Entry | Catalyst/ligand | Cat. mol % per C–Br |
Base
(24 equiv) |
Temp.
(ºC) |
Time |
Boronic acid/ester
(3 equiv per C–Br) |
Product detected by HRMS |
| 1 | Pd2dba3/SPhos | 6.25% | K3PO4 | 110 °C | 48 h | 14 | no |
| 2 | Pd2dba3/SPhos | 6.25% | K3PO4 | 130 ºC | 48 h | 14 | no |
| 3 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 110 °C | 48 h | 14 | no |
| 4 | Pd2dba3/Xantphos | 12.5% | Cs2CO3 | 110 °C | 48 h | 14 | no |
| 5 | Pd2dba3/Rac BINAP | 12.5% | Cs2CO3 | 110 °C | 48 h | 14 | no |
| 6 | Pd2dba3/SPhos | 100% | Cs2CO3 | 85 °C | 7 days | 14a | no |
| 7 | Pd(dppf)Cl2 | 2% | Cs2CO3 | 100 °C | 20 h | 20b | no |
| 8 | Pd(PPh3)4 | 5% | Cs2CO3 | 100 °C | 20 h | 20b | no |
| 9 | Pd(PPh3)4 | 12.5% | Na2CO3 | 120 °C | 2 h | 21c | no |
| 10 | Pd(PPh3)4 | 12.5% | Cs2CO3 | 100 °C | 48 h | 21 | no |
| 11 | Pd2dba3/SPhos | 12.5% | Cs2CO3 | 85 °C | 48 h | 22 | no |
| 12 | Pd2dba3/SPhos | 6.25% | Cs2CO3 | 85 °C | 24 h | 25 | no |
a12 equiv of boronic acid used in this reaction per C–Br. bAlternative procedure for Suzuki–Miyaura coupling [34]. cMicrowave irradiation instead of conventional heating was used [48].
Increasing the reaction temperature compared to the 85 °C used in the synthesis of the para-equivalent 26 gave no conversion and was accompanied by the formation of palladium black [49]. No reactivity was observed by either TLC or mass spectrometry when switching back to Pd2(dba)3 with three different ligands SPhos, XantPhos, and Rac-Binap (Table 3, entries 3–5). Increasing the time of reaction, catalyst loading, and equivalents of boronic acid significantly also resulted in no product formation (Table 3, entry 6).
Next, a change in the catalyst was investigated, based on a literature procedure which was developed by Johnstone and co-workers for the synthesis of meso-substituted aryl bis-pocket porphyrins [34]. Therein catalysts Pd(dppf)Cl2 and Pd(PPh3)3 were identified to be the most effective for accomplishing the Suzuki–Miyaura coupling at the ortho-position of the meso-phenyl position in sterically hindered planar porphyrins (Table 3, entries 7 and 8). The same success could not be replicated for OET-o-BrPPs with no reactivity being observed by TLC or by mass spectrometry. Likewise, a microwave-assisted coupling [48], resulted in no product formation (Table 3, entry 9). Thiophene-3-ylboronic acid (21) was also chosen for this reaction due to the smaller size compared to the phenyl- and p-tolylboronic acids 14 and 20. Using 21 as the starting material and the procedure by Droege et al. [34] it was anticipated the less steric substrate size would possibly allow conversion to occur; however, no product formation was observed (Table 3, entries 9 and 10). 4-Pyridylboronic acid pinacol ester (25) was also attempted; however, no product was formed. Vinylboronic acid ester 22, was also explored as a substrate, with multiple porphyrin products being observed by TLC and by 1H NMR. Desymmetrization of the porphyrin was also observed with the β-ethyl CH3 resonances splitting into two separate chemical environments; however, the identity of the product synthesized was not fully characterized. In future, if reactivity for OET-o-BrPPs were to be further explored a larger library of ligands whether biphenyl-based or other could be explored, or further changes in the pH of the solution. Enrichment to the αβαβ-atropisomer may also be favorable [50], as to alleviate the steric hindrance caused by the short distances (4.3–4.4 Å) between bromines in the α2β2-atropisomers (cf., Figure 5).
Borylation and further coupling of dodecasubstituted porphyrins
A Miyaura borylation was performed on porphyrin 13, using bis(pinacolato)diboron (B2Pin2), adapting a procedure from the literature [51]. This procedure was further optimized (Table 4) by utilizing conditions in the synthesis of 29 (Table 1, entry 9). Next, a reversed polarity Suzuki reaction was performed on the borolanyl porphyrin 46 (Scheme 5). This reaction yielded porphyrin 26 in a 53% yield and tetrapyrenylporphyrin 47 in a 36% yield, respectively. Compared to the synthesis of 26 by Suzuki coupling of para-bromo-phenylporphyrin 13 a significant increase in yield was observed. Furthermore, pyrene was installed on the para-phenyl position, showing large aromatic systems can also be installed through this route. Failure of the similar anthracenylboronic acid 15 to react in the presence of Cs2CO3 at 85 °C (Table 2, entry 2) shows reversing the polarity of the reaction can induce reactivity, where not previously possible.
Table 4: Optimization of the borylation of porphyrin 13 to yield 46.
| Entry | Catalyst mol % per C–Br bond | Catalyst | Equiv of B2Pin2 per C–Br bond | Temp. | Time | Product |
| 1 | 20% | Pd(dppf)Cl2 | 1 | 80 °C | 48 h | 0 |
| 2 | 20% | Pd(dppf)Cl2 | 10 | 80 °C | 48 h | 0 |
| 3 | 20% | Pd(dppf)Cl2 | 10 | 80 °C | 48 h | trace |
| 4 | 40% | Pd(dppf)Cl2 | 20 | 100 °C | 48 h | 30% |
Scheme 5: Borylation and subsequent Suzuki–Miyaura coupling of porphyrin 13.
Scheme 5: Borylation and subsequent Suzuki–Miyaura coupling of porphyrin 13.
X-ray crystal structure analysis
Despite the many examples in literature of the crystal structure and packing of nonplanar porphyrins [3,6,8,13,52,53], few examples of crystal structure and packing analysis exist for arm-extended porphyrins. One of the few examples are azide-porphyrin derivatives reported by Flanagan et al. [43]. Here, five crystal structures were obtained of meso-para-phenyl arm-extended porphyrins (26, 27, 28, 29, 33) and two crystal structures for meso-meta-phenyl derivatives 36 and 37. In addition, single crystal structures of 11 and 46 were determined. All structures were investigated using the NSD method [8,9], which allows a quantification and visualization of distortion modes.
It can be observed from the crystal structures that the porphyrins’ rings all exhibit the typical saddle-shaped conformation. Interestingly, substitution at the para- or meta-position can also induce partial ruffling of the porphyrin core (Table 5). Of all para-functionalized structures, porphyrin 33 bears the most similarity to that of compound 26, with minimal ruffling observed and the overall magnitudes of out-of-plane and in-plane distortions are comparable.
Table 5: Mean geometrical parameters of OET-meta/para-ArylPP and out-of-plane and in-plane distortion magnitudes.
| Compound | 26 | 27 | 28 | 29 | 33 | 36 | 37 | Units |
| pyrrole tilt | 28.0 | 28.4 | 31.2 | 32.9 | 29.0 | 29.0 | 31.1 | º |
|
N–N dist.
(adj) |
2.70 | 2.71 | 2.71 | 2.72 | 2.73 | 2.71 | 2.71 | Å |
|
N–N dist.
(opp) |
3.77 | 3.80 | 3.80 | 3.81 | 3.83 | 3.78 | 3.79 | Å |
| ΔCmesoa | 0.03 | 0.32 | 0.13 | 0.15 | 0.03 | 0.22 | 0.22 | Å |
| ΔCalphab | 0.5 | 0.47 | 0.51 | 0.53 | 0.5 | 0.5 | 0.5 | Å |
| ΔCbetac | 1.21 | 1.13 | 1.28 | 1.32 | 1.21 | 1.21 | 1.26 | Å |
| Δipd | 1.06 | 1.03 | 1.22 | 1.28 | 1.09 | 1.14 | 1.24 | Å |
| B1g | 0.07 | 0.05 | 0.02 | 0.00 | 0.06 | 0.12 | 0.04 | Å |
| Eu(x) | 0.08 | 0.00 | 0.07 | 0.00 | 0.05 | 0.00 | 0.05 | Å |
| A1g | 1.06 | 0.99 | 1.21 | 1.27 | 1.08 | 1.11 | 1.21 | Å |
| A2g | 0.00 | 0.27 | 0.11 | 0.17 | 0.04 | 0.21 | 0.22 | Å |
| Δoope | 3.73 | 3.58 | 3.93 | 4.06 | 3.72 | 3.78 | 3.91 | Å |
| B2U (sad) | 3.73 | 3.46 | 3.91 | 4.04 | 3.72 | 3.72 | 3.86 | Å |
| B1u (ruf) | 0.00 | 0.92 | 0.35 | 0.45 | 0.1 | 0.66 | 0.61 | Å |
| A2U (dom) | 0.11 | 0.06 | 0.01 | 0.00 | 0.04 | 0.14 | 0.04 | Å |
aAverage displacement of meso-carbon atoms from the xy plane, (C5, C10, C15, and C20) relative to the 24-atom mean porphyrin plane (mean plane defined as Δz = 0). bAverage displacement of α-carbon atoms from the xy plane (C1, C4, C6, C9, C11, C14, C16, C19) relative to the 24-atom mean porphyrin plane (Δz = 0). cAverage displacement of β-carbon atoms from the xy plane (C2, C3, C7, C8, C12, C13, C17, C18) relative to the mean porphyrin plane (Δz = 0). dAverage deviation of the 24-atom macrocycle (x,y coordinates) from the mean porphyrin plane, based on the least-squares method (mean plane defined as Δx and Δy = 0). eAverage deviation of the 24-atom macrocycle (z coordinates) with respect to the least-squares plane (mean plane defined as Δz = 0) [54].
With compound 26, no ruffling of the porphyrin ring is observed; however, with anthracene residues (27) a ruffling distortion of almost 1 Å is observed. This is not obvious at first, but differences in molecular symmetry [55] can be easily visualized using the neoplastic NSD plot [10] shown in Figure 3. Furthermore, the mean pyrrole ring tilt increases by 3–5° in the case of compounds 28 and 29 compared to that of compound 26. Saddle-shape distortion is reduced compared to that of biphenyl 26; this may be due to the proximity of the anthracene moiety to the β-ethyl positions, with C23–C14B within 3.63 Å and their respective hydrogen atoms 1.97 Å away from each other. However, this does not account for the increased ruffling observed in porphyrins 28 and 29, with similar distances to the β-ethyl groups as in porphyrin 26, and despite this there is almost 0.5 Å magnitude of ruffle distortion. This may also be due to crystal packing effects or the Ni(II) metal center [56,57], as well as the crystallization solvent [43]. It is not possible to ascertain whether steric effects of the β-ethyl and the anthracenyl carbons are causing the ruffling observed, and a full statistical model of a large library of dodecasubstituted porphyrins is needed to understand these observed effects.
![[1860-5397-20-234-3]](/bjoc/content/figures/1860-5397-20-234-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: View of the molecular structure of compounds 26 (top left) and 27 (top right) with atomic displacements at 50% probability and hydrogen atoms omitted for clarity. Bottom left: Neoplastic representation of the molecular symmetry of compound 26. Bottom right: Neoplastic representation of the molecular symmetry of compound 27.
Figure 3: View of the molecular structure of compounds 26 (top left) and 27 (top right) with atomic displacem...
When comparing meta-substituted porphyrins 36 and 37, ruffle distortion of the porphyrin ring is also observed. Interestingly in the case of the meta-anthracenyl derivative, the para-anthracenylporphyrin 27 experiences a magnitude of ≈0.3 Å less ruffling when compared to that of the meta-substituted porphyrin 37. In terms of meta-phenyl-substituted porphyrin 36 a contribution of ruffling is observed, but no ruffling is observed in the planar analogue.
Crystal packing analysis of arm-extended para-substituted porphyrins
Nonplanar porphyrins are known to form supramolecular assemblies [6], either through hydrogen-bonding networks or through π–π interactions. Examples of this can be seen in the trapping of Keggin-type heteropolyoxometalate (POM) through nonplanar Mo(V)–porphyrin complexes [58], or porphyrin nanotubes/nanochannels by intermolecular π–π interactions of the peripheral phenyl groups [59]. Additionally nonplanar supramolecular assemblies have found use in anion capture [12,15], and sensing [60], making the synthesis of these structures desirable from a supramolecular standpoint.
Two especially interesting crystal packing features were that observed in the structures of compound 27 and borylated porphyrin 46. In the case of porphyrin 27, when crystallized by slow evaporation from CDCl3, a crystal structure with a void diameter of approximately 5.8 Å was obtained (Figure 4). The void was measured from the Ni(II)...Ni(II) vector approximately perpendicular to the metals through the c-axis.
![[1860-5397-20-234-4]](/bjoc/content/figures/1860-5397-20-234-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Left: packing diagram of 27 viewed normal to the c-axis showing the channels in the lattice with the solvent molecule density removed using masking in OLEX2. Hydrogen atoms omitted for clarity. Right: packing diagram of 46, viewed normal to the c-axis, with bis(pinacolato)diboron occupying the cavities of the major 46. Hydrogen atoms omitted for clarity.
Figure 4: Left: packing diagram of 27 viewed normal to the c-axis showing the channels in the lattice with th...
Upon co-crystallization of borylated porphyrin 46 and bis(pinacolato)diboron, the accommodation of bis(pinacolato)diboron in the void of the lattice was observed (Figure 4). The crystal packing of this structure is quite similar to the supramolecular assembly of borylated porphyrin 5,10,15,20-tetrakis(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)porphyrin, where nitrobenzene accommodated tunnels of width of 7–8 Å [61]. The assembly of compound 46 also presents channel-type voids, making it part of only a few porphyrins appended with boronic ester groups to be structurally disseminated by X-ray crystallography [62,63]. Compound 46 was found to crystallize in a 1:1 ratio with bis(pinacolato)diboron, with a void size of 8–9 Å. The formation of channel-type lattice structures is thermodynamically unfavorable, when compared to tightly packed arrangements, similar to nitrobenzene, bis(pinacolato)diboron may be templating the formation of these channels [61]. However, more research is needed to understand the formation of these supramolecular assemblies.
X-ray crystal structure analysis of compound 11
As observed in the single crystal X-ray structure of 11 (Figure 5), the environment around the ortho-bromo-position is extremely sterically hindered.
![[1860-5397-20-234-5]](/bjoc/content/figures/1860-5397-20-234-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Left: view of part 0 2 in the molecular structure of the α2β2-atropisomer, 11 in the crystal, hydrogen atoms omitted for clarity. Displacement parameters shown at 50% probability and heteroatoms labelled only. Right: view of part 0 1 of the molecular structure of the α2β2-atropisomer, 11 in the crystal. Displacement parameters shown at 50% probability and heteroatoms labelled only.
Figure 5: Left: view of part 0 2 in the molecular structure of the α2β2-atropisomer, 11 in the crystal, hydro...
Figure 5, shows the Br…Br separations in the α2β2-atropsiomer of compound 11 to be 4.3–4.4 Å. While only an illustration of the situation in the solid phase this illustrates that coupling phenyl, thiophene or other aryl moieties at this position would be extremely difficult. Furthermore, the distance between the o-bromine atoms and the nearest carbon neighbor of the β-ethyl groups is 3.7 Å, further complicates the success of coupling at this position. As discussed previously, enrichment to the αβαβ, isomer may be necessary to remove the impact of opposing bromine atoms on the coupling reaction. Separation of the four individual atropisomers (αβαβ, α2β2, α3β, α4) has been accomplished before in dodecasubstituted porphyrins through Ni(II) metalation [15]. The core metalation effect prevents inner core N–H tautomerism [64] and thus increases the structural symmetry of the macrocycle [65,66], leading to more facile atropisomeric separation. However, in the case of compound 11 the atropisomers could not be separated due to low rotational barriers and similar polarities, even with Ni(II) metalation. There are many other methods available to achieve different desired atropisomeric ratios. These include thermal enrichment [67,68], photoracemization [69,70], axial-ligand coordination [71], precise separation techniques [50] or a combination of the procedures mentioned [72]. Many more examples of atropisomeric enrichment methods can be found in a 2024 review on atropisomerism by Maguire et al. [73] and could be further explored to isolate the αβαβ-atropisomer of porphyrin 11.
X-ray crystal structure analysis of compound 37
Interestingly the anthracenyl arm-extension on the meso-meta-phenyl position revealed a doubly sandwiched, intercalated dimeric structure, wherein by two anthracenyl units is sandwiched a single anthracenylphenyl arm whilst a anthracenylphenyl arm is sandwiched on the opposing side of the molecules in the same fashion (Figure 6). Support of the existence of this structure in solution was obtained from VT-NMR studies (Figure S51 and Figure S52 in Supporting Information File 1), with asymmetry observed in the β-ethyl CH3 resonances δH = 0.58 and 0.73 ppm and peak broadening in both the aromatic region and the {1H}13C NMR spectra. Coalescence of the β-ethyl resonances was observed when heating the sample to 70 °C and the same 1H NMR spectrum was observed after subsequent cooling of the sample as before heating. This indicates the thermodynamically favored dimeric structure to reassemble when cooling, reverting to the previous NMR trace.
![[1860-5397-20-234-6]](/bjoc/content/figures/1860-5397-20-234-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Schematic representation of porphyrin 37 showing a doubly intercalated structure.
Figure 6: Schematic representation of porphyrin 37 showing a doubly intercalated structure.
Conclusion
When considering sterically demanding systems with haloaryl and boronic acids as substrates for the Suzuki–Miyaura coupling, many may consider 2,6-alkyl-disubstituted phenyl rings as a model sterically demanding system to test the robust nature of both metal catalyst and ligand, for example, much work has been done on the synthesis of ortho-substituted biaryls, by the groups of Buchwald [74], Snieckus [75], Ackermann [76], and Tang [77] among others. Many of these examples have steric hindrance ‘adjacent’ to that of the reactive halogen/boronic site as opposed to the ‘adjacent’ and ‘opposite’ steric demand as seen with compound 11 with opposing bromines, coupled with the added complication of being a rotameric mixture, as well as adjacent hindrance of the nearby β-ethyl groups. Examples of palladium coupling on ‘opposing’ halogen atoms can be seen in the annulation of vic-bis(pinacolatoboryl)alkenes and -phenanthrenes [78]; yet, adjacent hindrance is not a problem in this case. Clearly, more work is required on the Suzuki–Miyaura coupling of molecules with sterically demanding ‘pockets’ with opposing and adjacent hindrance.
In conclusion, a library of arm-extended dodecasubstituted porphyrins was synthesized through classic C–C coupling reactions at the meso-phenyl position. It was found varying temperature and the pH of the solution are effective mitigations to overcome unfavorable reaction electronics or demanding sterics presented at the meso-phenyls’ meta- or para-position. Functionalization at the meso-phenyls’ ortho- position was not manageable and more research is needed to optimize conditions. Comparing the yields in coupling of borylated porphyrins and the halogenated analogues revealed a greater yield, when the polarity of the reaction was reversed; however, due to tedious synthesis and a lower yield over two steps, this synthetic approach is disadvantageous.
X-ray crystal structures were reported for almost half of these compounds. Crystal packing arrangements revealed this new library of arm-extended porphyrins as interesting candidates for the formation of supramolecular assemblies possibly capable of carrying out sensing and or capturing molecules of interest, as well as a dimeric intercalated structure.
Supporting Information
| Supporting Information File 1: Experimental methods, synthetic procedures, 1H, 11B and 13C NMR, VT-NMR, UV–vis, IR, HRMS (m/z)-APCI and HRMS (m/z)-LIFDI spectra and X-ray crystallographic data. | ||
| Format: PDF | Size: 12.7 MB | Download |
| Supporting Information File 2: Crystallographic information files for porphyrins 11 (tcd2100), 28 (tcd2038), 27 (tcd2036), 36 (tcd2056), 26 (tcd2017), 29 (tcd2127), 37 (tcd2277), 46 (tcd2153) and 33 (tcd2288). | ||
| Format: ZIP | Size: 39.3 KB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Brothers, P. J.; Senge, M. O., Eds. Fundamentals of Porphyrin Chemistry: A 21st Century Approach; John Wiley & Sons: Hoboken, NJ, USA, 2022. doi:10.1002/9781119129301
Return to citation in text: [1] -
Fleischer, E. B. J. Am. Chem. Soc. 1963, 85, 146–148. doi:10.1021/ja00885a007
Return to citation in text: [1] -
Senge, M. O. Chem. Commun. 2006, 243–256. doi:10.1039/b511389j
Return to citation in text: [1] [2] [3] [4] -
Olea, C., Jr.; Boon, E. M.; Pellicena, P.; Kuriyan, J.; Marletta, M. A. ACS Chem. Biol. 2008, 3, 703–710. doi:10.1021/cb800185h
Return to citation in text: [1] -
Senge, M. O.; MacGowan, S. A.; O'Brien, J. M. Chem. Commun. 2015, 51, 17031–17063. doi:10.1039/c5cc06254c
Return to citation in text: [1] -
Ishizuka, T.; Grover, N.; Kingsbury, C. J.; Kotani, H.; Senge, M. O.; Kojima, T. Chem. Soc. Rev. 2022, 51, 7560–7630. doi:10.1039/d2cs00391k
Return to citation in text: [1] [2] [3] [4] -
Haddad, R. E.; Gazeau, S.; Pécaut, J.; Marchon, J.-C.; Medforth, C. J.; Shelnutt, J. A. J. Am. Chem. Soc. 2003, 125, 1253–1268. doi:10.1021/ja0280933
Return to citation in text: [1] -
Kingsbury, C. J.; Senge, M. O. Coord. Chem. Rev. 2021, 431, 213760. doi:10.1016/j.ccr.2020.213760
Return to citation in text: [1] [2] [3] [4] -
Shelnutt, J. A. J. Porphyrins Phthalocyanines 2001, 5, 300–311. doi:10.1002/jpp.320
Return to citation in text: [1] [2] -
Kingsbury, C. J.; Senge, M. O. Angew. Chem., Int. Ed. 2024, 63, e202403754. doi:10.1002/anie.202403754
Return to citation in text: [1] [2] -
Norvaiša, K.; Yeow, K.; Twamley, B.; Roucan, M.; Senge, M. O. Eur. J. Org. Chem. 2021, 1871–1882. doi:10.1002/ejoc.202100154
Return to citation in text: [1] -
Stone, A.; Fleischer, E. B. J. Am. Chem. Soc. 1968, 90, 2735–2748. doi:10.1021/ja01013a001
Return to citation in text: [1] [2] -
Senge, M. O.; Forsyth, T. P.; Nguyen, L. T.; Smith, K. M. Angew. Chem., Int. Ed. Engl. 1995, 33, 2485–2487. doi:10.1002/anie.199424851
Return to citation in text: [1] [2] -
Cheng, B.; Munro, O. Q.; Marques, H. M.; Scheidt, W. R. J. Am. Chem. Soc. 1997, 119, 10732–10742. doi:10.1021/ja9716214
Return to citation in text: [1] -
Norvaiša, K.; Flanagan, K. J.; Gibbons, D.; Senge, M. O. Angew. Chem., Int. Ed. 2019, 58, 16553–16557. doi:10.1002/anie.201907929
Return to citation in text: [1] [2] [3] -
Roucan, M.; Kielmann, M.; Connon, S. J.; Bernhard, S. S. R.; Senge, M. O. Chem. Commun. 2018, 54, 26–29. doi:10.1039/c7cc08099a
Return to citation in text: [1] -
Cavalleri, M.; Damiano, C.; Manca, G.; Gallo, E. Chem. – Eur. J. 2023, 29, e202202729. doi:10.1002/chem.202202729
Return to citation in text: [1] -
Senge, M. O.; Renner, M. W.; Kalisch, W. W.; Fajer, J. J. Chem. Soc., Dalton Trans. 2000, 381–385. doi:10.1039/a905927j
Return to citation in text: [1] -
Senge, M. O.; Medforth, C. J.; Sparks, L. D.; Shelnutt, J. A.; Smith, K. M. Inorg. Chem. 1993, 32, 1716–1723. doi:10.1021/ic00061a030
Return to citation in text: [1] -
Hiroto, S.; Miyake, Y.; Shinokubo, H. Chem. Rev. 2017, 117, 2910–3043. doi:10.1021/acs.chemrev.6b00427
Return to citation in text: [1] -
Godlewski, B.; Baran, D.; de Robichon, M.; Ferry, A.; Ostrowski, S.; Malinowski, M. Org. Chem. Front. 2022, 9, 2396–2404. doi:10.1039/d1qo01909k
Return to citation in text: [1] -
Bakar, M. A.; Sergeeva, N. N.; Juillard, T.; Senge, M. O. Organometallics 2011, 30, 3225–3228. doi:10.1021/om200137p
Return to citation in text: [1] -
Locos, O. B.; Arnold, D. P. Org. Biomol. Chem. 2006, 4, 902–916. doi:10.1039/b516989e
Return to citation in text: [1] -
Shinokubo, H.; Osuka, A. Chem. Commun. 2009, 1011–1021. doi:10.1039/b817941g
Return to citation in text: [1] [2] -
Sergeeva, N. N.; Scala, A.; Bakar, M. A.; O’Riordan, G.; O’Brien, J.; Grassi, G.; Senge, M. O. J. Org. Chem. 2009, 74, 7140–7147. doi:10.1021/jo901535c
Return to citation in text: [1] -
Kato, K.; Kim, J. O.; Yorimitsu, H.; Kim, D.; Osuka, A. Chem. – Asian J. 2016, 11, 1738–1746. doi:10.1002/asia.201600424
Return to citation in text: [1] -
Sugita, N.; Hayashi, S.; Hino, F.; Takanami, T. J. Org. Chem. 2012, 77, 10488–10497. doi:10.1021/jo302122f
Return to citation in text: [1] -
Chen, J.; Aratani, N.; Shinokubo, H.; Osuka, A. Chem. – Asian J. 2009, 4, 1126–1133. doi:10.1002/asia.200900053
Return to citation in text: [1] -
Miyaura, N.; Yanagi, T.; Suzuki, A. Synth. Commun. 1981, 11, 513–519. doi:10.1080/00397918108063618
Return to citation in text: [1] -
Wang, K.; Osuka, A.; Song, J. ACS Cent. Sci. 2020, 6, 2159–2178. doi:10.1021/acscentsci.0c01300
Return to citation in text: [1] -
Peng, X.; Aratani, N.; Takagi, A.; Matsumoto, T.; Kawai, T.; Hwang, I.-W.; Ahn, T. K.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2004, 126, 4468–4469. doi:10.1021/ja0392972
Return to citation in text: [1] -
Aratani, N.; Osuka, A. Org. Lett. 2001, 3, 4213–4216. doi:10.1021/ol0168770
Return to citation in text: [1] -
Götz, D. C. G.; Bruhn, T.; Senge, M. O.; Bringmann, G. J. Org. Chem. 2009, 74, 8005–8020. doi:10.1021/jo901483q
Return to citation in text: [1] -
Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538
Return to citation in text: [1] [2] [3] [4] -
Pijeat, J.; Dappe, Y. J.; Thuéry, P.; Campidelli, S. Org. Biomol. Chem. 2018, 16, 8106–8114. doi:10.1039/c8ob02150c
Return to citation in text: [1] [2] -
Ferrero, S.; Barbero, H.; Miguel, D.; García-Rodríguez, R.; Álvarez, C. M. RSC Adv. 2020, 10, 36164–36173. doi:10.1039/d0ra07407a
Return to citation in text: [1] [2] -
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007
Return to citation in text: [1] -
Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S. K.; Liao, L.; Ambrogio, M. W.; Botros, Y. Y.; Duan, X.; Seki, S.; Stoddart, J. F.; Yaghi, O. M. Chem. Mater. 2011, 23, 4094–4097. doi:10.1021/cm201140r
Return to citation in text: [1] -
Muzzi, C. M.; Medforth, C. J.; Voss, L.; Cancilla, M.; Lebrilla, C.; Ma, J.-G.; Shelnutt, J. A.; Smith, K. M. Tetrahedron Lett. 1999, 40, 6159–6162. doi:10.1016/s0040-4039(99)01105-3
Return to citation in text: [1] -
Barton, D. H. R.; Zard, S. Z. J. Chem. Soc., Chem. Commun. 1985, 1098–1100. doi:10.1039/c39850001098
Return to citation in text: [1] -
Roth, S. D.; Shkindel, T.; Lightner, D. A. Tetrahedron 2007, 63, 11030–11039. doi:10.1016/j.tet.2007.08.041
Return to citation in text: [1] -
Lindsey, J. S.; Schreiman, I. C.; Hsu, H. C.; Kearney, P. C.; Marguerettaz, A. M. J. Org. Chem. 1987, 52, 827–836. doi:10.1021/jo00381a022
Return to citation in text: [1] -
Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963
Return to citation in text: [1] [2] [3] [4] [5] -
Hayes, H. L. D.; Wei, R.; Assante, M.; Geogheghan, K. J.; Jin, N.; Tomasi, S.; Noonan, G.; Leach, A. G.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2021, 143, 14814–14826. doi:10.1021/jacs.1c06863
Return to citation in text: [1] [2] -
Fasano, V.; McFord, A. W.; Butts, C. P.; Collins, B. S. L.; Fey, N.; Alder, R. W.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2020, 59, 22403–22407. doi:10.1002/anie.202007776
Return to citation in text: [1] -
Yamamoto, Y.; Matsumura, T.; Takao, N.; Yamagishi, H.; Takahashi, M.; Iwatsuki, S.; Ishihara, K. Inorg. Chim. Acta 2005, 358, 3355–3361. doi:10.1016/j.ica.2005.05.026
Return to citation in text: [1] -
Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. D.; King, E. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2017, 139, 13156–13165. doi:10.1021/jacs.7b07444
Return to citation in text: [1] [2] -
Garai, S.; Schaffer, P. C.; Laprairie, R. B.; Janero, D. R.; Pertwee, R. G.; Straiker, A.; Thakur, G. A. Bioorg. Med. Chem. 2021, 50, 116421. doi:10.1016/j.bmc.2021.116421
Return to citation in text: [1] [2] [3] -
Alimardanov, A.; Schmieder‐van de Vondervoort, L.; de Vries, A. H. M.; de Vries, J. G. Adv. Synth. Catal. 2004, 346, 1812–1817. doi:10.1002/adsc.200404210
Return to citation in text: [1] -
Lindsey, J. J. Org. Chem. 1980, 45, 5215. doi:10.1021/jo01313a042
Return to citation in text: [1] [2] -
Tang, X.-f.; Feng, S.-h.; Wang, Y.-k.; Yang, F.; Zheng, Z.-h.; Zhao, J.-n.; Wu, Y.-f.; Yin, H.; Liu, G.-z.; Meng, Q.-w. Tetrahedron 2018, 74, 3624–3633. doi:10.1016/j.tet.2018.05.023
Return to citation in text: [1] -
Senge, M. O.; Medforth, C. J.; Forsyth, T. P.; Lee, D. A.; Olmstead, M. M.; Jentzen, W.; Pandey, R. K.; Shelnutt, J. A.; Smith, K. M. Inorg. Chem. 1997, 36, 1149–1163. doi:10.1021/ic961156w
Return to citation in text: [1] -
Kingsbury, C. J.; Kielmann, M.; Twamley, B.; Senge, M. O. Molecules 2022, 27, 7060. doi:10.3390/molecules27207060
Return to citation in text: [1] -
Jentzen, W.; Song, X.-Z.; Shelnutt, J. A. J. Phys. Chem. B 1997, 101, 1684–1699. doi:10.1021/jp963142h
Return to citation in text: [1] -
Kingsbury, C. J.; Senge, M. O. Chem. Sci. 2024, 15, 13638–13649. doi:10.1039/d4sc01670j
Return to citation in text: [1] -
Brennan, T. D.; Scheidt, W. R.; Shelnutt, J. A. J. Am. Chem. Soc. 1988, 110, 3919–3924. doi:10.1021/ja00220a033
Return to citation in text: [1] -
Kozlowski, P. M.; Rush, T. S.; Jarzecki, A. A.; Zgierski, M. Z.; Chase, B.; Piffat, C.; Ye, B.-H.; Li, X.-Y.; Pulay, P.; Spiro, T. G. J. Phys. Chem. A 1999, 103, 1357–1366. doi:10.1021/jp9819700
Return to citation in text: [1] -
Yokoyama, A.; Kojima, T.; Fukuzumi, S. Dalton Trans. 2011, 40, 6445–6450. doi:10.1039/c0dt01708f
Return to citation in text: [1] -
Kojima, T.; Harada, R.; Nakanishi, T.; Kaneko, K.; Fukuzumi, S. Chem. Mater. 2007, 19, 51–58. doi:10.1021/cm062031k
Return to citation in text: [1] -
Norvaiša, K.; Kielmann, M.; Senge, M. O. ChemBioChem 2020, 21, 1793–1807. doi:10.1002/cbic.202000067
Return to citation in text: [1] -
Muniappan, S.; Lipstman, S.; Goldberg, I. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2008, 64, 177–179. doi:10.1107/s010827010800468x
Return to citation in text: [1] [2] -
Hata, H.; Shinokubo, H.; Osuka, A. J. Am. Chem. Soc. 2005, 127, 8264–8265. doi:10.1021/ja051073r
Return to citation in text: [1] -
Hyslop, A. G.; Kellett, M. A.; Iovine, P. M.; Therien, M. J. J. Am. Chem. Soc. 1998, 120, 12676–12677. doi:10.1021/ja982410h
Return to citation in text: [1] -
Urbani, M.; Torres, T. Chem. – Eur. J. 2014, 20, 16337–16349. doi:10.1002/chem.201403881
Return to citation in text: [1] -
Gouterman, M.; Wagnière, G. H.; Snyder, L. C. J. Mol. Spectrosc. 1963, 11, 108–127. doi:10.1016/0022-2852(63)90011-0
Return to citation in text: [1] -
Senge, M. O.; Ryan, A. A.; Letchford, K. A.; MacGowan, S. A.; Mielke, T. Symmetry 2014, 6, 781–843. doi:10.3390/sym6030781
Return to citation in text: [1] -
Hatano, K.; Anzai, K.; Kubo, T.; Tamai, S. Bull. Chem. Soc. Jpn. 1981, 54, 3518–3521. doi:10.1246/bcsj.54.3518
Return to citation in text: [1] -
Nishino, N.; Kobata, K.; Mihara, H.; Fujimoto, T. Chem. Lett. 1992, 21, 1991–1994. doi:10.1246/cl.1992.1991
Return to citation in text: [1] -
Freitag, R. A.; Mercer-Smith, J. A.; Whitten, D. G. J. Am. Chem. Soc. 1981, 103, 1226–1228. doi:10.1021/ja00395a045
Return to citation in text: [1] -
Freitag, R. A.; Whitten, D. G. J. Phys. Chem. 1983, 87, 3918–3925. doi:10.1021/j100243a026
Return to citation in text: [1] -
Mansour, A.; Belghith, Y.; Belkhiria, M. S.; Bujacz, A.; Guérineau, V.; Nasri, H. J. Porphyrins Phthalocyanines 2013, 17, 1094–1103. doi:10.1142/s1088424613500843
Return to citation in text: [1] -
Zimmer, B.; Bulach, V.; Drexler, C.; Erhardt, S.; Hosseini, M. W.; De Cian, A. New J. Chem. 2002, 26, 43–57. doi:10.1039/b104084g
Return to citation in text: [1] -
Maguire, S.; Strachan, G.; Norvaiša, K.; Donohoe, C.; Gomes-da-Silva, L. C.; Senge, M. O. Chem. – Eur. J. 2024, 30, e202401559. doi:10.1002/chem.202401559
Return to citation in text: [1] -
Yin, J.; Rainka, M. P.; Zhang, X.-X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 1162–1163. doi:10.1021/ja017082r
Return to citation in text: [1] -
Demchuk, O. M.; Yoruk, B.; Blackburn, T.; Snieckus, V. Synlett 2006, 2908–2913. doi:10.1055/s-2006-951538
Return to citation in text: [1] -
Ackermann, L.; Potukuchi, H. K.; Althammer, A.; Born, R.; Mayer, P. Org. Lett. 2010, 12, 1004–1007. doi:10.1021/ol1000186
Return to citation in text: [1] -
Zhao, Q.; Li, C.; Senanayake, C. H.; Tang, W. Chem. – Eur. J. 2013, 19, 2261–2265. doi:10.1002/chem.201203898
Return to citation in text: [1] -
Shimizu, M.; Nagao, I.; Tomioka, Y.; Hiyama, T. Angew. Chem., Int. Ed. 2008, 47, 8096–8099. doi:10.1002/anie.200803213
Return to citation in text: [1]
| 34. | Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538 |
| 35. | Pijeat, J.; Dappe, Y. J.; Thuéry, P.; Campidelli, S. Org. Biomol. Chem. 2018, 16, 8106–8114. doi:10.1039/c8ob02150c |
| 69. | Freitag, R. A.; Mercer-Smith, J. A.; Whitten, D. G. J. Am. Chem. Soc. 1981, 103, 1226–1228. doi:10.1021/ja00395a045 |
| 70. | Freitag, R. A.; Whitten, D. G. J. Phys. Chem. 1983, 87, 3918–3925. doi:10.1021/j100243a026 |
| 36. | Ferrero, S.; Barbero, H.; Miguel, D.; García-Rodríguez, R.; Álvarez, C. M. RSC Adv. 2020, 10, 36164–36173. doi:10.1039/d0ra07407a |
| 71. | Mansour, A.; Belghith, Y.; Belkhiria, M. S.; Bujacz, A.; Guérineau, V.; Nasri, H. J. Porphyrins Phthalocyanines 2013, 17, 1094–1103. doi:10.1142/s1088424613500843 |
| 65. | Gouterman, M.; Wagnière, G. H.; Snyder, L. C. J. Mol. Spectrosc. 1963, 11, 108–127. doi:10.1016/0022-2852(63)90011-0 |
| 66. | Senge, M. O.; Ryan, A. A.; Letchford, K. A.; MacGowan, S. A.; Mielke, T. Symmetry 2014, 6, 781–843. doi:10.3390/sym6030781 |
| 67. | Hatano, K.; Anzai, K.; Kubo, T.; Tamai, S. Bull. Chem. Soc. Jpn. 1981, 54, 3518–3521. doi:10.1246/bcsj.54.3518 |
| 68. | Nishino, N.; Kobata, K.; Mihara, H.; Fujimoto, T. Chem. Lett. 1992, 21, 1991–1994. doi:10.1246/cl.1992.1991 |
| 64. | Urbani, M.; Torres, T. Chem. – Eur. J. 2014, 20, 16337–16349. doi:10.1002/chem.201403881 |
| 43. | Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963 |
| 43. | Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963 |
| 42. | Lindsey, J. S.; Schreiman, I. C.; Hsu, H. C.; Kearney, P. C.; Marguerettaz, A. M. J. Org. Chem. 1987, 52, 827–836. doi:10.1021/jo00381a022 |
| 75. | Demchuk, O. M.; Yoruk, B.; Blackburn, T.; Snieckus, V. Synlett 2006, 2908–2913. doi:10.1055/s-2006-951538 |
| 43. | Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963 |
| 39. | Muzzi, C. M.; Medforth, C. J.; Voss, L.; Cancilla, M.; Lebrilla, C.; Ma, J.-G.; Shelnutt, J. A.; Smith, K. M. Tetrahedron Lett. 1999, 40, 6159–6162. doi:10.1016/s0040-4039(99)01105-3 |
| 73. | Maguire, S.; Strachan, G.; Norvaiša, K.; Donohoe, C.; Gomes-da-Silva, L. C.; Senge, M. O. Chem. – Eur. J. 2024, 30, e202401559. doi:10.1002/chem.202401559 |
| 40. | Barton, D. H. R.; Zard, S. Z. J. Chem. Soc., Chem. Commun. 1985, 1098–1100. doi:10.1039/c39850001098 |
| 41. | Roth, S. D.; Shkindel, T.; Lightner, D. A. Tetrahedron 2007, 63, 11030–11039. doi:10.1016/j.tet.2007.08.041 |
| 74. | Yin, J.; Rainka, M. P.; Zhang, X.-X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 1162–1163. doi:10.1021/ja017082r |
| 24. | Shinokubo, H.; Osuka, A. Chem. Commun. 2009, 1011–1021. doi:10.1039/b817941g |
| 37. | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007 |
| 36. | Ferrero, S.; Barbero, H.; Miguel, D.; García-Rodríguez, R.; Álvarez, C. M. RSC Adv. 2020, 10, 36164–36173. doi:10.1039/d0ra07407a |
| 38. | Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S. K.; Liao, L.; Ambrogio, M. W.; Botros, Y. Y.; Duan, X.; Seki, S.; Stoddart, J. F.; Yaghi, O. M. Chem. Mater. 2011, 23, 4094–4097. doi:10.1021/cm201140r |
| 72. | Zimmer, B.; Bulach, V.; Drexler, C.; Erhardt, S.; Hosseini, M. W.; De Cian, A. New J. Chem. 2002, 26, 43–57. doi:10.1039/b104084g |
| 35. | Pijeat, J.; Dappe, Y. J.; Thuéry, P.; Campidelli, S. Org. Biomol. Chem. 2018, 16, 8106–8114. doi:10.1039/c8ob02150c |
| 44. | Hayes, H. L. D.; Wei, R.; Assante, M.; Geogheghan, K. J.; Jin, N.; Tomasi, S.; Noonan, G.; Leach, A. G.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2021, 143, 14814–14826. doi:10.1021/jacs.1c06863 |
| 45. | Fasano, V.; McFord, A. W.; Butts, C. P.; Collins, B. S. L.; Fey, N.; Alder, R. W.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2020, 59, 22403–22407. doi:10.1002/anie.202007776 |
| 46. | Yamamoto, Y.; Matsumura, T.; Takao, N.; Yamagishi, H.; Takahashi, M.; Iwatsuki, S.; Ishihara, K. Inorg. Chim. Acta 2005, 358, 3355–3361. doi:10.1016/j.ica.2005.05.026 |
| 78. | Shimizu, M.; Nagao, I.; Tomioka, Y.; Hiyama, T. Angew. Chem., Int. Ed. 2008, 47, 8096–8099. doi:10.1002/anie.200803213 |
| 76. | Ackermann, L.; Potukuchi, H. K.; Althammer, A.; Born, R.; Mayer, P. Org. Lett. 2010, 12, 1004–1007. doi:10.1021/ol1000186 |
| 77. | Zhao, Q.; Li, C.; Senanayake, C. H.; Tang, W. Chem. – Eur. J. 2013, 19, 2261–2265. doi:10.1002/chem.201203898 |
| 34. | Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538 |
| 48. | Garai, S.; Schaffer, P. C.; Laprairie, R. B.; Janero, D. R.; Pertwee, R. G.; Straiker, A.; Thakur, G. A. Bioorg. Med. Chem. 2021, 50, 116421. doi:10.1016/j.bmc.2021.116421 |
| 48. | Garai, S.; Schaffer, P. C.; Laprairie, R. B.; Janero, D. R.; Pertwee, R. G.; Straiker, A.; Thakur, G. A. Bioorg. Med. Chem. 2021, 50, 116421. doi:10.1016/j.bmc.2021.116421 |
| 49. | Alimardanov, A.; Schmieder‐van de Vondervoort, L.; de Vries, A. H. M.; de Vries, J. G. Adv. Synth. Catal. 2004, 346, 1812–1817. doi:10.1002/adsc.200404210 |
| 44. | Hayes, H. L. D.; Wei, R.; Assante, M.; Geogheghan, K. J.; Jin, N.; Tomasi, S.; Noonan, G.; Leach, A. G.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2021, 143, 14814–14826. doi:10.1021/jacs.1c06863 |
| 47. | Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. D.; King, E. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2017, 139, 13156–13165. doi:10.1021/jacs.7b07444 |
| 34. | Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538 |
| 47. | Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. D.; King, E. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2017, 139, 13156–13165. doi:10.1021/jacs.7b07444 |
| 48. | Garai, S.; Schaffer, P. C.; Laprairie, R. B.; Janero, D. R.; Pertwee, R. G.; Straiker, A.; Thakur, G. A. Bioorg. Med. Chem. 2021, 50, 116421. doi:10.1016/j.bmc.2021.116421 |
| 51. | Tang, X.-f.; Feng, S.-h.; Wang, Y.-k.; Yang, F.; Zheng, Z.-h.; Zhao, J.-n.; Wu, Y.-f.; Yin, H.; Liu, G.-z.; Meng, Q.-w. Tetrahedron 2018, 74, 3624–3633. doi:10.1016/j.tet.2018.05.023 |
| 34. | Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538 |
| 1. | Brothers, P. J.; Senge, M. O., Eds. Fundamentals of Porphyrin Chemistry: A 21st Century Approach; John Wiley & Sons: Hoboken, NJ, USA, 2022. doi:10.1002/9781119129301 |
| 7. | Haddad, R. E.; Gazeau, S.; Pécaut, J.; Marchon, J.-C.; Medforth, C. J.; Shelnutt, J. A. J. Am. Chem. Soc. 2003, 125, 1253–1268. doi:10.1021/ja0280933 |
| 18. | Senge, M. O.; Renner, M. W.; Kalisch, W. W.; Fajer, J. J. Chem. Soc., Dalton Trans. 2000, 381–385. doi:10.1039/a905927j |
| 56. | Brennan, T. D.; Scheidt, W. R.; Shelnutt, J. A. J. Am. Chem. Soc. 1988, 110, 3919–3924. doi:10.1021/ja00220a033 |
| 57. | Kozlowski, P. M.; Rush, T. S.; Jarzecki, A. A.; Zgierski, M. Z.; Chase, B.; Piffat, C.; Ye, B.-H.; Li, X.-Y.; Pulay, P.; Spiro, T. G. J. Phys. Chem. A 1999, 103, 1357–1366. doi:10.1021/jp9819700 |
| 6. | Ishizuka, T.; Grover, N.; Kingsbury, C. J.; Kotani, H.; Senge, M. O.; Kojima, T. Chem. Soc. Rev. 2022, 51, 7560–7630. doi:10.1039/d2cs00391k |
| 19. | Senge, M. O.; Medforth, C. J.; Sparks, L. D.; Shelnutt, J. A.; Smith, K. M. Inorg. Chem. 1993, 32, 1716–1723. doi:10.1021/ic00061a030 |
| 4. | Olea, C., Jr.; Boon, E. M.; Pellicena, P.; Kuriyan, J.; Marletta, M. A. ACS Chem. Biol. 2008, 3, 703–710. doi:10.1021/cb800185h |
| 5. | Senge, M. O.; MacGowan, S. A.; O'Brien, J. M. Chem. Commun. 2015, 51, 17031–17063. doi:10.1039/c5cc06254c |
| 15. | Norvaiša, K.; Flanagan, K. J.; Gibbons, D.; Senge, M. O. Angew. Chem., Int. Ed. 2019, 58, 16553–16557. doi:10.1002/anie.201907929 |
| 55. | Kingsbury, C. J.; Senge, M. O. Chem. Sci. 2024, 15, 13638–13649. doi:10.1039/d4sc01670j |
| 2. | Fleischer, E. B. J. Am. Chem. Soc. 1963, 85, 146–148. doi:10.1021/ja00885a007 |
| 3. | Senge, M. O. Chem. Commun. 2006, 243–256. doi:10.1039/b511389j |
| 16. | Roucan, M.; Kielmann, M.; Connon, S. J.; Bernhard, S. S. R.; Senge, M. O. Chem. Commun. 2018, 54, 26–29. doi:10.1039/c7cc08099a |
| 17. | Cavalleri, M.; Damiano, C.; Manca, G.; Gallo, E. Chem. – Eur. J. 2023, 29, e202202729. doi:10.1002/chem.202202729 |
| 10. | Kingsbury, C. J.; Senge, M. O. Angew. Chem., Int. Ed. 2024, 63, e202403754. doi:10.1002/anie.202403754 |
| 8. | Kingsbury, C. J.; Senge, M. O. Coord. Chem. Rev. 2021, 431, 213760. doi:10.1016/j.ccr.2020.213760 |
| 10. | Kingsbury, C. J.; Senge, M. O. Angew. Chem., Int. Ed. 2024, 63, e202403754. doi:10.1002/anie.202403754 |
| 12. | Stone, A.; Fleischer, E. B. J. Am. Chem. Soc. 1968, 90, 2735–2748. doi:10.1021/ja01013a001 |
| 13. | Senge, M. O.; Forsyth, T. P.; Nguyen, L. T.; Smith, K. M. Angew. Chem., Int. Ed. Engl. 1995, 33, 2485–2487. doi:10.1002/anie.199424851 |
| 8. | Kingsbury, C. J.; Senge, M. O. Coord. Chem. Rev. 2021, 431, 213760. doi:10.1016/j.ccr.2020.213760 |
| 9. | Shelnutt, J. A. J. Porphyrins Phthalocyanines 2001, 5, 300–311. doi:10.1002/jpp.320 |
| 9. | Shelnutt, J. A. J. Porphyrins Phthalocyanines 2001, 5, 300–311. doi:10.1002/jpp.320 |
| 14. | Cheng, B.; Munro, O. Q.; Marques, H. M.; Scheidt, W. R. J. Am. Chem. Soc. 1997, 119, 10732–10742. doi:10.1021/ja9716214 |
| 54. | Jentzen, W.; Song, X.-Z.; Shelnutt, J. A. J. Phys. Chem. B 1997, 101, 1684–1699. doi:10.1021/jp963142h |
| 8. | Kingsbury, C. J.; Senge, M. O. Coord. Chem. Rev. 2021, 431, 213760. doi:10.1016/j.ccr.2020.213760 |
| 3. | Senge, M. O. Chem. Commun. 2006, 243–256. doi:10.1039/b511389j |
| 6. | Ishizuka, T.; Grover, N.; Kingsbury, C. J.; Kotani, H.; Senge, M. O.; Kojima, T. Chem. Soc. Rev. 2022, 51, 7560–7630. doi:10.1039/d2cs00391k |
| 8. | Kingsbury, C. J.; Senge, M. O. Coord. Chem. Rev. 2021, 431, 213760. doi:10.1016/j.ccr.2020.213760 |
| 13. | Senge, M. O.; Forsyth, T. P.; Nguyen, L. T.; Smith, K. M. Angew. Chem., Int. Ed. Engl. 1995, 33, 2485–2487. doi:10.1002/anie.199424851 |
| 52. | Senge, M. O.; Medforth, C. J.; Forsyth, T. P.; Lee, D. A.; Olmstead, M. M.; Jentzen, W.; Pandey, R. K.; Shelnutt, J. A.; Smith, K. M. Inorg. Chem. 1997, 36, 1149–1163. doi:10.1021/ic961156w |
| 53. | Kingsbury, C. J.; Kielmann, M.; Twamley, B.; Senge, M. O. Molecules 2022, 27, 7060. doi:10.3390/molecules27207060 |
| 3. | Senge, M. O. Chem. Commun. 2006, 243–256. doi:10.1039/b511389j |
| 11. | Norvaiša, K.; Yeow, K.; Twamley, B.; Roucan, M.; Senge, M. O. Eur. J. Org. Chem. 2021, 1871–1882. doi:10.1002/ejoc.202100154 |
| 43. | Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963 |
| 21. | Godlewski, B.; Baran, D.; de Robichon, M.; Ferry, A.; Ostrowski, S.; Malinowski, M. Org. Chem. Front. 2022, 9, 2396–2404. doi:10.1039/d1qo01909k |
| 6. | Ishizuka, T.; Grover, N.; Kingsbury, C. J.; Kotani, H.; Senge, M. O.; Kojima, T. Chem. Soc. Rev. 2022, 51, 7560–7630. doi:10.1039/d2cs00391k |
| 20. | Hiroto, S.; Miyake, Y.; Shinokubo, H. Chem. Rev. 2017, 117, 2910–3043. doi:10.1021/acs.chemrev.6b00427 |
| 58. | Yokoyama, A.; Kojima, T.; Fukuzumi, S. Dalton Trans. 2011, 40, 6445–6450. doi:10.1039/c0dt01708f |
| 59. | Kojima, T.; Harada, R.; Nakanishi, T.; Kaneko, K.; Fukuzumi, S. Chem. Mater. 2007, 19, 51–58. doi:10.1021/cm062031k |
| 43. | Flanagan, K. J.; Twamley, B.; Senge, M. O. Inorg. Chem. 2019, 58, 15769–15787. doi:10.1021/acs.inorgchem.9b01963 |
| 6. | Ishizuka, T.; Grover, N.; Kingsbury, C. J.; Kotani, H.; Senge, M. O.; Kojima, T. Chem. Soc. Rev. 2022, 51, 7560–7630. doi:10.1039/d2cs00391k |
| 29. | Miyaura, N.; Yanagi, T.; Suzuki, A. Synth. Commun. 1981, 11, 513–519. doi:10.1080/00397918108063618 |
| 30. | Wang, K.; Osuka, A.; Song, J. ACS Cent. Sci. 2020, 6, 2159–2178. doi:10.1021/acscentsci.0c01300 |
| 31. | Peng, X.; Aratani, N.; Takagi, A.; Matsumoto, T.; Kawai, T.; Hwang, I.-W.; Ahn, T. K.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2004, 126, 4468–4469. doi:10.1021/ja0392972 |
| 32. | Aratani, N.; Osuka, A. Org. Lett. 2001, 3, 4213–4216. doi:10.1021/ol0168770 |
| 33. | Götz, D. C. G.; Bruhn, T.; Senge, M. O.; Bringmann, G. J. Org. Chem. 2009, 74, 8005–8020. doi:10.1021/jo901483q |
| 27. | Sugita, N.; Hayashi, S.; Hino, F.; Takanami, T. J. Org. Chem. 2012, 77, 10488–10497. doi:10.1021/jo302122f |
| 61. | Muniappan, S.; Lipstman, S.; Goldberg, I. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2008, 64, 177–179. doi:10.1107/s010827010800468x |
| 28. | Chen, J.; Aratani, N.; Shinokubo, H.; Osuka, A. Chem. – Asian J. 2009, 4, 1126–1133. doi:10.1002/asia.200900053 |
| 15. | Norvaiša, K.; Flanagan, K. J.; Gibbons, D.; Senge, M. O. Angew. Chem., Int. Ed. 2019, 58, 16553–16557. doi:10.1002/anie.201907929 |
| 24. | Shinokubo, H.; Osuka, A. Chem. Commun. 2009, 1011–1021. doi:10.1039/b817941g |
| 25. | Sergeeva, N. N.; Scala, A.; Bakar, M. A.; O’Riordan, G.; O’Brien, J.; Grassi, G.; Senge, M. O. J. Org. Chem. 2009, 74, 7140–7147. doi:10.1021/jo901535c |
| 61. | Muniappan, S.; Lipstman, S.; Goldberg, I. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2008, 64, 177–179. doi:10.1107/s010827010800468x |
| 26. | Kato, K.; Kim, J. O.; Yorimitsu, H.; Kim, D.; Osuka, A. Chem. – Asian J. 2016, 11, 1738–1746. doi:10.1002/asia.201600424 |
| 62. | Hata, H.; Shinokubo, H.; Osuka, A. J. Am. Chem. Soc. 2005, 127, 8264–8265. doi:10.1021/ja051073r |
| 63. | Hyslop, A. G.; Kellett, M. A.; Iovine, P. M.; Therien, M. J. J. Am. Chem. Soc. 1998, 120, 12676–12677. doi:10.1021/ja982410h |
| 22. | Bakar, M. A.; Sergeeva, N. N.; Juillard, T.; Senge, M. O. Organometallics 2011, 30, 3225–3228. doi:10.1021/om200137p |
| 12. | Stone, A.; Fleischer, E. B. J. Am. Chem. Soc. 1968, 90, 2735–2748. doi:10.1021/ja01013a001 |
| 15. | Norvaiša, K.; Flanagan, K. J.; Gibbons, D.; Senge, M. O. Angew. Chem., Int. Ed. 2019, 58, 16553–16557. doi:10.1002/anie.201907929 |
| 23. | Locos, O. B.; Arnold, D. P. Org. Biomol. Chem. 2006, 4, 902–916. doi:10.1039/b516989e |
| 60. | Norvaiša, K.; Kielmann, M.; Senge, M. O. ChemBioChem 2020, 21, 1793–1807. doi:10.1002/cbic.202000067 |
© 2024 Cribbin et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.