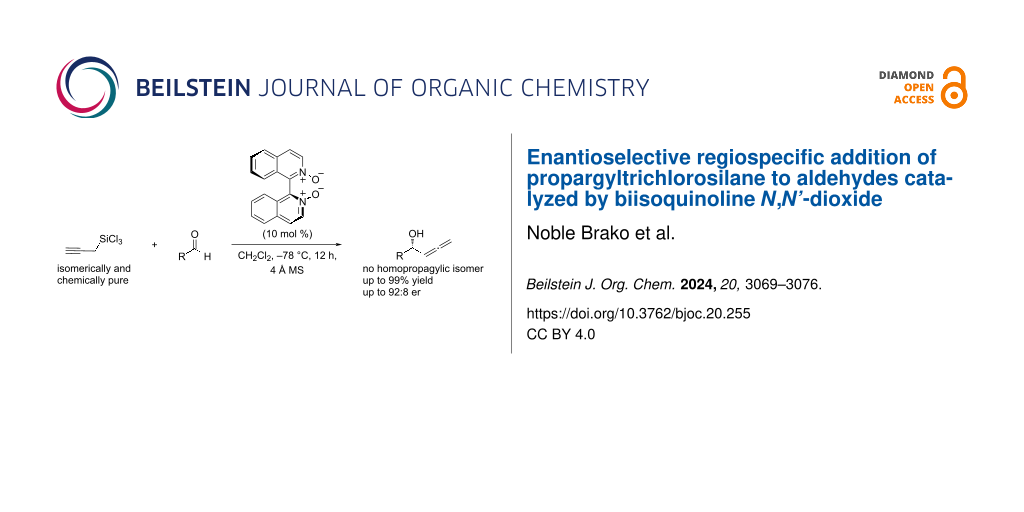
Abstract
Distilled propargyltrichlorosilane with >99% isomeric purity was prepared for the first time, and its asymmetric catalytic regiospecific addition reaction to aldehydes was developed through a systematic catalyst structure–reactivity and selectivity relationship study. The observed catalyst structure–enantioselectivity relationship of the present allenylation reaction was found exactly opposite to that of the analogous allylation reaction. The method provided eleven α-allenic alcohols in 22–99% yield with 61:39–92:8 enantiomeric ratios. Furthermore, possible mechanisms of propargyl–allenyl isomerization of propargyltrichlorosilane were computationally investigated.
Graphical Abstract
Introduction
Enantioenriched α-allenic alcohols are an important class of chiral building blocks used for the chemical synthesis of biologically relevant molecules [1-5]. Their strength comes from the rich synthetic versatility [6-9] and biological relevance [10] of the allene functionality. Accordingly, the development of efficient access to optically active chiral α-allenic alcohols continues to be of significant interest in organic chemistry [11-13]. The asymmetric catalytic addition of allenylation reagents to aldehydes provides direct access to chiral α-allenic alcohols in an enantioenriched form [11,12]. However, such metal/metalloid reagents and the corresponding metal catalyst-bound intermediates often equilibrate between possible regioisomeric forms and can undergo both, SE2 and SE2’ addition reactions, resulting in a mixture of homopropargylic alcohols and α-allenic alcohols [14-19], the separation of which is by no means trivial [20] (Scheme 1). Nonetheless, substituents at the carbon atom indicated by γ (R2) of these reagents have been shown to bias the metallotropic rearrangement and/or the kinetic reactivity of the competing regioisomeric intermediates toward electrophiles [14-19]. Consequently, all reported asymmetric catalytic aldehyde allenylation methods are currently limited to metal/metalloid reagents bearing R2 substituents [21-34], except for the methods with propargyltrichlorosilane [35,36] (Scheme 2). Other notable asymmetric catalytic approaches to prepare α-allenic alcohols (R2 = H) include the Corey–Bakshi–Shibata reduction of allenyl ketones [37], enzymatic [38-40], non-enzymatic [41] kinetic resolution of racemic α-allenic alcohols, and asymmetric 1,4-difunctionalization of borylenynes by catalytic conjugative cross-coupling [42].
Scheme 1: Metallotropic rearrangement and regioselectivity issues.
Scheme 1: Metallotropic rearrangement and regioselectivity issues.
Scheme 2: Asymmetric catalytic allenylation of aldehydes.
Scheme 2: Asymmetric catalytic allenylation of aldehydes.
Allenylation reagents that require regio-controlling auxiliaries such as a trimethylsilyl group (Scheme 2a) add steps before and/or after the reaction, thus they are less efficient in terms of atom- and step-economy [21-24,33,34]. In sharp contrast, propargyltrichlorosilane is configurationally stable and only reacts through the SE2’ mechanism under Lewis base-catalyzed conditions [43-45], although it was reported that distillation of propargyltrichlorosilane substantially isomerizes it to the thermodynamically more stable allenyltrichlorosilane that affords undesired homopropargylic alcohols [35,36] (Scheme 2b). Furthermore, Iseki [35] and Nakajima [36] evaluated only one chiral catalyst in their independent studies (i.e., no catalyst structure–reactivity and selectivity relationship study). In this context, we became interested in investigating propargyltrichlorosilane for the development of asymmetric catalytic allenylation methods [46]. It is worthy of note that propargyltrichlorosilane is an easy-to-handle liquid and only produces innoxious NaCl and SiO2 as easy-to-separate inorganic byproducts upon quenching with aqueous NaOH or NaHCO3 solutions.
Results and Discussion
Our group recently reported that N,N-diisopropylethylamine required for the synthesis of propargyltrichlorosilane isomerized it to allenyltrichlorosilane in the absence of solvents, and that removal of the amine before the distillation significantly suppressed the isomerization [46]. In this study, however, we could not prepare isomerically pure propargyltrichlorosilane on a preparative scale from propargyl chloride and CuCl by following Kobayashi’s protocol [45] (propargyltrichlorosilane/allenyltrichlorosilane = 10:1) while we fully reproduced the reported result (2 mmol scale, propargyltrichlorosilane/allenyltrichlorosilane = >99:1). In the same report, Kobayashi also described that the combination of propargyl bromide and CuF2 generated propargyltrichlorosilane faster than using propargyl chloride/CuCl without attenuating the selectivity. Although both protocols reported the same result with respect to the selectivity, we decided to test whether the combination of propargyl bromide and CuF2 could afford isomerically pure propargyltrichlorosilane on a preparative scale. To our delight, we were able to generate propargyltrichlorosilane free of allenyltrichlorosilane on a 50 mmol scale albeit in 43% chemical yield (determined by 1H NMR analysis of the reaction mixture using freshly distilled anhydrous methylene chloride as an internal standard, Scheme 3). There was no sign of allenyltrichlorosilane observable by 1H NMR in the reaction mixture, but a trace amount of it was detected after the distillation (propargyltrichlorosilane/allenyltrichlorosilane = 200:1 by 1H NMR spectroscopy, see Supporting Information File 1 for details).
Scheme 3: Selective preparation of propargyltrichlorosilane.
Scheme 3: Selective preparation of propargyltrichlorosilane.
With distilled propargyltrichlorosilane (>99% isomeric purity) in hand, we set out on our study on the allenylation reaction of benzaldehyde (1a) as model aldehyde with catalyst 3 (Scheme 4). Catalyst 3 was the only catalyst previously studied by Nakajima for propargyltrichlorosilane [36]. Ever since Nakajima reported the beneficial effect of N,N-diisopropylethylamine on the reaction rate of the aldehyde allylation reaction with allyltrichlorosilane [47], it has been routinely employed in analogous chlorosilane reactions [48-55]. However, a mechanistic basis of its role on the observed rate acceleration remains elusive while it certainly functions as a scavenger of HCl inherently present in chlorosilane reagents. Given our observation regarding propargyl–allenyl isomerization as discussed above, we wanted to avoid its use. Thus, we briefly tested whether 1) it is necessary for achieving a reasonable reaction rate, and 2) if it could be substituted with 4 Å molecular sieves as an acid scavenger. The reactions with catalyst 3 in the presence of either N,N-diisopropylethylamine or 4 Å molecular sieves gave identical results (99% yield, 78:22 er), and thus we decided to proceed with molecular sieves (Scheme 4).
Scheme 4: Evaluation of C2-symmetric catalysts with benzaldehyde (1a) as a model aldehyde. Reaction conditions: 1a (0.1 mmol), silane (0.15 mmol), catalyst (0.01 mmol), CH2Cl2 (0.4 mL); yields were determined by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane as an internal standard following workup and enantiomeric ratios were determined by HPLC on a chiral stationary phase.
Scheme 4: Evaluation of C2-symmetric catalysts with benzaldehyde (1a) as a model aldehyde. Reaction condition...
It is often the case that the narrower the chiral pocket of a catalyst is (i.e., less degree of conformational freedom for a bound substrate), the better is the enantioselectivity for analogous chlorosilane-mediated reactionss [47-49,51,54]. Therefore, we gradually narrowed the chiral pocket of catalysts from 3 to 4, 5 [53], and 6 [56,57]. To our surprise, the enantioselectivity consistently decreased as the chiral pocket became narrower while the reactivity remained the same. As such, we reduced the size of the substituents that craft the chiral pocket (7) and found that unsubstituted catalyst 8 was the most enantioselective. This observed catalyst structure–enantioselectivity relationship is exactly opposite to that for the analogous allylation reaction reported by Nakajima [47], thus it raises a possibility that the asymmetric induction mechanism could be fundamentally different between the present allenylation with propargyltrichlorosilane and the extensively investigated allylation with allyltrichlorosilane [58,59].
In light of the excellent reactivity and promising selectivity displayed by catalyst 8 for the model reaction, we proceeded to evaluate its ability to enantioselectively promote the allenylation of various aldehydes (Scheme 5). The catalyst tolerated all p-, m-, o-Cl-substituted benzaldehydes in terms of both reactivity and selectivity, affording the essentially same results as benzaldehyde (2b–d). Other electron-deficient substituents CF3 (2e) and Br (2f) did not adversely affect the reaction. However, electron-donating substituents (Me and MeO) on the benzene ring substantially lowered the chemical yields while they did not affect the enantioselectivity (2g,h). Likewise, electron-rich aldehydes 1i,j as well as the aliphatic aldehyde 1k provided moderate yields. These aldehydes gave substantially lower enantioselectivities than benzaldehydes, which may be attributable to that they have smaller steric demands in the vicinity of the carbonyl carbon atom than benzaldehyde. Importantly, we did not observe the corresponding homopropargylic alcohols [51] in all cases. Since this work is the first asymmetric catalysis study of isomerically pure propargyltrichlorosilane, it clearly demonstrated that this class of chiral Lewis bases regiospecifically catalyzed the addition of propargyltrichlorosilane to aldehydes, and that these catalysts did not induce the propargyl–allenyl metallotropic rearrangement albeit activating the C–Si bond. Thus, these findings underscore the importance of propargyltrichlorosilane as a regiospecific allenylation reagent and bode well for the development of new reactions.
Scheme 5: Evaluation of the extent to which (S)-8 catalyzed the allenylation reaction. Reaction conditions: aldehyde 1a–k (0.1 mmol), silane (0.15 mmol), (S)-8 (0.01 mmol), CH2Cl2 (0.4 mL). Enantiomeric ratios were determined by HPLC on a chiral stationary phase, yields were determined by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane as an internal standard following workup and isolated yields are given in parentheses. aReaction conditions: 1a (1.0 mmol), silane (1.5 mmol), (S)-8 (0.1 mmol), CH2Cl2 (4.0 mL).
Scheme 5: Evaluation of the extent to which (S)-8 catalyzed the allenylation reaction. Reaction conditions: a...
As we recently reported [46], we noticed during our initial investigation that propargyltrichlorosilane underwent isomerization to allenyltrichlorosilane in the presence of N,N-diisopropylethylamine upon standing after distillation (i.e., without solvent). Although the base-promoted propargyl–allenyl isomerization is well precedented in literature [60,61], we decided to investigate possible mechanisms of the propargyltrichlorosilane isomerization in the absence and presence of N,N-diisopropylethylamine using density functional theory (DFT) calculations (Figure 1).
![[1860-5397-20-255-1]](/bjoc/content/figures/1860-5397-20-255-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: A potential energy surface (PES) for the proposed mechanism for (a) isomerization of propargyltrichlorosilane without N,N-diisopropylethylamine and (b) with N,N-diisopropylethylamine. It includes Gibbs free energies (kcal/mol) and Mulliken charges (in parentheses).
Figure 1: A potential energy surface (PES) for the proposed mechanism for (a) isomerization of propargyltrich...
According to the DFT calculations, the free propargyltrichlorosilane (Iprop) can isomerize to allenyltrichlorosilane (Iall) with a prohibitively high barrier of 43.8 kcal/mol (Figure 1a). This suggests that the isomerization is not energetically feasible on its own. However, in the presence of N,N-diisopropylethylamine this process can occur readily. In the reactant (Rprop in Figure 1b), the nitrogen atom of N,N-diisopropylethylamine forms a hydrogen bond with the H1 proton of propargyltrichlorosilane and significantly activates the C1–H1 bond (1.11 Å). It is noteworthy that the nitrogen atom can also interact with the Si atom of propargyltrichlorosilane which is a stronger electrophile compared to the H1 atom. However, the bulky groups around nitrogen and three chlorine atoms coordinated to Si prevent a direct Si–N interaction. From Rprop, the amine group of N,N-diisopropylethylamine abstracts the H1 proton with a barrier of 14.2 kcal/mol to form an intermediate (IN1). The intermediate IN1 is unstable (endergonic by 14.0 kcal/mol) and will immediately stabilize to another intermediate (IN2) which is 1.2 kcal/mol lower in energy than IN1. The IN1→IN2 transformation is driven by the redistribution of the negative charge on C1 in IN1. In particular, the Mulliken charge on C1 reduces from −0.77e to −0.66e and the charge on C3 increases from −0.43e to −0.55e and facilitate Coulombic interaction between C3 and H1. In the next step, from IN2, in a barrier-less process the C3 atom abstracts the H1 proton that was acquired by the N atom in the previous step to generate allenyltrichlorosilane (Rall). Overall, the Rprop→Rall isomerization is almost thermoneutral, i.e., Rall being 1.7 kcal/mol exergonic from Rprop. These results show that the presence of N,N-diisopropylethylamine as base makes this process more energetically feasible by substantially stabilizing the transition states and intermediates in the pathway. These results are strongly supported by the experimental data. In a previous study, Hoveyda and co-workers proposed a similar mechanism for the isomerization of alkynes to allenes catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene [61].
Conclusion
In this study, we prepared distilled propargyltrichlorosilane with >99% isomeric purity for the first time, developed its asymmetric catalytic regiospecific addition reaction to aldehydes via a systematic catalyst structure–reactivity and selectivity relationship study, and computationally investigated possible mechanisms of N,N-diisopropylethylamine-promoted propargyl–allenyl isomerization of propargyltrichlorosilane. The observed catalyst structure–enantioselectivity relationship of the present allenylation reaction was found exactly opposite to that of the extensively investigated analogous allylation reaction, the findings of which raises a possibility that asymmetric induction mechanisms could be fundamentally different between the two transformations. Studies directed toward a better understanding of possible transition-state structures and the design of new catalysts to improve the results are currently underway in our laboratories.
Supporting Information
| Supporting Information File 1: Experimental details, characterization data, spectra, and HPLC traces. | ||
| Format: PDF | Size: 8.8 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063–1069. doi:10.1351/pac200880051063
Return to citation in text: [1] -
Alonso, J. M.; Almendros, P. Chem. Rev. 2021, 121, 4193–4252. doi:10.1021/acs.chemrev.0c00986
Return to citation in text: [1] -
Yoshida, M.; Shoji, Y.; Shishido, K. Org. Lett. 2009, 11, 1441–1443. doi:10.1021/ol9001637
Return to citation in text: [1] -
Wang, Y.; Hoen, R.; Hong, R. Synlett 2012, 23, 2729–2734. doi:10.1055/s-0032-1317566
Return to citation in text: [1] -
Roy, A.; Bhat, B. A.; Lepore, S. D. Org. Lett. 2015, 17, 900–903. doi:10.1021/ol503757h
Return to citation in text: [1] -
Yu, S.; Ma, S. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. doi:10.1002/anie.201101460
Return to citation in text: [1] -
Lechel, T.; Pfrengle, F.; Reissig, H.-U.; Zimmer, R. ChemCatChem 2013, 5, 2100–2130. doi:10.1002/cctc.201200875
Return to citation in text: [1] -
Adams, C. S.; Weatherly, C. D.; Burke, E. G.; Schomaker, J. M. Chem. Soc. Rev. 2014, 43, 3136–3163. doi:10.1039/c3cs60416k
Return to citation in text: [1] -
Muñoz, M. P. Chem. Soc. Rev. 2014, 43, 3164–3183. doi:10.1039/c3cs60408j
Return to citation in text: [1] -
Hoffmann‐Röder, A.; Krause, N. Angew. Chem., Int. Ed. 2004, 43, 1196–1216. doi:10.1002/anie.200300628
Return to citation in text: [1] -
Yamamoto, H.; Usanov, D. L. Propargyl and Allenyl Organometallics. Comprehensive Organic Synthesis, 2nd ed.; Elsevier, 2014; Vol. 2, pp 209–242. doi:10.1016/b978-0-08-097742-3.00206-8
Return to citation in text: [1] [2] -
Thaima, T.; Zamani, F.; Hyland, C. J. T.; Pyne, S. G. Synthesis 2017, 49, 1461–1480. doi:10.1055/s-0036-1588397
Return to citation in text: [1] [2] -
Huang, X.; Ma, S. Acc. Chem. Res. 2019, 52, 1301–1312. doi:10.1021/acs.accounts.9b00023
Return to citation in text: [1] -
Wisniewska, H. M.; Jarvo, E. R. J. Org. Chem. 2013, 78, 11629–11636. doi:10.1021/jo4019107
Return to citation in text: [1] [2] -
Lin, M.-J.; Loh, T.-P. J. Am. Chem. Soc. 2003, 125, 13042–13043. doi:10.1021/ja037410i
Return to citation in text: [1] [2] -
Guo, L.-N.; Gao, H.; Mayer, P.; Knochel, P. Chem. – Eur. J. 2010, 16, 9829–9834. doi:10.1002/chem.201000523
Return to citation in text: [1] [2] -
Fandrick, K. R.; Ogikubo, J.; Fandrick, D. R.; Patel, N. D.; Saha, J.; Lee, H.; Ma, S.; Grinberg, N.; Busacca, C. A.; Senanayake, C. H. Org. Lett. 2013, 15, 1214–1217. doi:10.1021/ol400124f
Return to citation in text: [1] [2] -
Zhang, R.; Xia, Y.; Yan, Y.; Ouyang, L. BMC Chem. 2022, 16, 14. doi:10.1186/s13065-022-00803-3
Return to citation in text: [1] [2] -
Dai, X.-L.; Ran, J.; Rajeshkumar, T.; Xu, Z.; Liu, S.; Lv, Z.; Maron, L.; Chen, Y.-H. Org. Lett. 2023, 25, 3060–3065. doi:10.1021/acs.orglett.3c00824
Return to citation in text: [1] [2] -
Fu, F.; Hoang, K. L. M.; Loh, T.-P. Org. Lett. 2008, 10, 3437–3439. doi:10.1021/ol801087s
Return to citation in text: [1] -
Yu, C.-M.; Yoon, S.-K.; Baek, K.; Lee, J.-Y. Angew. Chem., Int. Ed. 1998, 37, 2392–2395. doi:10.1002/(sici)1521-3773(19980918)37:17<2392::aid-anie2392>3.0.co;2-d
Return to citation in text: [1] [2] -
Inoue, M.; Nakada, M. Angew. Chem., Int. Ed. 2006, 45, 252–255. doi:10.1002/anie.200502871
Return to citation in text: [1] [2] -
Xia, G.; Yamamoto, H. J. Am. Chem. Soc. 2007, 129, 496–497. doi:10.1021/ja0679578
Return to citation in text: [1] [2] -
Reddy, L. R. Chem. Commun. 2012, 48, 9189–9191. doi:10.1039/c2cc34371a
Return to citation in text: [1] [2] -
Yu, C.-M.; Yoon, S.-K.; Lee, S.-J.; Lee, J.-Y.; Yoon, S.-K.; Kim, S. S. Chem. Commun. 1998, 2749–2750. doi:10.1039/a807940d
Return to citation in text: [1] -
Durán‐Galván, M.; Connell, B. T. Eur. J. Org. Chem. 2010, 2445–2448. doi:10.1002/ejoc.201000199
Return to citation in text: [1] -
Durán-Galván, M.; Worlikar, S. A.; Connell, B. T. Tetrahedron 2010, 66, 7707–7719. doi:10.1016/j.tet.2010.07.065
Return to citation in text: [1] -
Wang, M.; Khan, S.; Miliordos, E.; Chen, M. Adv. Synth. Catal. 2018, 360, 4634–4639. doi:10.1002/adsc.201801080
Return to citation in text: [1] -
Yanagisawa, A.; Bamba, K.; Kawada, A. ChemistrySelect 2018, 3, 13777–13781. doi:10.1002/slct.201802999
Return to citation in text: [1] -
Tap, A.; Blond, A.; Wakchaure, V. N.; List, B. Angew. Chem., Int. Ed. 2016, 55, 8962–8965. doi:10.1002/anie.201603649
Return to citation in text: [1] -
Zhong, F.; Xue, Q.-Y.; Yin, L. Angew. Chem., Int. Ed. 2020, 59, 1562–1566. doi:10.1002/anie.201912140
Return to citation in text: [1] -
Xu, G.; Wang, Z.; Shao, Y.; Sun, J. Org. Lett. 2021, 23, 5175–5179. doi:10.1021/acs.orglett.1c01712
Return to citation in text: [1] -
Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Nat. Commun. 2022, 13, 5036. doi:10.1038/s41467-022-32614-4
Return to citation in text: [1] [2] -
Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Angew. Chem., Int. Ed. 2022, 61, e202117114. doi:10.1002/anie.202117114
Return to citation in text: [1] [2] -
Iseki, K.; Kuroki, Y.; Kobayashi, Y. Tetrahedron: Asymmetry 1998, 9, 2889–2894. doi:10.1016/s0957-4166(98)00290-0
Return to citation in text: [1] [2] [3] -
Nakajima, M.; Saito, M.; Hashimoto, S. Tetrahedron: Asymmetry 2002, 13, 2449–2452. doi:10.1016/s0957-4166(02)00640-7
Return to citation in text: [1] [2] [3] [4] -
Yu, C.-M.; Kim, C.; Kweon, J.-H. Chem. Commun. 2004, 2494–2495. doi:10.1039/b407387h
Return to citation in text: [1] -
Yang, B.; Zhu, C.; Qiu, Y.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2016, 55, 5568–5572. doi:10.1002/anie.201601505
Return to citation in text: [1] -
Li, W.; Lin, Z.; Chen, L.; Tian, X.; Wang, Y.; Huang, S.-H.; Hong, R. Tetrahedron Lett. 2016, 57, 603–606. doi:10.1016/j.tetlet.2015.12.098
Return to citation in text: [1] -
Zhang, T.; Zhu, C. Synlett 2024, 35, 1170–1174. doi:10.1055/s-0042-1751525
Return to citation in text: [1] -
Wang, Y.; Zheng, K.; Hong, R. J. Am. Chem. Soc. 2012, 134, 4096–4099. doi:10.1021/ja300453u
Return to citation in text: [1] -
Law, C.; Kativhu, E.; Wang, J.; Morken, J. P. Angew. Chem., Int. Ed. 2020, 59, 10311–10315. doi:10.1002/anie.202001580
Return to citation in text: [1] -
Kobayashi, S.; Nishio, K. J. Am. Chem. Soc. 1995, 117, 6392–6393. doi:10.1021/ja00128a043
Return to citation in text: [1] -
Schneider, U.; Sugiura, M.; Kobayashi, S. Tetrahedron 2006, 62, 496–502. doi:10.1016/j.tet.2005.08.114
Return to citation in text: [1] -
Schneider, U.; Sugiura, M.; Kobayashi, S. Adv. Synth. Catal. 2006, 348, 323–329. doi:10.1002/adsc.200505379
Return to citation in text: [1] [2] -
Xu, C.; Nader, P.; Xavier, J.; Takenaka, N. Synlett 2023, 34, 2461–2464. doi:10.1055/s-0042-1751478
Return to citation in text: [1] [2] [3] -
Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S.-i. J. Am. Chem. Soc. 1998, 120, 6419–6420. doi:10.1021/ja981091r
Return to citation in text: [1] [2] [3] -
Shimada, T.; Kina, A.; Ikeda, S.; Hayashi, T. Org. Lett. 2002, 4, 2799–2801. doi:10.1021/ol026376u
Return to citation in text: [1] [2] -
Malkov, A. V.; Westwater, M.-M.; Gutnov, A.; Ramírez-López, P.; Friscourt, F.; Kadlčíková, A.; Hodačová, J.; Rankovic, Z.; Kotora, M.; Kočovský, P. Tetrahedron 2008, 64, 11335–11348. doi:10.1016/j.tet.2008.08.084
Return to citation in text: [1] [2] -
Kadlčíková, A.; Valterová, I.; Ducháčková, L.; Roithová, J.; Kotora, M. Chem. – Eur. J. 2010, 16, 9442–9445. doi:10.1002/chem.201001523
Return to citation in text: [1] -
Chen, J.; Captain, B.; Takenaka, N. Org. Lett. 2011, 13, 1654–1657. doi:10.1021/ol200102c
Return to citation in text: [1] [2] [3] -
Huang, Y.; Yang, L.; Shao, P.; Zhao, Y. Chem. Sci. 2013, 4, 3275–3281. doi:10.1039/c3sc50973g
Return to citation in text: [1] -
Reep, C.; Morgante, P.; Peverati, R.; Takenaka, N. Org. Lett. 2018, 20, 5757–5761. doi:10.1021/acs.orglett.8b02457
Return to citation in text: [1] [2] -
Vaganov, V. Y.; Fukazawa, Y.; Kondratyev, N. S.; Shipilovskikh, S. A.; Wheeler, S. E.; Rubtsov, A. E.; Malkov, A. V. Adv. Synth. Catal. 2020, 362, 5467–5474. doi:10.1002/adsc.202000936
Return to citation in text: [1] [2] -
Xu, C.; Nader, P.; Xavier, J.; Captain, B.; Takenaka, N. Tetrahedron 2023, 141, 133496. doi:10.1016/j.tet.2023.133496
Return to citation in text: [1] -
Sun, S.; Reep, C.; Zhang, C.; Captain, B.; Peverati, R.; Takenaka, N. Tetrahedron Lett. 2021, 81, 153338. doi:10.1016/j.tetlet.2021.153338
Return to citation in text: [1] -
Sun, S.; Xu, C.; Jarvis, J.; Nader, P.; Naumann, B.; Soliven, A.; Peverati, R.; Takenaka, N. Catalysts 2021, 11, 1103. doi:10.3390/catal11091103
Return to citation in text: [1] -
Fu, J.; Fujimori, S.; Denmark, S. E. Bifunctional Lewis Base Catalysis with Dual Activation of X 3 Si–Nu and C=O (n → σ*). In Lewis Base Catalysis in Organic Synthesis; Vedejs, E.; Denmark, S. E., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp 281–338. doi:10.1002/9783527675142.ch9
Return to citation in text: [1] -
Malkov, A. V.; Kočovský, P. Lewis Base-Catalyzed Reactions of SiX 3-Based reagents with C=O, C=N (n→σ*). In Lewis Base Catalysis in Organic Synthesis; Vedejs, E.; Denmark, S. E., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp 1011–1038. doi:10.1002/9783527675142.ch20
Return to citation in text: [1] -
Xing, Y.; Wei, Y.; Zhou, H. Curr. Org. Chem. 2012, 16, 1594–1608. doi:10.2174/138527212800840973
Return to citation in text: [1] -
Dabrowski, J. A.; Haeffner, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2013, 52, 7694–7699. doi:10.1002/anie.201303501
Return to citation in text: [1] [2]
| 56. | Sun, S.; Reep, C.; Zhang, C.; Captain, B.; Peverati, R.; Takenaka, N. Tetrahedron Lett. 2021, 81, 153338. doi:10.1016/j.tetlet.2021.153338 |
| 57. | Sun, S.; Xu, C.; Jarvis, J.; Nader, P.; Naumann, B.; Soliven, A.; Peverati, R.; Takenaka, N. Catalysts 2021, 11, 1103. doi:10.3390/catal11091103 |
| 47. | Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S.-i. J. Am. Chem. Soc. 1998, 120, 6419–6420. doi:10.1021/ja981091r |
| 58. | Fu, J.; Fujimori, S.; Denmark, S. E. Bifunctional Lewis Base Catalysis with Dual Activation of X 3 Si–Nu and C=O (n → σ*). In Lewis Base Catalysis in Organic Synthesis; Vedejs, E.; Denmark, S. E., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp 281–338. doi:10.1002/9783527675142.ch9 |
| 59. | Malkov, A. V.; Kočovský, P. Lewis Base-Catalyzed Reactions of SiX 3-Based reagents with C=O, C=N (n→σ*). In Lewis Base Catalysis in Organic Synthesis; Vedejs, E.; Denmark, S. E., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp 1011–1038. doi:10.1002/9783527675142.ch20 |
| 1. | Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063–1069. doi:10.1351/pac200880051063 |
| 2. | Alonso, J. M.; Almendros, P. Chem. Rev. 2021, 121, 4193–4252. doi:10.1021/acs.chemrev.0c00986 |
| 3. | Yoshida, M.; Shoji, Y.; Shishido, K. Org. Lett. 2009, 11, 1441–1443. doi:10.1021/ol9001637 |
| 4. | Wang, Y.; Hoen, R.; Hong, R. Synlett 2012, 23, 2729–2734. doi:10.1055/s-0032-1317566 |
| 5. | Roy, A.; Bhat, B. A.; Lepore, S. D. Org. Lett. 2015, 17, 900–903. doi:10.1021/ol503757h |
| 11. | Yamamoto, H.; Usanov, D. L. Propargyl and Allenyl Organometallics. Comprehensive Organic Synthesis, 2nd ed.; Elsevier, 2014; Vol. 2, pp 209–242. doi:10.1016/b978-0-08-097742-3.00206-8 |
| 12. | Thaima, T.; Zamani, F.; Hyland, C. J. T.; Pyne, S. G. Synthesis 2017, 49, 1461–1480. doi:10.1055/s-0036-1588397 |
| 21. | Yu, C.-M.; Yoon, S.-K.; Baek, K.; Lee, J.-Y. Angew. Chem., Int. Ed. 1998, 37, 2392–2395. doi:10.1002/(sici)1521-3773(19980918)37:17<2392::aid-anie2392>3.0.co;2-d |
| 22. | Inoue, M.; Nakada, M. Angew. Chem., Int. Ed. 2006, 45, 252–255. doi:10.1002/anie.200502871 |
| 23. | Xia, G.; Yamamoto, H. J. Am. Chem. Soc. 2007, 129, 496–497. doi:10.1021/ja0679578 |
| 24. | Reddy, L. R. Chem. Commun. 2012, 48, 9189–9191. doi:10.1039/c2cc34371a |
| 33. | Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Nat. Commun. 2022, 13, 5036. doi:10.1038/s41467-022-32614-4 |
| 34. | Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Angew. Chem., Int. Ed. 2022, 61, e202117114. doi:10.1002/anie.202117114 |
| 11. | Yamamoto, H.; Usanov, D. L. Propargyl and Allenyl Organometallics. Comprehensive Organic Synthesis, 2nd ed.; Elsevier, 2014; Vol. 2, pp 209–242. doi:10.1016/b978-0-08-097742-3.00206-8 |
| 12. | Thaima, T.; Zamani, F.; Hyland, C. J. T.; Pyne, S. G. Synthesis 2017, 49, 1461–1480. doi:10.1055/s-0036-1588397 |
| 13. | Huang, X.; Ma, S. Acc. Chem. Res. 2019, 52, 1301–1312. doi:10.1021/acs.accounts.9b00023 |
| 43. | Kobayashi, S.; Nishio, K. J. Am. Chem. Soc. 1995, 117, 6392–6393. doi:10.1021/ja00128a043 |
| 44. | Schneider, U.; Sugiura, M.; Kobayashi, S. Tetrahedron 2006, 62, 496–502. doi:10.1016/j.tet.2005.08.114 |
| 45. | Schneider, U.; Sugiura, M.; Kobayashi, S. Adv. Synth. Catal. 2006, 348, 323–329. doi:10.1002/adsc.200505379 |
| 10. | Hoffmann‐Röder, A.; Krause, N. Angew. Chem., Int. Ed. 2004, 43, 1196–1216. doi:10.1002/anie.200300628 |
| 41. | Wang, Y.; Zheng, K.; Hong, R. J. Am. Chem. Soc. 2012, 134, 4096–4099. doi:10.1021/ja300453u |
| 6. | Yu, S.; Ma, S. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. doi:10.1002/anie.201101460 |
| 7. | Lechel, T.; Pfrengle, F.; Reissig, H.-U.; Zimmer, R. ChemCatChem 2013, 5, 2100–2130. doi:10.1002/cctc.201200875 |
| 8. | Adams, C. S.; Weatherly, C. D.; Burke, E. G.; Schomaker, J. M. Chem. Soc. Rev. 2014, 43, 3136–3163. doi:10.1039/c3cs60416k |
| 9. | Muñoz, M. P. Chem. Soc. Rev. 2014, 43, 3164–3183. doi:10.1039/c3cs60408j |
| 42. | Law, C.; Kativhu, E.; Wang, J.; Morken, J. P. Angew. Chem., Int. Ed. 2020, 59, 10311–10315. doi:10.1002/anie.202001580 |
| 21. | Yu, C.-M.; Yoon, S.-K.; Baek, K.; Lee, J.-Y. Angew. Chem., Int. Ed. 1998, 37, 2392–2395. doi:10.1002/(sici)1521-3773(19980918)37:17<2392::aid-anie2392>3.0.co;2-d |
| 22. | Inoue, M.; Nakada, M. Angew. Chem., Int. Ed. 2006, 45, 252–255. doi:10.1002/anie.200502871 |
| 23. | Xia, G.; Yamamoto, H. J. Am. Chem. Soc. 2007, 129, 496–497. doi:10.1021/ja0679578 |
| 24. | Reddy, L. R. Chem. Commun. 2012, 48, 9189–9191. doi:10.1039/c2cc34371a |
| 25. | Yu, C.-M.; Yoon, S.-K.; Lee, S.-J.; Lee, J.-Y.; Yoon, S.-K.; Kim, S. S. Chem. Commun. 1998, 2749–2750. doi:10.1039/a807940d |
| 26. | Durán‐Galván, M.; Connell, B. T. Eur. J. Org. Chem. 2010, 2445–2448. doi:10.1002/ejoc.201000199 |
| 27. | Durán-Galván, M.; Worlikar, S. A.; Connell, B. T. Tetrahedron 2010, 66, 7707–7719. doi:10.1016/j.tet.2010.07.065 |
| 28. | Wang, M.; Khan, S.; Miliordos, E.; Chen, M. Adv. Synth. Catal. 2018, 360, 4634–4639. doi:10.1002/adsc.201801080 |
| 29. | Yanagisawa, A.; Bamba, K.; Kawada, A. ChemistrySelect 2018, 3, 13777–13781. doi:10.1002/slct.201802999 |
| 30. | Tap, A.; Blond, A.; Wakchaure, V. N.; List, B. Angew. Chem., Int. Ed. 2016, 55, 8962–8965. doi:10.1002/anie.201603649 |
| 31. | Zhong, F.; Xue, Q.-Y.; Yin, L. Angew. Chem., Int. Ed. 2020, 59, 1562–1566. doi:10.1002/anie.201912140 |
| 32. | Xu, G.; Wang, Z.; Shao, Y.; Sun, J. Org. Lett. 2021, 23, 5175–5179. doi:10.1021/acs.orglett.1c01712 |
| 33. | Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Nat. Commun. 2022, 13, 5036. doi:10.1038/s41467-022-32614-4 |
| 34. | Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Angew. Chem., Int. Ed. 2022, 61, e202117114. doi:10.1002/anie.202117114 |
| 37. | Yu, C.-M.; Kim, C.; Kweon, J.-H. Chem. Commun. 2004, 2494–2495. doi:10.1039/b407387h |
| 60. | Xing, Y.; Wei, Y.; Zhou, H. Curr. Org. Chem. 2012, 16, 1594–1608. doi:10.2174/138527212800840973 |
| 61. | Dabrowski, J. A.; Haeffner, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2013, 52, 7694–7699. doi:10.1002/anie.201303501 |
| 14. | Wisniewska, H. M.; Jarvo, E. R. J. Org. Chem. 2013, 78, 11629–11636. doi:10.1021/jo4019107 |
| 15. | Lin, M.-J.; Loh, T.-P. J. Am. Chem. Soc. 2003, 125, 13042–13043. doi:10.1021/ja037410i |
| 16. | Guo, L.-N.; Gao, H.; Mayer, P.; Knochel, P. Chem. – Eur. J. 2010, 16, 9829–9834. doi:10.1002/chem.201000523 |
| 17. | Fandrick, K. R.; Ogikubo, J.; Fandrick, D. R.; Patel, N. D.; Saha, J.; Lee, H.; Ma, S.; Grinberg, N.; Busacca, C. A.; Senanayake, C. H. Org. Lett. 2013, 15, 1214–1217. doi:10.1021/ol400124f |
| 18. | Zhang, R.; Xia, Y.; Yan, Y.; Ouyang, L. BMC Chem. 2022, 16, 14. doi:10.1186/s13065-022-00803-3 |
| 19. | Dai, X.-L.; Ran, J.; Rajeshkumar, T.; Xu, Z.; Liu, S.; Lv, Z.; Maron, L.; Chen, Y.-H. Org. Lett. 2023, 25, 3060–3065. doi:10.1021/acs.orglett.3c00824 |
| 38. | Yang, B.; Zhu, C.; Qiu, Y.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2016, 55, 5568–5572. doi:10.1002/anie.201601505 |
| 39. | Li, W.; Lin, Z.; Chen, L.; Tian, X.; Wang, Y.; Huang, S.-H.; Hong, R. Tetrahedron Lett. 2016, 57, 603–606. doi:10.1016/j.tetlet.2015.12.098 |
| 40. | Zhang, T.; Zhu, C. Synlett 2024, 35, 1170–1174. doi:10.1055/s-0042-1751525 |
| 61. | Dabrowski, J. A.; Haeffner, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2013, 52, 7694–7699. doi:10.1002/anie.201303501 |
| 20. | Fu, F.; Hoang, K. L. M.; Loh, T.-P. Org. Lett. 2008, 10, 3437–3439. doi:10.1021/ol801087s |
| 51. | Chen, J.; Captain, B.; Takenaka, N. Org. Lett. 2011, 13, 1654–1657. doi:10.1021/ol200102c |
| 14. | Wisniewska, H. M.; Jarvo, E. R. J. Org. Chem. 2013, 78, 11629–11636. doi:10.1021/jo4019107 |
| 15. | Lin, M.-J.; Loh, T.-P. J. Am. Chem. Soc. 2003, 125, 13042–13043. doi:10.1021/ja037410i |
| 16. | Guo, L.-N.; Gao, H.; Mayer, P.; Knochel, P. Chem. – Eur. J. 2010, 16, 9829–9834. doi:10.1002/chem.201000523 |
| 17. | Fandrick, K. R.; Ogikubo, J.; Fandrick, D. R.; Patel, N. D.; Saha, J.; Lee, H.; Ma, S.; Grinberg, N.; Busacca, C. A.; Senanayake, C. H. Org. Lett. 2013, 15, 1214–1217. doi:10.1021/ol400124f |
| 18. | Zhang, R.; Xia, Y.; Yan, Y.; Ouyang, L. BMC Chem. 2022, 16, 14. doi:10.1186/s13065-022-00803-3 |
| 19. | Dai, X.-L.; Ran, J.; Rajeshkumar, T.; Xu, Z.; Liu, S.; Lv, Z.; Maron, L.; Chen, Y.-H. Org. Lett. 2023, 25, 3060–3065. doi:10.1021/acs.orglett.3c00824 |
| 35. | Iseki, K.; Kuroki, Y.; Kobayashi, Y. Tetrahedron: Asymmetry 1998, 9, 2889–2894. doi:10.1016/s0957-4166(98)00290-0 |
| 36. | Nakajima, M.; Saito, M.; Hashimoto, S. Tetrahedron: Asymmetry 2002, 13, 2449–2452. doi:10.1016/s0957-4166(02)00640-7 |
| 46. | Xu, C.; Nader, P.; Xavier, J.; Takenaka, N. Synlett 2023, 34, 2461–2464. doi:10.1055/s-0042-1751478 |
| 36. | Nakajima, M.; Saito, M.; Hashimoto, S. Tetrahedron: Asymmetry 2002, 13, 2449–2452. doi:10.1016/s0957-4166(02)00640-7 |
| 35. | Iseki, K.; Kuroki, Y.; Kobayashi, Y. Tetrahedron: Asymmetry 1998, 9, 2889–2894. doi:10.1016/s0957-4166(98)00290-0 |
| 36. | Nakajima, M.; Saito, M.; Hashimoto, S. Tetrahedron: Asymmetry 2002, 13, 2449–2452. doi:10.1016/s0957-4166(02)00640-7 |
| 35. | Iseki, K.; Kuroki, Y.; Kobayashi, Y. Tetrahedron: Asymmetry 1998, 9, 2889–2894. doi:10.1016/s0957-4166(98)00290-0 |
| 47. | Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S.-i. J. Am. Chem. Soc. 1998, 120, 6419–6420. doi:10.1021/ja981091r |
| 48. | Shimada, T.; Kina, A.; Ikeda, S.; Hayashi, T. Org. Lett. 2002, 4, 2799–2801. doi:10.1021/ol026376u |
| 49. | Malkov, A. V.; Westwater, M.-M.; Gutnov, A.; Ramírez-López, P.; Friscourt, F.; Kadlčíková, A.; Hodačová, J.; Rankovic, Z.; Kotora, M.; Kočovský, P. Tetrahedron 2008, 64, 11335–11348. doi:10.1016/j.tet.2008.08.084 |
| 51. | Chen, J.; Captain, B.; Takenaka, N. Org. Lett. 2011, 13, 1654–1657. doi:10.1021/ol200102c |
| 54. | Vaganov, V. Y.; Fukazawa, Y.; Kondratyev, N. S.; Shipilovskikh, S. A.; Wheeler, S. E.; Rubtsov, A. E.; Malkov, A. V. Adv. Synth. Catal. 2020, 362, 5467–5474. doi:10.1002/adsc.202000936 |
| 53. | Reep, C.; Morgante, P.; Peverati, R.; Takenaka, N. Org. Lett. 2018, 20, 5757–5761. doi:10.1021/acs.orglett.8b02457 |
| 47. | Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S.-i. J. Am. Chem. Soc. 1998, 120, 6419–6420. doi:10.1021/ja981091r |
| 48. | Shimada, T.; Kina, A.; Ikeda, S.; Hayashi, T. Org. Lett. 2002, 4, 2799–2801. doi:10.1021/ol026376u |
| 49. | Malkov, A. V.; Westwater, M.-M.; Gutnov, A.; Ramírez-López, P.; Friscourt, F.; Kadlčíková, A.; Hodačová, J.; Rankovic, Z.; Kotora, M.; Kočovský, P. Tetrahedron 2008, 64, 11335–11348. doi:10.1016/j.tet.2008.08.084 |
| 50. | Kadlčíková, A.; Valterová, I.; Ducháčková, L.; Roithová, J.; Kotora, M. Chem. – Eur. J. 2010, 16, 9442–9445. doi:10.1002/chem.201001523 |
| 51. | Chen, J.; Captain, B.; Takenaka, N. Org. Lett. 2011, 13, 1654–1657. doi:10.1021/ol200102c |
| 52. | Huang, Y.; Yang, L.; Shao, P.; Zhao, Y. Chem. Sci. 2013, 4, 3275–3281. doi:10.1039/c3sc50973g |
| 53. | Reep, C.; Morgante, P.; Peverati, R.; Takenaka, N. Org. Lett. 2018, 20, 5757–5761. doi:10.1021/acs.orglett.8b02457 |
| 54. | Vaganov, V. Y.; Fukazawa, Y.; Kondratyev, N. S.; Shipilovskikh, S. A.; Wheeler, S. E.; Rubtsov, A. E.; Malkov, A. V. Adv. Synth. Catal. 2020, 362, 5467–5474. doi:10.1002/adsc.202000936 |
| 55. | Xu, C.; Nader, P.; Xavier, J.; Captain, B.; Takenaka, N. Tetrahedron 2023, 141, 133496. doi:10.1016/j.tet.2023.133496 |
| 45. | Schneider, U.; Sugiura, M.; Kobayashi, S. Adv. Synth. Catal. 2006, 348, 323–329. doi:10.1002/adsc.200505379 |
| 36. | Nakajima, M.; Saito, M.; Hashimoto, S. Tetrahedron: Asymmetry 2002, 13, 2449–2452. doi:10.1016/s0957-4166(02)00640-7 |
| 46. | Xu, C.; Nader, P.; Xavier, J.; Takenaka, N. Synlett 2023, 34, 2461–2464. doi:10.1055/s-0042-1751478 |
| 46. | Xu, C.; Nader, P.; Xavier, J.; Takenaka, N. Synlett 2023, 34, 2461–2464. doi:10.1055/s-0042-1751478 |
© 2024 Brako et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.