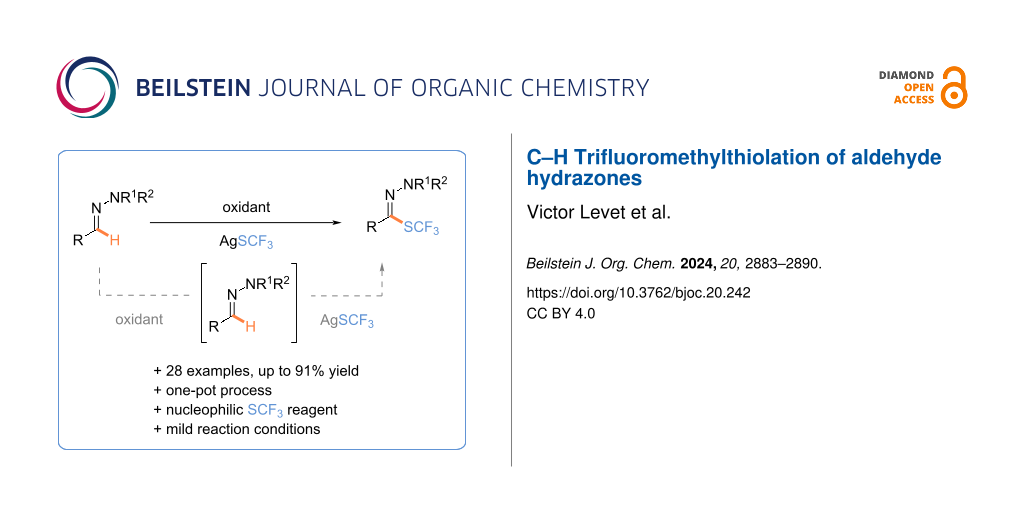
Abstract
The selective C–H trifluoromethylthiolation of aldehyde hydrazones afforded interesting fluorinated building blocks, which could be used as a synthetic platform. Starting from readily available (hetero)aromatic and aliphatic hydrazones, the formation of a C–SCF3 bond was achieved under oxidative and mild reaction conditions in the presence of the readily available AgSCF3 salt via a one-pot sequential process (28 examples, up to 91% yield). Mechanistic investigations revealed that AgSCF3 was the active species in the transformation.
Graphical Abstract
Introduction
Fluorinated molecules are of paramount importance [1-12] from industrial applications [13-15] to our daily lives thanks to the specific features [16] of the fluorine atom or the fluorinated groups. Aiming at pushing beyond the frontiers of knowledge in this very active research field, emergent fluorinated groups [17-20] such as the SCF3 moiety [21-51], an interesting fluorinated moiety with unique electron-withdrawing character and lipophilicity [52,53], have recently garnered interest from the scientific community. Various reagents and chemical transformations have been elaborated in this context over the years [21-51]. Despite these recent advances, the design of highly functionalized trifluoromethylthiolated molecules, which could be used as synthetic handles for synthesizing more complex molecules, is still appealing. In this context, we turned our attention to the trifluoromethylthiolated hydrazones, an interesting building block. Indeed, aldehyde hydrazones have been well studied and used in various transformations [54-64]. In consequence, a large number of transition-metal-catalyzed or radical-mediated processes for C–H functionalization of aldehyde hydrazones has flourished over the years.
An ideal scenario for a direct and sustainable synthetic route towards trifluoromethylthiolated hydrazones will be the direct C–H functionalization of the corresponding aldehyde hydrazone, an uncharted transformation to date. Forging a C–S bond by the direct C–H-bond functionalization of hydrazones is still underdeveloped. Except for transformations leading to the corresponding sulfur-containing heteroarenes, only a few methods have been developed (Scheme 1). In 1988, Lee and co-workers reported the synthesis of SR-containing hydrazones in a two-step process (chlorination [65] then reaction with thiols) from readily available aldehyde-derived hydrazones [66]. Wang et al. developed a method to access thiocyanated derivatives including an aldehyde hydrazone (a unique example) in 70% yield thanks to the in situ generation of SCN-succinimide from NCS and NH4SCN (Scheme 1) [67]. In the same vein, the group of Monteiro [68], then Hajra [69], independently, reported the synthesis of 5-thioxo-1,2,4-triazolium inner salts by the nucleophilic thiocyanation of N,N-dialkylhydrazonoyl bromides, in situ generated from aldehyde-derived hydrazones in the presence of an oxidant (NBS, (NH4)2S2O8), Scheme 1). In 2024, the synthesis of 2‑imino-1,3,4-thiadiazoles was achieved by cyclization of aryl hydrazones with aryl isothiocyanates promoted by elemental sulfur [70]. In the course of their studies for the thiocyanation of ketene dithioacetals, Yang, Wang and co-workers developed an electrochemical oxidization-based synthetic strategy to circumvent the need for external oxidants. In this context, a unique example of the thiocyanation of a hydrazone was depicted [71]. A key feature of the approach is to circumvent the need for external oxidants. In the same vein, the group of Hajra [72] and Yang [73], independently, investigated the electrochemical C–H sulfonylation of a library of aldehyde hydrazones using sodium sulfinates.
Scheme 1: State of the art and this work.
Scheme 1: State of the art and this work.
These seminal works brought interesting proofs of concept for the synthesis of SR-containing hydrazones. Inspired by these previous works and taking benefit from our in-home expertise to forge N–SCF3 bond (after chlorination/anion metathesis with AgSCF3 from the corresponding R1R2NH) [74], we assumed that a one-pot two-step process could be an efficient strategy for the trifluoromethylthiolation of hydrazones. Herein, the synthesis of trifluoromethylthiolated hydrazones from aldehyde hydrazones is depicted.
Results and Discussion
At the outset of the study, the morpholine hydrazone derived from 4-nitrobenzaldehyde was selected as a model substrate (Table 1). The latter was engaged in a two-step process: 1) halogenation to provide the corresponding N,N-hydrazonoyl bromide, which will then undergo an anion metathesis upon the addition of AgSCF3 to the reaction mixture. When the reaction was conducted in the presence of NBS in acetonitrile for 10 min, followed by the addition of AgSCF3, the desired product was isolated in 91% yield. A total selectivity for the formation of the Z isomer was observed as ascertained by 2D NMR (for more details, see Supporting Information File 1) [75]. Different reagents for the bromination or chlorination were also evaluated (Table 1, entries 1–3) and NBS was the most efficient one (Table 1, entry 1).
Table 1: Optimization of the reaction conditions.a
|
|
||
| Entry | Deviation from reaction conditions | Yield (%)b |
| 1 | none | 91 |
| 2 | N-bromophthalimide instead of NBS | 86c |
| 3 | NCS instead of NBS | ND |
aReaction conditions: hydrazone 1i (0.15 mmol, 1.0 equiv), oxidant (0.165 mmol, 1.1 equiv), in CH3CN (0.4 M), 20 °C, 10 min, then AgSCF3 (0.3 mmol, 2.0 equiv), under argon. bIsolated yields are given. cThe product was isolated with an inseparable impurity. ND = not determined.
With the best reaction conditions in hand, the nature of the hydrazone part was first investigated (Scheme 2). Under standard reaction conditions, electron-enriched hydrazones provided the expected products in high yields (2a, 3a, 4a). Note, that in the case of the N-tosylhydrazone, further optimization reactions were required (for more details, see Supporting Information File 1), and reducing the temperature for the halogenation reaction was beneficial to the outcome of the reaction, affording 5a in 55% yield. However, some other hydrazones 6a–8a were reluctant (for more details, see Supporting Information File 1) [75].
Scheme 2: Reaction conditions: hydrazone (0.3 mmol, 1.0 equiv), NBS (0.33 mmol, 1.1 equiv), in CH3CN (0.4 M), 20 °C, 10 min; then, AgSCF3 (0.6 mmol, 2.0 equiv), under argon. Isolated yields are given. aProducts 4a and 5a were isolated with an inseparable impurity. bReaction performed at 0 °C for the 1st step, and 20 °C for the 2nd one.
Scheme 2: Reaction conditions: hydrazone (0.3 mmol, 1.0 equiv), NBS (0.33 mmol, 1.1 equiv), in CH3CN (0.4 M),...
Then, the scope of the reaction was investigated using the hydrazones derived from morpholine (Scheme 3). Hydrazones derived from aromatic aldehydes (1a–p) were first investigated. It turned out that para-substituted compounds with electron-rich groups (e.g., OMe, OBn), halogens (Cl, Br, I), and electron-withdrawing groups (e.g., CF3) were smoothly trifluoromethylthiolated. In the same vein, meta- and ortho-substituted derivatives (1l–o) were converted into the corresponding fluorinated analogs. The functionalization of the 2,4-difluorophenyl derivative (1p) and the heteroaromatic compounds such as furan (1q) as well as pyridine (1r) derivatives went smoothly, with the lower yield obtained in the case of 2r being explained by a tedious purification. Interestingly, the methodology was successfully applied to the functionalization of aliphatic hydrazones 1s and 1t and even the hydrazone derived from citronellal 1u. The method was functional group-tolerant to various functional groups (nitro, CN, ester, alkenes) and halogens allowing an array of post-functionalization reactions. Finally, the trifluoromethylthiolation of molecules derived from compounds of interest was achieved to illustrate the synthetic utility of the method. Hence, the desired products 2v–x were efficiently isolated.
Scheme 3: Scope of the reaction. Reaction conditions: 1 (0.3 mmol, 1.0 equiv), NBS (0.33 mmol, 1.1 equiv) in CH3CN (0.4 M), 20 °C, 10 min; then, AgSCF3 (0.6 mmol, 2.0 equiv), 2 h, under argon. a0.15 mmol reaction scale. bProduct 2v was isolated with an inseparable impurity.
Scheme 3: Scope of the reaction. Reaction conditions: 1 (0.3 mmol, 1.0 equiv), NBS (0.33 mmol, 1.1 equiv) in ...
To get more insights into the transformation, additional experiments were conducted. First, the reaction was repeated in the presence of radical scavengers, namely 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) or di-tert-butylhydroxytoluene (BHT), and no significant impact on the outcome of the reaction was noticed (Scheme 4A). Pleasingly, the scale up of the reaction was smoothly conducted. Under standard reaction conditions, product 2a (1.2 g) was afforded starting from 1a (1 g), showcasing the robustness of the transformation (Scheme 4B). Intrigued about the nature of the active source of SCF3 in the transformation, experiments with different SCF3 sources were conducted. First, we hypothesized that trifluoromethylthiolated succinimide, which might be in situ generated from NBS and AgSCF3, could be the active species. When the reaction was carried out in the presence of this electrophilic source and 1a, no expected product was detected (Scheme 4C). Having in mind that in the presence of an oxidant, the SCF3 dimer (SCF3)2 might be generated, an additional test was realized. In the presence of NCS in THF, AgSCF3 was converted into the corresponding dimer in 5 min (monitored by 19F NMR). Then, the reaction was conducted in the presence of 1a in a THF/MeCN mixture (1:1) [75], but no product was detected (Scheme 4D). Based on these experiments and literature data [66], a two-step one-pot process was suggested based on 1) the bromination of the hydrazone 1 followed by 2) the anion metathesis in the presence of AgSCF3. Finally, to further illustrate the synthetic utility of the trifluoromethylthiolated hydrazones, product 2g was further functionalized. In the presence of 4-methylboronic acid, the arylation of 2g occurred and the expected product was isolated in 72% yield with the SCF3-hydrazone motif remaining untouched (Scheme 4E) [42].
Scheme 4: Mechanistic investigations and post-functionalization reactions. a19F NMR yields using α,α,α-trifluoroacetophenone as an internal standard. ND = not detected.
Scheme 4: Mechanistic investigations and post-functionalization reactions. a19F NMR yields using α,α,α-triflu...
Conclusion
In summary, a one-pot two-step process has been developed for the trifluoromethylthiolation of aldehyde hydrazones. A myriad of (hetero)aromatic and aliphatic hydrazones were efficiently functionalized including analogs of compounds of interest (28 examples, up to 91% yield) using readily available reagents, namely NBS and the nucleophilic reagent AgSCF3. This approach provides a straightforward access to an unprecedented class of trifluoromethylthiolated derivatives. This method offers new avenues for synthesizing a plethora of valuable SCF3-containing molecules using the synthetic potential of hydrazones in organic synthesis.
Experimental
General procedure for the preparation of trifluoromethylthiolated products 2–6: An oven-dried 10 mL reaction tube equipped with a stirring bar was charged with the hydrazone derivative (0.3 mmol, 1.0 equiv) and CH3CN (0.7 mL). The mixture was stirred until the solubilization of the reagent. Then, recrystallized NBS (58.7 mg, 0.33 mmol, 1.1 equiv) was added, and the reaction mixture was stirred for 5–10 minutes, after which, AgSCF3 (125.0 mg, 0.6 mmol, 2.0 equiv) was added. The reaction was stirred for another 2 hours at room temperature. α,α,α-Trifluoroacetophenone (42 μL, 0.3 mmol, 1.0 equiv) was added as an internal standard for determining the 19F NMR yield. The mixture was then filtered on a pad of celite and rinsed with CH2Cl2. The solution was then washed with brine twice (20 mL) and the organic layers were collected separately, dried over MgSO4, and concentrated in vacuo. The crude was purified by column chromatography on silica gel, flash chromatography to afford the desired product 2–6.
Supporting Information
| Supporting Information File 1: Full experimental procedures, characterization of products, details of mechanistic studies, and spectral data. | ||
| Format: PDF | Size: 4.7 MB | Download |
Funding
This work has been partially supported by University of Rouen Normandy, INSA Rouen Normandy, the Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, the graduate school for research XL-Chem (ANR-18-EURE-0020 XL CHEM), and Region Normandie. V.L. thanks the region Normandie for a doctoral fellowship. The French National Research Agency (ANR-21-CE07-0035-02 and ANR-22-CE92-0083) is gratefully acknowledged for generous financial support. R.B. thanks Normandy Region for a post-doctoral fellowship with the project NextFluoChem under the program «Normandie Recherche—Labels d’excellence» (n°23E02521).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496–3508. doi:10.1039/c0cs00221f
Return to citation in text: [1] -
Fujiwara, T.; O’Hagan, D. J. Fluorine Chem. 2014, 167, 16–29. doi:10.1016/j.jfluchem.2014.06.014
Return to citation in text: [1] -
Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832–2842. doi:10.1021/jm401375q
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Landelle, G.; Panossian, A.; Leroux, F. R. Curr. Top. Med. Chem. 2014, 14, 941–951. doi:10.2174/1568026614666140202210016
Return to citation in text: [1] -
Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2014, 20, 16830–16845. doi:10.1002/chem.201404537
Return to citation in text: [1] -
Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a
Return to citation in text: [1] -
Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441–5454. doi:10.1039/c6cs00351f
Return to citation in text: [1] -
Pan, Y. ACS Med. Chem. Lett. 2019, 10, 1016–1019. doi:10.1021/acsmedchemlett.9b00235
Return to citation in text: [1] -
Nobile, E.; Castanheiro, T.; Besset, T. Angew. Chem., Int. Ed. 2021, 60, 12170–12191. doi:10.1002/anie.202009995
Return to citation in text: [1] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476–2536. doi:10.3762/bjoc.9.287
Return to citation in text: [1] -
O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Chem. – Eur. J. 2016, 22, 16734–16749. doi:10.1002/chem.201603438
Return to citation in text: [1] -
Xiao, X.; Zheng, Z.-T.; Li, T.; Zheng, J.-L.; Tao, T.; Chen, L.-M.; Gu, J.-Y.; Yao, X.; Lin, J.-H.; Xiao, J.-C. Synthesis 2020, 52, 197–207. doi:10.1055/s-0039-1690714
Return to citation in text: [1] -
Pannecoucke, X.; Besset, T. Org. Biomol. Chem. 2019, 17, 1683–1693. doi:10.1039/c8ob02995d
Return to citation in text: [1] -
Besset, T.; Poisson, T. Extension to the SCF2H, SCH2F, and SCF2R Motifs (R = PO(OEt)2, CO2R, Rf). In Emerging Fluorinated Motifs; Ma, A.; Cahard, D., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp 449–475. doi:10.1002/9783527824342.ch16
Return to citation in text: [1] -
Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857
Return to citation in text: [1] [2] -
Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88
Return to citation in text: [1] [2] -
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b
Return to citation in text: [1] [2] -
Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e
Return to citation in text: [1] [2] -
Ye, K.-Y.; Zhang, X.; Dai, L.-X.; You, S.-L. J. Org. Chem. 2014, 79, 12106–12110. doi:10.1021/jo5019393
Return to citation in text: [1] [2] -
Lefebvre, Q.; Fava, E.; Nikolaienko, P.; Rueping, M. Chem. Commun. 2014, 50, 6617–6619. doi:10.1039/c4cc02060j
Return to citation in text: [1] [2] -
Liu, J.-B.; Xu, X.-H.; Chen, Z.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 897–900. doi:10.1002/anie.201409983
Return to citation in text: [1] [2] -
Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495
Return to citation in text: [1] [2] -
Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. doi:10.1002/anie.201505446
Return to citation in text: [1] [2] -
Yin, G.; Kalvet, I.; Schoenebeck, F. Angew. Chem., Int. Ed. 2015, 54, 6809–6813. doi:10.1002/anie.201501617
Return to citation in text: [1] [2] -
Li, X.; Zhao, J.; Zhang, L.; Hu, M.; Wang, L.; Hu, J. Org. Lett. 2015, 17, 298–301. doi:10.1021/ol5034018
Return to citation in text: [1] [2] -
Li, M.; Guo, J.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 264–267. doi:10.1021/acs.orglett.5b03433
Return to citation in text: [1] [2] -
Candish, L.; Pitzer, L.; Gómez‐Suárez, A.; Glorius, F. Chem. – Eur. J. 2016, 22, 4753–4756. doi:10.1002/chem.201600421
Return to citation in text: [1] [2] -
Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073
Return to citation in text: [1] [2] -
Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524
Return to citation in text: [1] [2] -
Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906–2909. doi:10.1021/acs.orglett.6b01257
Return to citation in text: [1] [2] -
Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846–5850. doi:10.1002/anie.201601713
Return to citation in text: [1] [2] -
Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790
Return to citation in text: [1] [2] -
Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k
Return to citation in text: [1] [2] -
Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a
Return to citation in text: [1] [2] -
Lübcke, M.; Yuan, W.; Szabó, K. J. Org. Lett. 2017, 19, 4548–4551. doi:10.1021/acs.orglett.7b02139
Return to citation in text: [1] [2] -
Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384
Return to citation in text: [1] [2] [3] -
Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 3693–3696. doi:10.1002/ejoc.201800418
Return to citation in text: [1] [2] -
Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Chem. Commun. 2018, 54, 9909–9912. doi:10.1039/c8cc05256e
Return to citation in text: [1] [2] -
Saravanan, P.; Anbarasan, P. Adv. Synth. Catal. 2018, 360, 2894–2899. doi:10.1002/adsc.201800366
Return to citation in text: [1] [2] -
Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227–4230. doi:10.1021/acs.orglett.8b01627
Return to citation in text: [1] [2] -
He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal. 2018, 8, 11741–11748. doi:10.1021/acscatal.8b04094
Return to citation in text: [1] [2] -
Lindberg, E.; Angerani, S.; Anzola, M.; Winssinger, N. Nat. Commun. 2018, 9, 3539. doi:10.1038/s41467-018-05916-9
Return to citation in text: [1] [2] -
Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690–12695. doi:10.1002/anie.201805859
Return to citation in text: [1] [2] -
Luo, Z.; Yang, X.; Tsui, G. C. Org. Lett. 2020, 22, 6155–6159. doi:10.1021/acs.orglett.0c02235
Return to citation in text: [1] [2] -
Doche, F.; Poisson, T.; Besset, T. ACS Catal. 2023, 13, 14112–14120. doi:10.1021/acscatal.3c03249
Return to citation in text: [1] [2] -
Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Xu, P.; Li, W.; Xie, J.; Zhu, C. Acc. Chem. Res. 2018, 51, 484–495. doi:10.1021/acs.accounts.7b00565
Return to citation in text: [1] -
Xu, X.; Zhang, J.; Xia, H.; Wu, J. Org. Biomol. Chem. 2018, 16, 1227–1241. doi:10.1039/c8ob00056e
Return to citation in text: [1] -
Brehme, R.; Enders, D.; Fernandez, R.; Lassaletta, J. M. Eur. J. Org. Chem. 2007, 5629–5660. doi:10.1002/ejoc.200700746
Return to citation in text: [1] -
Tatum, L. A.; Su, X.; Aprahamian, I. Acc. Chem. Res. 2014, 47, 2141–2149. doi:10.1021/ar500111f
Return to citation in text: [1] -
Lazny, R.; Nodzewska, A. Chem. Rev. 2010, 110, 1386–1434. doi:10.1021/cr900067y
Return to citation in text: [1] -
Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626–2704. doi:10.1021/cr100204f
Return to citation in text: [1] -
Kölmel, D. K.; Kool, E. T. Chem. Rev. 2017, 117, 10358–10376. doi:10.1021/acs.chemrev.7b00090
Return to citation in text: [1] -
Cabré, A.; Verdaguer, X.; Riera, A. Chem. Rev. 2022, 122, 269–339. doi:10.1021/acs.chemrev.1c00496
Return to citation in text: [1] -
Pair, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Angew. Chem., Int. Ed. 2013, 52, 5346–5349. doi:10.1002/anie.201300782
Return to citation in text: [1] -
Streit, A. D.; Zoll, A. J.; Hoang, G. L.; Ellman, J. A. Org. Lett. 2020, 22, 1217–1221. doi:10.1021/acs.orglett.0c00186
Return to citation in text: [1] -
Zhang, M.; Duan, Y.; Li, W.; Xu, P.; Cheng, J.; Yu, S.; Zhu, C. Org. Lett. 2016, 18, 5356–5359. doi:10.1021/acs.orglett.6b02711
Return to citation in text: [1] -
Patel, H. V.; Vyas, K. A.; Pandey, S. P.; Fernandes, P. S. Tetrahedron 1996, 52, 661–668. doi:10.1016/0040-4020(95)00916-7
Return to citation in text: [1] -
Lee, V. J.; Curran, W. V.; Fields, T. F.; Learn, K. J. Heterocycl. Chem. 1988, 25, 1873–1891. doi:10.1002/jhet.5570250651
Return to citation in text: [1] [2] -
Chen, Q.; Lei, Y.; Wang, Y.; Wang, C.; Wang, Y.; Xu, Z.; Wang, H.; Wang, R. Org. Chem. Front. 2017, 4, 369–372. doi:10.1039/c6qo00676k
Return to citation in text: [1] -
Prieto, A.; Uzel, A.; Bouyssi, D.; Monteiro, N. Eur. J. Org. Chem. 2017, 4201–4204. doi:10.1002/ejoc.201700819
Return to citation in text: [1] -
Mondal, S.; Samanta, S.; Hajra, A. Eur. J. Org. Chem. 2018, 1060–1066. doi:10.1002/ejoc.201701722
Return to citation in text: [1] -
Huynh, T. N.; Ong, K. T. N.; Dinh, P. T.; Nguyen, A. T.; Nguyen, T. T. J. Org. Chem. 2024, 89, 3202–3210. doi:10.1021/acs.joc.3c02675
Return to citation in text: [1] -
Wen, J.; Zhang, L.; Yang, X.; Niu, C.; Wang, S.; Wei, W.; Sun, X.; Yang, J.; Wang, H. Green Chem. 2019, 21, 3597–3601. doi:10.1039/c9gc01351b
Return to citation in text: [1] -
Sarkar, B.; Ghosh, P.; Hajra, A. Org. Lett. 2023, 25, 3440–3444. doi:10.1021/acs.orglett.3c00999
Return to citation in text: [1] -
Yang, Q.-L.; Lei, P.-P.; Hao, E.-J.; Zhang, B.-N.; Zhou, H.-H.; Li, W.-W.; Guo, H.-M. SynOpen 2023, 7, 535–547. doi:10.1055/s-0042-1751510
Return to citation in text: [1] -
Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Org. Chem. Front. 2016, 3, 620–624. doi:10.1039/c6qo00064a
Return to citation in text: [1] -
Note that when the standard reaction was conducted in a THF/MeCN mixture (1.3:1), 2a was isolated in 82% yield.
Return to citation in text: [1] [2] [3]
| 75. | Note that when the standard reaction was conducted in a THF/MeCN mixture (1.3:1), 2a was isolated in 82% yield. |
| 73. | Yang, Q.-L.; Lei, P.-P.; Hao, E.-J.; Zhang, B.-N.; Zhou, H.-H.; Li, W.-W.; Guo, H.-M. SynOpen 2023, 7, 535–547. doi:10.1055/s-0042-1751510 |
| 74. | Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Org. Chem. Front. 2016, 3, 620–624. doi:10.1039/c6qo00064a |
| 1. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 2. | Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496–3508. doi:10.1039/c0cs00221f |
| 3. | Fujiwara, T.; O’Hagan, D. J. Fluorine Chem. 2014, 167, 16–29. doi:10.1016/j.jfluchem.2014.06.014 |
| 4. | Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832–2842. doi:10.1021/jm401375q |
| 5. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 6. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 7. | Landelle, G.; Panossian, A.; Leroux, F. R. Curr. Top. Med. Chem. 2014, 14, 941–951. doi:10.2174/1568026614666140202210016 |
| 8. | Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2014, 20, 16830–16845. doi:10.1002/chem.201404537 |
| 9. | Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a |
| 10. | Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441–5454. doi:10.1039/c6cs00351f |
| 11. | Pan, Y. ACS Med. Chem. Lett. 2019, 10, 1016–1019. doi:10.1021/acsmedchemlett.9b00235 |
| 12. | Nobile, E.; Castanheiro, T.; Besset, T. Angew. Chem., Int. Ed. 2021, 60, 12170–12191. doi:10.1002/anie.202009995 |
| 21. | Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857 |
| 22. | Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88 |
| 23. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 24. | Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e |
| 25. | Ye, K.-Y.; Zhang, X.; Dai, L.-X.; You, S.-L. J. Org. Chem. 2014, 79, 12106–12110. doi:10.1021/jo5019393 |
| 26. | Lefebvre, Q.; Fava, E.; Nikolaienko, P.; Rueping, M. Chem. Commun. 2014, 50, 6617–6619. doi:10.1039/c4cc02060j |
| 27. | Liu, J.-B.; Xu, X.-H.; Chen, Z.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 897–900. doi:10.1002/anie.201409983 |
| 28. | Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495 |
| 29. | Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. doi:10.1002/anie.201505446 |
| 30. | Yin, G.; Kalvet, I.; Schoenebeck, F. Angew. Chem., Int. Ed. 2015, 54, 6809–6813. doi:10.1002/anie.201501617 |
| 31. | Li, X.; Zhao, J.; Zhang, L.; Hu, M.; Wang, L.; Hu, J. Org. Lett. 2015, 17, 298–301. doi:10.1021/ol5034018 |
| 32. | Li, M.; Guo, J.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 264–267. doi:10.1021/acs.orglett.5b03433 |
| 33. | Candish, L.; Pitzer, L.; Gómez‐Suárez, A.; Glorius, F. Chem. – Eur. J. 2016, 22, 4753–4756. doi:10.1002/chem.201600421 |
| 34. | Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073 |
| 35. | Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524 |
| 36. | Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906–2909. doi:10.1021/acs.orglett.6b01257 |
| 37. | Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846–5850. doi:10.1002/anie.201601713 |
| 38. | Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790 |
| 39. | Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k |
| 40. | Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a |
| 41. | Lübcke, M.; Yuan, W.; Szabó, K. J. Org. Lett. 2017, 19, 4548–4551. doi:10.1021/acs.orglett.7b02139 |
| 42. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 43. | Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 3693–3696. doi:10.1002/ejoc.201800418 |
| 44. | Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Chem. Commun. 2018, 54, 9909–9912. doi:10.1039/c8cc05256e |
| 45. | Saravanan, P.; Anbarasan, P. Adv. Synth. Catal. 2018, 360, 2894–2899. doi:10.1002/adsc.201800366 |
| 46. | Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227–4230. doi:10.1021/acs.orglett.8b01627 |
| 47. | He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal. 2018, 8, 11741–11748. doi:10.1021/acscatal.8b04094 |
| 48. | Lindberg, E.; Angerani, S.; Anzola, M.; Winssinger, N. Nat. Commun. 2018, 9, 3539. doi:10.1038/s41467-018-05916-9 |
| 49. | Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690–12695. doi:10.1002/anie.201805859 |
| 50. | Luo, Z.; Yang, X.; Tsui, G. C. Org. Lett. 2020, 22, 6155–6159. doi:10.1021/acs.orglett.0c02235 |
| 51. | Doche, F.; Poisson, T.; Besset, T. ACS Catal. 2023, 13, 14112–14120. doi:10.1021/acscatal.3c03249 |
| 71. | Wen, J.; Zhang, L.; Yang, X.; Niu, C.; Wang, S.; Wei, W.; Sun, X.; Yang, J.; Wang, H. Green Chem. 2019, 21, 3597–3601. doi:10.1039/c9gc01351b |
| 17. | Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Chem. – Eur. J. 2016, 22, 16734–16749. doi:10.1002/chem.201603438 |
| 18. | Xiao, X.; Zheng, Z.-T.; Li, T.; Zheng, J.-L.; Tao, T.; Chen, L.-M.; Gu, J.-Y.; Yao, X.; Lin, J.-H.; Xiao, J.-C. Synthesis 2020, 52, 197–207. doi:10.1055/s-0039-1690714 |
| 19. | Pannecoucke, X.; Besset, T. Org. Biomol. Chem. 2019, 17, 1683–1693. doi:10.1039/c8ob02995d |
| 20. | Besset, T.; Poisson, T. Extension to the SCF2H, SCH2F, and SCF2R Motifs (R = PO(OEt)2, CO2R, Rf). In Emerging Fluorinated Motifs; Ma, A.; Cahard, D., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp 449–475. doi:10.1002/9783527824342.ch16 |
| 72. | Sarkar, B.; Ghosh, P.; Hajra, A. Org. Lett. 2023, 25, 3440–3444. doi:10.1021/acs.orglett.3c00999 |
| 69. | Mondal, S.; Samanta, S.; Hajra, A. Eur. J. Org. Chem. 2018, 1060–1066. doi:10.1002/ejoc.201701722 |
| 13. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 14. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 15. | Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476–2536. doi:10.3762/bjoc.9.287 |
| 70. | Huynh, T. N.; Ong, K. T. N.; Dinh, P. T.; Nguyen, A. T.; Nguyen, T. T. J. Org. Chem. 2024, 89, 3202–3210. doi:10.1021/acs.joc.3c02675 |
| 65. | Patel, H. V.; Vyas, K. A.; Pandey, S. P.; Fernandes, P. S. Tetrahedron 1996, 52, 661–668. doi:10.1016/0040-4020(95)00916-7 |
| 67. | Chen, Q.; Lei, Y.; Wang, Y.; Wang, C.; Wang, Y.; Xu, Z.; Wang, H.; Wang, R. Org. Chem. Front. 2017, 4, 369–372. doi:10.1039/c6qo00676k |
| 66. | Lee, V. J.; Curran, W. V.; Fields, T. F.; Learn, K. J. Heterocycl. Chem. 1988, 25, 1873–1891. doi:10.1002/jhet.5570250651 |
| 54. | Xu, P.; Li, W.; Xie, J.; Zhu, C. Acc. Chem. Res. 2018, 51, 484–495. doi:10.1021/acs.accounts.7b00565 |
| 55. | Xu, X.; Zhang, J.; Xia, H.; Wu, J. Org. Biomol. Chem. 2018, 16, 1227–1241. doi:10.1039/c8ob00056e |
| 56. | Brehme, R.; Enders, D.; Fernandez, R.; Lassaletta, J. M. Eur. J. Org. Chem. 2007, 5629–5660. doi:10.1002/ejoc.200700746 |
| 57. | Tatum, L. A.; Su, X.; Aprahamian, I. Acc. Chem. Res. 2014, 47, 2141–2149. doi:10.1021/ar500111f |
| 58. | Lazny, R.; Nodzewska, A. Chem. Rev. 2010, 110, 1386–1434. doi:10.1021/cr900067y |
| 59. | Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626–2704. doi:10.1021/cr100204f |
| 60. | Kölmel, D. K.; Kool, E. T. Chem. Rev. 2017, 117, 10358–10376. doi:10.1021/acs.chemrev.7b00090 |
| 61. | Cabré, A.; Verdaguer, X.; Riera, A. Chem. Rev. 2022, 122, 269–339. doi:10.1021/acs.chemrev.1c00496 |
| 62. | Pair, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Angew. Chem., Int. Ed. 2013, 52, 5346–5349. doi:10.1002/anie.201300782 |
| 63. | Streit, A. D.; Zoll, A. J.; Hoang, G. L.; Ellman, J. A. Org. Lett. 2020, 22, 1217–1221. doi:10.1021/acs.orglett.0c00186 |
| 64. | Zhang, M.; Duan, Y.; Li, W.; Xu, P.; Cheng, J.; Yu, S.; Zhu, C. Org. Lett. 2016, 18, 5356–5359. doi:10.1021/acs.orglett.6b02711 |
| 68. | Prieto, A.; Uzel, A.; Bouyssi, D.; Monteiro, N. Eur. J. Org. Chem. 2017, 4201–4204. doi:10.1002/ejoc.201700819 |
| 42. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 21. | Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857 |
| 22. | Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88 |
| 23. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 24. | Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e |
| 25. | Ye, K.-Y.; Zhang, X.; Dai, L.-X.; You, S.-L. J. Org. Chem. 2014, 79, 12106–12110. doi:10.1021/jo5019393 |
| 26. | Lefebvre, Q.; Fava, E.; Nikolaienko, P.; Rueping, M. Chem. Commun. 2014, 50, 6617–6619. doi:10.1039/c4cc02060j |
| 27. | Liu, J.-B.; Xu, X.-H.; Chen, Z.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 897–900. doi:10.1002/anie.201409983 |
| 28. | Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495 |
| 29. | Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. doi:10.1002/anie.201505446 |
| 30. | Yin, G.; Kalvet, I.; Schoenebeck, F. Angew. Chem., Int. Ed. 2015, 54, 6809–6813. doi:10.1002/anie.201501617 |
| 31. | Li, X.; Zhao, J.; Zhang, L.; Hu, M.; Wang, L.; Hu, J. Org. Lett. 2015, 17, 298–301. doi:10.1021/ol5034018 |
| 32. | Li, M.; Guo, J.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 264–267. doi:10.1021/acs.orglett.5b03433 |
| 33. | Candish, L.; Pitzer, L.; Gómez‐Suárez, A.; Glorius, F. Chem. – Eur. J. 2016, 22, 4753–4756. doi:10.1002/chem.201600421 |
| 34. | Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073 |
| 35. | Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524 |
| 36. | Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906–2909. doi:10.1021/acs.orglett.6b01257 |
| 37. | Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846–5850. doi:10.1002/anie.201601713 |
| 38. | Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790 |
| 39. | Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k |
| 40. | Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a |
| 41. | Lübcke, M.; Yuan, W.; Szabó, K. J. Org. Lett. 2017, 19, 4548–4551. doi:10.1021/acs.orglett.7b02139 |
| 42. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 43. | Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 3693–3696. doi:10.1002/ejoc.201800418 |
| 44. | Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Chem. Commun. 2018, 54, 9909–9912. doi:10.1039/c8cc05256e |
| 45. | Saravanan, P.; Anbarasan, P. Adv. Synth. Catal. 2018, 360, 2894–2899. doi:10.1002/adsc.201800366 |
| 46. | Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227–4230. doi:10.1021/acs.orglett.8b01627 |
| 47. | He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal. 2018, 8, 11741–11748. doi:10.1021/acscatal.8b04094 |
| 48. | Lindberg, E.; Angerani, S.; Anzola, M.; Winssinger, N. Nat. Commun. 2018, 9, 3539. doi:10.1038/s41467-018-05916-9 |
| 49. | Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690–12695. doi:10.1002/anie.201805859 |
| 50. | Luo, Z.; Yang, X.; Tsui, G. C. Org. Lett. 2020, 22, 6155–6159. doi:10.1021/acs.orglett.0c02235 |
| 51. | Doche, F.; Poisson, T.; Besset, T. ACS Catal. 2023, 13, 14112–14120. doi:10.1021/acscatal.3c03249 |
| 75. | Note that when the standard reaction was conducted in a THF/MeCN mixture (1.3:1), 2a was isolated in 82% yield. |
| 52. | Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001 |
| 53. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 66. | Lee, V. J.; Curran, W. V.; Fields, T. F.; Learn, K. J. Heterocycl. Chem. 1988, 25, 1873–1891. doi:10.1002/jhet.5570250651 |
| 75. | Note that when the standard reaction was conducted in a THF/MeCN mixture (1.3:1), 2a was isolated in 82% yield. |
© 2024 Levet et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.