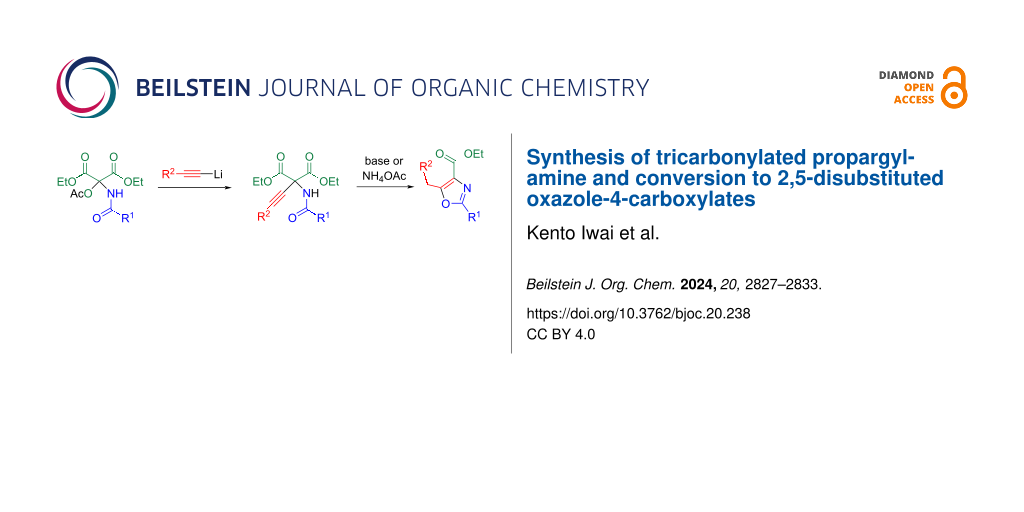
Abstract
The N,O-acetal derived from diethyl mesoxalate (DEMO) undergoes elimination of acetic acid upon treatment with a base, leading to the formation of N-acylimine in situ. Lithium acetylide readily attacks the imino group to afford N,1,1-tricarbonylated propargylamines. When the resulting propargylamine reacts with butyllithium, ring closure occurs between the ethynyl and carbamoyl groups, yielding 2,5-disubstituted oxazole-4-carboxylates. This cyclization also occurs when the propargylamine is heated with ammonium acetate, resulting in double activation.
Graphical Abstract
Introduction
Propargylamine is an important motif in the synthesis of heterocyclic compounds [1-4] and drug discovery [5,6] due to its multifunctionality, which includes a basic and nucleophilic amino group, an electrophilic and dipolarophilic triple bond, and an acidic propargyl methylene group. Among these, polycarbonylated propargylamines (PCPAs), specifically N,1-dicarbonylated or N,1,1-tricarbonylated propargylamines, are often used as model compounds to identify biologically active compounds [7-10] or their synthetic precursors [11-15] because of their easily modifiable dipeptide frameworks. Several methods exist for accessing PCPAs, such as the amination of 1-halo-1-alkynes [16,17], tandem reactions of α-imino esters with nucleophiles and electrophiles [18], and the nucleophilic addition of an acetylide to α-carbonylated N-acylimines (NAIs) [13,14,19-22]. To apply the latter method to the preparation of N,1,1-tricarbonylated propargylamines, the corresponding NAIs are necessary. However, the poor structural diversity of available NAIs limits the use of this method. For example, N,1-dicarbonylated NAIs derived from α-keto esters and N-acyl groups is limited to acetyl, benzoyl, and alkoxycarbonyl groups [15]. Conversely, NAIs derived from α-oxomalonic acid diester are more versatile. Nagao et al. prepared NAI 2 through the aza-Wittig reaction of diethyl mesoxalate (DEMO, diethyl α-oxomalonate). However, only the N-acetyl derivative has been employed (Scheme 1) [13,14].
Scheme 1: Synthesis of polyfunctionalized methane derivatives through successive nucleophilic additions to the central carbon atom of DEMO.
Scheme 1: Synthesis of polyfunctionalized methane derivatives through successive nucleophilic additions to th...
Recently, we have demonstrated that the central carbonyl group of DEMO is highly electrophilic, facilitating the nucleophilic addition of less reactive reagents such as acid amides [23-26]. When the reaction was conducted in the presence of acetic anhydride, the intermediately formed hemiacetal underwent acetylation, leading to N,O-acetals 1. In this method, an acid amide can be used as an amine masked with an acyl group. Subsequent elimination of acetic acid occurred to afford 2 in situ upon treatment with a base, enabling nucleophilic addition with various nucleophiles. This is because the imino carbon atom of 2 is also highly electrophilic, similar to DEMO [23-25]. This method offers an advantage over conventional methods as the N-acyl group can be modified by altering the acid amide. In this study, lithium acetylides were employed as nucleophiles to synthesize PCPAs, and their ring closures were also investigated.
Results and Discussion
NAI 2 can be generated by treating N,O-acetal 1 with a base, such as triethylamine. However, the addition of an amine was omitted because lithium acetylide functions both as nucleophile and base. When 1a was reacted with lithium acetylide, which was prepared from ethynylbenzene (3a) and butyllithium at 0 °C, the solution turned black, resulting in a complex reaction mixture (Table 1, entry 1). This complication persisted even when the reaction was conducted at −78 °C and then warmed to room temperature without addition of acetic acid. To address this, the reaction was performed at −78 °C, and acetic acid was added at the same temperature, yielding adduct 4a in 13% yield (Table 1, entry 2). The reaction yield was significantly influenced by the amounts of 3a and butyllithium used. The yield of 4a increased when a slight excess of acetylide was used (Table 1, entry 3). Solvation of lithium acetylide was also a critical factor in this reaction (Table 1, entry 4). Therefore, the reaction conditions in Table 1, entry 3 were determined to be optimal.
The optimized conditions were applied to various N,O-acetals 1 and alkynes 3 to determine the substrate scope of this protocol (Table 2). This reaction was effective with alkyl- and silyl-substituted alkynes 3b–d, yielding the corresponding adducts 4b–d, respectively (Table 2, entries 1–3). A significant advantage of this method is the high modifiability of the N-acyl group, achieved by altering the acid amide during the reaction with DEMO [23-25]. Specifically, aliphatic amide 1b can be used, which reacts with lithium acetylide (3a) to yield 4e (Table 2, entry 4).
Subsequently, ring closure utilizing the multifunctionality of 4 was examined (Table 3) [13,14]. To a dry THF solution of adduct 4a, butyllithium was added, and the reaction mixture was stirred at −78 °C for 5 min. Following the addition of acetic acid, the reaction mixture was concentrated and subjected to silica gel column chromatography, resulting in the isolation of ethyl 5-benzyl-2-(4-methylphenyl)oxazole-4-carboxylate (5a) in 13% yield (Table 3, entry 1). Several conditions were considered, but the yield of 5a did not improve. On the other hand, the addition of two equivalents of water increased the yield, indicating the important role of a stoichiometric amount of water (Table 3, entry 2). Indeed, using THF that had not been dried as purchased resulted in a significant increase of the yield to 82% (Table 3, entry 3). The choice of base was also crucial (Table 3, entries 3–5). While the yield of 5a was low with potassium tert-butoxide, a yield of 66% using lithium tert-butoxide was observed, suggesting that lithium ions activate for the ring closure. Under the conditions in Table 3, entry 3, adduct 4 underwent ring formation. This reaction was not influenced by the bulkiness of the substituent on the alkynyl group, yielding the corresponding oxazoles 5b–d (Table 3, entries 6–8). When N-propanoyl derivative 4e was used, cyclization proceeded similarly, yielding the corresponding oxazole 5e (Table 3, entry 9).
Table 3: Conversion of adducts 4 to oxazoles 5.
|
|
|||||
| entry | R1 | R2 | base | product | yield, % |
| 1a | p-MeC6H4 | Ph | BuLi | 5a | 13 |
| 2a,b | p-MeC6H4 | Ph | BuLi | 5a | 24 |
| 3 | p-MeC6H4 | Ph | BuLi | 5a | 82 |
| 4 | p-MeC6H4 | Ph | t-BuOK | 5a | 27 |
| 5 | p-MeC6H4 | Ph | t-BuOLi | 5a | 66 |
| 6 | p-MeC6H4 | Bu | BuLi | 5b | 45 |
| 7 | p-MeC6H4 | t-Bu | BuLi | 5c | 56 |
| 8 | p-MeC6H4 | Me3Si | BuLi | 5d | 52 |
| 9 | Et | Ph | BuLi | 5e | 70 |
aDry THF was used as solvent; b2 equiv of H2O were added.
When the reaction was quenched with deuterium oxide instead of acetic acid, monodeuterated oxazole 5a-d1 was obtained (Scheme 2). Based on these experimental results, a plausible mechanism was proposed, as shown in Scheme 3a. The 5-exo-dig ring closure is induced by O-attack of the amide moiety on the ethynyl group to form 6, during which a stoichiometric proton source (water in the solvent) is necessary. Subsequently, one of the ethoxycarbonyl groups at the 4-position is hydrolyzed to afford lithium carboxylate 7. In this step, the counter metal ion is considered to affect the activation of the ethoxycarbonyl group of 6. When the reaction mixture was warmed without the addition of acetic acid, a color change to black was observed, suggesting that decarboxylation accompanied by aromatization of the oxazole ring occurred during this process. Thus, protonation occurs, leading to oxazole 5 when the reaction mixture is warmed in the presence of large amounts of proton sources such as acetic acid or deuterium oxide. Although Nagao et al. proposed another mechanism, as illustrated in Scheme 3b [13,14], we cannot negate this mechanism because the reaction media and bases were different.
Scheme 2: Cyclization of 4a quenched by D2O.
Scheme 2: Cyclization of 4a quenched by D2O.
Scheme 3: Plausible mechanisms for the ring closure of 4.
Scheme 3: Plausible mechanisms for the ring closure of 4.
PCPA 4a was heated in the presence of methanesulfonic acid to undergo 6-endo-dig cyclization. However, hydration predominantly occurred, converting the ethynyl group to a phenacyl group, yielding 9 without any detectable cyclization product (Scheme 4). This hydration process is thought to proceed via two paths. The reaction is initiated by the protonation of the ethynyl group to generate the vinyl cation intermediate 10. Product 9 is directly formed by the attack of a water molecule on this cation, followed by tautomerism (path a). The intramolecular attack of an amide carbonyl on this cationic site in intermediate 10, leading to the formation of oxonium ion 11, is also possible (path b). After the addition of water, the formed hemiacetal 12 was hydrolyzed to give the hydrated product 9.
Scheme 4: Hydration of the ethynyl group of 4a.
Scheme 4: Hydration of the ethynyl group of 4a.
The less acidic ammonium acetate was effective for the ring closure of 4a (Table 4). When a solution of 4a and ammonium acetate was heated for 15 h, a 28% yield of 5a was obtained (Table 4, entry 1). Ammonium acetate dissociates into ammonia and acetic acid in an equilibrium upon heating, acting both as base and acid. This dual role activates the amide moiety and the ethynyl group, respectively. Using larger amounts of ammonium acetate substantially prolongs the reaction time due to its dissociation properties. Consequently, the yield of 5a increased to 92% by increasing the amount of ammonium acetate and extending the reaction time (Table 4, entries 2 and 3).
Conclusion
N,O-Acetals 1, derived from DEMO and acid amides, reacted with lithium acetylide to afford the corresponding adduct 4 through highly electrophilic NAIs. The N-acyl and alkynyl groups could be modified using acid amides and acetylides, respectively. When adduct 4 was treated with a base or ammonium acetate, ring closure proceeded to form a five-membered ring, accompanied by the elimination of the ethoxycarbonyl group.
2,5-Disubstituted oxazole-4-carboxylic acid derivatives are frequently found in biologically active compounds [27-31] and their synthetic intermediates [32-36]. Thus, this method, which enables modification at the 2- and 5-positions of oxazole-4-carboxylates, is a valuable tool for the study of these compounds.
Experimental
General
All reagents except for DEMO were purchased from commercial sources (Kanto Chemical Co., Inc. or Fujifilm Wako Pure Chemical Corp.) and used without further purification. Super dehydrated, stabilizer-free THF was used as solvent and purchased from Fujifilm Wako Pure Chemical Corp. DEMO was supplied by Kumiai Chemical Industry Co. Ltd. and purified by distillation. 1H and 13C{1H} NMR spectra were recorded on a JEOL JMN-ECZ400S spectrometer (400 MHz and 100 MHz, respectively) using TMS as internal standard. The assignments of the 13C{1H} NMR signals were reaffirmed by DEPT experiments. IR spectra were recorded with a JASCO FT/IR-4200 spectrometer equipped with an ATM detector. High-resolution mass spectra (HRMS) were obtained with a Bruker compact mass spectrometer APCI–TOF set at positive mode. The melting point was measured on an SRS-OptiMelt automated melting point system.
Preparation of N,O-acetal 1
In a manner analogous to that reported in reference [23], to a solution of DEMO (1.72 g, 10.0 mmol) in toluene (40 mL) were added 4-methylbenzamide (1.63 g, 12 mmol), 3 Å molecular sieves (3.4 g), and acetic anhydride (2.0 mL, 20 mmol). The resulting solution was heated at 100 °C for 4 h. After cooling to room temperature, the molecular sieves were filtered off, and the filtrate was washed with water (50 mL × 2). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to afford diethyl α-acetoxy-α-(4-methylbenzoylamino)malonate (1a, 2.86 g, 8.1 mmol, 81% yield) as white solid. When amide 1b was used, the experiment was conducted in the same way.
Synthesis of tricarbonylated propargylamines 4
Under argon atmosphere, a solution of ethynylbenzene (3a, 110 μL, 1.0 mmol) in THF (1 mL) was cooled to −50 °C. To this solution, a 1.6 M hexane solution of butyllithium (550 μL, 0.86 mmol) was added dropwise to afford lithium acetylide.
To a solution of N,O-acetal 1a (140.0 mg, 0.4 mmol) in THF (3 mL), the above-mentioned THF solution of butyllithium was added at −78 °C under argon atmosphere, and the resulting mixture was stirred for a further 1 h. After addition of acetic acid (0.1 mL), the mixture was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: hexane/ethyl acetate 70:30, Rf 0.55) to afford diethyl 2-[(4-methylbenzoyl)amino]-2-(phenylethynyl)propanedioate (4a, 122 mg, 0.31 mmol, 78% yield) as yellow oil. 1H NMR (400 MHz, CDCl3, δ) 7.78 (d, J = 8.0 Hz, 2H), 7.65 (br s, 1H), 7.48 (d, J = 8.0 Hz, 2H), 7.32–7.25 (m, 5H), 4.37 (q, J = 7.2 Hz, 4H), 2.40 (s, 3H), 1.35 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3, δ) 165.6 (C), 165.3 (C), 142.8 (C), 130.2 (C), 129.4 (CH), 128.9 (CH), 128.2 (CH), 127.5 (CH), 122.0 (C), 84.9 (C), 82.6 (C), 63.8 (CH2), 61.0 (C), 21.6 (CH3), 14.0 (CH3); IR (KBr, ATR) vmax: 1754, 1672, 1477, 1281, 1214, 1071, 751 cm−1; HRMS–APCI-TOF (m/z): [M + H]+ calcd for C23H24NO5, 394.1649; found, 394.1672.
When other alkynes and N,O-acetals were used, the experiments were conducted in the same way.
Cyclization of tricarbonylated propargylamine 4 leading to oxazoles 5
To a solution of propargylamine 4a (137 mg, 0.35 mmol) in THF (3 mL), 1.6 M butyllithium hexane solution (230 μL, 0.35 mmol) was added at −78 °C under argon atmosphere, and the resulting mixture was stirred for 5 min. After quenching with acetic acid (0.1 mL), the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: hexane/ethyl acetate 70:30, eluent for TLC: hexane/ethyl acetate 80:20, Rf 0.61) to afford ethyl 2-(4-methylphenyl)-5-(phenylmethyl)oxazole-4-carboxylate (5a, 92.2 mg, 0.29 mmol, 82% yield) as colorless oil. 1H NMR (400 MHz, CDCl3, δ) 7.88 (d, J = 8.4 Hz, 2H), 7.35–7.22 (m, 7H), 4.45 (s, 2H), 4.42 (q, J = 7.2 Hz, 2H), 2.39 (s, 3H), 1.40 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3, δ) 162.0 (C), 160.7 (C), 157.6 (C), 141.8 (C), 136.4 (C), 129.4 (CH), 128.5 (CH), 128.3 (C), 128.1 (CH), 126.8 (CH), 126.2 (CH), 123.4 (C), 60.9 (CH2), 31.6 (CH2), 20.2 (CH3), 13.3 (CH3); IR (KBr, ATR) vmax: 1735, 1710, 1178, 1087, 1054, 720 cm−1; HRMS–APCI-TOF (m/z): [M + H]+ calcd for C20H20NO3, 322.1438; found, 322.1458.
When other propargylamines were used, the experiments were conducted in the same way. In the deuteration experiment, the reaction was quenched with deuterium oxide (0.2 mL) instead of acetic acid. The decrease of the integral of the signal of the benzyl proton was confirmed by 1H NMR analysis.
Supporting Information
| Supporting Information File 1: Spectral data for 4, 5, and 9 as well as 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 686.7 KB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Bhoraniya, R. B.; Modha, S. G. ChemistryOpen 2023, 12, e202200223. doi:10.1002/open.202200223
Return to citation in text: [1] -
Das, A.; Waser, J. Tetrahedron 2022, 128, 133135. doi:10.1016/j.tet.2022.133135
Return to citation in text: [1] -
Gong, J.; Feng, H. Chem. Heterocycl. Compd. 2022, 58, 193–195. doi:10.1007/s10593-022-03071-0
Return to citation in text: [1] -
Vessally, E.; Babazadeh, M.; Hosseinian, A.; Edjlali, L.; Sreerama, L. Curr. Org. Chem. 2018, 22, 199–205. doi:10.2174/1385272821666170519113904
Return to citation in text: [1] -
Carneiro, A.; Uriarte, E.; Borges, F.; Matos, M. J. Future Med. Chem. 2023, 15, 211–224. doi:10.4155/fmc-2022-0243
Return to citation in text: [1] -
do Carmo Carreiras, M.; Ismaili, L.; Marco-Contelles, J. Bioorg. Med. Chem. Lett. 2020, 30, 126880. doi:10.1016/j.bmcl.2019.126880
Return to citation in text: [1] -
Szczerbiński, J.; Metternich, J. B.; Goubert, G.; Zenobi, R. Small 2020, 16, 1905197. doi:10.1002/smll.201905197
Return to citation in text: [1] -
Zhang, D.; Sun, M. B.; Lee, J.; Abdeen, A. A.; Kilian, K. A. J. Biomed. Mater. Res., Part A 2016, 104, 1212–1220. doi:10.1002/jbm.a.35661
Return to citation in text: [1] -
Cordova, A.; Woodrick, J.; Grindrod, S.; Zhang, L.; Saygideger-Kont, Y.; Wang, K.; DeVito, S.; Daniele, S. G.; Paige, M.; Brown, M. L. Bioconjugate Chem. 2016, 27, 1981–1990. doi:10.1021/acs.bioconjchem.5b00481
Return to citation in text: [1] -
Noichl, B. P.; Durkin, P. M.; Budisa, N. Biopolymers 2015, 104, 585–600. doi:10.1002/bip.22709
Return to citation in text: [1] -
Wayama, T.; Arai, Y.; Oguri, H. J. Org. Chem. 2022, 87, 5938–5951. doi:10.1021/acs.joc.2c00212
Return to citation in text: [1] -
Scheiner, M.; Sink, A.; Hoffmann, M.; Vrigneau, C.; Endres, E.; Carles, A.; Sotriffer, C.; Maurice, T.; Decker, M. J. Am. Chem. Soc. 2022, 144, 3279–3284. doi:10.1021/jacs.1c13492
Return to citation in text: [1] -
Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196
Return to citation in text: [1] [2] [3] [4] [5] -
Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8
Return to citation in text: [1] [2] [3] [4] [5] -
Nakamura, S.; Matsumoto, N.; Kibe, M.; Abe, K.; Takehara, T.; Suzuki, T. Adv. Synth. Catal. 2022, 364, 781–786. doi:10.1002/adsc.202101114
Return to citation in text: [1] [2] -
Egorova, A. V.; Viktorov, N. B.; Starova, G. L.; Dogadina, A. V. Russ. J. Gen. Chem. 2019, 89, 1765–1771. doi:10.1134/s1070363219090068
Return to citation in text: [1] -
Bachi, M. D.; Bar-Ner, N.; Stang, P. J.; Williamson, B. L. J. Org. Chem. 1993, 58, 7923–7924. doi:10.1021/jo00079a046
Return to citation in text: [1] -
Mizota, I.; Matsuda, Y.; Kamimura, S.; Tanaka, H.; Shimizu, M. Org. Lett. 2013, 15, 4206–4209. doi:10.1021/ol401934x
Return to citation in text: [1] -
Ghosh, S.; Biswas, K. RSC Adv. 2021, 11, 2047–2065. doi:10.1039/d0ra09392k
Return to citation in text: [1] -
Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Chem. Rev. 2017, 117, 14091–14200. doi:10.1021/acs.chemrev.7b00343
Return to citation in text: [1] -
Zani, L.; Bolm, C. Chem. Commun. 2006, 4263–4275. doi:10.1039/b607986p
Return to citation in text: [1] -
Arai, Y.; Oguri, H. Tetrahedron Lett. 2021, 78, 153283. doi:10.1016/j.tetlet.2021.153283
Return to citation in text: [1] -
Iwai, K.; Hikasa, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. J. Org. Chem. 2023, 88, 2207–2213. doi:10.1021/acs.joc.2c02647
Return to citation in text: [1] [2] [3] [4] -
Asahara, H.; Bonkohara, A.; Takagi, M.; Iwai, K.; Ito, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Org. Biomol. Chem. 2022, 20, 2282–2292. doi:10.1039/d1ob02482e
Return to citation in text: [1] [2] [3] -
Asahara, H.; Inoue, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Adv. Synth. Catal. 2016, 358, 2817–2828. doi:10.1002/adsc.201600436
Return to citation in text: [1] [2] [3] -
Köppen, J.; Matthies, D.; Siewers, S. Chem.-Ztg. 1987, 111, 247–248.
See for reactions of vicinal triketone with acid amide.
Return to citation in text: [1] -
Peng, T.; He, Y.; Wang, T.; Yu, J.; Ma, X.; Zhou, Z.; Sheng, Y.; Li, L.; Peng, H.; Li, S.; Zou, J.; Yuan, Y.; Zhao, Y.; Shi, H.; Li, F.; Liu, W.; Hu, K.; Lu, X.; Zhang, G.; Wang, F. J. Med. Chem. 2022, 65, 11010–11033. doi:10.1021/acs.jmedchem.2c00189
Return to citation in text: [1] -
Kim, Y.; Ma, C.; Park, S.; Shin, Y.; Lee, T.; Paek, J.; Kim, K. H.; Jang, G.; Cho, H.; Son, S.; Son, S.-H.; Lee, K. Y.; Lee, K.; Jung, Y. W.; Jeon, Y. H.; Byun, Y. Chem. – Asian J. 2021, 16, 3702–3712. doi:10.1002/asia.202100896
Return to citation in text: [1] -
Ke, S.; Fang, W.; Huang, W.; Zhang, Z.; Shi, L.; Wan, Z.; Wang, K.; Cao, C.; Huang, D. Bioorg. Med. Chem. Lett. 2020, 30, 127245. doi:10.1016/j.bmcl.2020.127245
Return to citation in text: [1] -
Moraski, G. C.; Markley, L. D.; Chang, M.; Cho, S.; Franzblau, S. G.; Hwang, C. H.; Boshoff, H.; Miller, M. J. Bioorg. Med. Chem. 2012, 20, 2214–2220. doi:10.1016/j.bmc.2012.02.025
Return to citation in text: [1] -
Chen, H.-J.; Wang, W.-L.; Wang, G.-F.; Shi, L.-P.; Gu, M.; Ren, Y.-D.; Hou, L.-F.; He, P.-L.; Zhu, F.-H.; Zhong, X.-G.; Tang, W.; Zuo, J.-P.; Nan, F.-J. ChemMedChem 2008, 3, 1316–1321. doi:10.1002/cmdc.200800136
Return to citation in text: [1] -
Pankova, A. S. J. Org. Chem. 2022, 87, 11121–11130. doi:10.1021/acs.joc.2c01365
Return to citation in text: [1] -
Prashanth, S.; Adarsh, D. R.; Bantu, R.; Sridhar, B.; Reddy, B. V. S. Tetrahedron Lett. 2022, 113, 154252. doi:10.1016/j.tetlet.2022.154252
Return to citation in text: [1] -
Oberheide, A.; Arndt, H.-D. Adv. Synth. Catal. 2021, 363, 1132–1136. doi:10.1002/adsc.202001262
Return to citation in text: [1] -
Qi, Z.; Wang, S. Org. Lett. 2021, 23, 8549–8553. doi:10.1021/acs.orglett.1c03252
Return to citation in text: [1] -
Babaoglu, E.; Hilt, G. Chem. – Eur. J. 2020, 26, 8879–8884. doi:10.1002/chem.202001465
Return to citation in text: [1]
| 23. | Iwai, K.; Hikasa, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. J. Org. Chem. 2023, 88, 2207–2213. doi:10.1021/acs.joc.2c02647 |
| 1. | Bhoraniya, R. B.; Modha, S. G. ChemistryOpen 2023, 12, e202200223. doi:10.1002/open.202200223 |
| 2. | Das, A.; Waser, J. Tetrahedron 2022, 128, 133135. doi:10.1016/j.tet.2022.133135 |
| 3. | Gong, J.; Feng, H. Chem. Heterocycl. Compd. 2022, 58, 193–195. doi:10.1007/s10593-022-03071-0 |
| 4. | Vessally, E.; Babazadeh, M.; Hosseinian, A.; Edjlali, L.; Sreerama, L. Curr. Org. Chem. 2018, 22, 199–205. doi:10.2174/1385272821666170519113904 |
| 16. | Egorova, A. V.; Viktorov, N. B.; Starova, G. L.; Dogadina, A. V. Russ. J. Gen. Chem. 2019, 89, 1765–1771. doi:10.1134/s1070363219090068 |
| 17. | Bachi, M. D.; Bar-Ner, N.; Stang, P. J.; Williamson, B. L. J. Org. Chem. 1993, 58, 7923–7924. doi:10.1021/jo00079a046 |
| 27. | Peng, T.; He, Y.; Wang, T.; Yu, J.; Ma, X.; Zhou, Z.; Sheng, Y.; Li, L.; Peng, H.; Li, S.; Zou, J.; Yuan, Y.; Zhao, Y.; Shi, H.; Li, F.; Liu, W.; Hu, K.; Lu, X.; Zhang, G.; Wang, F. J. Med. Chem. 2022, 65, 11010–11033. doi:10.1021/acs.jmedchem.2c00189 |
| 28. | Kim, Y.; Ma, C.; Park, S.; Shin, Y.; Lee, T.; Paek, J.; Kim, K. H.; Jang, G.; Cho, H.; Son, S.; Son, S.-H.; Lee, K. Y.; Lee, K.; Jung, Y. W.; Jeon, Y. H.; Byun, Y. Chem. – Asian J. 2021, 16, 3702–3712. doi:10.1002/asia.202100896 |
| 29. | Ke, S.; Fang, W.; Huang, W.; Zhang, Z.; Shi, L.; Wan, Z.; Wang, K.; Cao, C.; Huang, D. Bioorg. Med. Chem. Lett. 2020, 30, 127245. doi:10.1016/j.bmcl.2020.127245 |
| 30. | Moraski, G. C.; Markley, L. D.; Chang, M.; Cho, S.; Franzblau, S. G.; Hwang, C. H.; Boshoff, H.; Miller, M. J. Bioorg. Med. Chem. 2012, 20, 2214–2220. doi:10.1016/j.bmc.2012.02.025 |
| 31. | Chen, H.-J.; Wang, W.-L.; Wang, G.-F.; Shi, L.-P.; Gu, M.; Ren, Y.-D.; Hou, L.-F.; He, P.-L.; Zhu, F.-H.; Zhong, X.-G.; Tang, W.; Zuo, J.-P.; Nan, F.-J. ChemMedChem 2008, 3, 1316–1321. doi:10.1002/cmdc.200800136 |
| 11. | Wayama, T.; Arai, Y.; Oguri, H. J. Org. Chem. 2022, 87, 5938–5951. doi:10.1021/acs.joc.2c00212 |
| 12. | Scheiner, M.; Sink, A.; Hoffmann, M.; Vrigneau, C.; Endres, E.; Carles, A.; Sotriffer, C.; Maurice, T.; Decker, M. J. Am. Chem. Soc. 2022, 144, 3279–3284. doi:10.1021/jacs.1c13492 |
| 13. | Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196 |
| 14. | Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8 |
| 15. | Nakamura, S.; Matsumoto, N.; Kibe, M.; Abe, K.; Takehara, T.; Suzuki, T. Adv. Synth. Catal. 2022, 364, 781–786. doi:10.1002/adsc.202101114 |
| 32. | Pankova, A. S. J. Org. Chem. 2022, 87, 11121–11130. doi:10.1021/acs.joc.2c01365 |
| 33. | Prashanth, S.; Adarsh, D. R.; Bantu, R.; Sridhar, B.; Reddy, B. V. S. Tetrahedron Lett. 2022, 113, 154252. doi:10.1016/j.tetlet.2022.154252 |
| 34. | Oberheide, A.; Arndt, H.-D. Adv. Synth. Catal. 2021, 363, 1132–1136. doi:10.1002/adsc.202001262 |
| 35. | Qi, Z.; Wang, S. Org. Lett. 2021, 23, 8549–8553. doi:10.1021/acs.orglett.1c03252 |
| 36. | Babaoglu, E.; Hilt, G. Chem. – Eur. J. 2020, 26, 8879–8884. doi:10.1002/chem.202001465 |
| 7. | Szczerbiński, J.; Metternich, J. B.; Goubert, G.; Zenobi, R. Small 2020, 16, 1905197. doi:10.1002/smll.201905197 |
| 8. | Zhang, D.; Sun, M. B.; Lee, J.; Abdeen, A. A.; Kilian, K. A. J. Biomed. Mater. Res., Part A 2016, 104, 1212–1220. doi:10.1002/jbm.a.35661 |
| 9. | Cordova, A.; Woodrick, J.; Grindrod, S.; Zhang, L.; Saygideger-Kont, Y.; Wang, K.; DeVito, S.; Daniele, S. G.; Paige, M.; Brown, M. L. Bioconjugate Chem. 2016, 27, 1981–1990. doi:10.1021/acs.bioconjchem.5b00481 |
| 10. | Noichl, B. P.; Durkin, P. M.; Budisa, N. Biopolymers 2015, 104, 585–600. doi:10.1002/bip.22709 |
| 13. | Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196 |
| 14. | Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8 |
| 5. | Carneiro, A.; Uriarte, E.; Borges, F.; Matos, M. J. Future Med. Chem. 2023, 15, 211–224. doi:10.4155/fmc-2022-0243 |
| 6. | do Carmo Carreiras, M.; Ismaili, L.; Marco-Contelles, J. Bioorg. Med. Chem. Lett. 2020, 30, 126880. doi:10.1016/j.bmcl.2019.126880 |
| 13. | Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196 |
| 14. | Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8 |
| 13. | Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196 |
| 14. | Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8 |
| 23. | Iwai, K.; Hikasa, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. J. Org. Chem. 2023, 88, 2207–2213. doi:10.1021/acs.joc.2c02647 |
| 24. | Asahara, H.; Bonkohara, A.; Takagi, M.; Iwai, K.; Ito, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Org. Biomol. Chem. 2022, 20, 2282–2292. doi:10.1039/d1ob02482e |
| 25. | Asahara, H.; Inoue, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Adv. Synth. Catal. 2016, 358, 2817–2828. doi:10.1002/adsc.201600436 |
| 15. | Nakamura, S.; Matsumoto, N.; Kibe, M.; Abe, K.; Takehara, T.; Suzuki, T. Adv. Synth. Catal. 2022, 364, 781–786. doi:10.1002/adsc.202101114 |
| 23. | Iwai, K.; Hikasa, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. J. Org. Chem. 2023, 88, 2207–2213. doi:10.1021/acs.joc.2c02647 |
| 24. | Asahara, H.; Bonkohara, A.; Takagi, M.; Iwai, K.; Ito, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Org. Biomol. Chem. 2022, 20, 2282–2292. doi:10.1039/d1ob02482e |
| 25. | Asahara, H.; Inoue, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Adv. Synth. Catal. 2016, 358, 2817–2828. doi:10.1002/adsc.201600436 |
| 13. | Sano, S.; Shimizu, H.; Kim, K.; Lee, W. S.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2006, 54, 196–203. doi:10.1248/cpb.54.196 |
| 14. | Nagao, Y.; Kim, K.; Sano, S.; Kakegawa, H.; Lee, W. S.; Shimizu, H.; Shiro, M.; Katunuma, N. Tetrahedron Lett. 1996, 37, 861–864. doi:10.1016/0040-4039(95)02284-8 |
| 19. | Ghosh, S.; Biswas, K. RSC Adv. 2021, 11, 2047–2065. doi:10.1039/d0ra09392k |
| 20. | Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Chem. Rev. 2017, 117, 14091–14200. doi:10.1021/acs.chemrev.7b00343 |
| 21. | Zani, L.; Bolm, C. Chem. Commun. 2006, 4263–4275. doi:10.1039/b607986p |
| 22. | Arai, Y.; Oguri, H. Tetrahedron Lett. 2021, 78, 153283. doi:10.1016/j.tetlet.2021.153283 |
| 18. | Mizota, I.; Matsuda, Y.; Kamimura, S.; Tanaka, H.; Shimizu, M. Org. Lett. 2013, 15, 4206–4209. doi:10.1021/ol401934x |
| 23. | Iwai, K.; Hikasa, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. J. Org. Chem. 2023, 88, 2207–2213. doi:10.1021/acs.joc.2c02647 |
| 24. | Asahara, H.; Bonkohara, A.; Takagi, M.; Iwai, K.; Ito, A.; Yoshioka, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Org. Biomol. Chem. 2022, 20, 2282–2292. doi:10.1039/d1ob02482e |
| 25. | Asahara, H.; Inoue, K.; Tani, S.; Umezu, K.; Nishiwaki, N. Adv. Synth. Catal. 2016, 358, 2817–2828. doi:10.1002/adsc.201600436 |
| 26. |
Köppen, J.; Matthies, D.; Siewers, S. Chem.-Ztg. 1987, 111, 247–248.
See for reactions of vicinal triketone with acid amide. |
© 2024 Iwai et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.